Abstract
The strong catalytic performance, eco-friendly reaction systems, and selectivity of enzyme-based biocatalysts are extremely interesting. Immobilization has been shown to be a good way to improve enzyme stability and recyclability. Chitosan-incorporated metal oxides, among other support matrices, are an intriguing class of support matrices for the immobilization of various enzymes. Herein, the cross-linked chitosan/zinc oxide nanocomposite (CS/ZnO) was synthesized and further improved by adding iron oxide (Fe2O3) nanoparticles. The final cross-linked CS/ZnO/Fe2O3 nanocomposite was used as an immobilized support for catalase and is characterized by SEM, EDS, and FTIR. The nanocomposite CS/ZnO/Fe2O3 enhanced the biocompatibility and immobilized system properties. CS/ZnO/Fe2O3 achieved a higher immobilization yield (84.32%) than CS/ZnO (37%). After 10 repeated cycles, the remaining immobilized catalase activity of CS/ZnO and CS/ZnO/Fe2O3 was 14% and 45%, respectively. After 60 days of storage at 4 °C, the remaining activity of immobilized enzyme onto CS/ZnO and CS/ZnO/Fe2O3 was found to be 32% and 47% of its initial activity. The optimum temperature was noticed to be broad at 25–30 °C for the immobilized enzyme and 25 °C for the free enzyme. Compared with the free enzyme optimum pH (7.0), the optimum pH for the immobilized enzyme was 7.5. The Km and Vmax values for the free and immobilized enzyme on CS/ZnO, and the immobilized enzyme on CS/ZnO/Fe2O3, were found to be 91.28, 225.17, and 221.59 mM, and 10.45, 15.87, and 19.92 µmole ml−1, respectively. Catalase immobilization on CS/ZnO and CS/ZnO/Fe2O3 offers better stability than free catalase due to the enzyme’s half-life. The half-life of immobilized catalase on CS/ZnO/Fe2O3 was between 31.5 and 693.2 min.
1. Introduction
Biopolymer-based organic–inorganic nanocomposites gain excellent properties from both components as a result of synergy. The properties include biodegradability, biocompatibility, renewability, enhanced mechanical properties, and non-toxicity. Hence, these nanocomposite materials are of considerable attention [1,2,3,4,5]. Chitosan is an amino polysaccharide formed by chitin deacetylation and consists of randomly distributed units of (1,4)-linked-glucosamine and N-acetyl-d-glucosamine [6]. At present, chitosan is a versatile, easily processable cationic biomaterial that can be widely used in medical, antibacterial dressings, tissue engineering and enzyme immobilization applications [7,8,9]. The incorporation of chitosan–inorganic nanocomposites improves their weak mechanical strength, antibacterial properties, adsorption, and drug-delivery properties [10,11,12]. Nanoparticles may have some disadvantages, including externally exposed enzymes, and proteolysis may occur.
ZnO nanoparticles have been extensively researched for several technical applications such as cancer treatment catalysis, chemical absorbance, and antibacterial, cosmetic and pharmaceutical industries [13,14]. Nevertheless, because of the lack of proper functional groups, naked ZnO nanoparticles cannot sufficiently stabilize enzymes. It has been reported that a ZnO/chitosan nanocomposite was used successfully in food packaging [15]. ZnO/chitosan nanocomposites have never been used as supports for the catalase enzyme, to the best of our knowledge. However, ZnO/chitosan can be effective to immobilize enzymes, but the immobilization yield is low due to the lack of stability of the resulting beads, and the leakage of enzymes which occurred due to the loose structure of the nanocomposite beads. It was envisioned that adding different contents of iron oxide (Fe2O3) nanoparticles into a ZnO/chitosan nanocomposite would lead to beads with a more compact membrane and network, thus making it difficult for the enzyme to leak out from the beads and improve the stability.
Enzymes are versatile, highly effective, and eco-friendly catalysts that operate under mild conditions [16]. Free enzymes experience operational challenges (generation of toxic by-products, temperature variation, and pH). Enzyme immobilization is the ultimate solution, allowing enzyme recovery (an essential prerequisite for enzyme reuse). It also provides stability to enzymes against changing conditions in a reaction media, because enzymes are responsive to reaction changes and are thus denatured in adverse conditions [17,18,19].
Therefore, immobilization provides multiple advantages over free enzymes under identical reaction conditions, enhancing catalytic efficiency and enzyme recycling [20,21]. Catalase (EC 1.11.1.6) is a heme-containing metalloenzyme and is regarded as one of the most common enzymes in plant and animal tissues. Catalase, which decomposes harmful hydrogen peroxide to water and molecular oxygen, has been used for a long time in industry [22,23].
Catalase has a short shelf life and poor operating stability, which reduces its potential for use. The technique for overcoming these problems is to immobilize catalase on solid supports, e.g., on a ZnO/chitosan nanocomposite incorporated with Fe2O3. The aim of this study was to immobilize catalase on a ZnO/Fe2O3 chitosan nanocomposite. Catalase, primarily used in the food industry, was chosen as a model enzyme for the immobilization experiment; the morphological characters, kinetic parameters, reusability, and storage stability of the immobilized enzyme were investigated.
2. Results and Discussion
2.1. Immobilization of Catalase Enzyme
In the present study, to obtain the suitable beads as supports for catalase, different concentrations of ZnO Np (0.2–0.6 g) were mixed with 2% chitosan, obtaining suitable beads (Table S1, supplementary material). Catalase was immobilized on ZnO/chitosan at different pH values (6, 7, and 8) (Table S1). The maximum immobilization yield of catalase (37%) was detected at 0.4g ZnO Np and pH 8.0, with 183.5 unit/g support and 417 units of immobilized catalase/mg protein loaded (Table 1). The lowering in immobilization yield could be due to a lack of stability of the resulting beads and enzyme leakage due to the loose nanocomposite bead structure. To improve the immobilization yield, different concentrations of iron (III) oxide (0.1–0.3 g) were mixed with 0.4g ZnO Np and 2% chitosan. The maximum immobilization yield of catalase (84.32%) was detected at 0.2g Fe2O3 Np and pH 8.0, with 500 unit/g support and 855 units of immobilized catalase/mg protein loaded (Table 1). The improvement in immobilization enzyme can be attributed to the strong interaction between the amino group in chitosan with iron (III) oxide and zinc oxide [24]. In previous work, chitosan activated with cyanuric chloride was used as a support for peroxidase, with the maximum immobilization of 60% [9]. Pinheiro et al. (2020) reported that lipase B was immobilized on chitosan activated with divinyl sulfone with immobilization yield of 68.13% [25].

Table 1.
Effect of zinc oxide and iron (III) oxide nanoparticles on the immobilization process.
