A Novel Chitosan Nanosponge as a Vehicle for Transepidermal Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
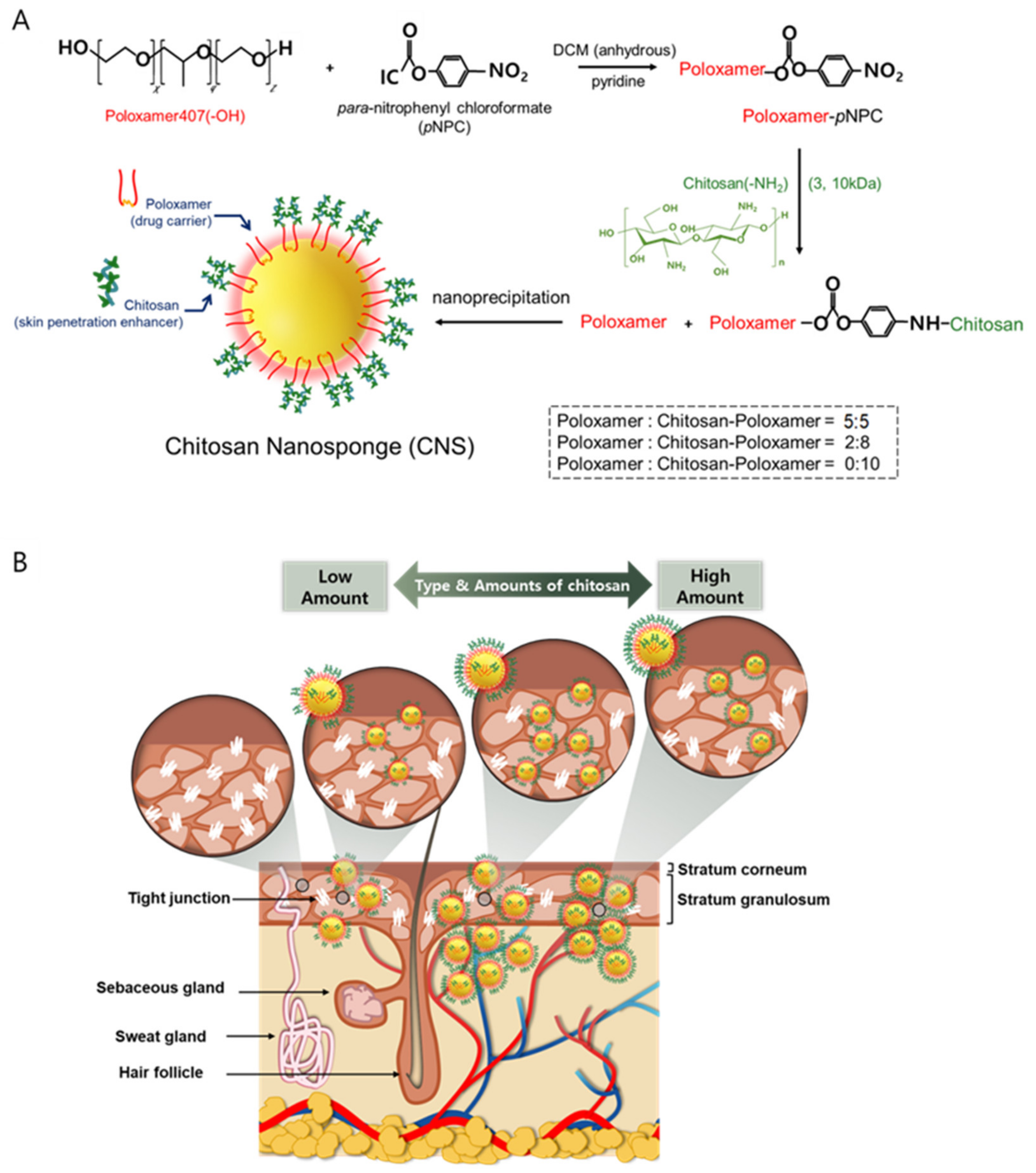
2.2. Preparation of Chitosan-Conjugated Poloxamers
2.3. Production of the CNS
2.4. Ninhydrin Assay
2.5. CNS Stability Analysis
2.6. In Vitro Cytotoxicity Test
2.7. Skin Permeation of Drugs through CNSs
3. Results and Discussion
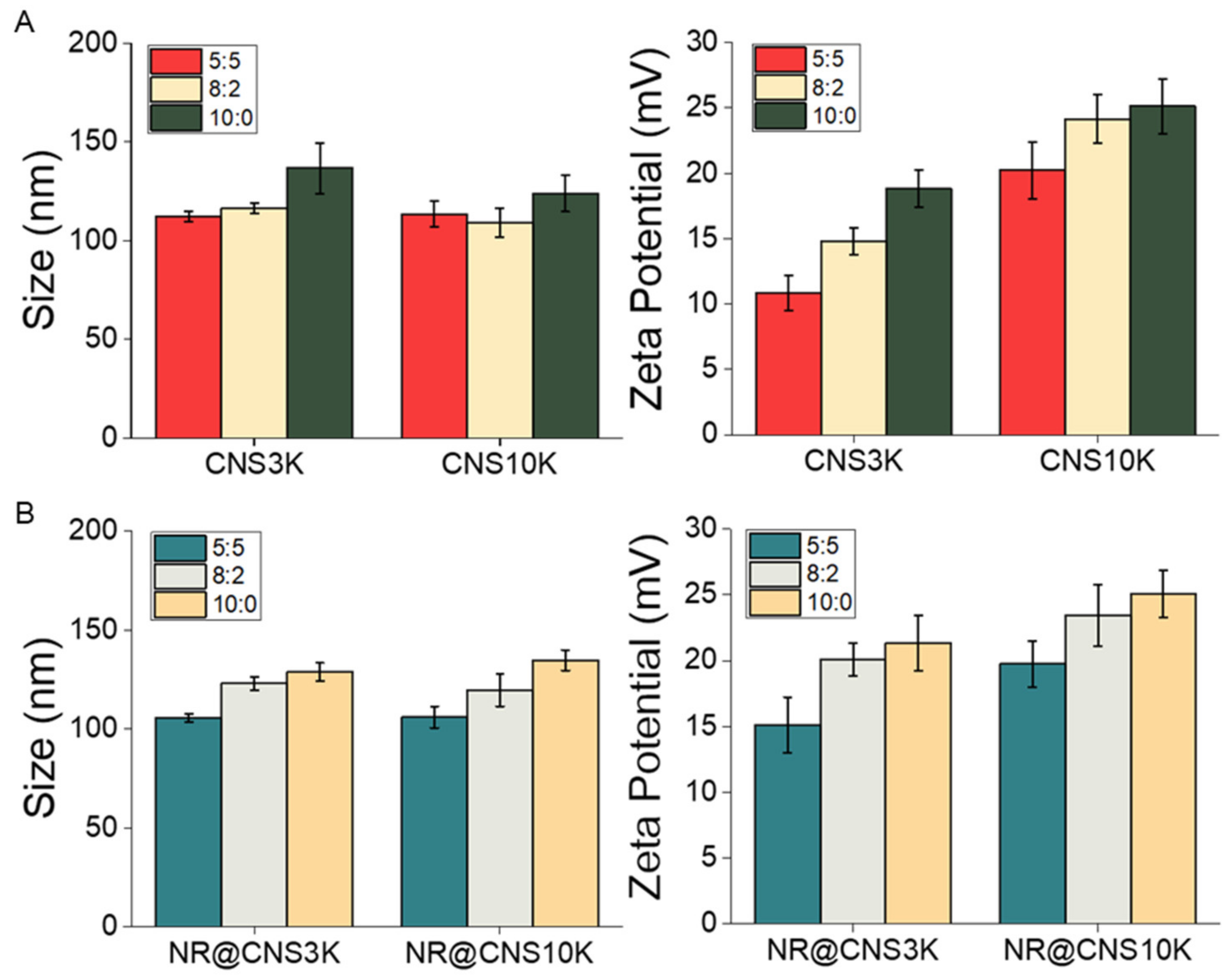
3.1. Preparation and Physicochemical Characteristics of CNSs
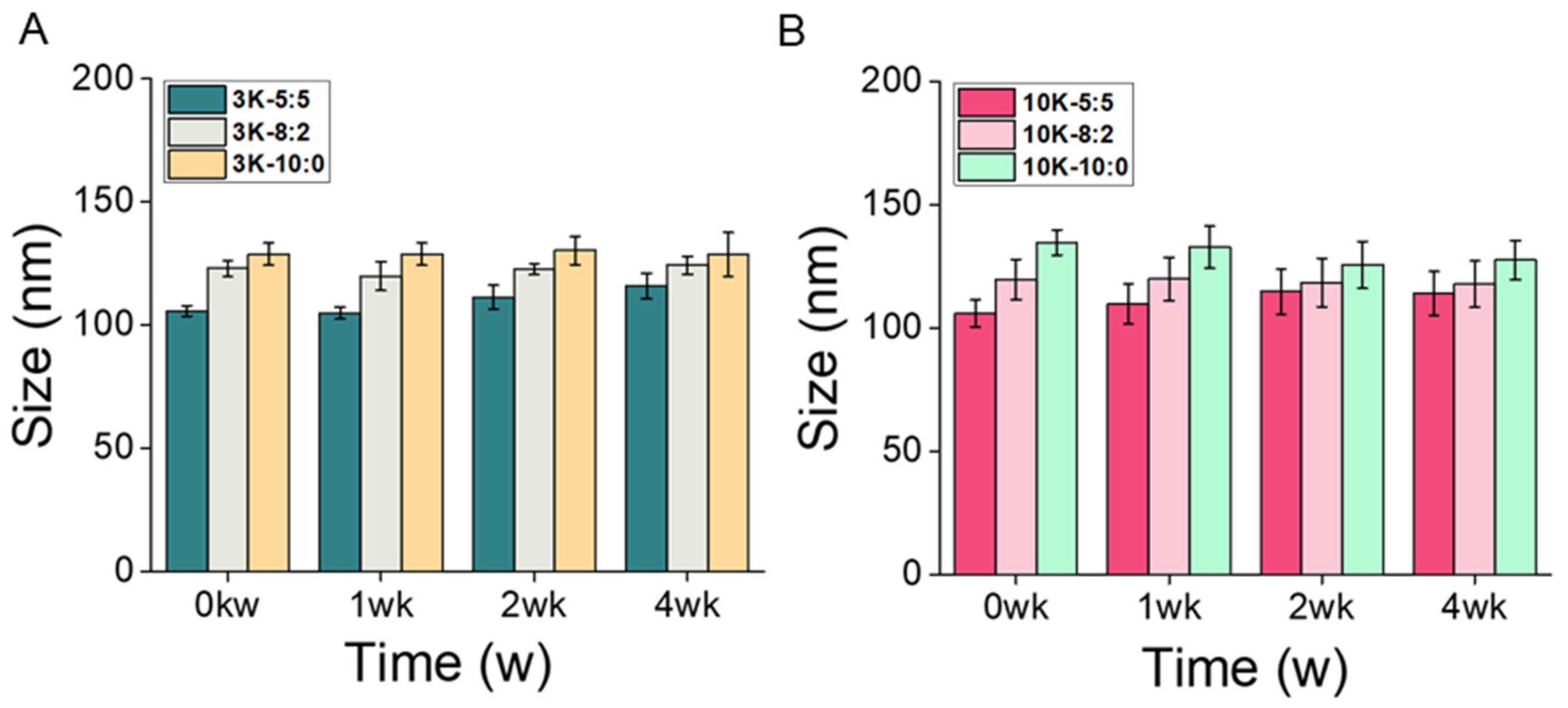
3.2. CNS Stability
3.3. In Vitro Cytotoxicity of CNS
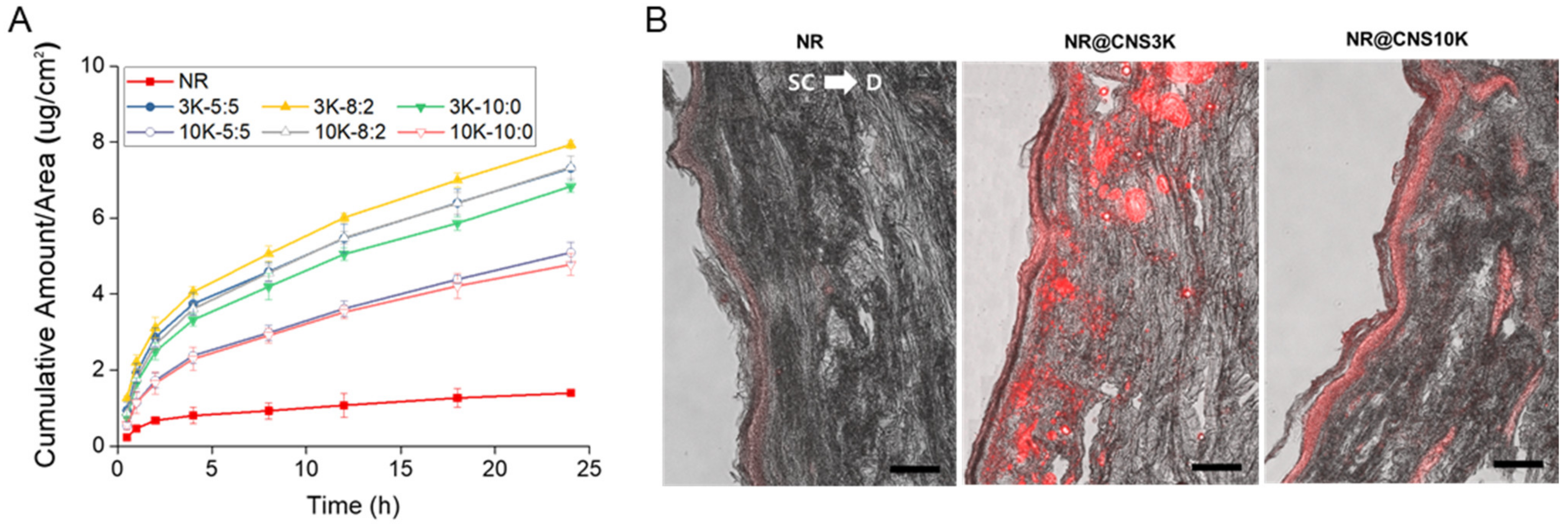
3.4. In Vitro Skin Permeation of NR through CNSs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jain, S.; Jain, P.; Umamaheshwari, R.B.; Jain, N.K. Transfersomes—A novel vesicular carrier for enhanced transdermal delivery: Development, characterization, and performance evaluation. Drug Dev. Ind. Pharm. 2003, 29, 1013–1026. [Google Scholar] [CrossRef]
- Panchognula, R. Transdermal delivery of drugs. Ind. J. Pharmacol. 1997, 140, 140–156. [Google Scholar]
- Gaikwad, A.K. Transdermal drug delivery system: Formulation aspects and evaluation. Compr. J. Pharm. Sci. 2013, 1, 1–10. [Google Scholar]
- Talib, S.; Ahmed, N.; Khan, D.; Khan, G.M.; Rehman, A. Chitosan-chondroitin based artemether loaded nanoparticles for transdermal drug delivery system. J. Drug. Deliv. Sci. Technol. 2021, 61, 102281. [Google Scholar] [CrossRef]
- Zhou, X.; Hao, Y.; Yuan, L.; Pradhan, S.; Shrestha, K.; Pradhan, O.; Liu, H.; Li, W. Nano-formulations for transdermal drug delivery: A review. Chin. Chem. Lett. 2018, 29, 1713–1724. [Google Scholar] [CrossRef]
