Characterization and Caco-2 Cell Transport Assay of Chito-Oligosaccharides Nano-Liposomes Based on Layer-by-Layer Coated
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
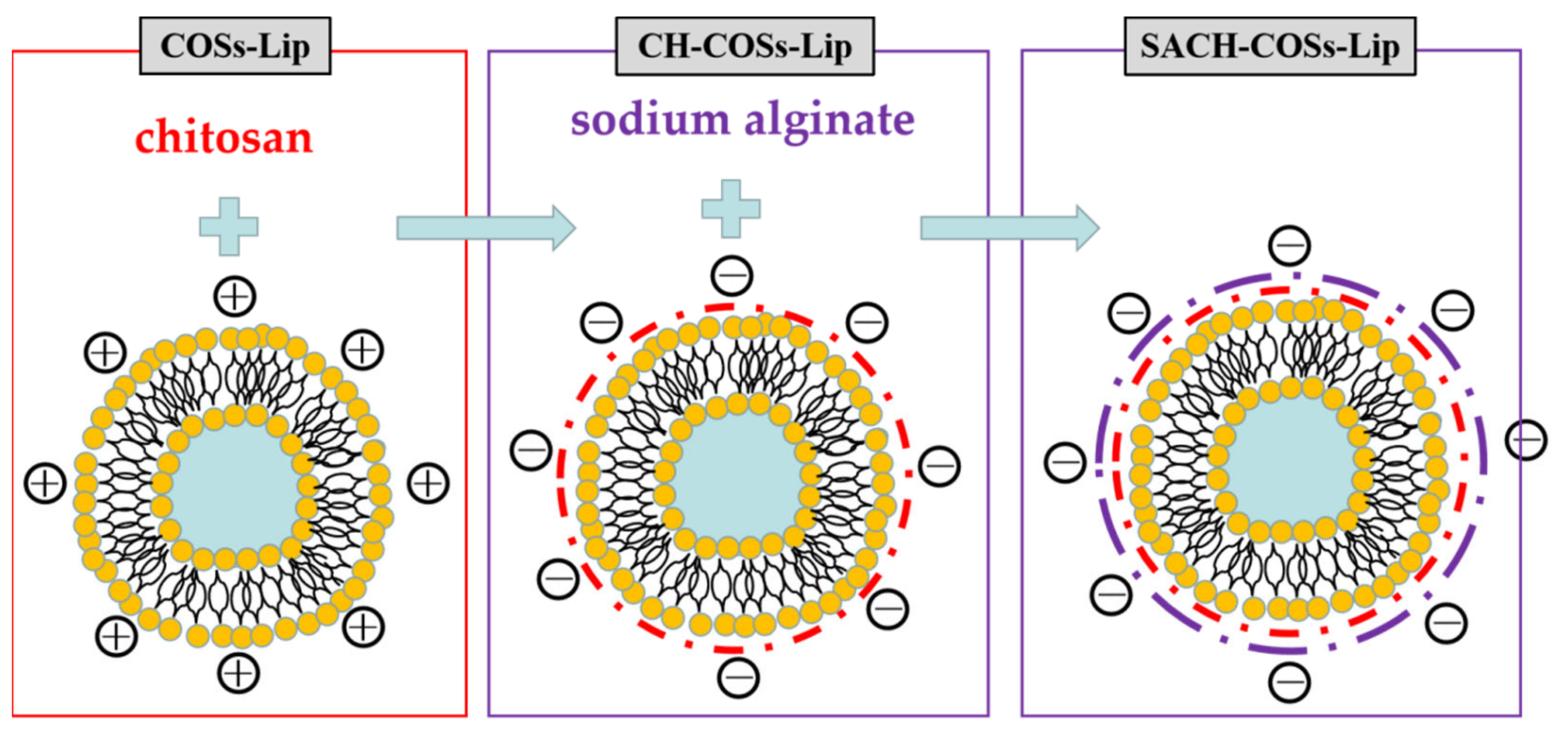
2.2. Preparation of Liposomes
2.3. Characterization of the Liposomes
2.3.1. Determination of Encapsulation Efficiency
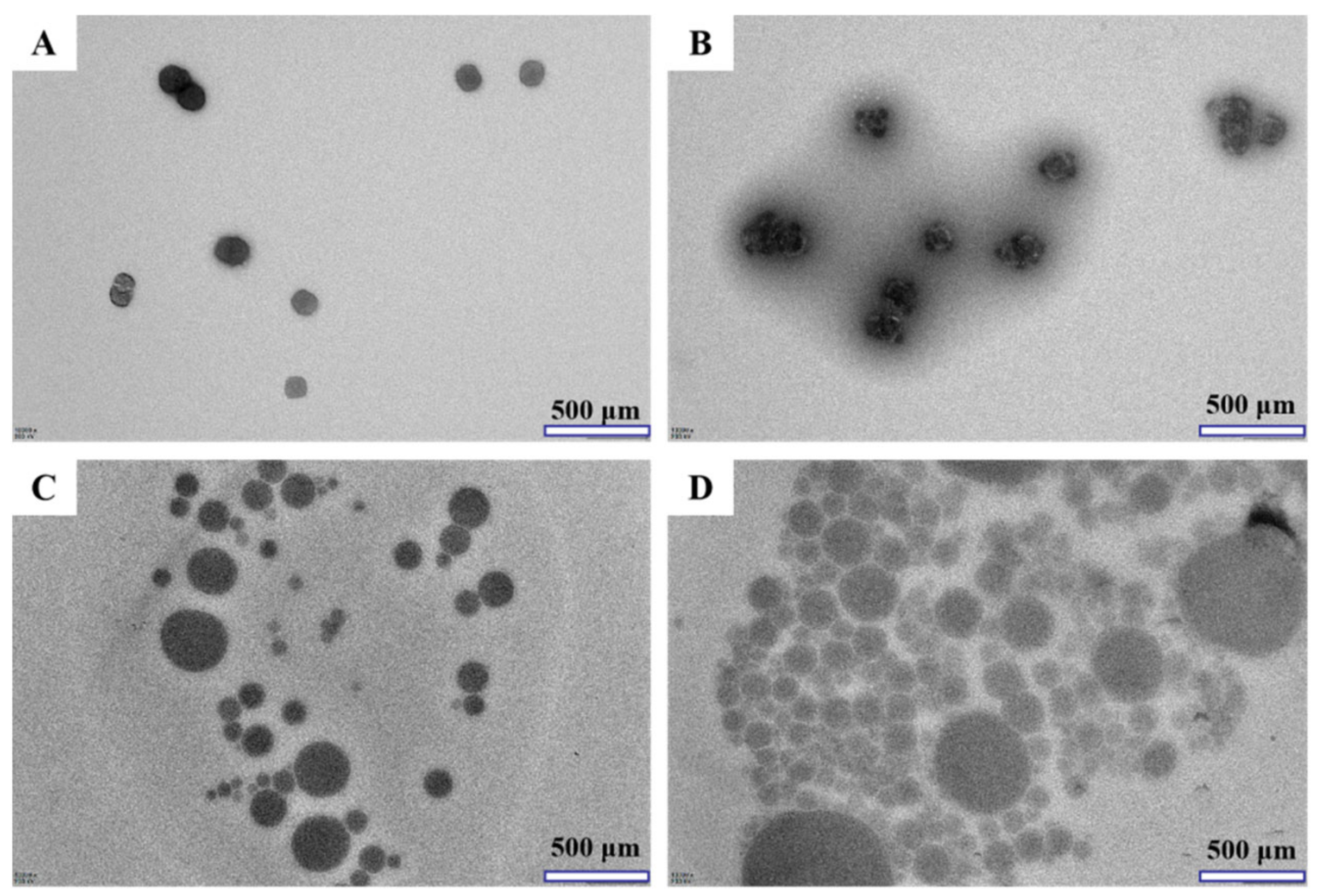
2.3.2. Transmission Electron Microscopy (TEM) Analysis
2.3.3. ζ-Potential, Z-Average Diameter (Dz), and Polydispersity Index (PDI) Analysis
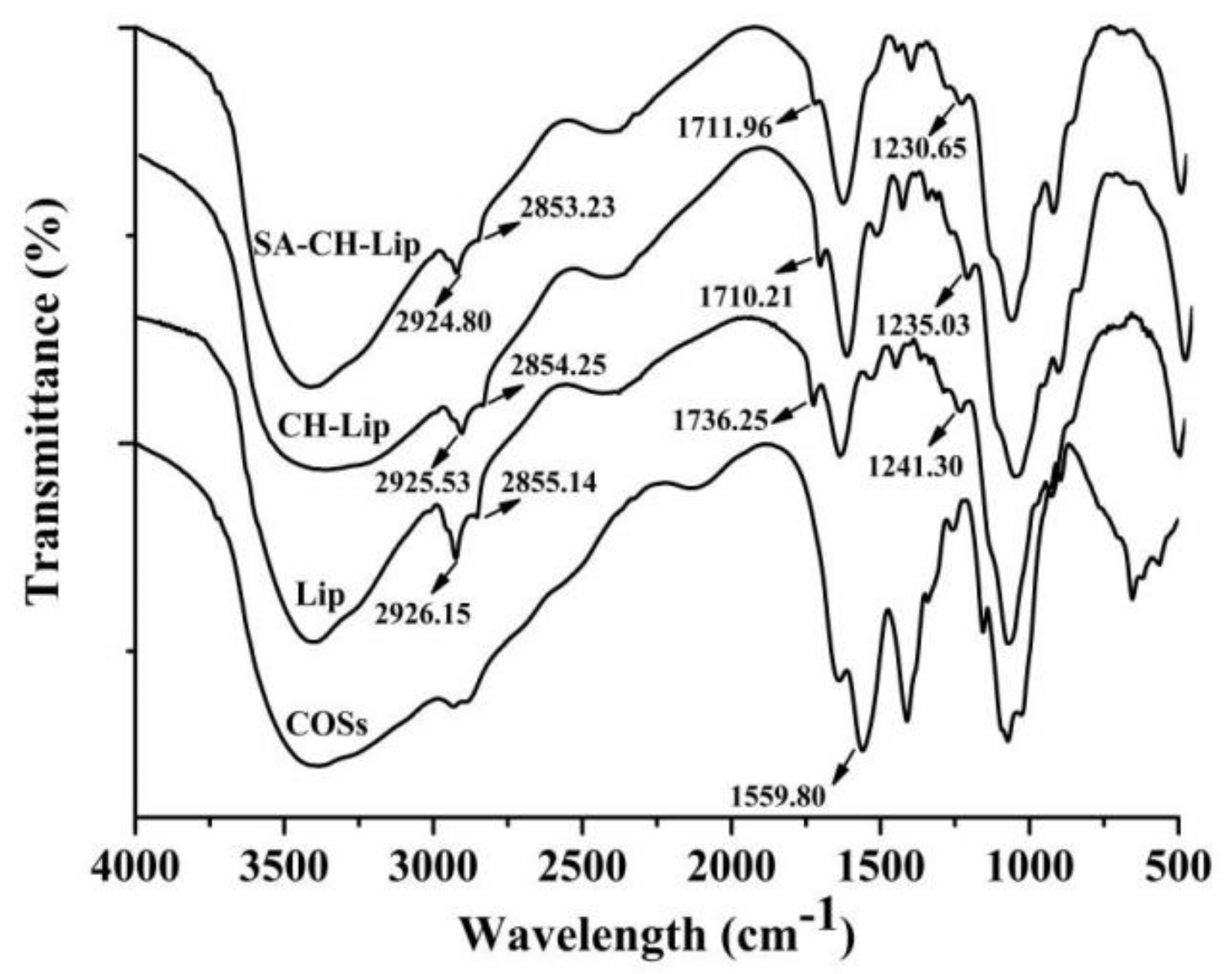
2.3.4. Fourier Transform Infrared (FTIR) Spectra Analysis
2.3.5. X-ray Diffraction (XRD) Analysis
2.4. In Vitro Digestive Stability
2.5. Absorption Characteristic of the Liposomes in Caco-2 Cells
2.5.1. MTT Assay
2.5.2. Caco-2 Cell Transport Assay
2.6. Statistical Analysis
3. Results
3.1. Characterization of the Liposomes
3.1.1. Encapsulation Efficiency
3.1.2. Dz, PDI, and ζ-Potential
3.1.3. Microstructure by TEM Analysis
3.1.4. Fourier Transform Infrared (FTIR) Spectra Analysis
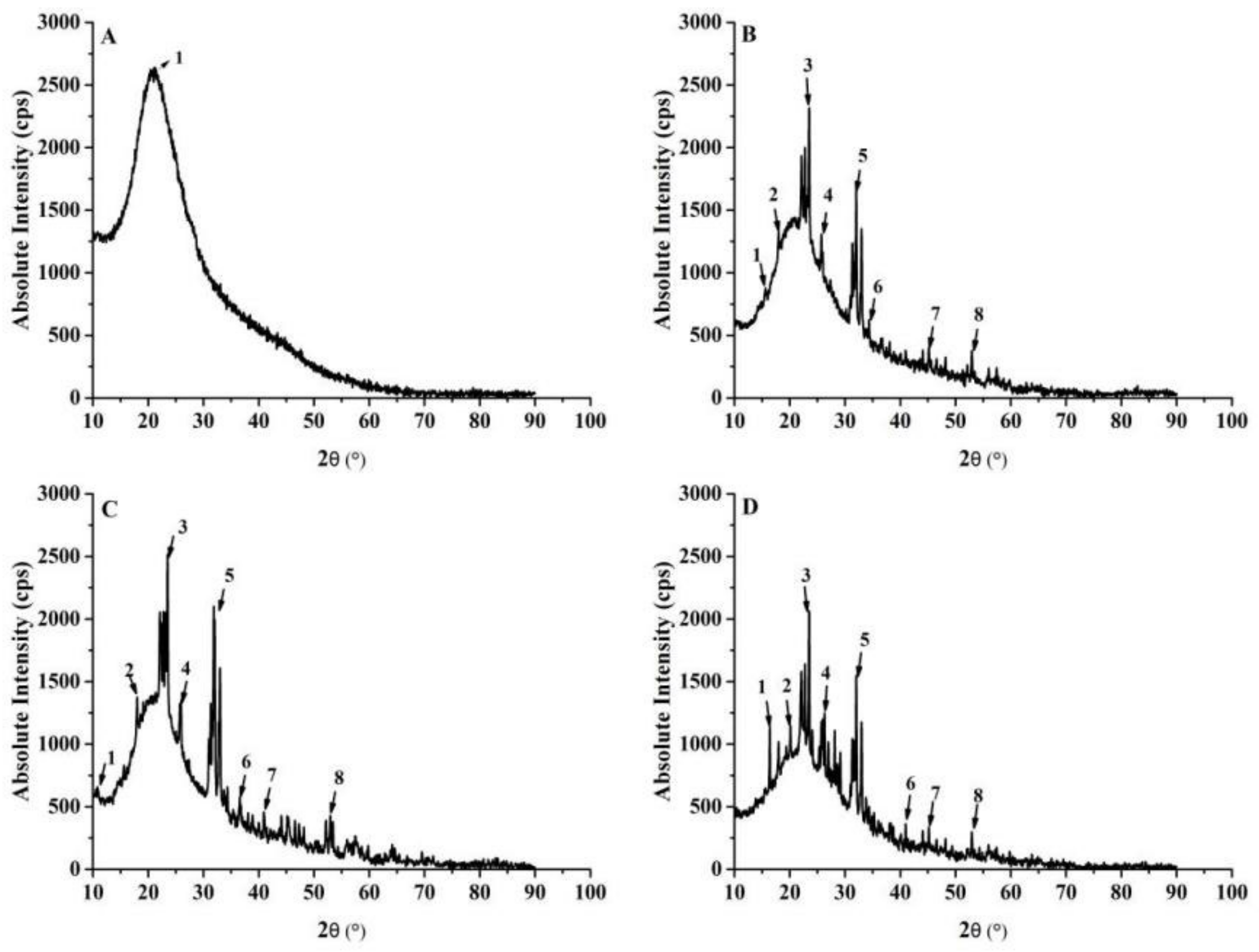
3.1.5. XRD Analysis
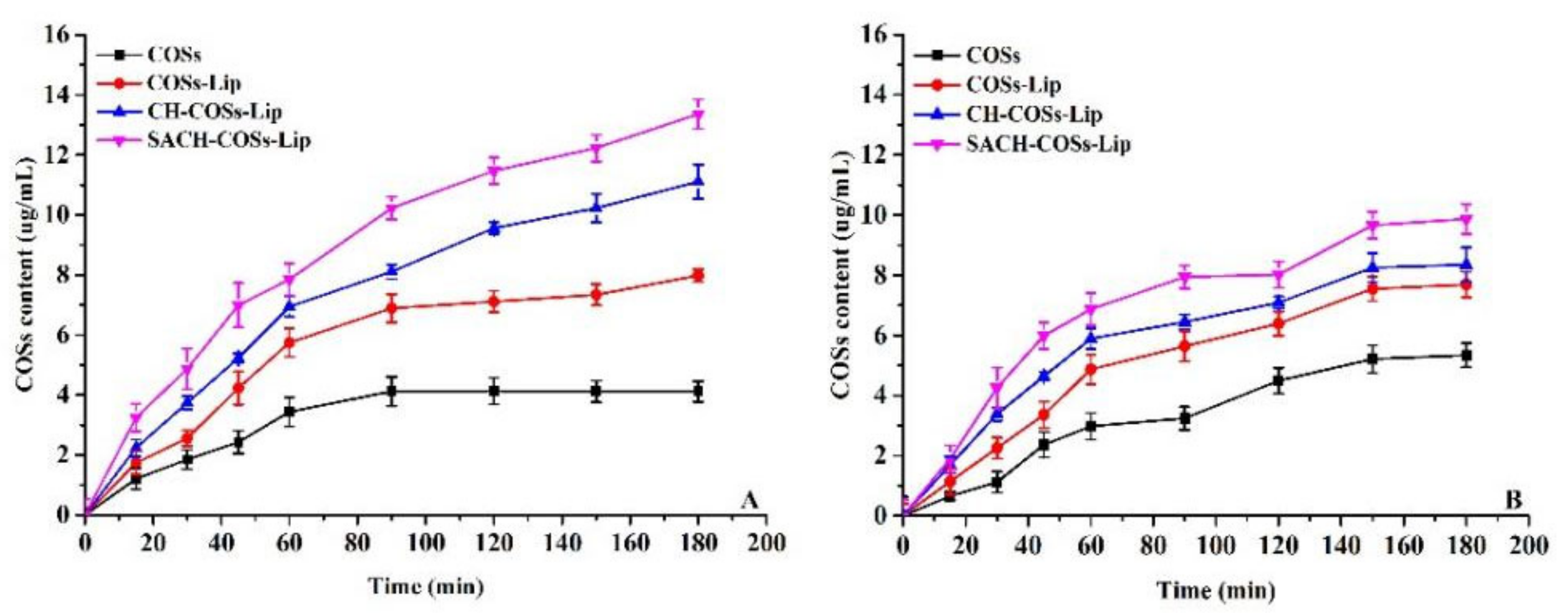
3.2. In Vitro Simulated Digestion Analysis
3.3. Cell Experiment
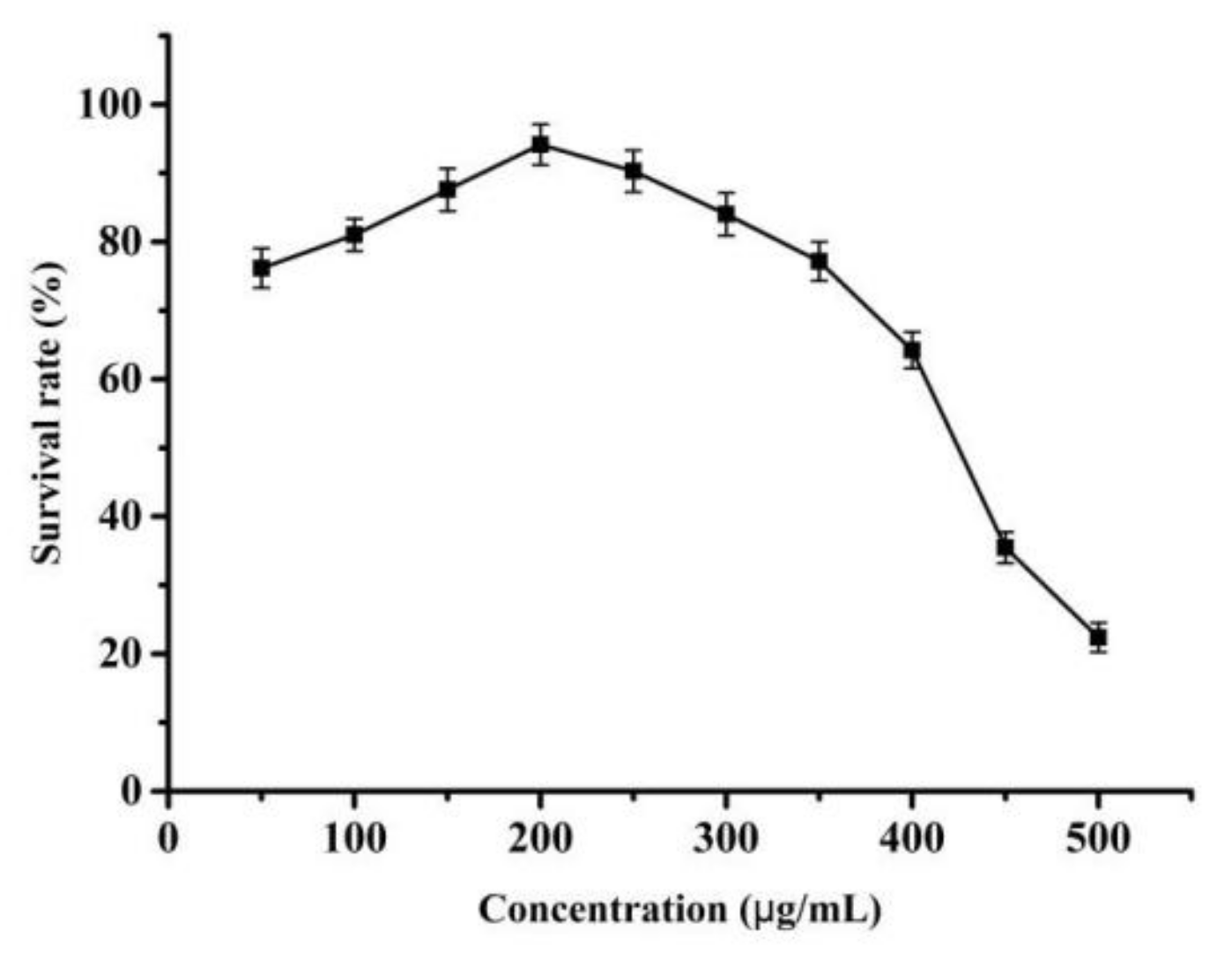
3.3.1. MTT Assay Results
3.3.2. Transport Rate of the Nano-Liposomes onto Caco-2 Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, X.; Zheng, J.; Jiao, S.; Cheng, G.; Feng, C.; Du, Y.; Liu, H. A review on the preparation of chitosan oligosaccharides and application to human health, animal husbandry and agricultural production. Carbohyd. Polym. 2019, 220, 60–70. [Google Scholar] [CrossRef]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Khan, T.M.; Akabar, M.D.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef]
