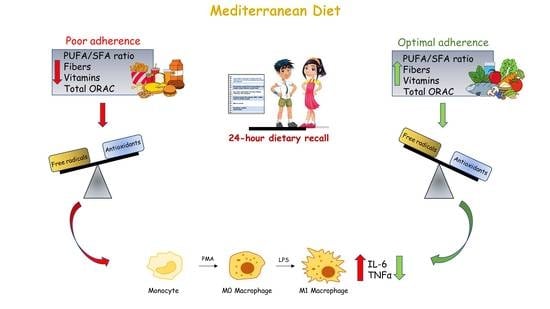
Potential Antioxidant and Anti-Inflammatory Properties of Serum from Healthy Adolescents with Optimal Mediterranean Diet Adherence: Findings from DIMENU Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Anthropometric Parameters and Bioelectrical Impedance Analysis
2.3. KIDMED Score
2.4. Dietary Assessment by 24 h Recall
2.5. Biochemical and Hormonal Measurements
2.6. Cell Culture and Experimental Treatments
2.7. Cytokine Measurement
2.8. ROM and BAP Assays
2.9. Statistical Analysis
3. Results
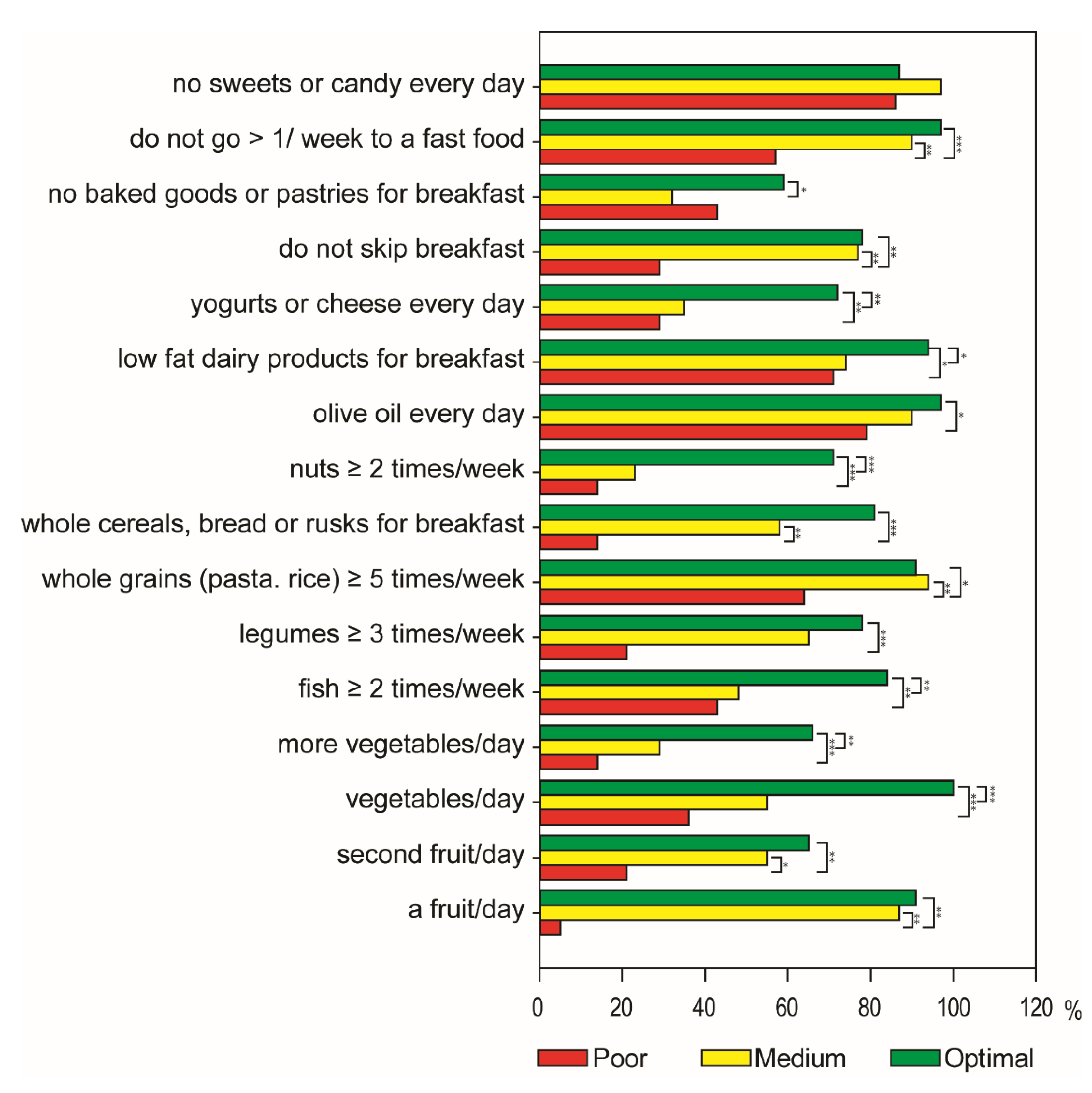
3.1. Characteristics of Participants and Adherence to the Mediterranean Diet
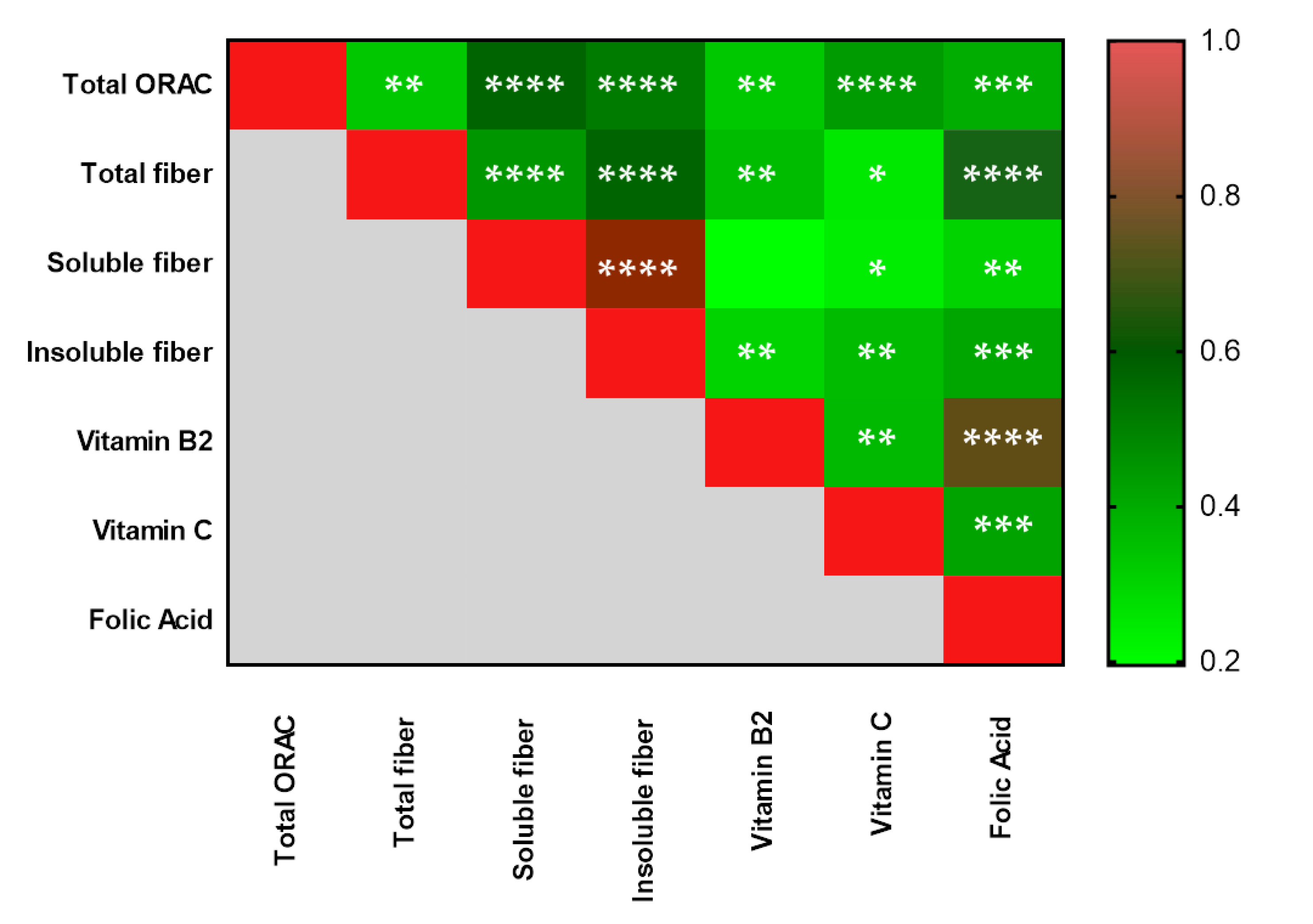
3.2. Dietary Intake Assessment by a 24 h Recall in Adolescents
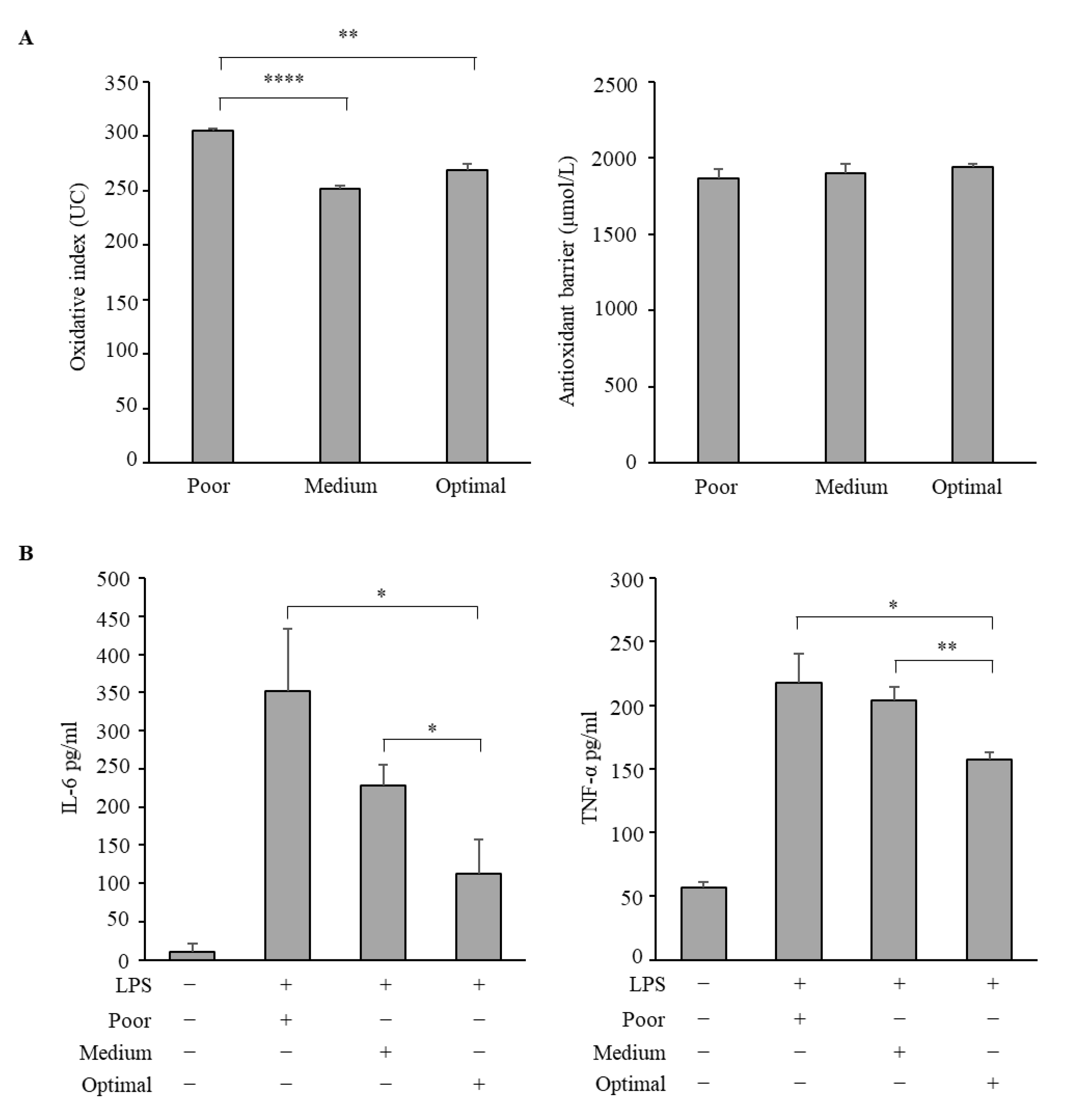
3.3. Antioxidative and Anti-Inflammatory Properties of Serum Samples from Adolescents Classified According to the Three Mediterranean Diet Adherence Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godos, J.; Galvano, F. Insights on Mediterranean Diet from the SUN Cohort: Cardiovascular and Cognitive Health. Nutrients 2020, 12, 1332. [Google Scholar] [CrossRef] [PubMed]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef] [PubMed]
- Nomikos, T.; Fragopoulou, E.; Antonopoulou, S.; Panagiotakos, D.B. Mediterranean diet and platelet-activating factor; a systematic review. Clin. Biochem. 2018, 60, 1–10. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Lagiou, P. Healthy Traditional Mediterranean Diet: An Expression of Culture, History, and Lifestyle. Nutr. Rev. 1997, 55, 383–389. [Google Scholar] [CrossRef]
- Davis, C.R.; Bryan, J.; Hodgson, J.M.; Murphy, K.J. Definition of the Mediterranean Diet; A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef]
- Echouffo-Tcheugui, J.B.; Ahima, R.S. Does diet quality or nutrient quantity contribute more to health? J. Clin. Investig. 2019, 129, 3969–3970. [Google Scholar] [CrossRef]
- Schulze, M.B.; A Martínez-González, M.; Fung, T.T.; Lichtenstein, A.H.; Forouhi, N. Food based dietary patterns and chronic disease prevention. BMJ 2018, 361, k2396. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Mente, A.; De Koning, L.; Shannon, H.S.; Anand, S.S. A Systematic Review of the Evidence Supporting a Causal Link between Dietary Factors and Coronary Heart Disease. Arch. Intern. Med. 2009, 169, 659–669. [Google Scholar] [CrossRef] [Green Version]