- Iqbal, B.; Ali, J.; Baboota, S. Recent advances and development in epidermal and dermal drug deposition enhancement technology. Int. J. Dermatol. 2018, 57, 646–660. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.; Jose, S.; Mukund, V.P.B.; Vasudevan, D.T. Transferosomes—A vesicular transdermal delivery system for enhanced drug permeation. J. Adv. Pharm. Technol. Res. 2011, 2, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Knepp, V.M.; Szoka, F.C., Jr.; Guy, R.H. Controlled drug release from a novel liposomal delivery system. II. Transdermal delivery characteristics. J. Control. Release 1990, 12, 25–30. [Google Scholar] [CrossRef]
- Lin, H.; Xie, Q.; Huang, X.; Ban, J.; Wang, B.; Wei, X.; Chen, Y.; Lu, Z. Increased skin permeation efficiency of imperatorin via charged ultradeformable lipid vesicles for transdermal delivery. Int. J. Nanomed. 2018, 13, 831–842. [Google Scholar] [CrossRef] [Green Version]
- Ahad, A.; Al-Saleh, A.A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I.; Raish, M.; Yassin, A.E.B.; Alam, M.A. Formulation and characterization of novel soft nanovesicles for enhanced transdermal delivery of eprosartan mesylate. Saudi Pharm. J. 2017, 25, 1040–1046. [Google Scholar] [CrossRef]
- Takeuchi, I.; Kagawa, A.; Makino, K. Skin permeability and transdermal delivery route of 30-nm cyclosporin A-loaded nanoparticles using PLGA-PEG-PLGA triblock copolymer. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124866. [Google Scholar] [CrossRef]
- Prausnitz, M.R. A practical assessment of transdermal drug delivery by skin electroporation. Adv. Drug Deliv. Rev. 1999, 35, 61–76. [Google Scholar] [CrossRef]
- Nawaz, A.; Wong, T.W. Microwave as skin permeation enhancer for transdermal drug delivery of chitosan-5-fluorouracil nanoparticles. Carbohydr. Polym. 2017, 157, 906–919. [Google Scholar] [CrossRef]
