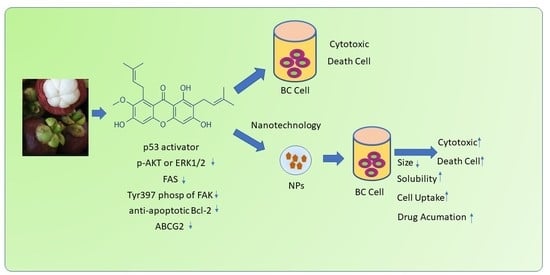
α-Mangostin Nanoparticles Cytotoxicity and Cell Death Modalities in Breast Cancer Cell Lines
Abstract
1. Introduction
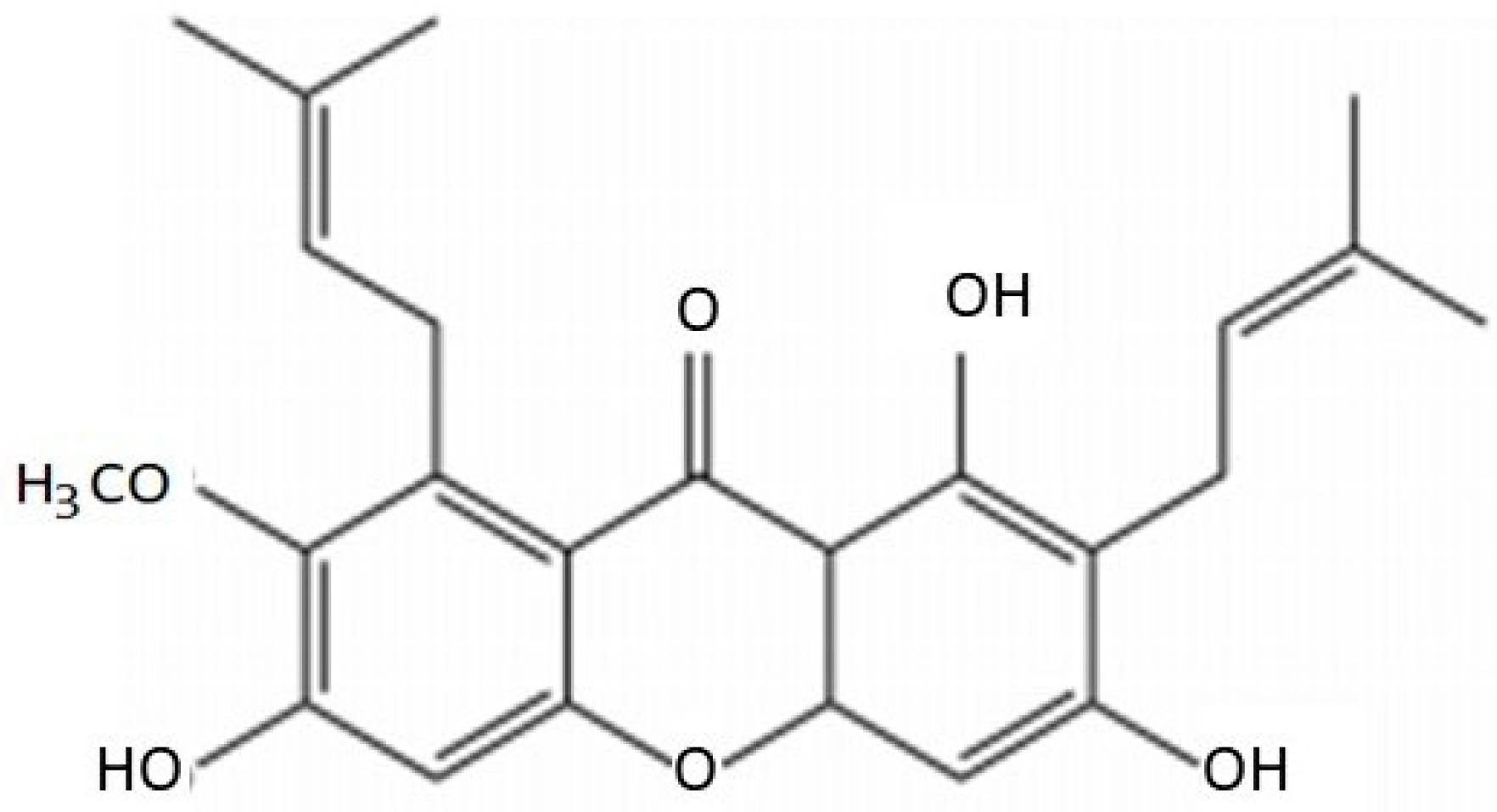
2. Chemical Characteristics of AMG
3. The Activity of AMG in Breast Cancer
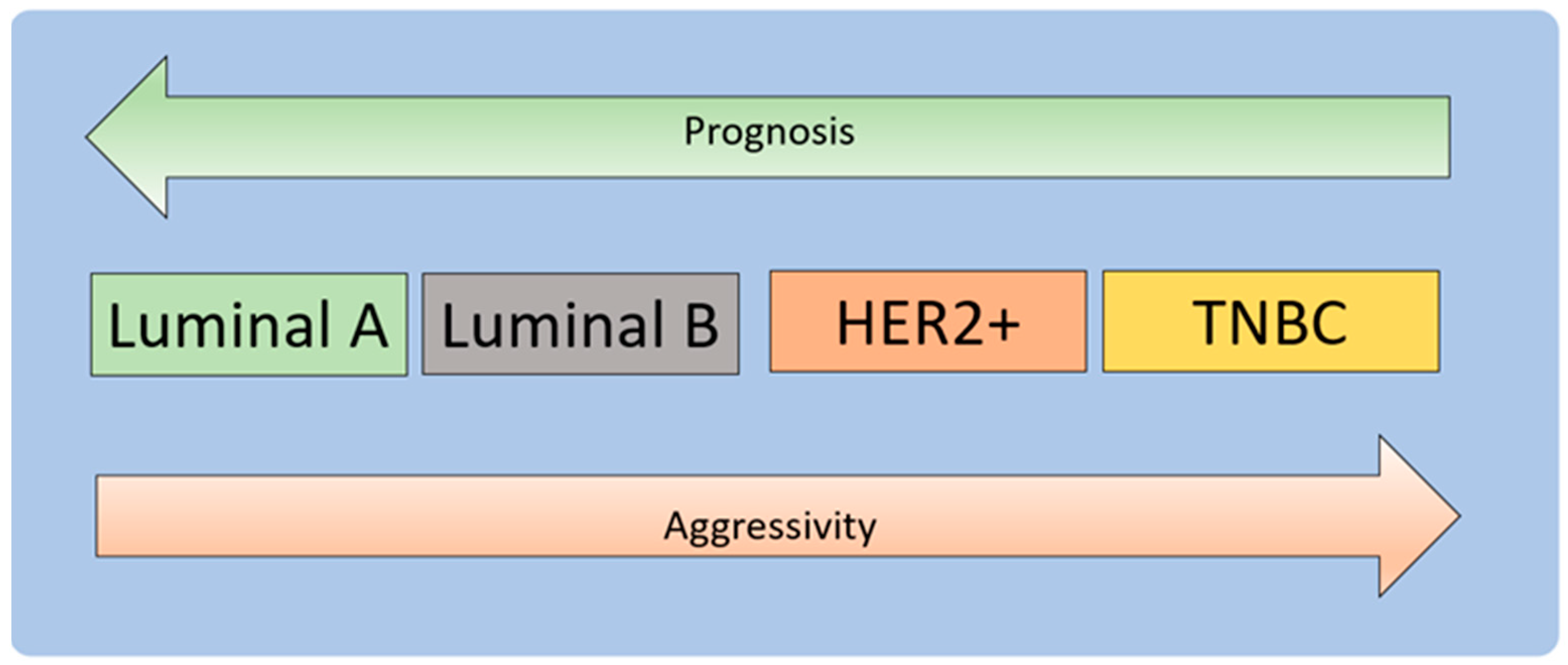
3.1. Molecular Development of Breast Cancer
3.2. Mechanisms of Molecules Drug Resistance (MDR)
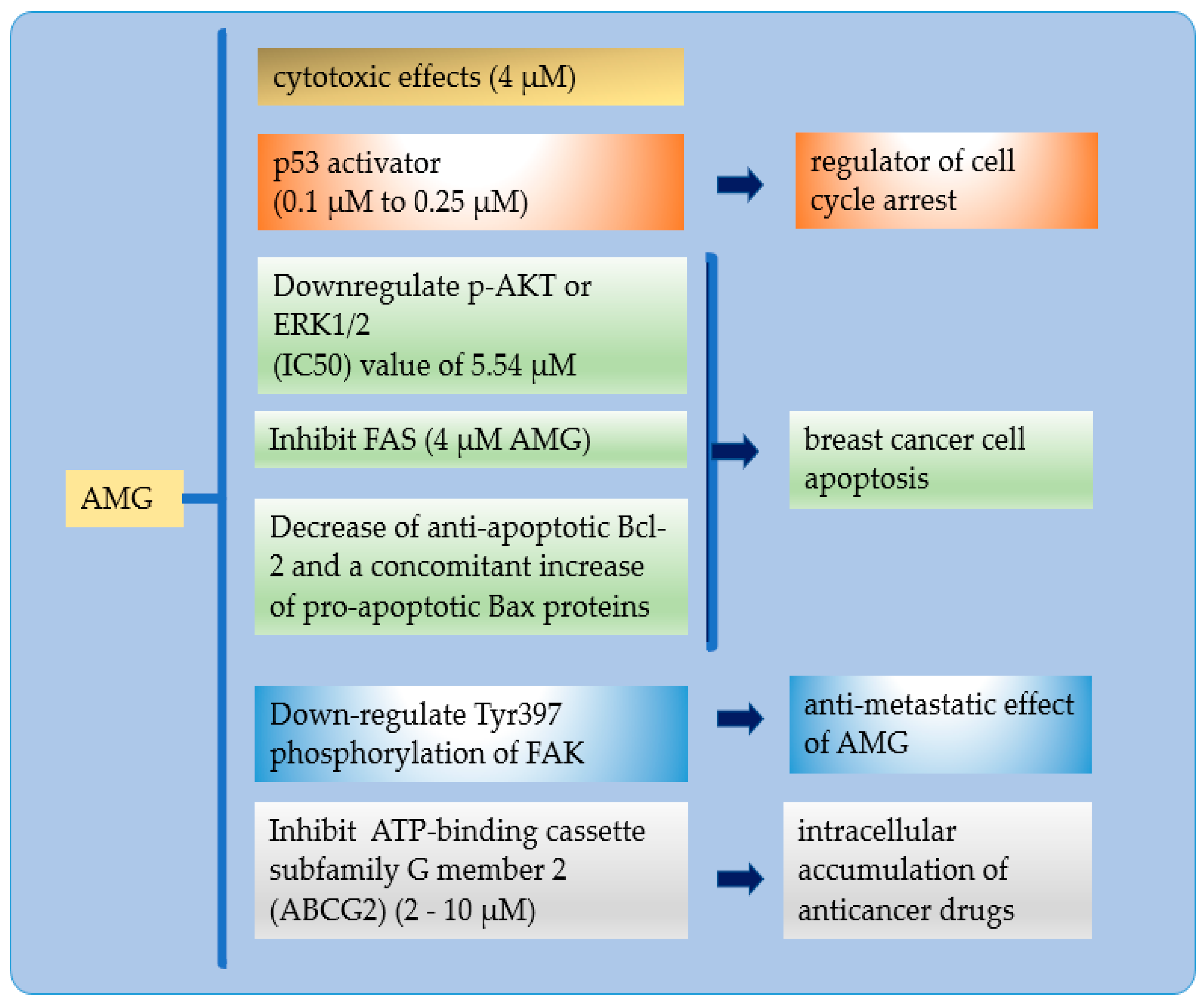
3.3. Cytotoxicity
3.4. Mechanisms of Cell Death
4. Molecular Cell Death Mechanisms of AMG in Cancer Cells
4.1. In Vitro Effect of AMG in Breast Cancer
4.2. In Vivo Effect of AMG in Breast Cancer
5. Nanotechnology of AMG in Breast Cancer
6. Perspective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ziai, J.; Gilbert, H.N.; Foreman, O.; Eastham-Anderson, J.; Chu, F.; Huseni, M.; Kim, J.M. CD8+ T cell infiltration in breast and colon cancer: A histologic and statistical analysis. PLoS ONE 2018, 13, e0190158. [Google Scholar] [CrossRef]
- Van Oost, V. Study of alpha mangostin as a chemoprotective agent for breast cancer via activation of the p53 pathway. Elaia 2018, 2, 16. [Google Scholar]
- Shan, T.; Ma, Q.; Guo, K.; Liu, J.; Li, W.; Wang, F.; Wu, E. Xanthones from Mangosteen Extracts as Natural Chemopreventive Agents: Potential Anticancer Drugs. Curr. Mol. Med. 2011, 11, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Baktiar Laskar, Y.; Meitei Lourembam, R.; Behari Mazumder, P. Herbal Remedies for Breast Cancer Prevention and Treatment. Med. Plants - Use Prev. Treat. Dis. 2020, 1–22. [Google Scholar] [CrossRef]
- Ullah, I.; Khalil, A.T.; Ali, M.; Iqbal, J.; Ali, W.; Alarifi, S.; Shinwari, Z.K. Green-Synthesized Silver Nanoparticles Induced Apoptotic Cell Death in MCF-7 Breast Cancer Cells by Generating Reactive Oxygen Species and Activating Caspase 3 and 9 Enzyme Activities. Oxid. Med. Cell. Longev. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Park, I.S.; Cho, J.H.; Han, Y.; Lee, K.W.; Song, Y.S. Targeting Cancer Stem Cells with Phytoceuticals for Cancer Therapy; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128161517. [Google Scholar]
- Bonafè, F.; Pazzini, C.; Marchionni, S.; Guarnieri, C.; Muscari, C. Complete Disaggregation of MCF-7-derived Breast Tumour Spheroids with Very Low Concentrations of α -Mangostin Loaded in CD44 Thioaptamer-tagged Nanoparticles. Int. J. Med. Sci. 2019, 16, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.J.; Gu, Q.L.; Yang, K.; Ming, X.J.; Wang, J.X. Anticarcinogenic Effects of α -Mangostin: A Review. Planta Med. 2017, 83, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Scolamiero, G.; Pazzini, C.; Bonafè, F.; Guarnieri, C.; Muscari, C. Effects of α-mangostin on viability, growth and cohesion of multicellular spheroids derived from human breast cancer cell lines. Int. J. Med. Sci. 2018, 15, 23–30. [Google Scholar] [CrossRef]
- Chen, G.; Li, Y.; Wang, W.; Deng, L. Bioactivity and pharmacological properties of α-mangostin from the mangosteen fruit: A review. Expert Opin. Ther. Pat. 2018, 28, 415–427. [Google Scholar] [CrossRef]
- Minute, L.; Teijeira, A.; Sanchez-Paulete, A.R.; Ochoa, M.C.; Alvarez, M.; Otano, I.; Etxeberrria, I.; Bolaños, E.; Azpilikueta, A.; Garasa, S.; et al. Cellular cytotoxicity is a form of immunogenic cell death. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Sánchez, M.L.; Sandoval-Ramírez, J.; Sánchez-Sánchez, L. Steroidal Saponins and Cell Death in Cancer Chapter. In Cell Death—Autophagy, Apoptosis and Necrosis; INTECH: London, UK, 2015; Volume 32, pp. 331–352. [Google Scholar]
- Ahmad, J.; Wahab, R.; Siddiqui, M.A.; Saquib, Q.; Al-Khedhairy, A.A. Cytotoxicity and cell death induced by engineered nanostructures (quantum dots and nanoparticles) in human cell lines. J. Biol. Inorg. Chem. 2020, 25, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Du, X.; Wang, Q.; Liu, J.; Zhang, E.; Sai, L.; Peng, C.; Lavin, M.F.; Yeo, A.J.; Yang, X.; et al. Mechanism of cell death induced by silica nanoparticles in hepatocyte cells is by apoptosis. Int. J. Mol. Med. 2019, 44, 903–912. [Google Scholar] [CrossRef]