- Eri, T.; Akinori, K.; Satoshi, W.; Masayoshi, S.; Yasusato, S.; Yasutada, I.; Hideaki, S.; Vaclav, M.; Peter, O.B.; Fumitaka, O. Acidic chitinase-chitin complex is dissociated in a competitive manner by acetic acid: Purification of natural enzyme for supplementation purposes. Int. J. Mol. Sci. 2018, 19, 362. [Google Scholar]
- Chatchai, M.; Preedajit, W.; Saravut, S.; Aekkacha, M.; Pawin, P.; Tharinee, M.; Rath, P.; Varanuj, C. Activation of AMPK by chitosan oligosaccharide in intestinal epithelial cells: Mechanism of action and potential applications in intestinal disorders. Biochem. Pharmacol. 2015, 96, 225–236. [Google Scholar]
- Bruna, F.; Dara, G.; Francisca, R.; Natália, M.; Tito, J.; Luiz, P. Production of low molecular weight chitosan by acid and oxidative pathways: Effect on physicochemical properties. Food Res. Int. 2019, 123, 88–94. [Google Scholar]
- Shukla, S.; Jadaun, A.; Arora, V.; Sinha, R.K.; Biyani, N.; Jain, V.K. In vitro toxicity assessment of chitosan oligosaccharide coated iron oxide nanoparticles. Toxicol. Rep. 2015, 2, 27–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natali, O.; Sandra, A.; Diana, S.; Diego, V.; Paola, O.; Claudia, O. Isolation of chitosan from Ganoderma lucidum mushroom for biomedical applications. J. Mater. Sci. Mater. Med. 2015, 26, 135. [Google Scholar]