2.2. ATR–FTIR
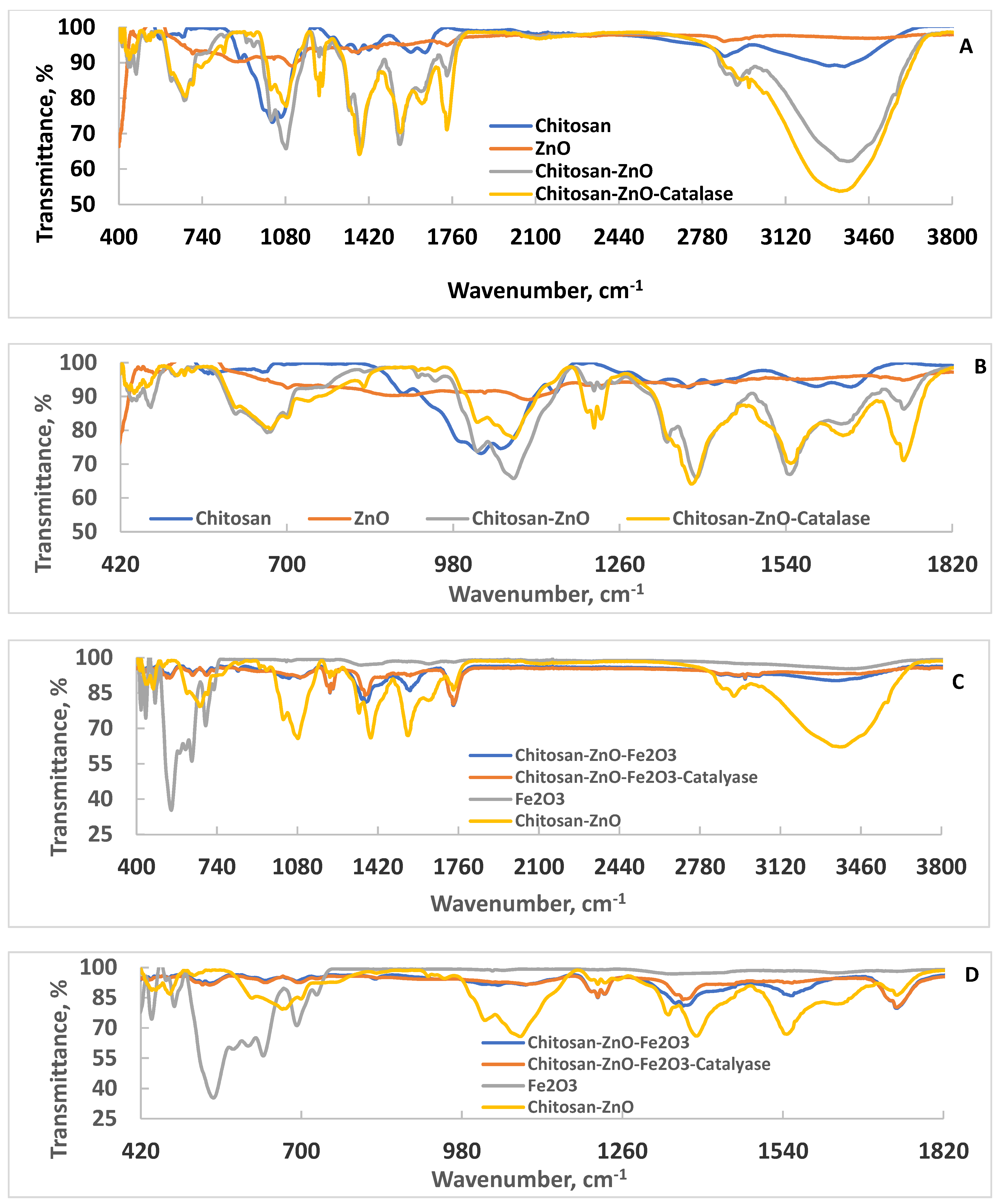
The ATR–FTIR spectra of chitosan, ZnO, chitosan–ZnO, and chitosan–ZnO–catalase are shown Figure 1. The typical characteristic vibration peaks of amide I (C=O stretch), II (C–N stretch, NH bend), and III (C–N stretch, NH bend) of chitosan appeared at 1379, 1596 and 1651 cm−1, respectively. Two NH stretching peaks due to amino groups appeared at 3306 and 3361 cm−1. The peak that appeared at 3878 cm−1 was due to the CH stretching vibration of CH2. The glucoside linkage (C–O–C) stretching peak appeared at 1028 cm−1 [9,26]. For ZnO nanoparticles, the stretching vibration due to Zn–O appeared at 410 (strong) and 480 (weak) cm−1 [27,28]. Mixing ZnO nanoparticles with chitosan changed the characteristic peaks of both components. It is clearly observed that the strong stretching Zn–O peak disappeared, whereas the weak one changed and its intensity increased with the appearance of new peaks, and blue shift of the C–O–C peak indicated mutual interactions between ZnO nanoparticles and chitosan matrix that led to the formation of a gel-like structure, as also indicated by the increased intensity of the amino and hydroxyl groups present in chitosan (3382 cm−1). Upon catalase immobilization, changes in the vibration peaks further occurred. The broad peak due to amino and hydroxyl groups was enlarged and appeared with a redshift at 3350 cm−1. Additionally, the C–O–C peak and ZnO peaks blue-shifted after catalase immobilization. These data suggest that the nanocomposite could immobilize the catalase enzyme. Interestingly, when the chitosan–ZnO nanocomposite was mixed with Fe2O3 nanoparticles, the obtained nanocomposite was compacted, and its ATR–FTIR pattern appeared completely different after adding Fe2O3 (compare Figure 1A,B with Figure 1C,D), indicating good compatibility and interactions of Fe2O3 and the chitosan–ZnO nanocomposite. Figure 1C,D show that the broad peak intensity due to amino and hydroxyl groups present in the chitosan–ZnO nanocomposite decreased after mixing the sample with Fe2O3 nanoparticles. Additionally, the amide II and the peaks due to Fe2O3 that appeared in the range 418–728 [29] almost disappeared upon mixing Fe2O3 nanoparticles with the chitosan–ZnO nanocomposite. Interestingly, upon catalase immobilization onto the chitosan–ZnO–Fe2O3 nanocomposite sample, the ATR–FTIR changed to reveal new peaks and the appearance of strong, broad peaks due to NH2, amide and OH groups present in the catalase, strong amide bands, and reappearing C–O–C peaks, confirming the success of catalase enzyme immobilization.
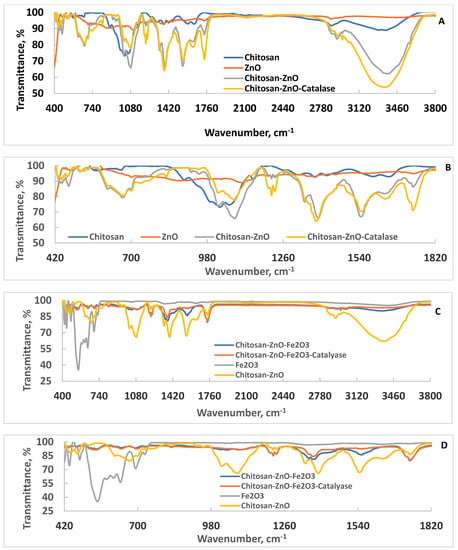
Figure 1.
ATR–FTIR spectra of chitosan, ZnO, chitosan–ZnO, and chitosan–ZnO–catalase ((A), full scale; (B), expanded region), and ATR–FTIR of chitosan–ZnO–Fe2O3, chitosan–ZnO–Fe2O3–catalase, Fe2O3, and chitosan–ZnO ((C), full scale; (D), expanded region).