- Wiedersberg, S.; Guy, R.H. Transdermal drug delivery: 30+ years of war and still fighting! J. Control. Release 2014, 190, 150–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musazzi, U.M.; Santini, B.; Selmin, F.; Marini, V.; Corsi, F.; Allevi, R.; Ferretti, A.M.; Prosperi, D.; Cilurzo, F.; Colombo, M.; et al. Impact of semi-solid formulations on skin penetration of iron oxide nanoparticles. J. Nanobiotechnol. 2017, 14, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathur, V.; Satrawala, Y.; Rajput, M.S. Physical and chemical penetration enhancers in transdermal drug delivery system. Asian J. Pharm. 2010, 4, 173. [Google Scholar] [CrossRef]
- Magnusson, B.M.; Walters, K.A.; Roberts, M.S. Veterinary drug delivery: Potential for skin penetration enhancement. Adv. Drug Deliv. Rev. 2001, 50, 205–227. [Google Scholar] [CrossRef]
- Zsikó, S.; Csányi, E.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Berkó, S. Methods to evaluate skin penetration in vitro. Sci. Pharm. 2019, 87, 19. [Google Scholar] [CrossRef] [Green Version]
- Lončarević, A.; Ivanković, M.; Rogina, A. Lysozyme-Induced Degradation of Chitosan: The Characterisation of Degraded Chitosan Scaffold. J. Tissue Repair Regen. 2017, 19, 177. [Google Scholar] [CrossRef] [Green Version]
- Han, T.; Nwe, N.; Furuike, T.; Tokura, S.; Tamura, H. Methods of N-acetylated chitosan scaffolds and its In-vitro biodegradation by lysozyme. J. Biomed. Eng. 2012, 5, 15–23. [Google Scholar]
- Petzold, G.; Gianelli, M.P.; Bugueño, G.; Celan, R.; Pavez, C.; Orellana, P. Encapsulation of liquid smoke flavoring in ca-alginate and ca-alginate-chitosan beads. J. Food Sci. Technol. 2014, 51, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Hwang, Y.; Oh, H.; Kim, S.; Kim, J.-H.; Lee, J.-H.; Shin, Y.C.; Tae, G.; Choi, W.I. A novel chitosan nanocapsule for enhanced skin penetration of cyclosporin A and effective hair growth in vivo. Nano Res. 2019, 12, 3024–3030. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolytecomplexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef] [PubMed]