- Menni, C.; Zierer, J.; Pallister, T.; Jackson, M.A.; Long, T.; Mohney, R.P.; Steves, C.; Spector, T.D.; Valdes, A.M. Omega-3 fatty acids correlate with gut microbiome diversity and production of N-carbamylglutamate in middle aged and elderly women. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, I.; Hughes, M.; Rowsell, R.; Cockerell, R.; Pipingas, A.; Crewther, S.; Crewther, D. Omega-3 supplementation improves cognition and modifies brain activation in young adults. Hum. Psychopharmacol. Clin. Exp. 2014, 29, 133–144. [Google Scholar] [CrossRef]
- Kuroda, T.; Ohta, H.; Onoe, Y.; Tsugawa, N.; Shiraki, M. Intake of omega-3 fatty acids contributes to bone mineral density at the hip in a younger Japanese female population. Osteoporos. Int. 2017, 28, 2887–2891. [Google Scholar] [CrossRef]
- Sayon-Orea, C.; Razquin, C.; Bulló, M.; Corella, D.; Fitó, M.; Romaguera, D.; Vioque, J.; Alonso-Gómez, Á.M.; Wärnberg, J.; Martínez, J.A.; et al. Effect of a Nutritional and Behavioral Intervention on Energy-Reduced Mediterranean Diet Adherence Among Patients With Metabolic Syndrome. JAMA 2019, 322, 1486–1499. [Google Scholar] [CrossRef]
- Di Pietro, N.; Marcovecchio, M.L.; Di Silvestre, S.; De Giorgis, T.; Cordone, V.G.P.; Lanuti, P.; Chiarelli, F.; Bologna, G.; Mohn, A.; Pandolfi, A. Plasma from pre-pubertal obese children impairs insulin stimulated Nitric Oxide (NO) bioavailability in endothelial cells: Role of ER stress. Mol. Cell. Endocrinol. 2017, 443, 52–62. [Google Scholar] [CrossRef]
- Di Pietrantonio, N.; Palmerini, C.; Pipino, C.; Baldassarre, M.P.A.; Bologna, G.; Mohn, A.; Giannini, C.; Lanuti, P.; Chiarelli, F.; Pandolfi, A.; et al. Plasma from obese children increases monocyte-endothelial adhesion and affects intracellular insulin signaling in cultured endothelial cells: Potential role of mTORC1-S6K1. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2021, 1867, 166076. [Google Scholar] [CrossRef] [PubMed]
- Morelli, C.; Avolio, E.; Galluccio, A.; Caparello, G.; Manes, E.; Ferraro, S.; De Rose, D.; Santoro, M.; Barone, I.; Catalano, S.; et al. Impact of Vigorous-Intensity Physical Activity on Body Composition Parameters, Lipid Profile Markers, and Irisin Levels in Adolescents: A Cross-Sectional Study. Nutrients 2020, 12, 742. [Google Scholar] [CrossRef] [Green Version]
- Formoso, G.; Pipino, C.; Baldassarre, M.P.A.; Del Boccio, P.; Zucchelli, M.; D’Alessandro, N.; Tonucci, L.; Cichelli, A.; Pandolfi, A.; Di Pietro, N. An Italian Innovative Small-Scale Approach to Promote the Conscious Consumption of Healthy Food. Appl. Sci. 2020, 10, 5678. [Google Scholar] [CrossRef]