2.3. SEM–EDX
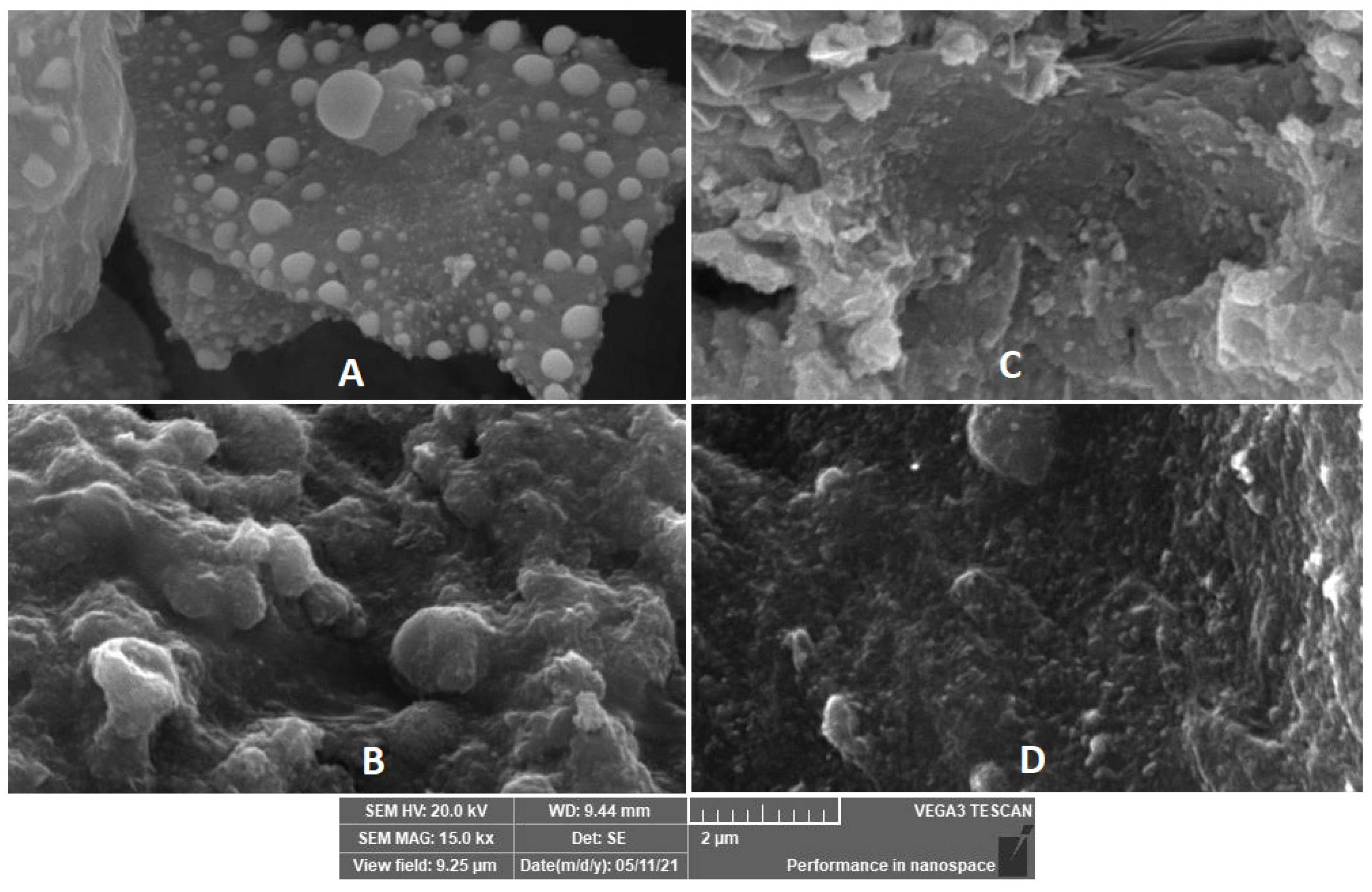
The textures of the nanocomposites are shown in Figure 2A–D. It is clear that the rough chitosan matrix is embedded with white spherical ZnO nanoparticles in Figure 2A. Upon adding the catalase enzyme, new spongy-like spheres appeared, indicating the successful immobilization. Similarly, Figure 2C is entirely different from Figure 2A, due to the coverage of Fe2O3 that compacted the morphology with the disappearance of the white spheres of ZnO. Upon catalase enzyme immobilization on sample C, a new morphology was obtained, in which spongy-like spheres due to catalase enzymes were observed (sample D). Additionally, EDX as a tool for surface elemental analysis was performed for all samples, and the results indicated the presence of all elements present in these samples to further confirm the synthesis of these nanocomposites and the success of enzyme immobilization (see Figures S4–S7 EDX samples A, B, C, and D, supplementary material).
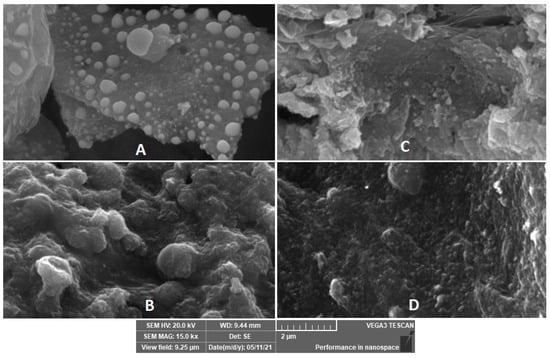
Figure 2.
SEM images of chitosan–ZnO (A), chitosan–ZnO–catalase (B), chitosan–ZnO–Fe2O3 (C), and chitosan–ZnO–Fe2O3–catalase (D).
2.4. Reusability and Storage Stability
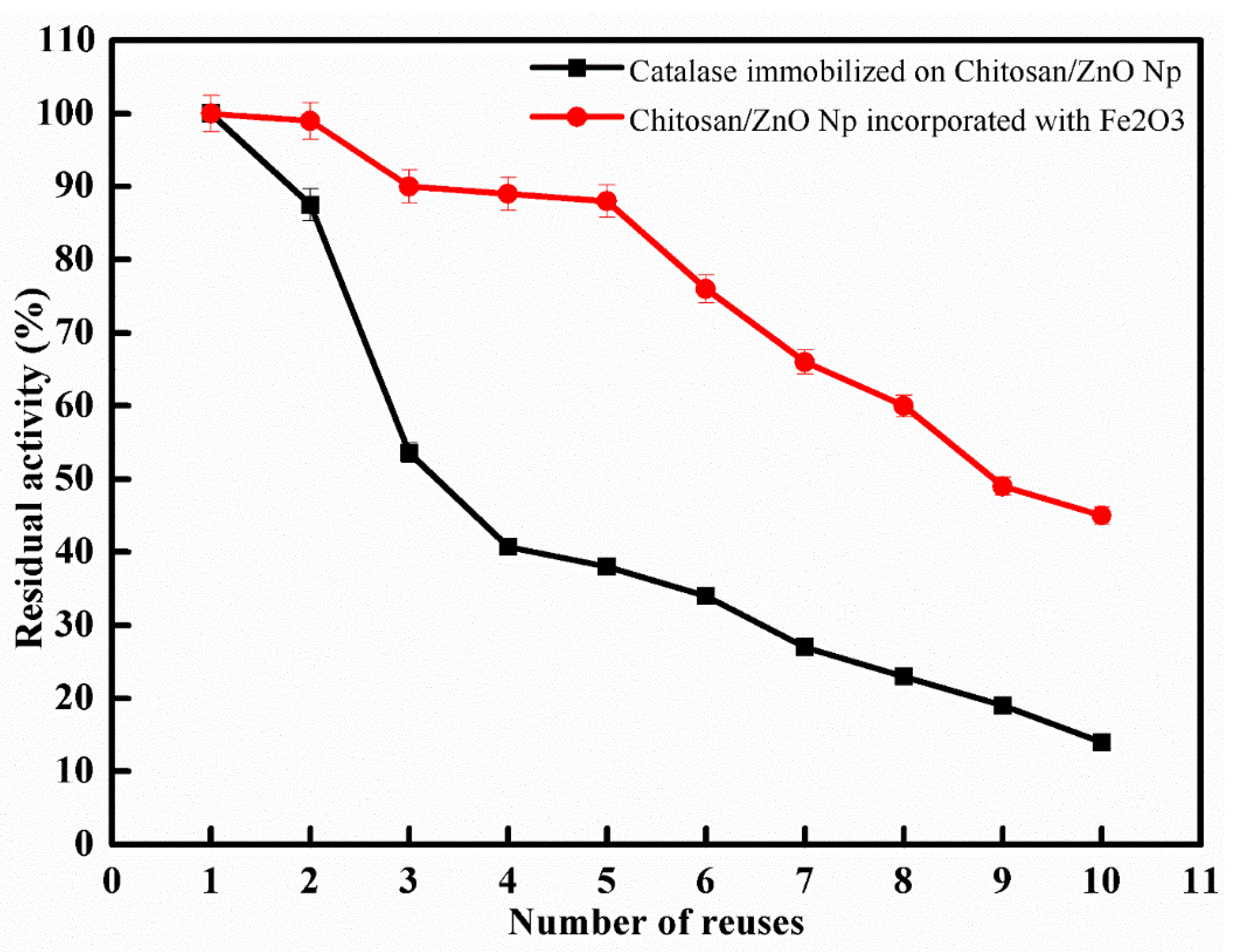
The most critical aspect of immobilized enzymes for both economical and industrial applications is their reusability. The reusability study was carried out under optimal conditions, and enzyme activity was investigated for ten reuses. As shown in Figure 3, all samples showed a moderate decrease in enzymatic activity up to 10 cycle applications. The result showed that the immobilized enzyme onto CS/ZnO and CS/ZnO/Fe2O3 retained 38% and 88% of its original activity after five cycles while retaining 14% and 45% of its original activity after 10 cycles. The biocatalyst produced via incorporating Fe2O3 with CS/ZnO improved the reusability. The reduction in enzymatic activity observed during recycling could be attributed to frequent interactions between the substrate and the active site of the immobilized enzyme, distorting the active site, resulting in activity loss [30,31,32]. In several studies, immobilized catalase on Eupergit C lost about 50% of its activity after 22 cycles [33], but when using chitosan beads and chitosan bentonite beads as supports for catalase, the enzyme retained about 50% and 70% of its original activity after 20 cycles, respectively [34].
