Characterization of Citrusnobilis Peel Methanolic Extract for Antioxidant, Antimicrobial, and Anti-Inflammatory Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Modified Solvent Evaporated Citrus Nobilis Peel Dry Extract
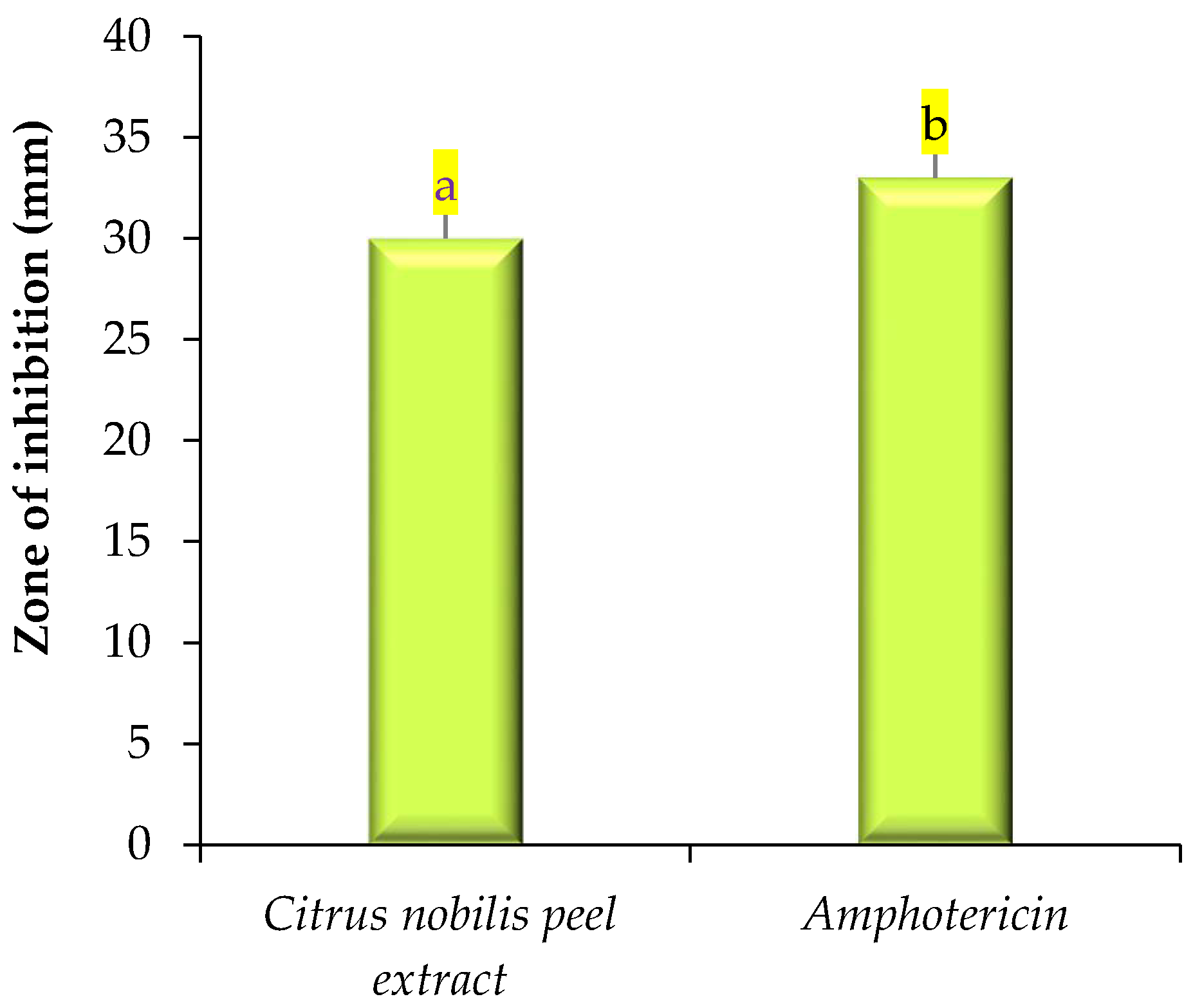
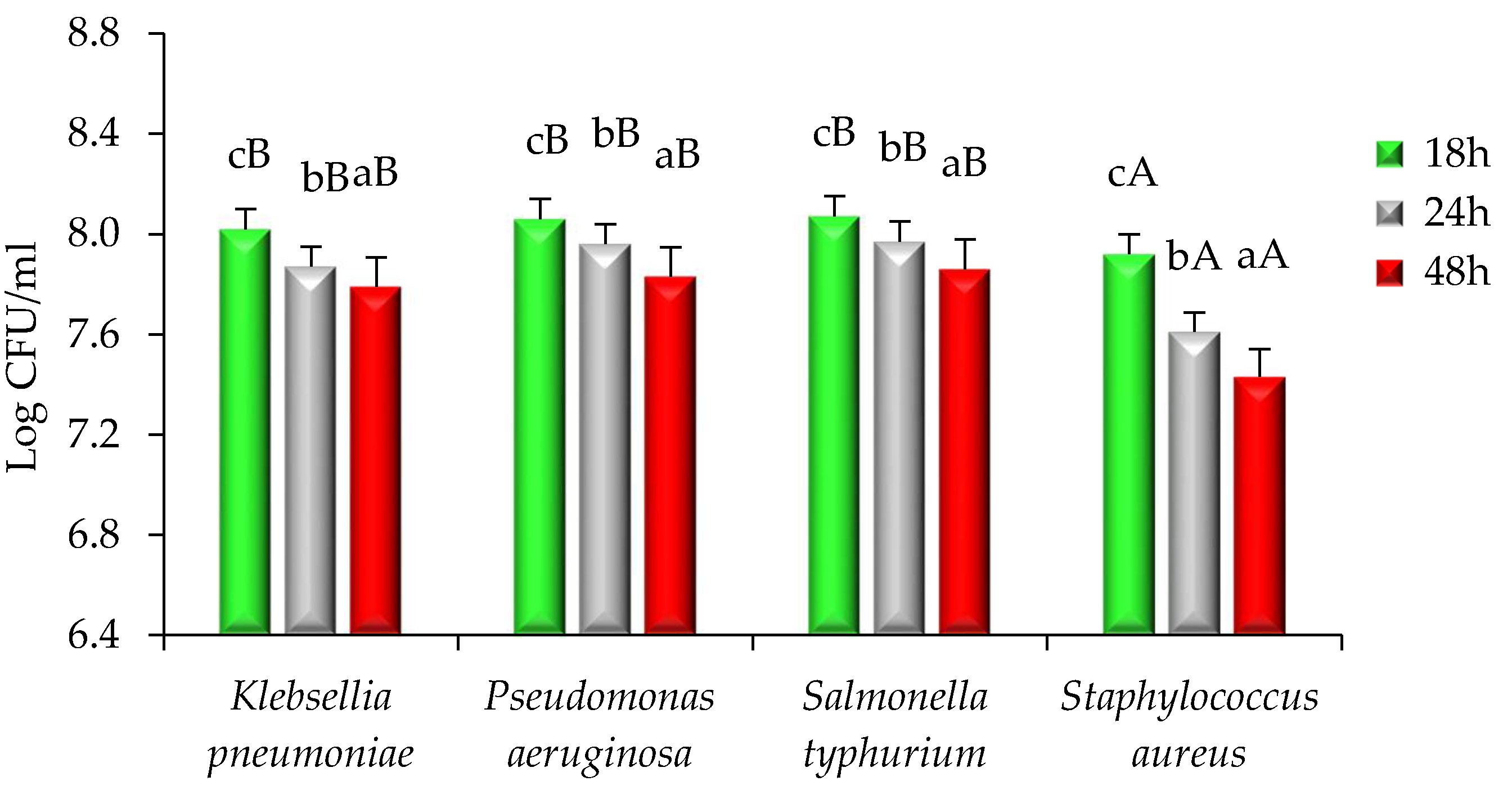
2.2. Antimicrobial Activity
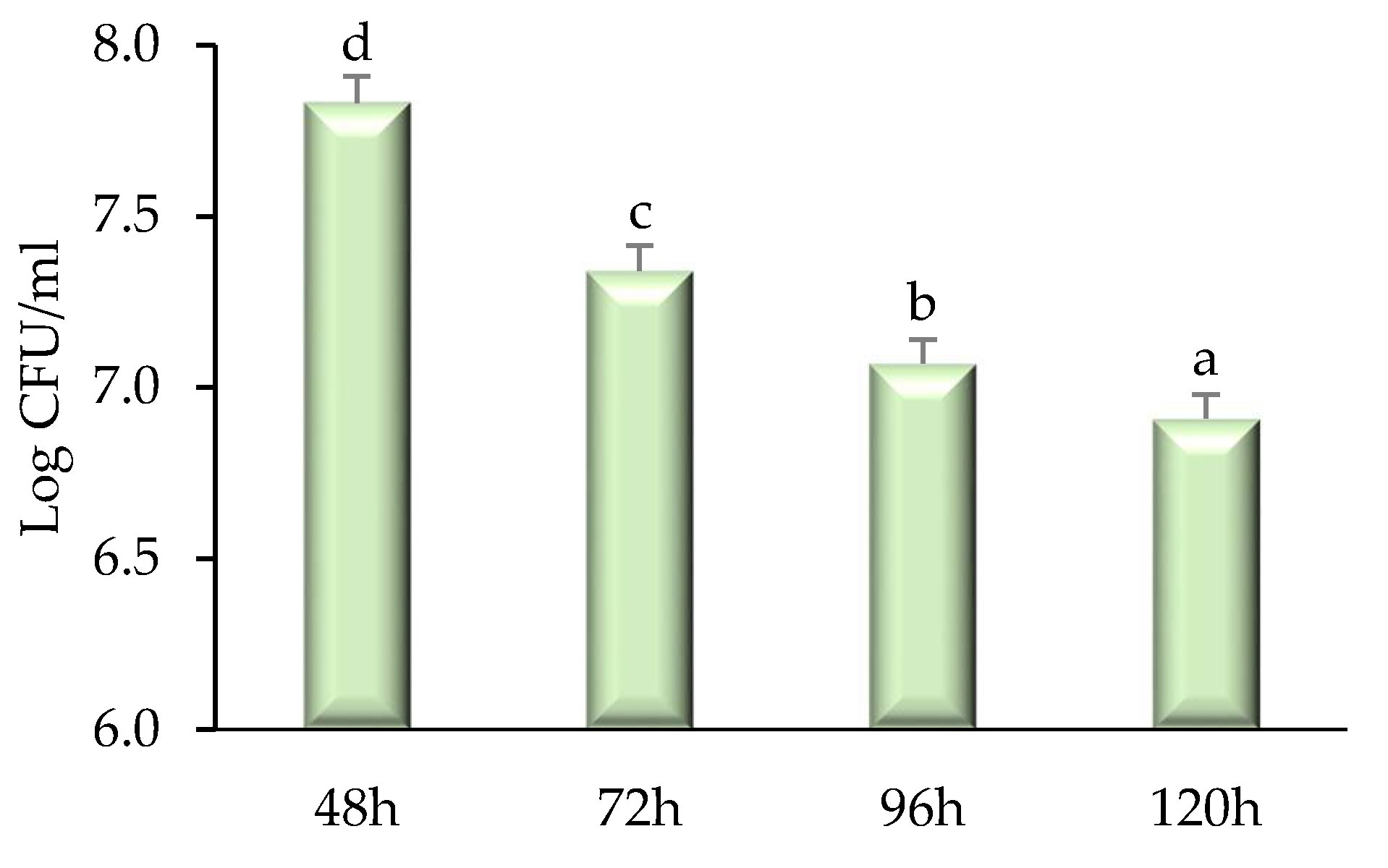
2.3. Time–Kill Kinetics
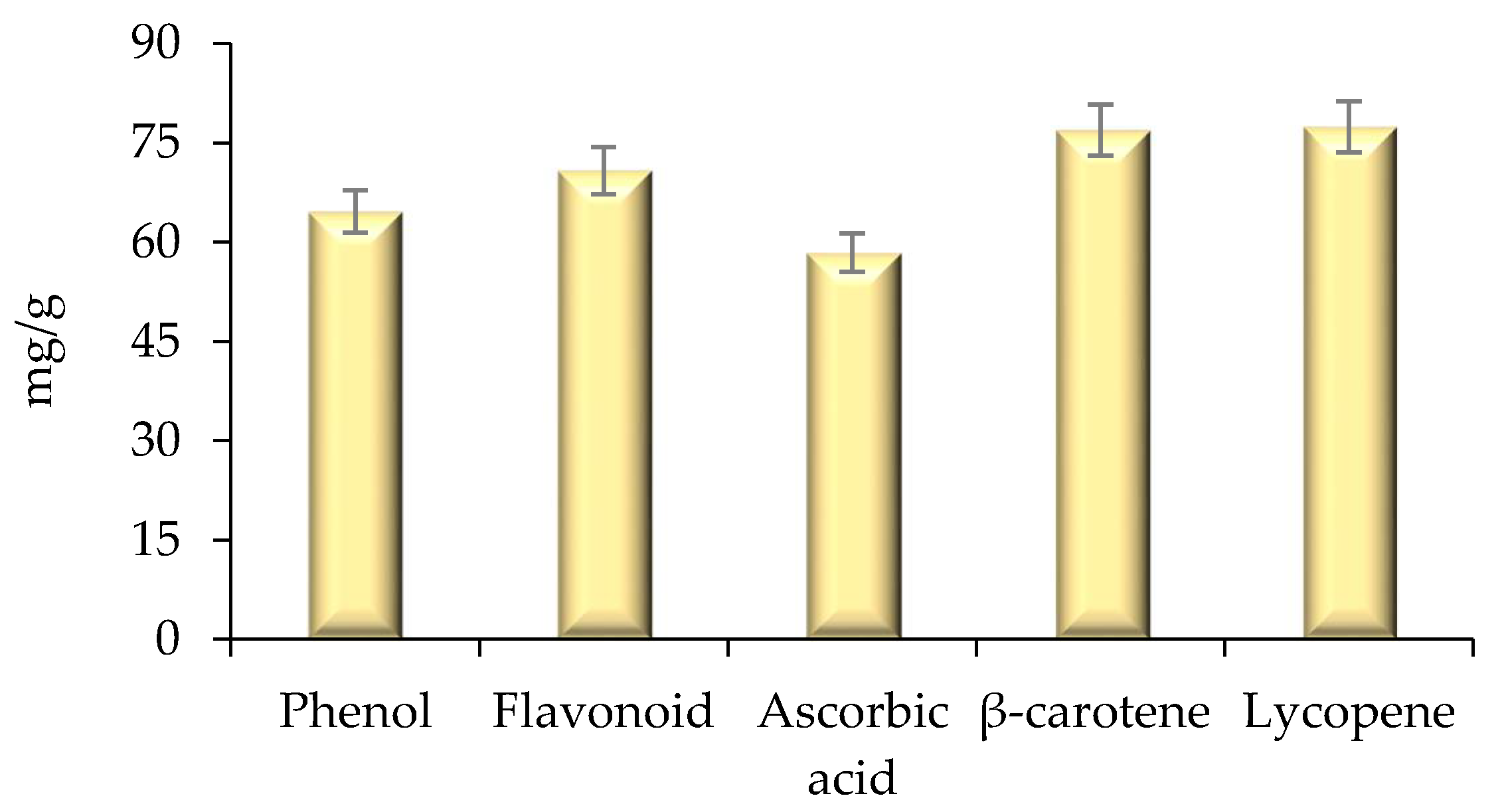
2.4. Estimation of Bioactive Compounds and Their Antioxidant Activity
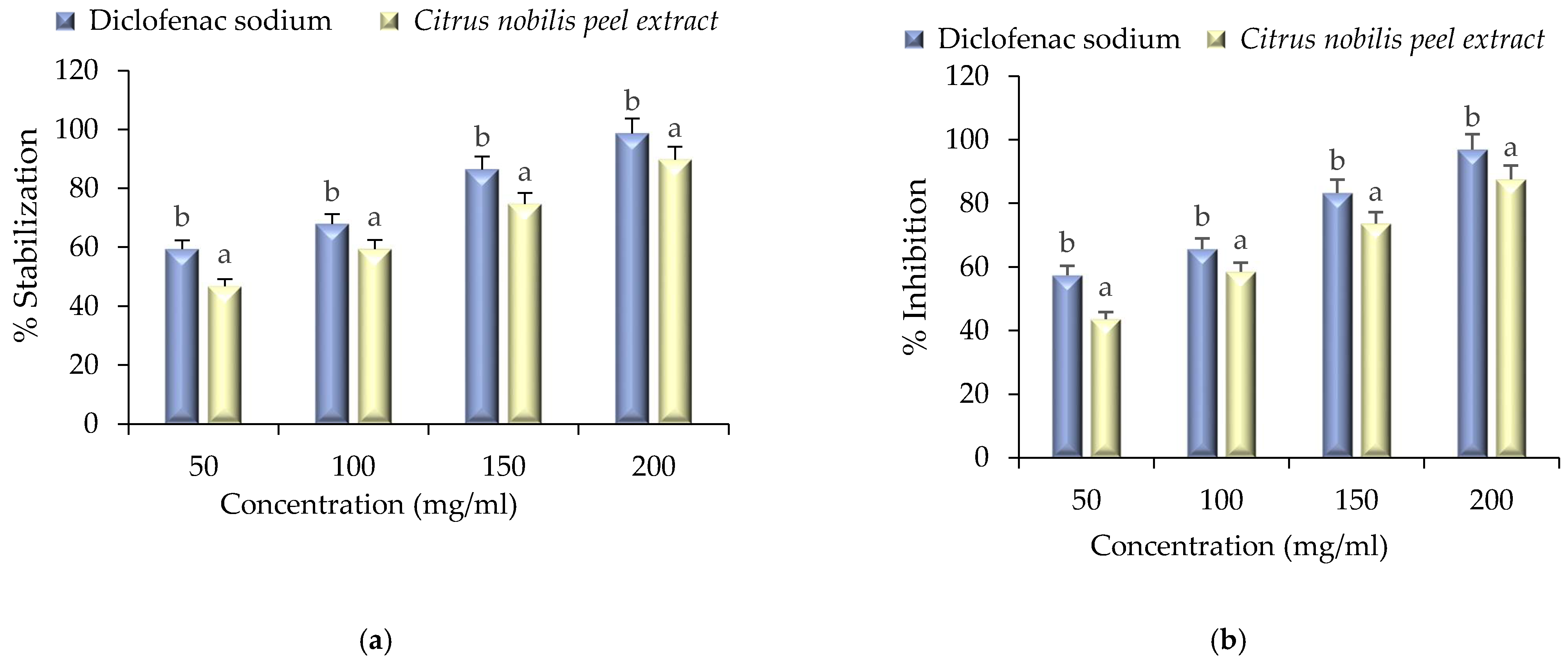
2.5. Anti-Inflammatory Activity
3. Materials and Methods
3.1. Phytochemical Assays
3.1.1. Chemical Reagents
3.1.2. Preparation of Peel Extract
3.1.3. Characterization of Extract
3.1.4. In Vitro Antimicrobial Activity
3.1.5. Quantification of Phenolics
3.1.6. In Vitro Antioxidant Activity
3.1.7. In Vitro Anti-Inflammatory Properties
3.2. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Domingo, C.; Tadeo, F.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the origin and evolution of Citrus. Nat. Cell Biol. 2018, 554, 311–316. [Google Scholar] [CrossRef] [Green Version]