- Seyfarth, F.; Schliemann, S.; Elsner, P.; Hipler, C. Antifungal effect of highand low-molecular-weight chitosan hydrochloride, carboxymethyl chitosan, chitosan oligosaccharide and N-acetyl-D-glucosamine against Candida albicans, Candida krusei and Candida glabrata. Int. J. Pharm. 2008, 353, 139–148. [Google Scholar]
- Sun, T.; Qin, Y.; Xu, H.; Xie, J.; Hu, D.; Xue, B.; Hua, X. Antibacterial activities and preservative effect of chitosan oligosaccharide Maillard reaction products on Penaeus vannamei. Int. J. Biol. Macromol. 2017, 105, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Hong, S.H.; Yoo, S.J.; Baek, K.S.; Jeon, Y.J.; Choung, S.Y. Differential effects of chitooligosaccharides on serum cytokine levels in aged subjects. J. Med. Food. 2006, 9, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Fukui, T.; Atomi, H.; Imanaka, T. Characterization of an exo-betaD-glucosaminidase involved in a novel chitinolytic pathway from the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. J. Bacteriol. 2003, 185, 5175–5181. [Google Scholar] [CrossRef] [Green Version]
- Benchamas, G.; Huang, L.; Huang, Y.; Huang, L. Preparation and biological activities of chitosan oligosaccharides. Trends Food Sci. Technol. 2021, 107, 38–44. [Google Scholar] [CrossRef]
- Kim, S. Liposomal drug delivery system. J. Pharm. Invest. 2016, 46, 387–392. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, S. Liposomes in drug delivery: progress and limitations. Int. J. Pharm. 1997, 154, 123–140. [Google Scholar] [CrossRef]