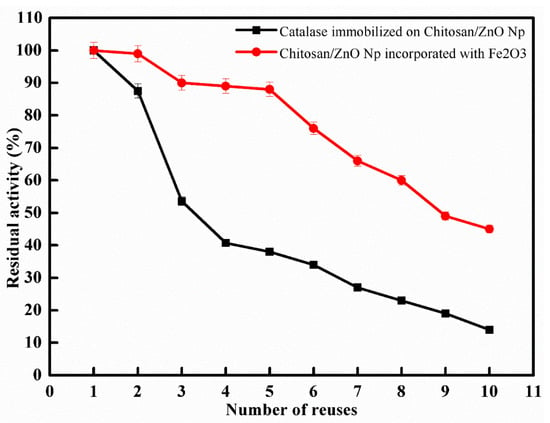
Figure 3.
Reuse of immobilized catalase on CS/ZnO and CS/ZnO/Fe2O3 (means ± S.E, n = 3).
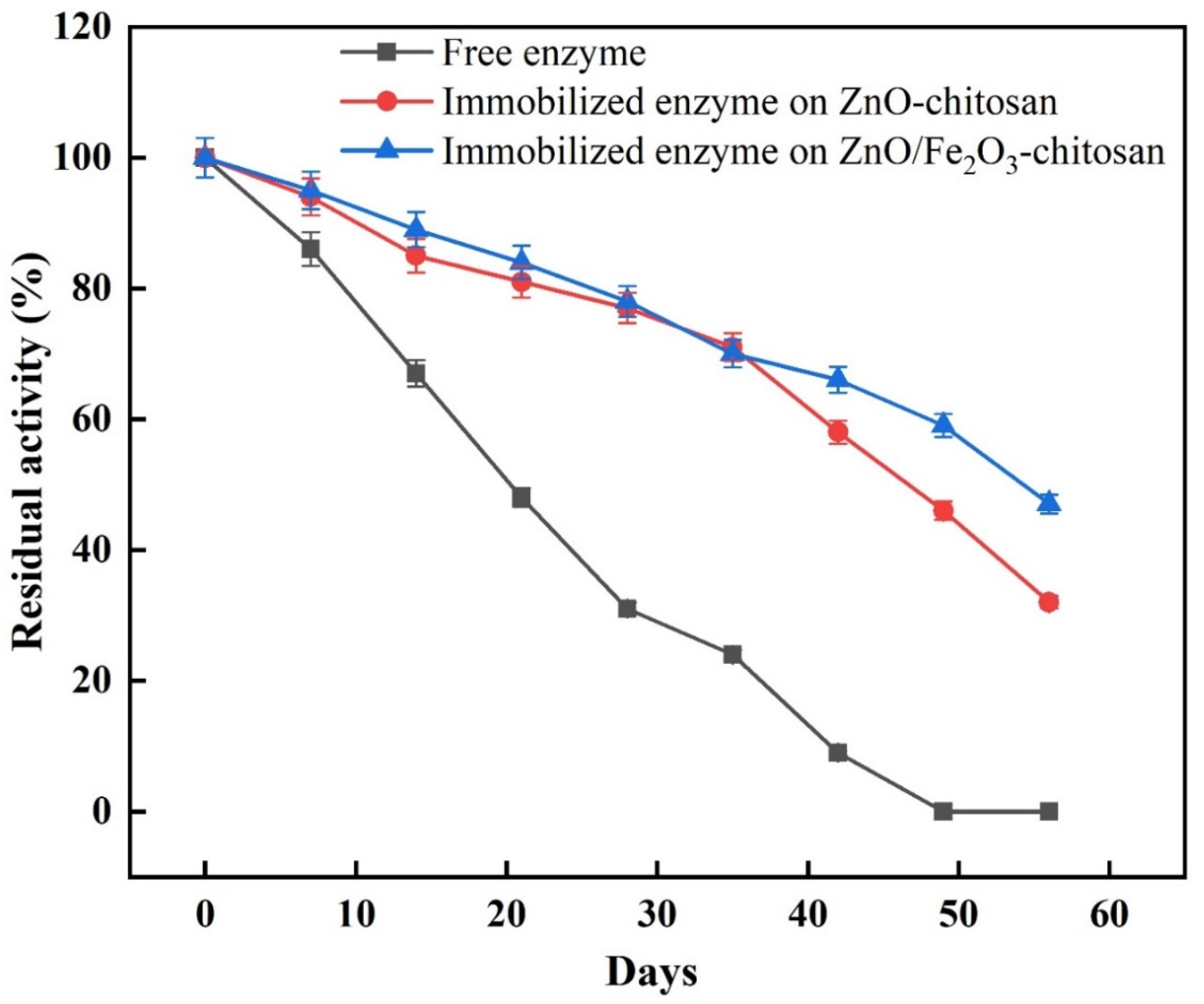
To determine storage stability, the activities of free and immobilized enzymes were determined after two months of storage at 4 °C. The activity was determined every 7 days. The immobilization process improved the enzyme’s storage stability (Figure 4). The results showing that the immobilized enzyme onto CS/ZnO and CS/ZnO/Fe2O3 exhibited 46% and 59% of its original activity, respectively, after 7 weeks of storage, whereas the free enzyme, over the same period, lost its activity. Therefore, immobilization significantly reduces enzyme deactivation and enhances the enzyme stability. In several studies, immobilized catalase on copper-adsorbed chitosan beads lost about 50% of its activity after 60 days [22], but when using chitosan bentonite beads as a support for catalase, the enzyme retained about 60% of its activity after 60 days [34].
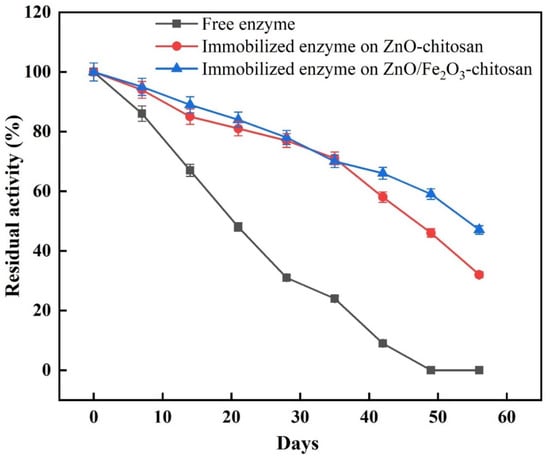
Figure 4.
Effect of storage stability on the free and immobilized enzyme at 4 °C.