- El Menshawe, S.F.E.; Aboud, H.M.; Elkomy, M.H.; Kharshoum, R.M.; Abdeltwab, A.M. A novel nanogel loaded with chitosan decorated bilosomes for transdermal delivery of terbutaline sulfate: Artificial neural network optimization, in vitro characterization and in vivo evaluation. Drug Deliv. Transl. Res. 2020, 10, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Al-Kassas, R.; Wen, J.; Cheng, A.E.M.; Kim, A.M.J.; Liu, S.S.M.; Yu, J. Transdermal delivery of propranolol hydrochloride through chitosan nanoparticles dispersed in mucoadhesive gel. Carbohydr. Polym. 2016, 153, 176–186. [Google Scholar] [CrossRef]
- Abdel-Hafez, S.M.; Hathout, R.M.; Sammour, O.A. Tracking the transdermal penetration pathways of optimized curcumin-loaded chitosan nanoparticles via confocal laser scanning microscopy. Int. J. Biol. Macromol. 2018, 108, 753–764. [Google Scholar] [CrossRef]
- Nair, S.S. Chitosan-based transdermal drug delivery systems to overcome skin barrier functions. J. Drug Deliv. Ther. 2019, 9, 266–270. [Google Scholar] [CrossRef] [Green Version]
- Hasanovic, A.; Zehl, M.; Reznicek, G.; Valenta, C. Chitosan-tripolyphosphate nanoparticles as a possible skin drug delivery system for aciclovir with enhanced stability. J. Pharm. Pharmacol. 2009, 61, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Lim, S.J.; Lee, M.K. Chitosan-coated liposomes to stabilize and enhance transdermal delivery of indocyanine green for photodynamic therapy of melanoma. Carbohydr. Polym. 2019, 224, 115–143. [Google Scholar] [CrossRef]