- Rowland, N.; Woodley, F. The stability of liposomes in vitro to pH, bile salts and pancreatic lipase. Biochim. Biophys. Acta 1980, 620, 400–409. [Google Scholar] [CrossRef]
- Wu, W.; Lu, Y.; Qi, J. Oral delivery of liposomes. Ther. Deliv. 2015, 6, 1239–1241. [Google Scholar] [CrossRef]
- Deshpande, P.; Biswas, S.; Torchilin, P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Vecchio, D.; Li, J.; Zhu, J.; Zhang, Q.; Fu, V.; Li, J.; Soracha, T.; Lu, D.; Zhang, L. Hydrogel containing nanoparticle-stabilized liposomes for topical antimicrobial delivery. ACS Nano. 2014, 8, 2900–2907. [Google Scholar] [CrossRef]
- Hermala, F.; Frischa, B.; Spechtb, A.; Bourel-Bonneta, L.; Heurtaulta, B. Development and characterization of layer-by-layer coated liposomes with poly(L-lysine) and poly(L-glutamic acid) to increase their resistance in biological media. Int. J. Pharmaceut. 2020, 586, 119568. [Google Scholar] [CrossRef]
- Balanc, B.; Trifkovic, K.; Dordevic, V.; Markovic, S.; Pjanovic, R.; Nedovic, B. Novel resveratrol delivery systems based on alginate-sucrose and alginate-chitosan microbeads containing liposomes. Food Hydrocoll. 2016, 61, 832–842. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.; Ye, A.; Peng, S.; Wei, F.; Liu, C.; Han, J. Environmental stress stability of microencapsules based on liposomes decorated with chitosan and sodium alginate. Food Chem. 2016, 196, 396–404. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; Leporatti, S.; El-Kemary, M.A. Extraction of chlorophyll and carotenoids loaded into chitosan as potential targeted therapy and bio imaging agents for breast carcinoma. Int. J. Biol. Macromol. 