2.5. Biochemical and Physicochemical Characterization
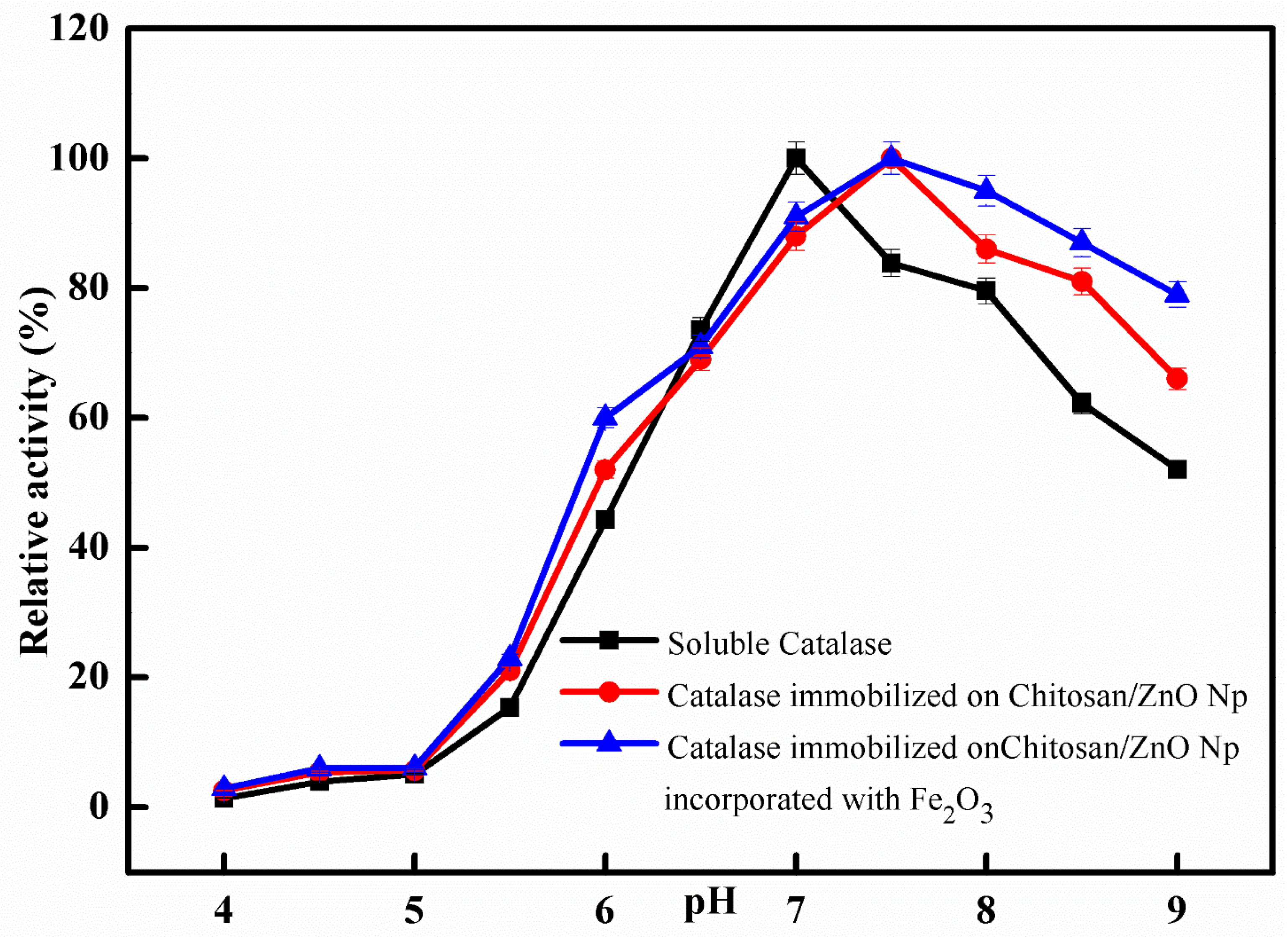
The impact of pH on the catalytic activity of free and immobilized catalase was comparatively studied at different pH values from 4.0 to 9.0. The obtained results are depicted in the graph shown in Figure 5; the optimum pH for free catalase activity was identified at pH 7.0, while it was shifted to pH 7.5 after immobilization. The activity of immobilized catalase on CS/ZnO/Fe2O3 was greater than that of immobilized catalase on CS/ZnO and free catalase at a series of pH scales (4.0–9.0), according to the results of both free and immobilized catalase. The optimum activity exhibited by free catalase and immobilized on chitosan bentonite was recorded at pH 7.5 and 8.0, respectively [34]. For comparison, the optimum activity exhibited by free catalase and immobilized on Eupergit C was recorded at pH 7.5 and 7.0, respectively [22]. The supporting secondary interactions between the immobilized enzyme on nanoparticles and the chitosan matrix are believed to be responsible for the pH shift towards alkaline values after immobilization [35,36].
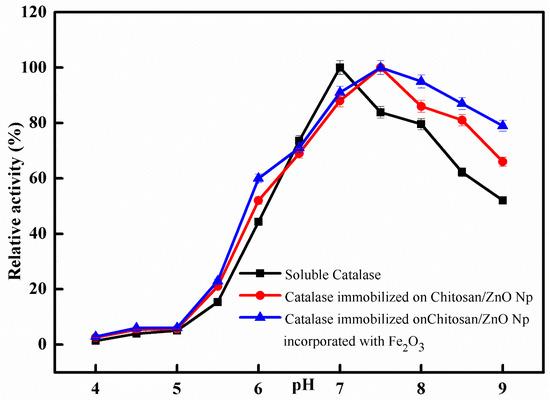
Figure 5.
Optimum pH for free and immobilized catalase. Each point represents the mean of three experiments ± S.E.
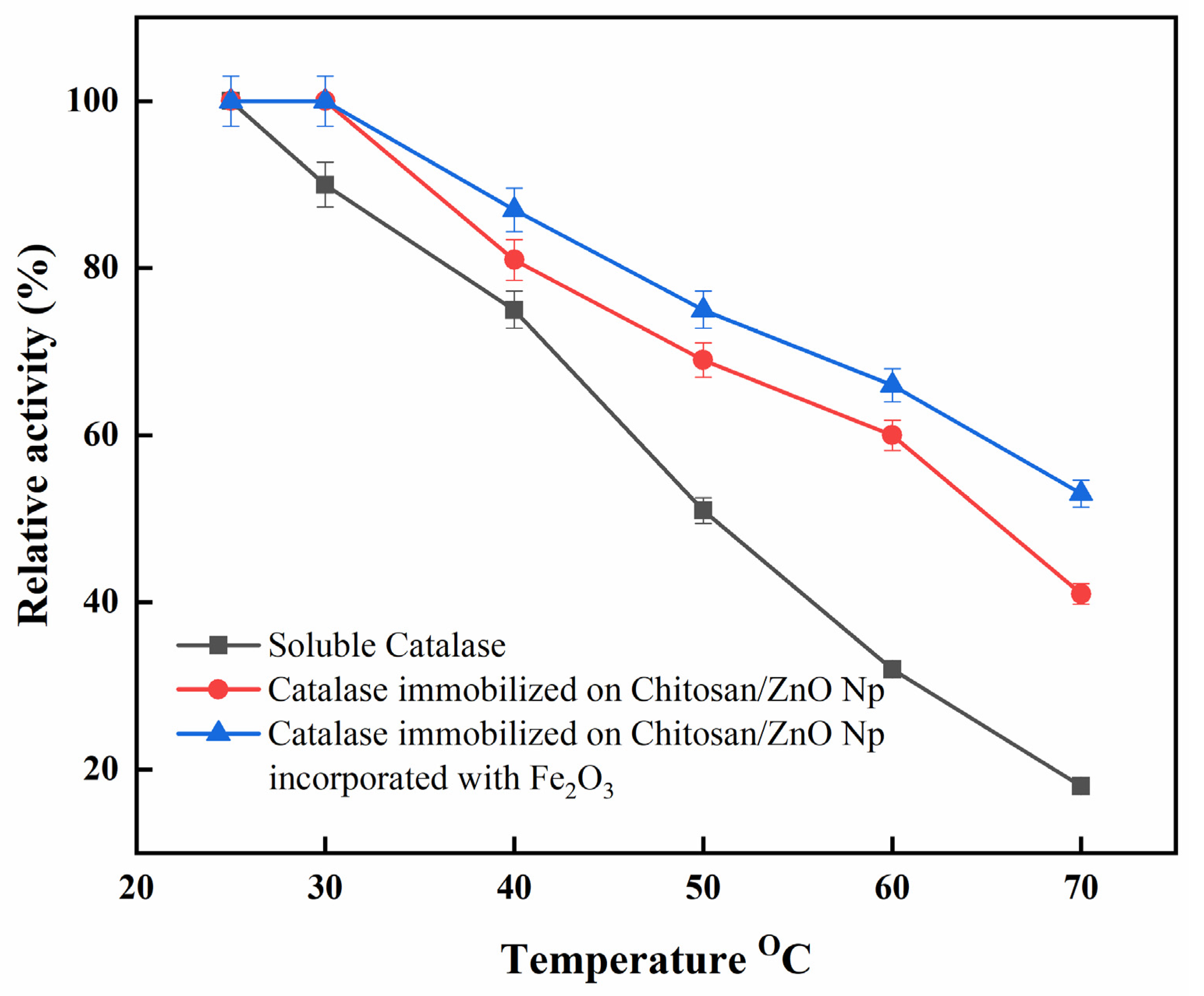
Figure 6 shows the influence of temperature on the activities of immobilized and free catalase. Data were standardized with respect to the highest activity exhibited by free and immobilized catalase in ideal conditions to better compare the behavior of catalase in different temperature conditions. The optimum relative activity of free catalase was recorded at 25 °C. However, immobilized catalase had a broad highest relative activity, approximately between 25 and 30 °C. The immobilized catalase on CS/ZnO/Fe2O3 also showed better thermal stability at 60 °C (~66%) and 70 °C (~53%) as compared to immobilized catalase on CS/ZnO [60 °C (~60%) and 70 °C (~41%)] and free catalase [60 °C (~32%) and 70 °C (~18%)]. Alptekin et al. demonstrated that the maximum catalytic activity for free catalase was recorded at 25 °C, whereas it shifted to 40 °C in the case of immobilized catalase onto Eupergit C [33]. In addition, at 40–70 °C, the activity of the immobilized catalase onto CS/ZnO/Fe2O3 was shown to be less temperature-sensitive than that of immobilized catalase onto CS/ZnO and free catalase. The temperature shift was caused by changes in the physical and chemical properties of the immobilized enzyme. Furthermore, the formation of the binding of immobilized catalases through amino groups might reduce conformational flexibility and lead to increased molecule activation energy to reorganize the proper conformation of substrate binding [37].