- Galluccio, A.; Caparello, G.; Avolio, E.; Manes, E.; Ferraro, S.; Giordano, C.; Sisci, D.; Bonofiglio, D. Self-Perceived Physical Activity and Adherence to the Mediterranean Diet in Healthy Adolescents during COVID-19: Findings from the DIMENU Pilot Study. Healthcare 2021, 9, 622. [Google Scholar] [CrossRef] [PubMed]
- Detopoulou, P.; Demopoulos, C.; Antonopoulou, S. Micronutrients, Phytochemicals and Mediterranean Diet: A Potential Protective Role against COVID-19 through Modulation of PAF Actions and Metabolism. Nutrients 2021, 13, 462. [Google Scholar] [CrossRef]
- Perez-Araluce, R.; Martinez-Gonzalez, M.; Fernández-Lázaro, C.; Bes-Rastrollo, M.; Gea, A.; Carlos, S. Mediterranean diet and the risk of COVID-19 in the ‘Seguimiento Universidad de Navarra’ cohort. Clin. Nutr. 2021. [Google Scholar] [CrossRef]
- Ponzo, V.; Pellegrini, M.; D’Eusebio, C.; Bioletto, F.; Goitre, I.; Buscemi, S.; Frea, S.; Ghigo, E.; Bo, S. Mediterranean Diet and SARS-COV-2 Infection: Is There Any Association? A Proof-of-Concept Study. Nutrients 2021, 13, 1721. [Google Scholar] [CrossRef]
- Rifas-Shiman, S.L.; Willett, W.C.; Lobb, R.; Kotch, J.; Dart, H.; Gillman, M.W. PrimeScreen, a brief dietary screening tool: Reproducibility and comparability with both a longer food frequency questionnaire and biomarkers. Public Health Nutr. 2001, 4, 249–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritenbaugh, C.; Aickin, M.; Taren, D.; Teufel, N.; Graver, E.; Woolf, K.; Alberts, D.S. Use of a food frequency questionnaire to screen for dietary eligibility in a randomized cancer prevention phase III trial. Cancer Epidemiol. Biomark. Prev. 1997, 6, 347–354. [Google Scholar]
- Morelli, C.; Avolio, E.; Galluccio, A.; Caparello, G.; Manes, E.; Ferraro, S.; Caruso, A.; De Rose, D.; Barone, I.; Adornetto, C.; et al. Nutritional Education Program and Physical Activity Im-prove the Adherence to the Mediterranean Diet: Impact on Inflammatory Biomarker Levels in Healthy Adolescents from the DIMENU Longitudinal Study. Front. Nutr. 2021, 8, 422. [Google Scholar] [CrossRef]
- Cabrera, S.G.; Fernández, N.H.; Hernández, C.R.; Nissensohn, M.; Román-Viñas, B.; Serra-Majem, L. Kidmed test; prevalence of low adherence to the mediterranean diet in children and young; a systematic review. Nutr. Hosp. 2015, 32, 2390–2399. [Google Scholar] [CrossRef]