- Ke, X.; Shen, L. Molecular targeted therapy of cancer: The progress and future prospect. Front. Lab. Med. 2017, 1, 69–75. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Muchtaridi, M.; Wijaya, C.A. Anticancer potential of α-mangostin. Asian J. Pharm. Clin. Res. 2017, 10, 440–445. [Google Scholar] [CrossRef]
- Klein-Júnior, L.C.; Campos, A.; Niero, R.; Corrêa, R.; Vander Heyden, Y.; Filho, V.C. Xanthones and Cancer: From Natural Sources to Mechanisms of Action. Chem. Biodivers. 2020, 17. [Google Scholar] [CrossRef]
- Ryu, H.W.; Curtis-Long, M.J.; Jung, S.; Jin, Y.M.; Cho, J.K.; Ryu, Y.B.; Lee, W.S.; Park, K.H. Xanthones with neuraminidase inhibitory activity from the seedcases of Garcinia mangostana. Bioorganic Med. Chem. 2010, 18, 6258–6264. [Google Scholar] [CrossRef] [PubMed]
- Asasutjarit, R.; Meesomboon, T.; Adulheem, P.; Kittiwisut, S.; Sookdee, P.; Samosornsuk, W.; Fuongfuchat, A. Physicochemical properties of alpha-mangostin loaded nanomeulsions prepared by ultrasonication technique. Heliyon 2019, 5, e02465. [Google Scholar] [CrossRef]
- Pérez-Soto, E.; Estanislao-Gómez, C.C.; Pérez-Ishiwara, D.G.; Ramirez-Celis, C.; Gómez-García, M.d.C. Cytotoxic Effect and Mechanisms from Some Plant-Derived Compounds in Breast Cancer. In Cytotoxicity—Definition, Identification, and Cytotoxic Compounds; IntechOpen: London, UK, 2019; pp. 1–20. [Google Scholar]
- Demïrel, M.A.; Süntar, İ. The Role of Secondary Metabolites on Gynecologic Cancer Therapy: Some Pathways and Mechanisms. Turkish J. Pharm. Sci. 2017, 14, 324–334. [Google Scholar] [CrossRef]
- Shin, S.A.; Moon, S.Y.; Kim, W.Y.; Paek, S.M.; Park, H.H.; Lee, C.S. Structure-Based Classification and Anti-Cancer Effects of Plant Metabolites. Int. J. Mol. Sci. 2018, 19, 2651. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Kumari, A.; Rahman, N.; Mandave, P. Anticancer Activities of Plant Secondary Metabolites: Rice Callus Suspension Culture as a New Paradigm. Rice Sci. 2021, 28, 13–30. [Google Scholar] [CrossRef]
- Beutler, J.A. Natural Products as a Foundation for Drug Discovery. Curr. Protoc. Pharmacol. 2019, 86, e67. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, M. The Success of Natural Products in Drug Discovery. Pharmacol Pharm. 2013, 04, 17–31. [Google Scholar] [CrossRef]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef] [PubMed]
- Marzaimi, I.N.; Aizat, W.M. Current Review on Mangosteen Usages in Antiinflammation and Other Related Disorders, 2nd ed.; Elsevier Inc: Amsterdam, The Netherlands, 2019; ISBN 9780128138205. [Google Scholar]
- Helm, K.; Beyreis, M.; Mayr, C.; Ritter, M.; Jakab, M.; Kiesslich, T.; Plaetzer, K. In Vitro Cell Death Discrimination and Screening Method by Simple and Cost-Effective Viability Analysis. Cell. Physiol. Biochem. 2017, 41, 1011–1019. [Google Scholar] [CrossRef]
- Lang, G.T.; Jiang, Y.Z.; Shi, J.X.; Yang, F.; Li, X.G.; Pei, Y.C.; Zhang, C.H.; Ma, D.; Xiao, Y.; Hu, P.C.; et al. Characterization of the genomic landscape and actionable mutations in Chinese breast cancers by clinical sequencing. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Zubair, M.; Wang, S.; Ali, N. Advanced Approaches to Breast Cancer Classification and Diagnosis. Front. Pharmacol. 2021, 11, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, S.; Aarnoutse, R.; Ziemons, J.; Kooreman, L.; Boleij, A.; Smidt, M. Exploring the Potential of Breast Microbiota as Biomarker for Breast Cancer and Therapeutic Response. Am. J. Pathol. 2021, 191, 968–982. [Google Scholar] [CrossRef]
- Szymiczek, A.; Lone, A.; Akbari, M.R. Molecular intrinsic versus clinical subtyping in breast cancer: A comprehensive review. Clin. Genet. 2021, 99, 613–637. [Google Scholar] [CrossRef]
- Amirkhani Namagerdi, A.; d’Angelo, D.; Ciani, F.; Iannuzzi, C.A.; Napolitano, F.; Avallone, L.; De Laurentiis, M.; Giordano, A. Triple-Negative Breast Cancer Comparison with Canine Mammary Tumors from Light Microscopy to Molecular Pathology. Front. Oncol. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Dai, X.; Li, T.; Bai, Z.; Yang, Y.; Liu, X.; Zhan, J.; Shi, B. Breast cancer intrinsic subtype classification, clinical use and future trends. Am. J. Cancer Res. 2015, 5, 2929–2943. [Google Scholar]