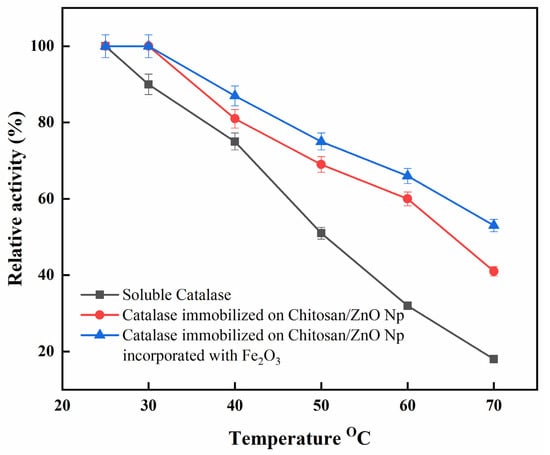
Figure 6.
Optimum temperature for free and immobilized catalase. Each point represents the mean of three experiments ± S.E.
To evaluate the effect of immobilization on the kinetic parameters of the enzymatic reaction, the parameters were calculated for free and immobilized catalase. The Michaelis–Menten model of enzyme kinetics was used to evaluate the initial velocities of experiments at different hydrogen peroxide concentrations (17.5–28 mM). The apparent Michaelis constant (Km) and maximum velocity of the reaction (Vmax) were calculated using the Lineweaver–Burk process, as shown in Table 2 and Figure S1. The Km values for free catalase, immobilized catalase on CS/ZnO, and immobilized catalase on CS/ZnO/Fe2O3, were calculated to be 91.28, 225.17, and 221.59 mM, respectively. Vmax was estimated as 10.45, 15.87, and 19.92 µmole ml−1 for free, immobilized catalase on CS/ZnO, and immobilized catalase on CS/ZnO/Fe2O3, respectively. Table 2 shows that the kinetics of the bound catalase changed as expected when enzymes were immobilized. It is well recognized that Vmax eflects the fundamental properties of immobilized enzymes and is influenced by diffusion constraints, whereas Km reflects the enzyme’s effective properties and is influenced by both partition and diffusion effects [22]. The higher Km values of immobilized enzyme were due to either the enzyme’s conformational changes, which reduced the probability of forming a substrate–enzyme complex, or the substrate’s accessibility to the immobilized enzyme’s active sites.

Table 2.
Kinetic parameters of free enzyme and immobilized enzymes.
The half-life and thermal stability of free and immobilized catalase were evaluated at temperatures ranging from 30 to 70 °C and time intervals of 0, 10, 20, 30, 40, 50, 60, and 70 min, respectively. Results for temperatures of 30–70 °C are presented in Figure S2. Table 3 shows the half-life (t1/2) and deactivation constant values (Kd) for all temperature profiles, as calculated from the graph’s slope. Table 3 shows that, in general, the immobilization of catalase on CS/ZnO and CS/ZnO/Fe2O3 provided more stability than free catalase in terms of enzyme half-life (t1/2). In comparison to free catalase, the t1/2 values at temperature ranges from 30 °C to 60 °C are the largest. However, the CS/ZnO/Fe2O3 matrix as a support for enzymes provided more stability. The half-life (t1/2) values of the immobilized enzyme on CS/ZnO/Fe2O3 at temperatures 30, 40, 50, and 60 °C were 693, 345, 231, and 173 min, respectively, in comparison to the half-lives of the immobilized enzyme on CS/ZnO of 347, 231, 77, and 46 min, respectively, at the same temperature. There was significant correlation between the temperature and deactivation constant (Kd) values. The deactivation constant (Kd) pace for free and immobilized enzyme on CS/ZnO and CS/ZnO/Fe2O3 increased more than 7.0-, 6.5, and 4-fold, respectively, when the temperature was raised from 30 to 60 °C. As shown in Table 3, the activation energies (Ea) of free and immobilized enzymes on CS/ZnO and CS/ZnO/Fe2O3 were determined to be 36.82, 15.94, and 12.41 kJ mol−1, respectively, by using the Arrhenius equation (Figure S3). High activation energy (Ea) values indicate that the enzyme is more sensitive to temperature changes [38]. The fact that the apparent activation energy (Ea) decreases confirms that the immobilized enzyme is regulated by mass transfer rather than kinetics. Furthermore, the observed decrease would suggest that the enzyme has undergone a conformational transition, which may explain the observed increased value of Vmax [39]. Furthermore, such low activation energy (Ea) values for immobilized enzymes indicate that only a small amount of energy is required to form the activated complex of substrate hydrolysis, indicating an efficient hydrolytic capability, as mentioned by Bibi et al. [40]. A similar decrease in Ea was reported by Lamb and Stuckey for the immobilization of β-galactosidase on colloidal liquid aphrons [39], and Esawy and Combet-Blanc for immobilized milk-clotting enzyme [41].

Table 3.
Half-life (t1/2), deactivation constant (Kd) and activation energy (Ea) of free enzyme and immobilized form.
3. Materials and Methods
Zinc oxide nanoparticles, 40 wt.% in ethanol, <100 nm particle size (ZnO Np), iron (III) oxide nano powder, <50 nm particle size, hydrogen peroxide (30%), chitosan, and catalase from bovine livers were obtained from Sigma-Aldrich (St. Louis, MO, USA). Mono- and disodium phosphate, and all other chemicals, were supplied by Beijing Solarbio Technology Co., Ltd.
3.1. Synthesis of ZnO/Chitosan and ZnO/Fe2O3 Chitosan Nanocomposites as an Enzyme Support
To prepare the ZnO/chitosan nanocomposite, 2 g of chitosan was dissolved in 100 mL of 1% acetic acid to obtain a standard 2% chitosan solution. Different concentrations of ZnO were mixed with 10 mL of 2% (w/v) chitosan solution and sonicated for 10 min at room temperature. The beads obtained were washed by distilled water and dried at 80 °C for 2 h.
To synthesize the ZnO/Fe2O3 chitosan nanocomposite, 1 mL of ZnO (representing 0.4 g ZnO) was mixed with 10 mL of 2% (w/v) chitosan solution and sonicated for 10 min at room temperature, followed by mixing different concentrations of iron (III) oxide (0.1, 0.2, 0.3 g, which represented 5%, 10%, and 15% Fe2O3) with continued sonication for another 10 min at room temperature. The ZnO/Fe2O3 chitosan nanocomposites obtained were washed by distilled water and dried at 80 °C for 2 h.
3.2. Immobilization Procedure
Immobilization was carried out by mixing catalase enzyme end-over-end at 110 rpm with 20 mM sodium acetate buffer (pH 6.0) or Tris–HCl (pH 7.0 or 8.0) and 1 g CS/ZnO and 1 g CS/ZnO incorporated with different concentrations of iron (III) oxide (5%, 10%, and 15% Fe2O3 nanoparticles). Immobilization reactions by adsorption occurred at room temperature overnight [18]. The supernatant was removed, the nanocomposites of CS/ZnO and CS/ZnO/Fe2O3 were dried at room temperature, and the amount of immobilization was investigated.
3.3. Catalase Assay
The activity of free and immobilized catalase was assessed using the method of Aebi [42]. In brief, 2 mL of substrate was made by mixing 25 mM H2O2 in a 75 mM phosphate buffer (pH 7.0) with 0.1 mL free enzyme or 5 mg immobilized enzyme beads. The absorbance was registered for 1 min at 240 nm. For immobilized catalase, the reaction was terminated with the removal of CS/ZnO/Fe2O3 beads from a reaction mixture. The immobilization yield, catalase activity (unit/g support), and specific activity (unit/mg protein) were defined as follows:
The amount of enzyme that caused a change of 0.1 in absorbance per minute was defined as one unit of enzyme activity.