2021, 182, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.N. Optimally designed theranostic system based folic acids and chitosan as a promising mucoadhesive delivery system for encapsulating curcumin LbL nano-template against invasiveness of breast cancer. Int. J. Biol Macromol. 2021, 182, 1981–1993. [Google Scholar] [CrossRef] [PubMed]
- Haidar, S.; Hamdy, C.; Tabrizian, M. Protein release kinetics for core-shell hybrid nanoparticles based on the layer-by-layer assembly of alginate and chitosan on liposomes. Biomaterials 2008, 29, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, M.; Yang, Y.; Lei, B.; Ma, S.; Yu, Y. Influence of nutrients on the bioaccessibility and transepithelial transport of polybrominated diphenyl ethers measured using an in vitro method and Caco-2 cell monolayers. Ecotox. Environ. Saf. 2021, 208, 111569. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.; Lee, Y.C. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, X.; Li, H.; Du, N.; Song, S.; Hou, W. Preparation and characterization of (betamethasone sodium phosphate intercalated layered double hydroxide) @liposome nanocomposites. Colloid Surf. A 2017, 529, 824–831. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Liu, W.; Li, T.; Liu, C. Improved physical and in vitro digestion stability of a polyelectrolyte delivery system based on layer-by-layer self-assembly alginate-chitosan-coated nanoliposomes. J. Agr. Food Chem. 2013, 61, 4133–4144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Lee, H.; Song, G.; Kim, Y.; Han, H. Enhanced oral absorption of sorafenib via the layer-by-layer deposition of a pH-sensitive polymer and glycol chitosan on the liposome. Int. J. Pharmaceut. 2018, 544, 14–20. [Google Scholar] [CrossRef]