- Zhang, M.H.; Man, H.T.; Zhao, X.D.; Dong, N.; Ma, S.L. Estrogen receptor-positive breast cancer molecular signatures and therapeutic potentials (Review). Biomed. Reports 2014, 2, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Matutino, A.; Joy, A.A.; Brezden-Masley, C.; Chia, S.; Verma, S. Hormone receptor-positive, HER2-negative metastatic breast cancer: Redrawing the lines. Curr. Oncol. 2018, 25, S131–S141. [Google Scholar] [CrossRef]
- Sadeghi, F.; Asgari, M.; Matloubi, M.; Ranjbar, M.; Karkhaneh Yousefi, N.; Azari, T.; Zaki-Dizaji, M. Molecular contribution of BRCA1 and BRCA2 to genome instability in breast cancer patients: Review of radiosensitivity assays. Biol. Proced. Online 2020, 22, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Gorodetska, I.; Kozeretska, I.; Dubrovska, A. BRCA genes: The role in genome stability, cancer stemness and therapy resistance. J. Cancer 2019, 10, 2109–2127. [Google Scholar] [CrossRef] [PubMed]
- Hedau, S.; Batra, M.; Singh, U.R.; Bharti, A.C.; Ray, A.; Das, B.C. Expression of BRCA1 and BRCA2 proteins and their correlation with clinical staging in breast cancer. J. Cancer Res. Ther. 2015, 11, 158–163. [Google Scholar] [CrossRef]
- Blandino, G.; Di Agostino, S. New therapeutic strategies to treat human cancers expressing mutant p53 proteins. J. Exp. Clin. Cancer Res. 2018, 37, 1–13. [Google Scholar] [CrossRef]
- Zilfou, J.T.; Lowe, S.W. Tumor suppressive functions of p53. Cold Spring Harb. Perspect. Biol. 2009, 1, 1–12. [Google Scholar] [CrossRef]
- Tamaki, T.; Naomoto, Y.; Kimura, S.; Kawashima, R.; Shirakawa, Y.; Shigemitsu, K.; Yamatsuji, T.; Haisa, M.; Gunduz, M.; Tanaka, N. Apoptosis in normal tissues induced by anti-cancer drugs. J. Int. Med. Res. 2003, 31, 6–16. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y. Targeting p53 for novel anticancer therapy. Transl. Oncol. 2010, 3, 1–12. [Google Scholar] [CrossRef]
- Chang, P.Y.; Peng, S.F.; Lee, C.Y.; Lu, C.C.; Tsai, S.C.; Shieh, T.M.; Wu, T.S.; Tu, M.G.; Chen, M.Y.; Yang, J.S. Curcumin-loaded nanoparticles induce apoptotic cell death through regulation of the function of MDR1 and reactive oxygen species in cisplatin-resistant CAR human oral cancer cells. Int. J. Oncol. 2013, 43, 1141–1150. [Google Scholar] [CrossRef]
- Macus, T.K.; Liu, Z.; Wei, Y.; Lin-Lee, Y.C.; Tatebe, S.; Mills, G.B.; Unate, H. Induction of human MDR1 gene expression by 2-acetylaminofluorene is mediated by effectors of the phosphoinositide 3-kinase pathway that activate NF-κB signaling. Oncogene 2002, 21, 1945–1954. [Google Scholar] [CrossRef]
- Robinson, K.; Tiriveedhi, V. Perplexing Role of P-Glycoprotein in Tumor Microenvironment. Front. Oncol. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Li, P. Advances in chitosan-based nanoparticles for oncotherapy. Carbohydr. Polym. 2019, 222, 115004. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.P.; Hsiao, S.H.; Murakami, M.; Lu, Y.J.; Li, Y.Q.; Huang, Y.H.; Hung, T.H.; Ambudkar, S.V.; Wu, Y.S. Alpha-Mangostin Reverses Multidrug Resistance by Attenuating the Function of the Multidrug Resistance-Linked ABCG2 Transporter. Mol. Pharm. 2017, 14, 2805–2814. [Google Scholar] [CrossRef]
- Stojak, M.; Mazur, L.; Opydo-Chanek, M.; Ukawska, M.; Oszczapowicz, I. In vitro induction of apoptosis and necrosis by new derivatives of daunorubicin. Anticancer Res. 2013, 33, 4439–4444. [Google Scholar]
- Kasko, A.M. Polymeric Biomaterials with Engineered Degradation. J. Polym. Sci. Part A 2013, 3531–3566. [Google Scholar] [CrossRef]
- Shahneh, F.Z.; Valiyari, S.; Azadmehr, A.; Hajiaghaee, R.; Yaripour, S.; Bandehagh, A.; Baradaran, B. Inhibition of growth and induction of apoptosis in fibrosarcoma cell lines by echinophora platyloba DC: In vitro analysis. Adv. Pharmacol. Sci. 2013, 2013. [Google Scholar] [CrossRef]
- Anilkumar, T.V.; Sarraf, C.E.; Hunt, T.; Alison, M.R. The nature of cytotoxic drug _ induced cell death in murine intestinal crypts. Br. J. Cancer 1992, 65, 552–558. [Google Scholar] [CrossRef][Green Version]
- Moghadam, S.A.; Rezania, V.; Tuszynski, J.A. Cell death and survival due to cytotoxic exposure modelled as a two-state Ising system. R. Soc. Open Sci. 2020, 7. [Google Scholar] [CrossRef]