3.4. Characterization
Scanning electron microscopy (SEM) (Quanta FEG 250, FEI Co., Hillsboro, OR, USA) and energy-dispersive X-ray spectroscopy (EDX) of nanocomposites with and without catalase enzymes were measured on an SEM Quanta FEG 250, FEI Co., working at 20 KV. Then, the fabric was coated with gold, fixed with stubs of Quanta holders, and examined under vacuum. Attenuated total reflectance Fourier-transform infrared spectroscopy (ATR–FTIR, PerkinElmer Spectrum 100) was used to study the chemical composition of the nanocomposites.
3.5. Reusability and Storage Stability of the Immobilized Catalase
The immobilized enzyme activity was evaluated in the reusability studies as described above. The immobilized enzyme was withdrawn and washed several times with 75 mM phosphate buffer (pH 7.0) in the following operating cycle, and a fresh substrate was added. At specific intervals, the reaction was carried out continuously for 10 cycles of use. The storage stability of the immobilized and free enzyme was evaluated by measuring the activities of the enzymes for 60 days at 4 °C, at fixed intervals.
3.6. Biochemical and Physicochemical Characterization of the Free and Immobilized Catalase
3.6.1. The Effect of pH and Temperature
The following buffers were used to determine the activity of free and immobilized catalase: 75 Mm sodium acetate buffer (pH 4.0–5.5), sodium phosphate buffer (pH 6.0–8.0), and Tris–HCL buffer (pH 8.5–9.5). The reaction was carried out as described above. The optimum temperature of free and immobilized enzyme was determined by performing the reaction at a temperature between 30 and 70 °C with an interval of 10 °C. The highest enzyme activity in each set was assigned a value of 100%.
3.6.2. Thermal Stability and Half-Life of Free and Immobilized Enzyme
Catalase activities of free and immobilized forms were monitored in temperatures ranging from 30 to 70 °C, and residual activities were measured from sterile aliquots withdrawn at periodic intervals using the following equation [8]:
where E and E0 represent the activities at time t (min) and time t = 0 min. The results were obtained by plotting a graph of time (t) on the X-axis against -ln (Residual catalase activity) on the Y-axis. The graph’s slope determined the enzyme Kd, and t1/2 was computed as:
The half-life of the enzyme (t1/2) was defined as the duration after which the activity of the enzyme decreased to half the original activity [43].
3.6.3. Kinetic Constant
To determine the Km and Vmax values, Lineweaver–Burk plots were applied using different concentrations of H2O2 as a substrate (17.5–30 mM).
4. Conclusions
The results demonstrate that immobilizing catalase on CS/ZnO/Fe2O3 at pH 8.0 is a very effective procedure, because the biocatalyst was much more stable (reused five times with 88% of its activity) than immobilization on CS/ZnO (after five reuses, it lost 62% of its activity). Additionally, study of the enzyme–support reaction showed that the best pH was 7.5 and the best temperature was 25–30 °C. Thus, the biocatalyst (catalase on CS/ZnO/Fe2O3) was chosen because it presented better performance regarding thermal inactivation (t1/2 = 693 min) at 30 °C. This biocatalyst exhibited low activation energy (Ea = 12.41), deactivation constant value (Kd = 4-fold) and high affinity to the substrate (Km = 221.59 mM). Based on the obtained results, the CS/ZnO/Fe2O3 system is a very promising support for catalase immobilization, resulting in excellent stabilization.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/catal11070820/s1, Figure S1: Kinetic parameters of immobilized and free catalase. Each point represents the mean of three experiments ± SE, Figure S2: Thermal stability and half-life for temperatures of 30–70 °C, Figure S3: Determination of the activation energy based on Arrhenius plots Figures S4–S7: EDX analysis of samples A–D, Table S1: The effect of ionic strength on the immobilization process.
Author Contributions
R.M.E.-S., N.S.E.A. and Y.Q.A. contributed to the ideas, executed all the experiments, analyzed and interpreted the data, as well as wrote, reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Deanship of Scientific Research (DSR), King Abdulaziz University.
Data Availability Statement
Data are provided as Supplementary Materials.
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant No. (DF-672-130-1441). The authors, therefore, gratefully acknowledge DSR technical and financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gan, J.; Bagheri, A.R.; Aramesh, N.; Gul, I.; Franco, M.; Almulaiky, Y.Q.; Bilal, M. Covalent organic frameworks as emerging host platforms for enzyme immobilization and robust biocatalysis–A review. Int. J. Biol. Macromol. 2020, 167, 502–515. [Google Scholar]
- Ma, Y.; Ling, L.; Guo, Y.; Fu, Y.; Shao, Q.; Wu, T.; Guo, S.; Sun, K.; Guo, X.; Wujcik, E.K. Porous lignin based poly(acrylic acid)/organo-montmorillonite nanocomposites: Swelling behaviors and rapid removal of Pb(II) ions. Polymer 2017, 128, 12–23. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, S.; Chen, L.; Jiang, D.; Shao, Q.; Zhang, B.; Zhai, Z.; Wang, C.; Zhao, M.; Ma, Y. Electrically insulated epoxy nanocomposites reinforced with synergistic core–shell SiO2@MWCNTs and montmorillonite bifillers. Macromol. Chem. Phys. 2017, 218, 1700357. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, M.; Li, J.; Yu, J.; Sun, S.; Ge, S.; Guo, X.; Xie, F.; Jiang, B.; Wujcik, E.K. Silver nanoparticles/graphene oxide decorated carbon fiber synergistic reinforcement in epoxy-based composites. Polymer 2017, 131, 263–271. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.; Zhang, J.; Shao, Q.; Li, J.; Li, H.; Lin, B.; Yu, M.; Chen, S.; Guo, Z. Excellent corrosion protection performance of epoxy composite coatings filled with silane functionalized silicon nitride. J. Polym. Res. 2018, 25, 130. [Google Scholar] [CrossRef]
- Casal, E.; Corzo, N.; Moreno, F.J.; Olano, A. Selective recovery of glycosylated caseinmacropeptide with chitosan. J. Agric. Food Chem. 2005, 53, 1201–1204. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Iqbal, M.; Hu, H.; Zhang, X. Chitosan beads immobilized manganese peroxidase catalytic potential for detoxification and decolorization of textile effluent. Int. J. Biol. Macromol. 2016, 89, 181–189. [Google Scholar] [CrossRef]
- Çetinus, Ş.A.; Öztop, H.N. Immobilization of catalase into chemically crosslinked chitosan beads. Enz. Microb. Technol. 2003, 32, 889–894. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Malki, A.L.; Kumosani, T.A.; El-Shishtawy, R.M. Horseradish peroxidase and chitosan: Activation, immobilization and comparative results. Int. J. Biol. Macromol. 2013, 60, 295–300. [Google Scholar] [CrossRef]
- Ifuku, S.; Ikuta, A.; Egusa, M.; Kaminaka, H.; Izawa, H.; Morimoto, M.; Saimoto, H. Preparation of high-strength transparent chitosan film reinforced with surfacedeacetylated chitin nanofibers. Carbohydr. Polym. 2013, 98, 1198–1202. [Google Scholar] [CrossRef]
- Gu, H.; Xu, X.; Zhang, H.; Liang, C.; Lou, H.; Ma, C.; Li, Y.; Guo, Z.; Gu, J. Chitosan-coatedmagnetite with covalently grafted polystyrene based carbon nanocomposites for hexavalent chromium adsorption. Eng. Sci. 2018, 1, 46–54. [Google Scholar] [CrossRef]
- He, Y.; Yang, S.; Liu, H.; Shao, Q.; Chen, Q.; Lu, C.; Jiang, Y.; Liu, C.; Guo, Z. Reinforced carbon fiber laminates with oriented carbon nanotube epoxy nanocomposites: Magnetic field assisted alignment and cryogenic temperaturemechanical properties. J. Colloid Interface Sci. 2018, 517, 40–51. [Google Scholar] [CrossRef]