- Gionfriddo, G.; Plastina, P.; Augimeri, G.; Catalano, S.; Giordano, C.; Barone, I.; Morelli, C.; Giordano, F.; Gelsomino, L.; Sisci, D.; et al. Modulating Tumor-Associated Macrophage Polarization by Synthetic and Natural PPARγ Ligands as a Potential Target in Breast Cancer. Cells 2020, 9, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Russa, D.; Pellegrino, D.; Montesanto, A.; Gigliotti, P.; Perri, A.; La Russa, A.; Bonofiglio, R. Oxidative Balance and Inflammation in Hemodialysis Patients: Biomarkers of Cardiovascular Risk? Oxidative Med. Cell. Longev. 2019, 2019, 8567275. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, E.; La Russa, D.; Pellegrino, D. Impaired Oxidative Status Is Strongly Associated with Cardiovascular Risk Factors. Oxidative Med. Cell. Longev. 2017, 2017, 6480145. [Google Scholar] [CrossRef] [Green Version]
- Novak, D.; Štefan, L.; Prosoli, R.; Emeljanovas, A.; Mieziene, B.; Milanović, I.; Janic, S.R. Mediterranean Diet and Its Correlates among Adolescents in Non-Mediterranean European Countries: A Population-Based Study. Nutrients 2017, 9, 177. [Google Scholar] [CrossRef] [Green Version]
- Galan-Lopez, P.; Ries, F.; Gisladottir, T.; Domínguez, R.; Sánchez-Oliver, A.J. Healthy Lifestyle: Relationship between Mediterranean Diet, Body Composition and Physical Fitness in 13 to 16-Years Old Icelandic Students. Int. J. Environ. Res. Public Heal. 2018, 15, 2632. [Google Scholar] [CrossRef] [Green Version]
- Grosso, G.; Galvano, F. Mediterranean diet adherence in children and adolescents in southern European countries. NFS J. 2016, 3, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Caparello, G.; Galluccio, A.; Giordano, C.; Lofaro, D.; Barone, I.; Morelli, C.; Sisci, D.; Catalano, S.; Andò, S.; Bonofiglio, D. Adherence to the Mediterranean diet pattern among university staff: A cross-sectional web-based epidemiological study in Southern Italy. Int. J. Food Sci. Nutr. 2019, 71, 581–592. [Google Scholar] [CrossRef]
- Geelen, A.; Souverein, O.W.; Busstra, M.C.; De Vries, J.H.; Veer, P.V. ‘T Comparison of approaches to correct intake–health associations for FFQ measurement error using a duplicate recovery biomarker and a duplicate 24 h dietary recall as reference method. Public Health Nutr. 2014, 18, 226–233. [Google Scholar] [CrossRef] [Green Version]
- Kipnis, V.; Midthune, D.; Freedman, L.; Bingham, S.; E Day, N.; Riboli, E.; Ferrari, P.; Carroll, R.J. Bias in dietary-report instruments and its implications for nutritional epidemiology. Public Health Nutr. 2002, 5, 915–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragaee, S.; Abdel-Aal, E.-S.M.; Noaman, M. Antioxidant activity and nutrient composition of selected cereals for food use. Food Chem. 2006, 98, 32–38. [Google Scholar] [CrossRef]