- Kademi, H.I.; Garba, U. Citrus peel essential oils: A review on composition and antimicrobial activities. Int. J. Food Saf. Nutr. Publ. Health Technol. 2017, 9, 38–44. [Google Scholar]
- Tada, A.; Miura, H. The Relationship between Vitamin C and Periodontal Diseases: A Systematic Review. Int. J. Environ. Res. Public Heal. 2019, 16, 2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Molecules 2007, 12, 1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bains, A.; Chawla, P. In vitro bioactivity, antimicrobial and anti-inflammatory efficacy of modified solvent evaporation assisted Trametes versicolor extract. 3 Biotech 2020, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Ind. J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Bains, A.; Tripathi, A. Evaluation of antioxidant and anti-Inflammatory properties of aqueous extract of wild mushrooms collected from himachal pradesh. Asian J. Pharm. Clin. Res. 2017, 10, 467–472. [Google Scholar] [CrossRef] [Green Version]
- Bains, A.; Chawla, P.; Tripathi, A.; Sadh, P.K. A comparative study of antimicrobial and anti-inflammatory efficiency of modified solvent evaporated and vacuum oven dried bioactive components of Pleurotus floridanus. J. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Chawla, P.; Kumar, N.; Kaushik, R.; Dhull, S.B. Synthesis, characterization and cellular mineral absorption of nanoemulsions of Rhododendron arboreum flower extracts stabilized with gum arabic. J. Food Sci. Technol. 2019, 56, 5194–5203. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Citrus peels waste as a source of value-added compounds: Extraction and quantification of bioactive polyphenols. Food Chem. 2019, 295, 289–299. [Google Scholar] [CrossRef]
- Pathak, P.; Mandavgane, S.; Kulkarni, B.D. Fruit Peel Waste:Characterization and its Potential Uses. Curr. Sci. 2017, 113, 444–454. [Google Scholar] [CrossRef]