- Orrenius, S.; Nicotera, P.; Zhivotovsky, B. Cell death mechanisms and their implications in toxicology. Toxicol. Sci. 2011, 119, 3–19. [Google Scholar] [CrossRef]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application (Review). Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Kumar, N.; Hammerschmid, D.; Privat-Maldonado, A.; Dewilde, S.; Bogaerts, A. Synergistic Effects of Melittin and Plasma Treatment: A Promising Approach for Cancer Therapy. Cancers 2019, 11, 1109. [Google Scholar] [CrossRef]
- Istifli, E.S.; Hüsunet, M.T.; Ila, H.B. Cell Division, Cytotoxicity, and the Assays Used in the Detection of Cytotoxicity. In Cytotoxicity—Definition, Identification, and Cytotoxic Compounds; Istifli, E.S., Ila, H.B., Eds.; IntechOpen: London, UK, 2019; pp. 1–120. ISBN 9781789847543. [Google Scholar]
- Dasari, A.; Choi, J.-S.; Berdis, A.J. Chapter 7—Chemotherapeutic Intervention by Inhibiting DNA Polymerases A2—Kelley, Mark R., 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 978-0-12-803582-5. [Google Scholar]
- Nappi, A.J.; Ottaviani, E. Cytotoxicity and cytotoxic molecules in invertebrates. BioEssays 2000, 22, 469–480. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.Ö.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy 2019, 15, 4–33. [Google Scholar] [CrossRef]
- Lemasters, J.J. Molecular mechanisms of cell death. Mol. Pathol. Mol. Basis Hum. Dis. 2018, 1–24. [Google Scholar] [CrossRef]
- Cogo, F.; Poreba, M.; Rut, W.; Groborz, K.; Smyth, P.; Johnston, M.C.; Williams, R.; Longley, D.B.; Burden, R.E.; Salvesen, G.S.; et al. Development of an advanced nanoformulation for the intracellular delivery of a caspase-3 selective activity-based probe. Nanoscale 2019, 11, 742–751. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; El-Deiry, W.S. Overview of cell death signaling pathways. Cancer Biol. Ther. 2005, 4, 147–171. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Jeong, H.; Yu, S.W. Autophagy as a decisive process for cell death. Exp. Mol. Med. 2020, 52, 921–930. [Google Scholar] [CrossRef]
- Coleman, W.B.; Tsongalis, G.J. Moleculor Pathology; Elsevier Inc.: Amsterdam, Netherlands, 2017. [Google Scholar]
- Ibrahim, M.Y.; Hashim, N.M.; Mariod, A.A.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Arbab, I.A. α-Mangostin from Garcinia mangostana Linn: An updated review of its pharmacological properties. Arab. J. Chem. 2016, 9, 317–329. [Google Scholar] [CrossRef]
- Moongkarndi, P.; Jaisupa, N.; Samer, J.; Kosem, N.; Konlata, J.; Rodpai, E.; Pongpan, N. Comparison of the biological activity of two different isolates from mangosteen. J. Pharm. Pharmacol. 2014, 66, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Atluri, N.; Holur, R.; Thirumalanadhuni, V.; Palempalli, M.D. Modulation of Pro-Inflammatory Genes By α—Mangostin From Garcinia mangostana. Int. J. Pharm. Sci. Invent. 2014, 3, 23–29. [Google Scholar]
- Kurose, H.; Shibata, M.A.; Iinuma, M.; Otsuki, Y. Alterations in cell cycle and induction of apoptotic cell death in breast cancer cells treated with -mangostin extracted from mangosteen pericarp. J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.Y.; Hashim, N.M.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Kamalidehghan, B.; Ghaderian, M.; Dehghan, F.; Ali, L.Z.; Karimian, H.; et al. Involvement of NF-κB and HSP70 signaling pathways in the apoptosis of MDA-MB-231 cells induced by a prenylated xanthone compound, α-mangostin, From Cratoxylum arborescens. Drug Des. Devel. Ther. 2014, 8, 2193–2211. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, Y.B.; Ko, K.C.; Shi, M.D.; Liao, Y.C.; Chiang, T.A.; Wu, P.F.; Shih, Y.X.; Shih, Y.W. α-Mangostin, a novel dietary xanthone, suppresses TPA-mediated MMP-2 and MMP-9 expressions through the erk signaling pathway in mcf-7 human breast adenocarcinoma cells. J. Food Sci. 2010, 75. [Google Scholar] [CrossRef]