- Almalik, A.; Alradwan, I.; Kalam, M. Effect of cryoprotection on particle size stability and preservation of chitosan nanoparticles with and without hyaluronate or alginate coating. Saudi Pharmaceut. J. 2017, 25, 861. [Google Scholar] [CrossRef]
- Mohammed, A.; Sibghatullah, S.; Gamaleldin, H.; Abdullah, A.; Osman, Y.; Mohamed, B. Chitosan-coated flexible liposomes magnify the anticancer activity and bioavailability of docetaxel: Impact on composition. Molecules 2019, 24, 250. [Google Scholar]
- Zhou, F.; Xu, T.; Zhao, Y.; Song, C.; Zhang, L.; Wu, X.; Lu, B. Chitosan-coated liposomes as delivery systems for improving the stability and oral bioavailability of acteoside. Food Hydrocoll. 2018, 83, 17–24. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, R.; Liu, L.; Chi, J.; Huang, F.; Dong, L.; Ma, Q.; Jia, X.; Zhang, M. Preparation, stability and antioxidant capacity of nano liposomes loaded with procyandins from lychee pericarp. J. Food Eng. 2020, 284, 110065. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; Zhu, L.; Gan, Q.; Le, X. Temperature-dependent structure stability and in vitro release of chitosan-coated curcumin liposome. Food Res. Int. 2015, 74, 97–105. [Google Scholar] [CrossRef]
- Liu, W.; Lu, J.; Ye, A.; Xu, Q.; Tian, M.; Kong, Y.; Wei, F.; Han, J. Comparative performances of Lactoferrin-loaded liposomes under, in vitro, adult and infant digestion models. Food Chem. 2018, 78, 366–373. [Google Scholar] [CrossRef]