- Chawla, P.; Kumar, N.; Bains, A.; Dhull, S.B.; Kumar, M.; Kaushik, R.; Punia, S. Gum arabic capped copper nanoparticles: Synthesis, characterization, and applications. Int. J. Biol. Macromol. 2020, 146, 232–242. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [Green Version]
- Safdar, M.N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A.A. Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J. Food Drug Anal. 2017, 25, 488–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, M.; Saeed, M.T. Potential application of waste fruit peels (orange, yellow lemon and banana) as wide range natural antimicrobial agent. J. King Saud Univ. Sci. 2020, 32, 805–810. [Google Scholar] [CrossRef]
- Chika, K.; Ifeanyichukwu, I.; Malachy, U.; Benigna, O.; Adaora, C.E.; Araka, O.; Adikwu, M. Phenotypic detection of AmpC enzymes and antimicrobial susceptibility of Klebsiella spp. isolated from abattoir. Int. J. Appl. Microbiol. Biotechnol. Res. 2016, 4, 117–121. [Google Scholar]
- Chutia, M.; Bhuyan, P.D.; Pathak, M.; Sarma, T.; Boruah, P. Antifungal activity and chemical composition of Citrus reticulata Blanco essential oil against phytopathogens from North East India. LWT 2009, 42, 777–780. [Google Scholar] [CrossRef]