- Huang, W.; Liang, Y.; Ma, X. Alpha-mangostin induces endoplasmic reticulum stress and autophagy which count against fatty acid synthase inhibition mediated apoptosis in human breast cancer cells. Cancer Cell Int. 2019, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Tian, W.; Ma, X. Alpha-mangostin inhibits intracellular fatty acid synthase and induces apoptosis in breast cancer cells. Mol. Cancer 2014, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jittiporn, K.; Suwanpradid, J.; Patel, C.; Rojas, M.; Thirawarapan, S.; Moongkarndi, P.; Suvitayavat, W.; Caldwell, R.B. Anti-angiogenic actions of the mangosteen polyphenolic xanthone derivative α-mangostin. Microvasc. Res. 2014, 93, 72–79. [Google Scholar] [CrossRef]
- Li, G.; Thomas, S.; Johnson, J.J. Polyphenols from the mangosteen (Garcinia mangostana) fruit for breast and prostate cancer. Front. Pharmacol. 2013, 4 JUN, 1–4. [Google Scholar] [CrossRef]
- Leão, M.; Gomes, S.; Pedraza-Chaverri, J.; MacHado, N.; Sousa, E.; Pinto, M.; Inga, A.; Pereira, C.; Saraiva, L. α-mangostin and gambogic acid as potential inhibitors of the p53-MDM2 interaction revealed by a yeast approach. J. Nat. Prod. 2013, 76, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.J.; Tait, S.W.G. Targeting BCL-2 regulated apoptosis in cancer. Open Biol. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Naseri, M.H.; Mahdavi, M.; Davoodi, J.; Tackallou, H.S.; Goudarzvand, M.; Neishabouri, S.H. Up regulation of Bax and down regulation of Bcl2 during 3-NC mediated apoptosis in human cancer cells. Cancer Cell Int. 2015, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Hsieh, S.C.; Lin, C.L.; Lin, Y.S.; Tsai, J.P.; Hsieh, Y.H. Alpha-Mangostin Suppresses the Metastasis of Human Renal Carcinoma Cells by Targeting MEK/ERK Expression and MMP-9 Transcription Activity. Cell. Physiol. Biochem. 2017, 44, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, Y.; Takada, T.; Suzuki, H. Inhibitors of human ABCG2: From technical background to recent updates with clinical implications. Front. Pharmacol. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Pisco, A.O.; Jackson, D.A.; Huang, S. Reduced intracellular drug accumulation in drug-resistant leukemia cells is not only solely due to MDR-mediated efflux but also to decreased uptake. Front. Oncol. 2014, 4, 1–9. [Google Scholar] [CrossRef]
- Kigen, G.; Edwards, G. Intracellular accumulation of Praziquantel in T lymphoblastoid cell lines, CEM (parental) and CEMvbl(P-gp-overexpressing). BMC Pharmacol. Toxicol. 2016, 17, 1–11. [Google Scholar] [CrossRef]
- Shibata, M.A.; Iinuma, M.; Morimoto, J.; Kurose, H.; Akamatsu, K.; Okuno, Y.; Akao, Y.; Otsuki, Y. α-Mangostin extracted from the pericarp of the mangosteen (Garcinia mangostana Linn) reduces tumor growth and lymph node metastasis in an immunocompetent xenograft model of metastatic mammary cancer carrying a p53 mutation. BMC Med. 2011, 9, 69. [Google Scholar] [CrossRef]
- Aukkanimart, R.; Boonmars, T.; Sriraj, P.; Sripan, P.; Songsri, J.; Ratanasuwan, P.; Laummaunwai, P.; Boueroy, P.; Khueangchaingkhwang, S.; Pumhirunroj, B.; et al. In Vitro and In Vivo Inhibitory Effects of α-Mangostin on Cholangiocarcinoma Cells and Allografts. Asian Pac. J. Cancer Prev. 2017, 18, 707–713. [Google Scholar] [CrossRef]
- Imai, S.; Arai, T.; Yamada, T.; Niwa, M. Improved In Vitro-In Vivo Correlation by Using the Unbound-Fraction-Adjusted IC50 for Breast Cancer Resistance Protein Inhibition. Pharm. Res. 2020, 37, 1–11. [Google Scholar] [CrossRef]
- Chahal, R.; Nanda, A.; Akkol, E.K.; Sobarzo-sánchez, E.; Arya, A.; Kaushik, D.; Dutt, R.; Bhardwaj, R.; Rahman, M.H.; Mittal, V. Ageratum conyzoides L. And its secondary metabolites in the management of different fungal pathogens. Molecules 2021, 26, 2933. [Google Scholar] [CrossRef]
- Larayetan, R.; Ololade, Z.S.; Ogunmola, O.O.; Ladokun, A. Phytochemical Constituents, Antioxidant, Cytotoxicity, Antimicrobial, Antitrypanosomal, and Antimalarial Potentials of the Crude Extracts of Callistemon citrinus. Evidence-based Complement. Altern. Med. 2019, 2019. [Google Scholar] [CrossRef]
- Hu, X.; Wu, T.; Bao, Y.; Zhang, Z. Nanotechnology based therapeutic modality to boost anti-tumor immunity and collapse tumor defense. J. Control. Release 2017, 256, 26–45. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Batool, R.; Mahmood, T.; Ali, B.; Khalil, A.T.; Kanwal, S.; Shah, S.A.; Ahmad, R. Potential phytocompounds for developing breast cancer therapeutics: Nature’s healing touch. Eur. J. Pharmacol. 2018, 827, 125–148. [Google Scholar] [CrossRef]