- Bożena, P.; Lucjan, M.; Barbara, Z.; Roman, P.; Antoni, G.; Wiesław, G. FTIR, 1H NMR and EPR spectroscopy studies on the interaction of flavone apigenin with dipalmitoyl phosphatidylcholine liposomes. Biochim. Biophys. Acta 2013, 1828, 518–527. [Google Scholar]
- Guo, X.; Wang, Y.; Qin, Y.; Shen, P.; Peng, Q. Structures, properties and application of alginic acid: A review. Int. J. Biol. Macromol. 2020, 162, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, Q.; Chu, L.; Xia, Q. Liposome-chitosan hydrogel bead delivery system for the encapsulation of linseed oil and quercetin: Preparation and in vitro characterization studies. LWT Food Sci. Technol. 2020, 117, 108615. [Google Scholar] [CrossRef]
- Kumara, N.; Bhat, R.; Prasad, S. Why chitosan could be apt candidate for glaucoma drug delivery - An overview. Int. J. Biol. Macromol. 2021, 176, 47–65. [Google Scholar] [CrossRef]
- Liu, W.; Kong, Y.; Ye, A.; Shen, P.; Dong, L.; Xu, X.; Hou, Y.; Wang, Y.; Jin, Y.; Han, J. Preparation, formation mechanism and in vitro dynamic digestion behavior of quercetin-loaded liposomes in hydrogels. Food Hydrocoll. 2020, 104, 105743. [Google Scholar] [CrossRef]

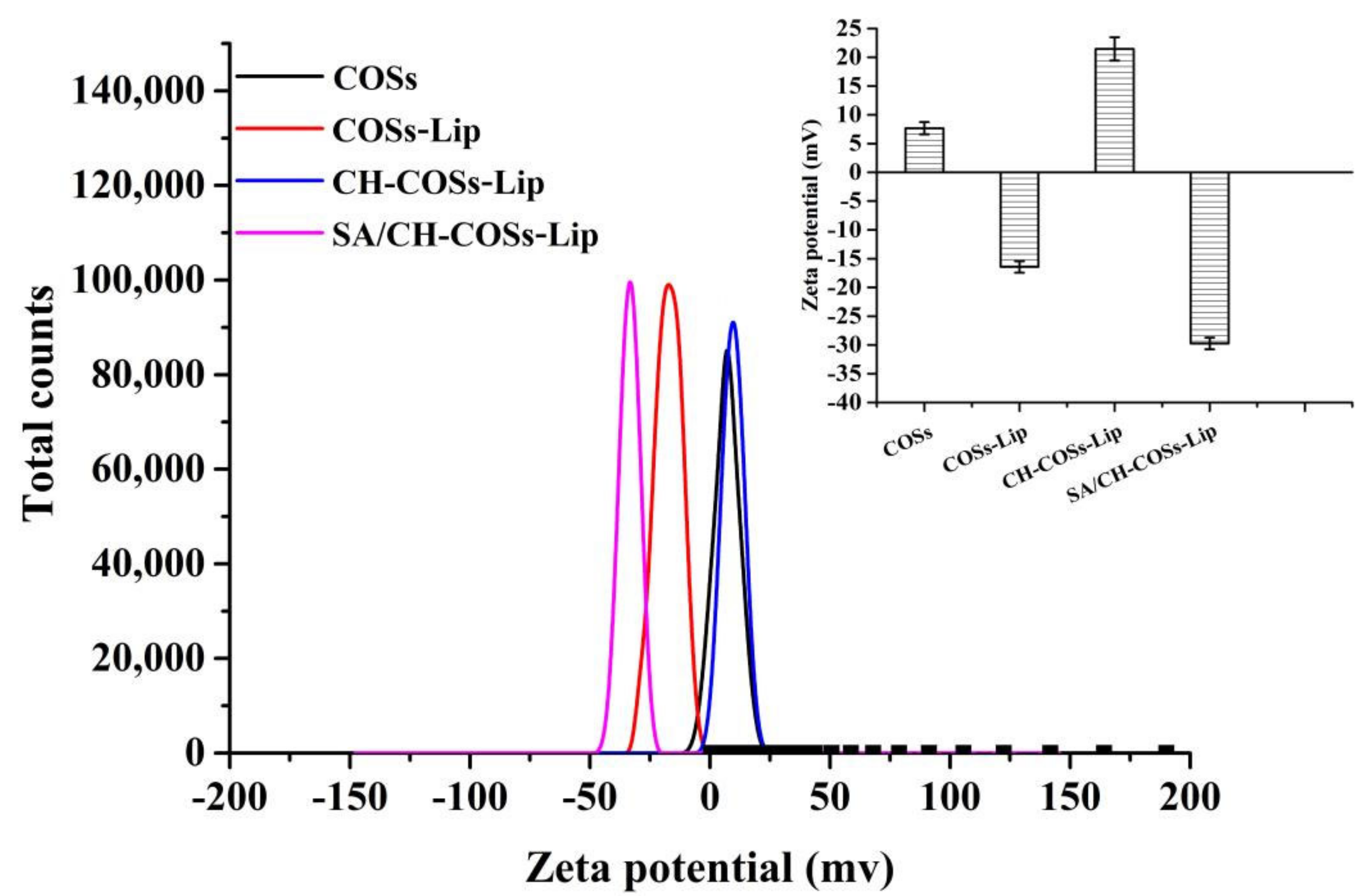

| Group | Dz (nm) | PDI | Encapsulation Efficiency (%) |
|---|---|---|---|
| COSs | 187.30 ± 5.20 a | 0.118 ± 0.097 a | – |
| COSs-Lip | 201.44 ± 4.68 b | 0.227 ± 0.145 b | 61.23 ± 2.09 c |
| CH-COSs-Lip | 256.47 ± 5.62 c | 0.283 ± 0.134 c | 82.34 ± 4.53 b |