- Takeuchi, I.; Takeshita, T.; Suzuki, T.; Makino, K. Iontophoretic transdermal delivery using chitosan-coated PLGA nanoparticles for positively charged drugs. Colloids Surf. B Biointerfaces 2017, 160, 520–526. [Google Scholar] [CrossRef]
- Chung, Y.I.; Kim, J.C.; Kim, Y.H.; Tae, G.; Lee, S.Y.; Kim, K.; Kwon, I.C. The effect of surface functionalization of PLGA nanoparticles by heparin- or chitosan-conjugated pluronic on tumor targeting. J. Control. Release 2010, 143, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Choi, W.I.; Kim, Y.H.; Tae, G.; Lee, S.Y.; Kim, K.; Kwon, I.C. In-vivo tumor targeting of pluronic-based nano-carriers. J. Control. Release 2010, 147, 109–117. [Google Scholar] [CrossRef]
- Sun, S.W.; Lin, Y.C.; Weng, Y.M.; Chen, M.J. Efficiency improvements on Ninhydrin method for amino acid quantification. J. Food Compos. Anal. 2006, 19, 112–117. [Google Scholar] [CrossRef]
- Khan, Z. Chitosan capped Au@Pd@Ag trimetallic nanoparticles: Synthesis, stability, capping action and adsorbing activities. Int. J. Biol. Macromol. 2020, 153, 545–560. [Google Scholar] [CrossRef] [PubMed]
- MacCuspie, R.I. Colloidal stability of silver nanoparticles in biologically relevant conditions. J. Nanopart. Res. 2011, 13, 2893–2908. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, D.Y.; Kim, J.I.; Kin, P.Y. In vitro cytotoxicity tests on cultured human skin fibroblasts to predict skin irritation potential of surfactants. Toxicol. Vitro 2000, 14, 345–349. [Google Scholar] [CrossRef]
- Choi, W.I.; Lee, J.H.; Kim, J.Y.; Kim, J.C.; Kim, Y.H.; Tae, G. Efficient skin permeation of soluble protein via flexible and functional nano-carrier. J. Control. Release 2012, 157, 272–278. [Google Scholar] [CrossRef]