- Vasilopoulou, E.; Georga, K.; Joergensen, M.; Naska, A.; Trichopoulou, A. The Antioxidant Properties of Greek Foods and the Flavonoid Content of the Mediterranean Menu. Curr. Med. Chem. Immunol. Endocr. Metab. Agents 2005, 5, 33–45. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly) phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pecora, F.; Persico, F.; Argentiero, A.; Neglia, C.; Esposito, S. The Role of Micronutrients in Support of the Immune Response against Viral Infections. Nutrients 2020, 12, 3198. [Google Scholar] [CrossRef]
- Ayuso, J.M.; on behalf of theHELENA Study group; Valtueña, J.; Huybrechts, I.; Breidenassel, C.; Cuenca-García, M.; De Henauw, S.; Stehle, P.; Kafatos, A.; Kersting, M.; et al. Fruit and vegetables consumption is associated with higher vitamin intake and blood vitamin status among European adolescents. Eur. J. Clin. Nutr. 2017, 71, 458–467. [Google Scholar] [CrossRef]
- Camps, J. Oxidative Stress and Inflammation in Non-communicable Diseases—Molecular Mechanisms and Perspectives in Therapeutics. In Proceedings of the Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2014. [Google Scholar]
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA damage and disease: Induction, repair and significance. Mutat. Res. Mutat. Res. 2004, 567, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Hashimoto, T.; Tsuda, Y.; Kitaoka, T.; Kyotani, S. Evaluation of oxidative stress and antioxidant capacity in healthy children. J. Chin. Med Assoc. 2019, 82, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.; Willoughby, D.A.; Gilroy, D. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Rev. Immunol. 2002, 2, 787–795. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total Sample | Girls | Boys |
| Subjects (number) | 77 | 36 | 41 |
| Age (years) | 15.77 ± 1.07 | 15.72 ± 1.13 | 15.80 ± 1.03 |
| Weight (Kg) | 64.89 ± 12.59 | 58.55 ± 7.83 | 70.45 ± 13.4 |
| Height (cm) | 167.9 ± 8.21 | 161.54 ± 5.63 | 173.53 ± 5.6 |
| BMI (Kg/m2) | 22.87 ± 3.39 | 22.41 ± 2.93 | 23.26 ± 3.74 |
| Overweight (%) | 24.67 | 9.09 | 15.58 |
| Obesity (%) | 3.89 | 1.3 | 2.59 |
| Waist (cm) | 22.87 ± 3.39 | 22.41 ± 2.93 | 23.26 ± 3.74 |
| Hip (cm) | 73.23 ± 9.04 | 68.80 ± 6.38 | 77.10 ± 9.3 |
| Waist/hip ratio | 95.75 ± 8.05 | 95.08 ± 7.34 | 96.32 ± 8.67 |
| BCM (%) | 54.45 ± 3.73 | 53.38 ± 2.92 | 56.26 ± 3.43 |
| FM (Kg) | 14.37 ± 6.57 | 16.09 ± 5.55 | 12.85 ± 7.07 |
| FFM (Kg) | 50.78 ± 9.81 | 42.55 ± 3.47 | 58.02 ± 7.60 |