- Pro, E.; Mo, S.; Jb, O.; Ij, O. Comparative study on the antimicrobial effects of essential oils from peels of three citrus fruits. MOJ Biol. Med. 2019, 4, 49–54. [Google Scholar] [CrossRef]
- Do, Q.-D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Karsheva, M.; Kirova, E.; Alexandrova, S. Natural antioxidants from citrus mandarin peels. Extraction of polyphenols; effect of operational conditions on total polyphenols contents and antioxidant activity. J. Chem. Technol. Metall. 2013, 48, 35–41. [Google Scholar]
- Shin, H.-S.; Kang, S.-I.; Yoon, S.-A.; Ko, H.-C.; Kim, S.-J. Sinensetin Attenuates LPS-Induced Inflammation by Regulating the Protein Level of IκB-α. Biosci. Biotechnol. Biochem. 2012, 76, 847–849. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Chen, D.; Yu, C.; Lv, B.; Peng, J.; Wang, J.; Lin, Y. Citrus nobiletin ameliorates experimental colitis by reducing inflammation and restoring impaired intestinal barrier function. Mol. Nutr. Food Res. 2015, 59, 829–842. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, X.; Chen, H.; He, K.; Liu, Y.; Gong, J.; Gong, J. Nobiletin attenuates lipopolysaccharide/D-galactosamine-induced liver injury in mice by activating the Nrf2 antioxidant pathway and subsequently inhibiting NF-κB-mediated cytokine production. Mol. Med. Rep. 2016, 14, 5595–5600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Li, S.; Wei, C.-C.; Huang, J.; Pan, M.-H.; Shahidi, F.; Ho, C.-T. Anti-inflammatory effects of polymethoxyflavones from citrus peels: A review. J. Food Bioact. 2018, 3, 76–86. [Google Scholar] [CrossRef] [Green Version]
- Majeed, H.; Liu, F.; Hategekimana, J.; Sharif, H.R.; Qi, J.; Ali, B.; Bian, Y.-Y.; Ma, J.; Yokoyama, W.; Zhong, F. Bactericidal action mechanism of negatively charged food grade clove oil nanoemulsions. Food Chem. 2016, 197, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Najda, A. Zawartość wybranych metabolitów wtórnych i zdolność przeciwutleniająca ziela Mentha × piperita L. var. officinalis Sole f. pallescens Camus suszonego próżniowo. Przemysł Chem. 2020, 1, 125–128. [Google Scholar] [CrossRef]