| SA/CH-COSs-Lip | 331.19 ± 6.75 d | 0.331 ± 0.085 d | 94.45 ± 3.08 a |
| Group | PappAB (×10−6 cm/s) | PappBA (×10−6 cm/s) |
|---|---|---|
| COSs | 14.46 ± 0.34 d | 4.31 ± 0.29 a |
| COSs-Lip | 20.07 ± 2.65 c | 4.87 ± 0.34 a |
| CH-COSs-Lip | 24.44 ± 1.57 b | 4.90 ± 0.43 a |
| SA/CH-COSs-Lip | 28.89 ± 3.41 a | 4.76 ± 0.28 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, T.; Jia, A.; Yao, M.; Zhang, M.; Sun, C.; Shi, Y.; Liu, X.; Sun, J.; Liu, C. Characterization and Caco-2 Cell Transport Assay of Chito-Oligosaccharides Nano-Liposomes Based on Layer-by-Layer Coated. Molecules 2021, 26, 4144. https://doi.org/10.3390/molecules26144144
Cui T, Jia A, Yao M, Zhang M, Sun C, Shi Y, Liu X, Sun J, Liu C. Characterization and Caco-2 Cell Transport Assay of Chito-Oligosaccharides Nano-Liposomes Based on Layer-by-Layer Coated. Molecules. 2021; 26(14):4144. https://doi.org/10.3390/molecules26144144
Chicago/Turabian StyleCui, Tingting, Airong Jia, Mengke Yao, Miansong Zhang, Chanchan Sun, Yaping Shi, Xue Liu, Jimin Sun, and Changheng Liu. 2021. "Characterization and Caco-2 Cell Transport Assay of Chito-Oligosaccharides Nano-Liposomes Based on Layer-by-Layer Coated" Molecules 26, no. 14: 4144. https://doi.org/10.3390/molecules26144144
APA StyleCui, T., Jia, A., Yao, M., Zhang, M., Sun, C., Shi, Y., Liu, X., Sun, J., & Liu, C. (2021). Characterization and Caco-2 Cell Transport Assay of Chito-Oligosaccharides Nano-Liposomes Based on Layer-by-Layer Coated. Molecules, 26(14), 4144. https://doi.org/10.3390/molecules26144144