| PhA (°) | 6.34 ± 0.90 | 6.01 ± 0.93 | 6.63 ± 0.78 |
| TBW (%) | 58.09 ± 6.68 | 54.59 ± 5.54 | 61.17 ± 6.10 |
| General metabolic, Health, and Inflammatory Biomarkers | |||
| Glucose (mg/dL) | 79.71 ± 6.76 | 77.77 ± 6.73 | 81.41 ± 6.39 |
| Insulin (mU/L) | 10.90 ± 7.81 | 9.50 ± 5.67 | 12.13 ± 9.18 |
| HOMA-IR | 2.16 ± 1.56 | 1.84 ± 1.1 | 2.39 ± 1.86 |
| TG (mg/dL) | 75.26 ± 61.94 | 70.67 ± 59.45 | 79.29 ± 64.50 |
| Total Cholesterol (mg/dL) | 150.83 ± 27.71 | 158 ± 28.35 | 144.54 ± 25.86 |
| LDL (mg/dL) | 85.51 ± 22.77 | 88.72 ± 25.65 | 82.68 ± 19.78 |
| HDL (mg/dL) | 50.21 ± 11.53 | 55.27 ± 11.89 | 45.76 ± 9.24 |
| Creatinine (mg/dL) | 0.89 ± 0.12 | 0.81 ± 0.09 | 0.96 ± 0.09 |
| Urea nitrogen (mg/dL) | 30.06 ± 6.48 | 28 ± 4.89 | 31.88 ± 7.18 |
| Uric acid (mg/dL) | 4.86 ± 1.40 | 4.26 ± 1.03 | 5.39 ± 1.42 |
| Total bilirubin (mg/dL) | 1.01 ± 0.56 | 0.84 ± 0.31 | 1.16 ± 0.69 |
| Direct bilirubin (mg/dL) | 027 ± 0.09 | 0.24 ± 0.06 | 0.30 ± 0.10 |
| ESR (mm/h) | 17.01 ± 11.01 | 23.28 ± 11.22 | 11.51 ± 7.35 |
| CRP (mg/L) | 1.20 ± 0.64 | 1.19 ± 0.60 | 1.21 ± 0.68 |
| Adherence to the MD | |||
| KIDMED Score (mean ± SD) | 6.71 ± 2.58 | 6.5 ± 2.74 | 6.9 ± 2.46 |
| KIDMED Score | Total Sample |
|---|---|
| Poor adherence (≤3) | 7 (9%) |
| Medium adherence (4–7) | 37 (48%) |
| Optimal adherence (≥8) | 33 (43%) |
| Adherence to the Mediterranean Diet | ||||
|---|---|---|---|---|
| Primary Energy Sources | Poor | Medium | Optimal | p-Value |
| Total Energy (kcal) | 1437.85 ± 386.31 | 1716 ± 520.79 | 1707.42 ± 441.85 | 0.34 * 0.37 ¥ 0.10 § |
| Total Fat (g) | 72.10 ± 19.62 | 79.23 ± 25.79 | 76.12 ± 19.62 | 0.70 * 0.93 ¥ 0.88 § |
| Total Carbohydrate (g) | 137.15 ± 44.02 | 175.65 ± 76.12 | 169.26 ± 71.02 | 0.40 * 0.53 ¥ 0.93 § |
| Total protein (g) | 55.45 ± 28.92 | 70.43 ± 26.65 | 81.31 ± 24.22 | 0.34 * 0.05 ¥ 0.19 § |
| Animal Protein (g) | 31.30 ± 22.87 | 40.60 ± 27.21 | 49.99 ± 24.46 | 0.66 * 0.19 ¥ 0.28 § |
| Vegetable Protein (g) | 18.23 ± 10.22 | 22.65 ± 12.18 | 23.80 ± 8.82 | 0.58 * 0.43 ¥ 0.89 § |
| Fats | ||||
| SFA (g) | 38.86 ± 33.03 | 45.71 ± 20.15 | 48.18 ± 22.60 | 0.74 * 0.58 ¥ 0.89 § |
| MUFA (g) | 39.65 ± 10.98 | 38.14 ± 16.25 | 52.07 ± 78.08 | 0.10 * 0.84 ¥ 0.51 § |
| PUFA (g) | 35.61 ± 33.03 | 42.46 ± 20.15 | 44.93 ± 22.60 | 0.74 * 0.58 ¥ 0.89 § |
| Vegetable Fats (g) | 47.66 ± 10.87 | 43.11 ± 17.81 | 43.54 ± 17.68 | 0.80 * 0.83 ¥ 0.99 § |
| Animal Fats (g) | 14.97 ± 12.25 | 30.96 ± 24.01 | 26.06 ± 23.58 | 0.22 * 0.48 ¥ 0.65 § |