- Ing, L.Y.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int. J. Biomater. 2012, 2012, 632698. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, E.; Brandtzae, O.K.; Vehus, T.; Roberg-Larsen, H.; Bogoeva, V.; Ademi, O.; Hildahl, J.; Lundanes, E.; Wilson, S.R. A critical evaluation of Amicon Ultra centrifugal filters for separating proteins, drugs and nanoparticles in biosamples. J. Pharm. Biomed. Anal. 2016, 120, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.S.; Ramos, I.I.; Fernandes, S.R.; Barreiros, L.; Lima, S.A.C.; Reis, S.; Domingues, M.R.M.; Segundo, M.A. Insights on Ultrafiltration-Based Separation for the Purification and Quantification of Methotrexate in Nanocarriers. Molecules 2020, 25, 1879. [Google Scholar] [CrossRef] [Green Version]
- Araujo, H.C.; Silva, A.C.G.; Paião, L.I.; Magario, M.K.W.; Frasnelli, S.C.T.; Oliveira, S.H.P.; Pessan, J.P.; Monteiro, D.R. Antimicrobial, antibiofilm and cytotoxic effects of a colloidal nanocarrier composed by chitosan-coated iron oxide nanoparticles loaded with chlorhexidine. J. Dent. 2020, 101, 103453. [Google Scholar] [CrossRef]
- Contri, R.V.; Fiel, L.A.; Alnasif, N.; Pohlmann, A.R.; Guterres, S.S.; Schäfer-Korting, M. Skin penetration and dermal tolerability of acrylic nanocapsules: Influence of the surface charge and a chitosan gel used as vehicle. Int. J. Pharm. 2016, 507, 12–20. [Google Scholar] [CrossRef]
- Zhang, N.; Said, A.; Wischke, C.; Kral, V.; Brodwolf, R.; Volz, P.; Boreham, A.; Gerecke, C.; Li, W.; Neffe, A.T.; et al. Poly[acrylonitrile-co-(N-vinyl pyrrolidone)] nanoparticles–Composition-dependent skin penetration enhancement of a dye probe and biocompatibility. Eur. J. Pharm. Biopharm. 2017, 116, 66–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Liu, D.; Liu, H.; Yang, Q.; Yao, K.; Wang, X.; Wang, L.; Yang, X. Effect of Low Molecular Weight Chitosans on Drug Permeation through Mouse Skin: 1. Transdermal Delivery of Baicalin. J. Pharm. Sci. 2010, 99, 2991–2998. [Google Scholar] [CrossRef] [PubMed]