- Ivanova, N.; Gugleva, V.; Dobreva, M.; Pehlivanov, I.; Stefanov, S.; Andonova, V. Novel Applications of Nanoparticles in Nature and Building Materials. In Novel Nanomaterials; IntechOpen: London, UK, 2016; Volume i, p. 13. [Google Scholar]
- Baig, N.; Kammakakam, I.; Falath, W.; Kammakakam, I. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 1–29. [Google Scholar] [CrossRef]
- Grewal, I.K.; Singh, S.; Arora, S.; Sharma, N. Polymeric nanoparticles for breast cancer therapy: A comprehensive review. Biointerface Res. Appl. Chem. 2021, 11, 11151–11171. [Google Scholar] [CrossRef]
- Bashir, S.; Aamir, M.; Sarfaraz, R.M.; Hussain, Z.; Sarwer, M.U.; Mahmood, A.; Akram, M.R.; Qaisar, M.N. Fabrication, characterization and in vitro release kinetics of tofacitinib-encapsulated polymeric nanoparticles: A promising implication in the treatment of rheumatoid arthritis. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 449–458. [Google Scholar] [CrossRef]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine Scale-up Technologies: Feasibilities and Challenges. Ageing Int. 2014, 15, 1527–1534. [Google Scholar] [CrossRef]
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric nanocarriers of drug delivery systems in cancer therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef]
- Bertoni, S.; Passerini, N.; Albertini, B. Nanomaterials for Oral Drug Administration; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128180389. [Google Scholar]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 1–24. [Google Scholar] [CrossRef]
- Fathi, M.; Abdolahinia, E.D.; Barar, J.; Omidi, Y. Smart stimuli-responsive biopolymeric nanomedicines for targeted therapy of solid tumors. Nanomedicine 2020, 15, 2171–2200. [Google Scholar] [CrossRef] [PubMed]
- Aisha, A.F.A.; Abdulmajid, A.M.S.; Ismail, Z.; Alrokayan, S.A.; Abu-Salah, K.M. Development of Polymeric Nanoparticles of Garcinia mangostana Xanthones in Eudragit RL100/RS100 for Anti-Colon Cancer Drug Delivery. J. Nanomater. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Yu, T.; Huang, X.; Liu, J.; Fu, Q.; Wang, B.; Qian, Z. Polymeric nanoparticles encapsulating ␣ -mangostin inhibit the growth and metastasis in colorectal cancer. Appl. Mater. Today 2019, 16, 351–366. [Google Scholar] [CrossRef]
- Wathoni, N.; Rusdin, A.; Motoyama, K.; Joni, I.M.; Lesmana, R.; Muchtaridi, M. Nanoparticle drug delivery systems for α-mangostin. Nanotechnol. Sci. Appl. 2020, 13, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Sriyanti, I.; Edikresnha, D.; Rahma, A.; Munir, M.M.; Rachmawati, H.; Khairurrijal, K. Mangosteen pericarp extract embedded in electrospun PVP nanofiber mats: Physicochemical properties and release mechanism of α-mangostin. Int. J. Nanomedicine 2018, 13, 4927–4941. [Google Scholar] [CrossRef]
- Wathoni, N.; Meylina, L.; Rusdin, A.; Fouad, A.; Mohammed, A.; Tirtamie, D.; Herdiana, Y.; Motoyama, K.; Panatarani, C.; Joni, I.M.; et al. The Potential Cytotoxic Activity Enhancement of α -Mangostin in Chitosan-Kappa Carrageenan-Loaded Nanoparticle against MCF-7 Cell Line. Polymer 2021, 13, 1–13. [Google Scholar]
- Pham, D.T.; Saelim, N.; Tiyaboonchai, W. Alpha mangostin loaded crosslinked silk fibroin-based nanoparticles for cancer chemotherapy. Colloids Surf B Biointerfaces 2019, 181, 705–713. [Google Scholar] [CrossRef]
- Dissanayake, S.; Denny, W.A.; Gamage, S.; Sarojini, V. Recent developments in anticancer drug delivery using cell penetrating and tumor targeting peptides. J. Control. Release 2017, 250, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Bazylinska, U.; Saczko, J. Nanoemulsion-templated polylelectrolyte multifunctional nanocapsules for DNA entrapment and bioimaging. Colloids Surf B Biointerfaces 2016, 137, 191–202. [Google Scholar] [CrossRef]
- Mcmahon, K.M.; Foit, L.; Angeloni, N.L.; Giles, F.J.; Gordon, L.I.; Thaxton, C.S. Nanotechnology-Based Precision Tools for the Detection and Treatment of Cancer. Cancer Treat Res 2015, 166, 129–150. [Google Scholar] [CrossRef]