| Omega-3 Fatty Acids (g) | 0.68 ± 0.23 | 1.16 ± 0.89 | 1.20 ± 0.74 | 0.32 * 0.27 ¥ 0.98 § |
| Omega-6 Fatty Acids (g) | 6.69 ± 2.90 | 7.99 ± 5.29 | 7.57 ± 4.42 | 0.78 * 0.90 ¥ 0.93 § |
| EPA (g) | 0.025 ± 0.04 | 0.14 ± 0.24 | 0.07 ± 0.14 | 0.30 * 0.70 ¥ 0.36 § |
| DHA (g) | 0.02 ± 0.02 | 0.22 ± 0.69 | 0.14 ± 0.28 | 0.60 * 0.85 ¥ 0.75 § |
| PUFA:SFA ratio | 0.82 ± 0.13 | 0.90 ± 0.07 | 0.90 ± 0.06 | 0.05 * 0.04 ¥ 0.96 § |
| Cholesterol (mg) | 150.89 ± 114.63 | 233.55 ± 173.76 | 230.69 ± 175.99 | 0.47 * 0.50 ¥ 0.99 § |
| Carbohydrates | ||||
| Starch (g) | 94.74 ± 37.64 | 104.73 ± 62.28 | 100.95 ± 51.90 | 0.90 * 0.96 ¥ 0.96 § |
| Soluble sugars (g) | 21.97 ± 13.24 | 55.37 ± 33.23 | 55.019 ± 22.35 | 0.01 * 0.01 ¥ 0.99 § |
| Glycemic Index | 184.34 ± 330.02 | 59.75 ± 15.21 | 59.064 ± 16.17 | 0.01 * 0.01 ¥ 0.99 § |
| Glycemic Load | 70.92 ± 55.15 | 83.93 ± 54.39 | 82.64 ± 47.83 | 0.81 * 0.85 ¥ 0.99 § |
| Fibers | ||||
| Total dietary fiber | 9.90 ± 4.90 | 13.32 ± 5.77 | 16.21 ± 5.37 | 0.30 * 0.02 ¥ 0.08 § |
| Soluble dietary fiber | 0.97 ± 1.10 | 2.08 ± 1.25 | 2.64 ± 1.59 | 0.14 * 0.01 ¥ 0.23 § |
| Insoluble dietary fiber | 3.27 ± 2.14 | 6.15 ± 4.68 | 8.043 ± 4.49 | 0.26 * 0.03 ¥ 0.18 § |
| Vitamins | ||||
| Vitamin A eq. Retinol | 666.35 ± 563.48 | 922.60 ± 705.33 | 1147.81 ± 807.09 | 0.68 * 0.26 ¥ 0.42 § |
| Vitamin B1 (mg) | 0.78 ± 0.30 | 1.52 ± 3.32 | 0.95 ± 0.27 | 0.72 * 0.98 ¥ 0.57 § |
| Vitamin B2 (mg) | 0.79 ± 0.44 | 1.02 ± 0.40 | 1.35 ± 0.50 | 0.43 * 0.01 ¥ 0.01 § |
| Vitamin B3 (mg) | 13.37 ± 7.04 | 14.71 ± 8.24 | 18.74 ± 7.77 | 0.91 * 0.24 ¥ 0.09 § |
| Vitamin B5 (mg) | 1.63 ± 1.16 | 2.33 ± 1.62 | 3.2 ± 1.87 | 0.59 * 0.07 ¥ 0.09 § |
| Vitamin B6 (mg) | 1.35 ± 0.66 | 1.69 ± 1.35 | 1.98 ± 0.60 | 0.70 * 0.32 ¥ 0.50 § |
| Vitamin B8 (µg) | 6.54 ± 5.77 | 12.78 ± 11.76 | 16.46 ± 11.68 | 0.38 * 0.09 ¥ 0.37 § |
| Folic Acid (µg) | 165.46 ± 108.23 | 200.82 ± 100 | 263.29 ± 105.32 | 0.68 * 0.06 ¥ 0.04 § |
| Vitamin B12 (µg) | 1.45 ± 1.41 | 3.28 ± 2.70 | 3.90 ± 2.64 | 0.21 * 0.07 ¥ 0.59 § |
| Vitamin C (mg) | 33.25 ± 37.75 | 84.58 ± 49.05 | 108.60 ± 72.64 | 0.10 * 0.01 ¥ 0.22 § |
| Vitamin K (µg) | 0.00 ± 0.00 | 2.48 ± 5.14 | 3.86 ± 6.64 | 0.53 * 0.22 ¥ 0.56 § |
| Vitamin D (µg) | 0.85 ± 0.58 | 2.87 ± 6.77 | 2.65 ± 3.87 | 0.63 * 0.70 ¥ 0.98 § |
| Vitamin E (mg) | 10.69 ± 2.77 | 13.25 ± 6.77 | 12.79 ± 4.27 | 0.51 * 0.63 ¥ 0.94 § |
| Total ORAC (µmol TE) | 1599.86 ± 1625.11 | 4196.97 ± 3069.62 | 6236.43 ± 5973.07 | 0.35 * 0.04 ¥ 0.16 § |
| Minerals | ||||
| Calcium (mg) | 334.13 ± 243.55 | 543.93 ± 338.73 | 571.26 ± 266.03 | 0.62 * 0.91 ¥ 0.65 § |