- Najda, A.; Błaszczyk, L.; Winiarczyk, K.; Dyduch, J.; Tchórzewska, D. Comparative studies of nutritional and health-enhancing properties in the “garlic-like” plant Allium ampeloprasum var. ampeloprasum (GHG-L) and A. sativum. Sci. Hortic. 2016, 201, 247–255. [Google Scholar] [CrossRef]
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. Nippon. Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, R.; Chawla, P.; Kumar, N.; Janghu, S.; Lohan, A. Effect of premilling treatments on wheat gluten extraction and noodle quality. Food Sci. Technol. Int. 2018, 24, 627–636. [Google Scholar] [CrossRef]
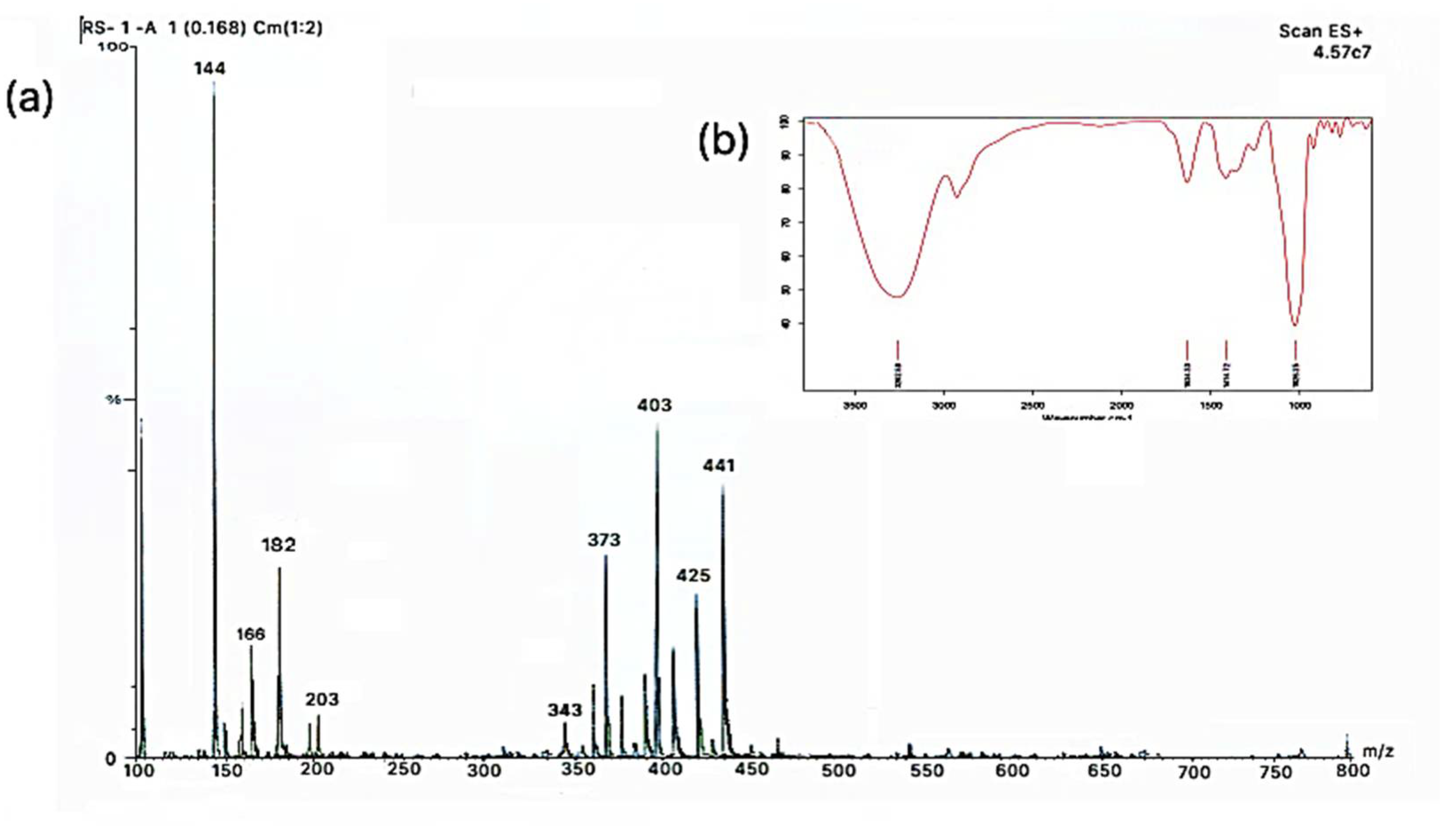


| Molecular Weight (g) | Compound Detected |
|---|---|
| 144 | Limonene |
| 166 | 4-Butoxy phenol |
| 182 | 3-(1-Piperidinyl methyl) phenol |
| 203 | Anthocyanin |
| 343 | Eupatorine |
| 373 | Sinensetin/ Tangeretin |
| 403 | Nobiletin |