- Ogiso, T.; Yamaguchi, T.; Iwaki, M.; Tanino, T.; Miyake, Y. Effect of positively and negatively charged liposomes on skin permeation of drugs. J. Drug Target. 2001, 9, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Yuan, M.; Chen, C.; Wang, L.; Tang, Z.; Fan, Y.; Liu, X.; Ma, Y.; Gan, Y. Berberine-Loaded Thiolated Pluronic F127 Polymeric Micelles for Improving Skin Permeation and Retention. Int. J. Nanomed. 2020, 15, 9987–10005. [Google Scholar] [CrossRef]


| Types of CNS | Amount of Chitosan (wt.%) | ||
|---|---|---|---|
| 5:5 | 8:2 | 10:0 | |
| CNS3K | 15.1 ± 2.7 | 24.3 ± 3.7 | 31.3 ± 2.1 |
| CNS10K | 29.5 ± 2.2 | 47.4 ± 2.9 | 59.4 ± 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.S.; Oh, H.; Kim, S.; Lee, J.-H.; Shin, Y.C.; Choi, W.I. A Novel Chitosan Nanosponge as a Vehicle for Transepidermal Drug Delivery. Pharmaceutics 2021, 13, 1329. https://doi.org/10.3390/pharmaceutics13091329
Lee JS, Oh H, Kim S, Lee J-H, Shin YC, Choi WI. A Novel Chitosan Nanosponge as a Vehicle for Transepidermal Drug Delivery. Pharmaceutics. 2021; 13(9):1329. https://doi.org/10.3390/pharmaceutics13091329
Chicago/Turabian StyleLee, Jin Sil, Hyeryeon Oh, Sunghyun Kim, Jeung-Hoon Lee, Yong Chul Shin, and Won Il Choi. 2021. "A Novel Chitosan Nanosponge as a Vehicle for Transepidermal Drug Delivery" Pharmaceutics 13, no. 9: 1329. https://doi.org/10.3390/pharmaceutics13091329
APA StyleLee, J. S., Oh, H., Kim, S., Lee, J.-H., Shin, Y. C., & Choi, W. I. (2021). A Novel Chitosan Nanosponge as a Vehicle for Transepidermal Drug Delivery. Pharmaceutics, 13(9), 1329. https://doi.org/10.3390/pharmaceutics13091329