- Sepand, M.R.; Ranjbar, S.; Kempson, I.M.; Akbariani, M.; Muganda, W.C.A.; Müller, M.; Ghahremani, M.H.; Raoufi, M. Targeting non-apoptotic cell death in cancer treatment by nanomaterials: Recent advances and future outlook. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102243. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Joni, I.M.; Muchtaridi, M. Chitosan-Based Nanoparticles of Targeted Drug Delivery System in Breast Cancer Treatmen. Polymers 2021, 13, 1717. [Google Scholar] [CrossRef]
- Peng, F.; Setyawati, M.I.; Tee, J.K.; Ding, X.; Wang, J.; Nga, M.E.; Ho, H.K.; Leong, D.T. Nanoparticles promote in vivo breast cancer cell intravasation and extravasation by inducing endothelial leakiness. Nat. Nanotechnol. 2019, 14, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.K.; Yu, W.; Shrivastava, A.; Shankar, S.; Srivastava, R.K. α-Mangostin-encapsulated PLGA nanoparticles inhibit pancreatic carcinogenesis by targeting cancer stem cells in human, and transgenic (KrasG12D, and KrasG12D/tp53R270H) mice. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Saeed, N.A.; Hamzah, I.H.; Mahmood, S.I. The applications of nano-medicine in the breast cancer therapy. J. Phys. Conf. Ser. 2021, 1853. [Google Scholar] [CrossRef]
- Tariq, H.; Bokhari, S.A.I. Surface-functionalised hybrid nanoparticles for targeted treatment of cancer. IET Nanobiotechnol. 2020, 14, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, D.; Shen, J.; Wang, Q. A Review of Mesoporous Silica Nanoparticle Delivery Systems in Chemo-Based Combination Cancer Therapies. Front. Chem. 2020, 8, 1–17. [Google Scholar] [CrossRef]
- Shao, J.; Fang, Y.; Zhao, R.; Chen, F.; Yang, M.; Jiang, J.; Chen, Z.; Yuan, X.; Jia, L. Evolution from small molecule to nano-drug delivery systems: An emerging approach for cancer therapy of ursolic acid. Asian J. Pharm. Sci. 2020, 15, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Mout, R.; Moyano, D.F.; Rana, S.; Rotello, V.M. Surface functionalization of nanoparticles for nanomedicine. Chem Soc Rev. 2012, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wu, Z.; Li, X.; Xiao, L.; Yang, M.; Li, Y.; Duan, J.; Sun, Z. The Size-dependent Cytotoxicity of Amorphous Silica Nanoparticles: A Systematic Review of in vitro Studies. Int. J. Nanomed. 2020, 15, 9089–9113. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [PubMed]
- Güncüm, E.; Işıklan, N.; Anlaş, C.; Ünal, N.; Bulut, E.; Bakırel, T. Development and characterization of polymeric-based nanoparticles for sustained release of amoxicillin–an antimicrobial drug. Artif. Cells Nanomed. Biotechnol. 2018, 46, 964–973. [Google Scholar] [CrossRef]
- Choi, M.E.; Price, D.R.; Ryter, S.W.; Choi, A.M.K. Necroptosis: A crucial pathogenic mediator of human disease. JCI Insight 2019, 4, 1–16. [Google Scholar] [CrossRef]
- Peltonen, L.; Hirvonen, J. Drug nanocrystals – Versatile option for formulation of poorly soluble materials. Int. J. Pharm. 2018, 537, 73–83. [Google Scholar] [CrossRef]
- Song, B.; Zhou, T.; Liu, J.; Shao, L.Q. Involvement of Programmed Cell Death in Neurotoxicity of Metallic Nanoparticles: Recent Advances and Future Perspectives. Nanoscale Res. Lett. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, Y. A mechanistic overview of herbal medicine and botanical compounds to target transcriptional factors in Breast cancer. Pharmacol. Res. 2018, 130, 292–302. [Google Scholar] [CrossRef]
- Human, A.; Cancer, L.; Ja, M. Increased Cytotoxic Efficacy of Protocatechuic Acid in A549 Human Lung Cancer Delivered via Hydrophobically Modified-Chitosan Nanoparticles as an Anticancer Modality. Polymers 2020, 12, 1–24. [Google Scholar]
- Čulen, M.; Tuszyński, P.K.; Polak, S.; Jachowicz, R.; Mendyk, A.; Dohnal, J. Development of in vitro - In vivo correlation/relationship modeling approaches for immediate release formulations using compartmental dynamic dissolution data from “golem”: A novel apparatus. Biomed Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Mohamed, M.E.F.; Trueman, S.; Othman, A.A.; Han, J.H.; Ju, T.R.; Marroum, P. Development of In Vitro–In Vivo Correlation for Upadacitinib Extended-Release Tablet Formulation. AAPS J. 2019, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, H.; Asim Farooq, M.; Batool, Z.; Ahsan, A.; Syed, A. Significance of In-Vitro and In-Vivo Correlation in Drug Delivery System. Res. Pharm. Heal. Sci. 2018, 4, 523–531. [Google Scholar] [CrossRef]