| 425 | Vitexin/Iso-vitexin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, A.; Najda, A.; Bains, A.; Nurzyńska-Wierdak, R.; Chawla, P. Characterization of Citrusnobilis Peel Methanolic Extract for Antioxidant, Antimicrobial, and Anti-Inflammatory Activity. Molecules 2021, 26, 4310. https://doi.org/10.3390/molecules26144310
Malik A, Najda A, Bains A, Nurzyńska-Wierdak R, Chawla P. Characterization of Citrusnobilis Peel Methanolic Extract for Antioxidant, Antimicrobial, and Anti-Inflammatory Activity. Molecules. 2021; 26(14):4310. https://doi.org/10.3390/molecules26144310
Chicago/Turabian StyleMalik, Anjali, Agnieszka Najda, Aarti Bains, Renata Nurzyńska-Wierdak, and Prince Chawla. 2021. "Characterization of Citrusnobilis Peel Methanolic Extract for Antioxidant, Antimicrobial, and Anti-Inflammatory Activity" Molecules 26, no. 14: 4310. https://doi.org/10.3390/molecules26144310
APA StyleMalik, A., Najda, A., Bains, A., Nurzyńska-Wierdak, R., & Chawla, P. (2021). Characterization of Citrusnobilis Peel Methanolic Extract for Antioxidant, Antimicrobial, and Anti-Inflammatory Activity. Molecules, 26(14), 4310. https://doi.org/10.3390/molecules26144310