| Phosphorus (mg) | 657.19 ± 184.33 | 848 ± 426.36 | 986.47 ± 405.55 | 0.49 * 0.13 ¥ 0.34 § |
| Iodium (µg) | 23.45 ± 19.43 | 80.36 ± 115.18 | 75.83 ± 66.92 | 0.29 * 0.36 ¥ 0.98 § |
| Sodium(mg) | 1131.22 ± 1008.09 | 1319.69 ± 1137.96 | 1274.45 ± 1060.31 | 0.91 * 0.95 ¥ 0.98 § |
| Iron (mg) | 5.98 ± 2.04 | 8.83 ± 5.47 | 9.22 ± 2.85 | 0.25 * 0.17 ¥ 0.92 § |
| Magnesium (mg) | 118.26 ± 56.14 | 183.51 ± 120.74 | 217.01 ± 95.75 | 0.30 * 0.07 ¥ 0.39 § |
| Selenium (µg) | 15.48 ± 9.47 | 35.6 ± 42.62 | 50.421 ± 48.61 | 0.51 * 0.14 ¥ 0.34 § |
| Potassium (mg) | 1651.16 ± 829.74 | 2216.68 ± 866.57 | 2762.54 ± 787.02 | 0.23 * 0.01 ¥ 0.02 § |
| Zinc (mg) | 5.60 ± 1.40 | 8.79 ± 5.03 | 8.957 ± 3.43 | 0.16 * 0.14 ¥ 0.98 § |
| Water (g) | 471.43 ± 292 | 767.21 ± 466.76 | 983.22 ± 507.56 | 0.29 * 0.03 ¥ 0.15 § |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Augimeri, G.; Galluccio, A.; Caparello, G.; Avolio, E.; La Russa, D.; De Rose, D.; Morelli, C.; Barone, I.; Catalano, S.; Andò, S.; et al. Potential Antioxidant and Anti-Inflammatory Properties of Serum from Healthy Adolescents with Optimal Mediterranean Diet Adherence: Findings from DIMENU Cross-Sectional Study. Antioxidants 2021, 10, 1172. https://doi.org/10.3390/antiox10081172
Augimeri G, Galluccio A, Caparello G, Avolio E, La Russa D, De Rose D, Morelli C, Barone I, Catalano S, Andò S, et al. Potential Antioxidant and Anti-Inflammatory Properties of Serum from Healthy Adolescents with Optimal Mediterranean Diet Adherence: Findings from DIMENU Cross-Sectional Study. Antioxidants. 2021; 10(8):1172. https://doi.org/10.3390/antiox10081172
Chicago/Turabian StyleAugimeri, Giuseppina, Angelo Galluccio, Giovanna Caparello, Ennio Avolio, Daniele La Russa, Daniela De Rose, Catia Morelli, Ines Barone, Stefania Catalano, Sebastiano Andò, and et al. 2021. "Potential Antioxidant and Anti-Inflammatory Properties of Serum from Healthy Adolescents with Optimal Mediterranean Diet Adherence: Findings from DIMENU Cross-Sectional Study" Antioxidants 10, no. 8: 1172. https://doi.org/10.3390/antiox10081172
APA StyleAugimeri, G., Galluccio, A., Caparello, G., Avolio, E., La Russa, D., De Rose, D., Morelli, C., Barone, I., Catalano, S., Andò, S., Giordano, C., Sisci, D., & Bonofiglio, D. (2021). Potential Antioxidant and Anti-Inflammatory Properties of Serum from Healthy Adolescents with Optimal Mediterranean Diet Adherence: Findings from DIMENU Cross-Sectional Study. Antioxidants, 10(8), 1172. https://doi.org/10.3390/antiox10081172