- Juan, A.; Cimas, F.J.; Bravo, I.; Pandiella, A.; Ocaña, A.; Alonso-Moreno, C. Antibody conjugation of nanoparticles as therapeutics for breast cancer treatment. Int. J. Mol. Sci. 2020, 21, 6018. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bahreyni, A.; Mohamud, Y.; Luo, H. Emerging nanomedicines for effective breast cancer immunotherapy. J. Nanobiotechnol. 2020, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, P.Y.; Hettiarachchi, S.D.; Zhou, Y.; Ouhtit, A.; Seven, E.S.; Oztan, C.Y.; Celik, E.; Leblanc, R.M. Nanoparticle-mediated targeted drug delivery for breast cancer treatment. Biochim. Biophys. Acta-Rev. Cancer 2019, 1871, 419–433. [Google Scholar] [CrossRef]
- Yu, Z.; Gao, L.; Chen, K.; Zhang, W.; Zhang, Q.; Li, Q.; Hu, K. Nanoparticles: A New Approach to Upgrade Cancer Diagnosis and Treatment. Nanoscale Res. Lett. 2021, 16. [Google Scholar] [CrossRef] [PubMed]
| Anticancer Activity | Cell Models/Methods | Delivery | IC50/Concentration | Molecular Mechanisms | Reference |
|---|---|---|---|---|---|
| Cytotoxicity | SKBR3/The MTT assay/intracellular ROS level induced by H2 O2 | AMG | 8.21 μg/mL (ED50) | ↓ cancer cell production | [70] |
| Water-soluble extracts | 160 μg/mL (ED50) | ↓ cancer cell production | |||
| intracellular ROS level induced by H2O | AMG | 20 μg/mL | ↓ ROS | ||
| Water-soluble extracts | 50 μg/ml | ↓ ROS | |||
| Attenuation of inflammation | MDA-MB-231 | 10 μM AMG −20 μM AMG | iNOS protein ↓, mRNA expressions COX-2↓, NO↓, PGE2production from PEG2↓ | [71] | |
| Induction of cell cycle arrest | MDA-MB-231 cells | 12 μM–20 μM AMG | 20 μM IC50 (24 h), 16 μM (48 h) | G1-arrest, p21CIP1expression↑, cyclins expression↓, CDC(s) expression↓, CDKs expression↓, PCNA↓, CHEK2 expression↑ | [72] |
| Induction of the apoptotic signaling pathway | MDA-MB-231/The MTT assay | 20 μM AMG | 20 μM IC50 (24 h), 16 μM (48 h) | Mitochondrial membrane potential↓, caspase-7, caspase-8, caspase-9 and caspase-3 expression↑, ROS↑, Bcl-2 expression↓, Bax expression↑, Hsp70 protein expression↓, NF-κB translocation↓, PARP cleavage, Cytochrome c ↑ | [72,73] |
| MCF-7, MCF-10A(normal cell)/ The MTT assay | 20 µg/mL AMG | IC50 10.26 ± 0.25 µg/mL (MCF-7), >0.30 µg/mL (MCF 10 A) | |||
| MCF-7 he MTT assay | 1 mg/mL in DMSO of AMG | IC50 8–16 μM AMG after 24, under 6 μM AMG not give cytotoxicity | Caspases-8, caspase-9, and caspase−7 expressions↑, cytochrome c release, PARP cleavage, Bax expression↑, p53 expression↑, Bcl-2 expression↓, Bid↓, ERα expression↓ | [74] | |
| MDA-MB, MCF 7/ | 24 h of exposure to AMG | 4 μM AMG for 24 h, (IC50) value of 5.54 μM | Fatty acid synthase (FAS) ↓, ER stress↑, Autophagy inhibition ↑, LC3II/LC3I↑, p62 ↑ | [74,75,76] | |
| Inhibition of angiogenic and metastatic progression | Bovine retinal endothelial cells | 1.4–8 μM AMG | VEGF-induced phosphorylation of VEGFR2 and ERK1/2-MAPK↓ | [77] | |
| MCF-7/The MTT assay | 20 µg/mL AMG | IC50 2 μM AMG | TNF-α-induced NF-κB translocation↓, NF-κB↓, c-Fos↓, and c-Jun↓ | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. α-Mangostin Nanoparticles Cytotoxicity and Cell Death Modalities in Breast Cancer Cell Lines. Molecules 2021, 26, 5119. https://doi.org/10.3390/molecules26175119
Herdiana Y, Wathoni N, Shamsuddin S, Muchtaridi M. α-Mangostin Nanoparticles Cytotoxicity and Cell Death Modalities in Breast Cancer Cell Lines. Molecules. 2021; 26(17):5119. https://doi.org/10.3390/molecules26175119
Chicago/Turabian StyleHerdiana, Yedi, Nasrul Wathoni, Shaharum Shamsuddin, and Muchtaridi Muchtaridi. 2021. "α-Mangostin Nanoparticles Cytotoxicity and Cell Death Modalities in Breast Cancer Cell Lines" Molecules 26, no. 17: 5119. https://doi.org/10.3390/molecules26175119
APA StyleHerdiana, Y., Wathoni, N., Shamsuddin, S., & Muchtaridi, M. (2021). α-Mangostin Nanoparticles Cytotoxicity and Cell Death Modalities in Breast Cancer Cell Lines. Molecules, 26(17), 5119. https://doi.org/10.3390/molecules26175119