- Wang, R.H.; Xin, J.H.; Tao, X.M. UV-blocking property of dumbbell-shaped ZnO crystallites on cotton fabrics. Inorg. Chem. 2005, 44, 3926–3930. [Google Scholar] [CrossRef]
- Selvarajan, E.; Mohanasrinivasan, V.; Subathra, C.; George, P. Immobilization of β-galactosidase from Lactobacillus plantarum HF571129 on ZnO nanoparticles: Characterization and lactose hydrolysis. Bioprocess. Biosyst. Eng. 2015, 38, 1655–1669. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Kim, J.; Grate, J.W.; Wang, P. Nanobiocatalysis and its potential applications. Trends Biotechnol. 2008, 26, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Noreen, S.; Asgher, M.; Hussain, F.; Iqbal, A. Performance improvement of Ca-alginate bead cross-linked laccase from Trametes versicolor IBL-04. BioResources 2016, 11, 558–572. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Harbi, M.H.; Almulaiky, Y.Q.; Ibrahim, I.H.; El-Shishtawy, R.M. Immobilization of horseradish peroxidase on Fe3O4 magnetic nanoparticles. Electron. J. Biotechnol. 2017, 27, 84–90. [Google Scholar] [CrossRef]
- Lin, J.; Fan, L.; Miao, R.; Le, X.; Chen, S.; Zhou, X. Enhancing catalytic performance of laccase via immobilization on chitosan/CeO2 microspheres. Int. J. Biol. Macromol. 2015, 78, 1–8. [Google Scholar] [CrossRef]
- Aldhahri, M.; Almulaiky, Y.Q.; El-Shishtawy, R.M.; Al-Shawafi, W.M.; Salah, N.; Alshahrie, A.; Alzahrani, H.A. Ultra-thin 2D CuO nanosheet for HRP immobilization supported by encapsulation in a polymer matrix: Characterization and dye degradation. Catal. Lett. 2021, 151, 232–246. [Google Scholar] [CrossRef]
- Almulaiky, Y.Q.; Al-Harbi, S.A. A novel peroxidase from Arabian balsam (Commiphora gileadensis) stems: Its purification, characterization and immobilization on a carboxymethylcellulose/Fe3O4 magnetic hybrid material. Int. J. Biol. Macromol. 2019, 133, 767–774. [Google Scholar] [CrossRef]
- Çetinus, Ş.A.; Şahin, E.; Saraydin, D. Preparation of Cu (II) adsorbed chitosan beads for catalase immobilization. Food Chem. 2009, 114, 962–969. [Google Scholar] [CrossRef]
- Czechowska, E.; Ranoszek-Soliwoda, K.; Tomaszewska, E.; Pudlarz, A.; Celichowski, G.; Gralak-Zwolenik, D.; Grobelny, J. Comparison of the antioxidant activity of catalase immobilized on gold nanoparticles via specific and non-specific adsorption. Colloids Surf. B Biointerface 2018, 171, 707–714. [Google Scholar] [CrossRef]
- Ghaffari, S.B.; Sarrafzadeh, M.H.; Salami, M.; Khorramizadeh, M.R. A pH-sensitive delivery system based on N-succinyl chitosan-ZnO nanoparticles for improving antibacterial and anticancer activities of curcumin. Int. J. Biol. Macromol. 2020, 151, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, B.B.; Rios, N.S.; Aguado, E.R.; Fernandez-Lafuente, R.; Freire, T.M.; Fechine, P.B.; Gonçalves, L.R. Chitosan activated with divinyl sulfone: A new heterofunctional support for enzyme immobilization. Application in the immobilization of lipase B from Candida antarctica. Int. J. Biol. Macromol. 2019, 130, 798–809. [Google Scholar] [CrossRef] [PubMed]
- El-Shishtawy, R.M.; Mohamed, S.A.; Asiri, A.M.; Ahmed, N.S.E. Synthesis of hemicyanine-based chitosan ligands in dye-affinity chromatography for the purification of chewing stick peroxidase. Int. J. Biol. Macromol. 2020, 148, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Singh, V.; Sharma, Y.C. Methyl transesterification of waste cooking oil using a laboratory synthesized reusable heterogeneous base catalyst: Process optimization and homogeneity study of catalyst. Energy Convers. Manag. 2017, 148, 1438–1452. [Google Scholar] [CrossRef]
- Hong, R.Y.; Li, J.H.; Chen, L.L.; Liu, D.Q.; Li, H.Z.; Zheng, Y.; Ding, J.J. Synthesis, surface modification and photocatalytic property of ZnO nanoparticles. Powder Technol. 2009, 189, 426–432. [Google Scholar] [CrossRef]
- Hwang, S.W.; Umar, A.; Dar, G.N.; Kim, S.H.; Badran, R.I. Synthesis and Characterization of Iron Oxide Nanoparticles for Phenyl Hydrazine Sensor Applications. Sens. Lett. 2014, 12, 97–101. [Google Scholar] [CrossRef]
- Almulaiky, Y.Q.; Aqlan, F.M.; Aldhahri, M.; Baeshen, M.; Khan, T.J.; Khan, K.A.; Alayafi, A.A. α-Amylase immobilization on amidoximated acrylic microfibres activated by cyanuric chloride. R. Soc. Open Sci. 2018, 5, 172164. [Google Scholar] [CrossRef] [PubMed]
- Defaei, M.; Taheri-Kafrani, A.; Miroliaei, M.; Yaghmaei, P. Improvement of stability and reusability of α-amylase immobilized on naringin functionalized magnetic nanoparticles: A robust nanobiocatalyst. Int. J. Biol. Macromol. 2018, 113, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Al-Najada, A.R.; Almulaiky, Y.Q.; Aldhahri, M.; El-Shishtawy, R.M.; Mohamed, S.A.; Baeshen, M.; Al-Harbi, S.A. Immobilisation of α-amylase on activated amidrazone acrylic fabric: A new approach for the enhancement of enzyme stability and reusability. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Alptekin, Ö.; Tükel, S.S.; Yıldırım, D.; Alagöz, D. Immobilization of catalase onto Eupergit C and its characterization. J. Mol. Catal. B Enzym. 2010, 64, 177–183. [Google Scholar] [CrossRef]
- Kaushal, J.; Singh, G.; Arya, S.K. Immobilization of catalase onto chitosan and chitosan–bentonite complex: A comparative study. Biotechnol. Rep. 2018, 18, e00258. [Google Scholar] [CrossRef] [PubMed]
- Abdulaal, W.H.; Almulaiky, Y.Q.; El-Shishtawy, R.M. Encapsulation of HRP enzyme onto a magnetic Fe3O4 Np–PMMA film via casting with sustainable biocatalytic activity. Catalysts 2020, 10, 181. [Google Scholar] [CrossRef]
- Almulaiky, Y.Q.; El-Shishtawy, R.M.; Aldhahri, M.; Mohamed, S.A.; Afifi, M.; Abdulaal, W.H.; Mahyoub, J.A. Amidrazone modified acrylic fabric activated with cyanuric chloride: A novel and efficient support for horseradish peroxidase immobilization and phenol removal. Int. J. Biol. Macromol. 2019, 140, 949–958. [Google Scholar] [CrossRef]
- Mazlan, S.Z.; Hanifah, S.A. Effects of temperature and pH on immobilized laccase activity in conjugated methacrylate-acrylate microspheres. Int. J. Polym. Sci. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Zeyadi, M.; Almulaiky, Y.Q. A novel peroxidase from Ziziphus jujuba fruit: Purification, thermodynamics and biochemical characterization properties. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Lamb, S.B.; Stuckey, D.C. Enzyme immobilization on colloidal liquid aphrons (CLAs): The influence of system parameters on activity. Enzym. Microb. Technol. 2000, 26, 574–581. [Google Scholar] [CrossRef]
- Bibi, Z.; Shahid, F.; Ali, S.; Qader, U.; Aman, A. Agar-agar entrapment increases the stability of endo-β-1, 4-xylanase for repeated biodegradation of xylan. Int. J. Biol. Macromol. 2015, 75, 121–127. [Google Scholar] [CrossRef]
- Esawy, M.A.; Combet-Blanc, Y. Immobilization of Bacillus licheniformis 5A1 milk-clotting enzyme and characterization of its enzyme properties. World J. Microbiol. Biotechnol. 2006, 22, 197–200. [Google Scholar] [CrossRef]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis, 2nd ed.; Bergmayer, H., Ed.; Verlag Chemie International: Deerfield Beach, FL, USA, 1981; pp. 673–684. [Google Scholar]
- Gohel, S.D.; Singh, S.P. Characteristics and thermodynamics of a thermostable protease from a salt-tolerant alkaliphilic actinomycete. Int. J. Biol. Macromol. 2013, 56, 20–27. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).