Evaluation of Plasmatic Procalcitonin in Healthy, and in Systemic Inflammatory Response Syndrome (SIRS) Negative or Positive Colic Horses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. PCT Evaluation
2.3. Statistical Analysis
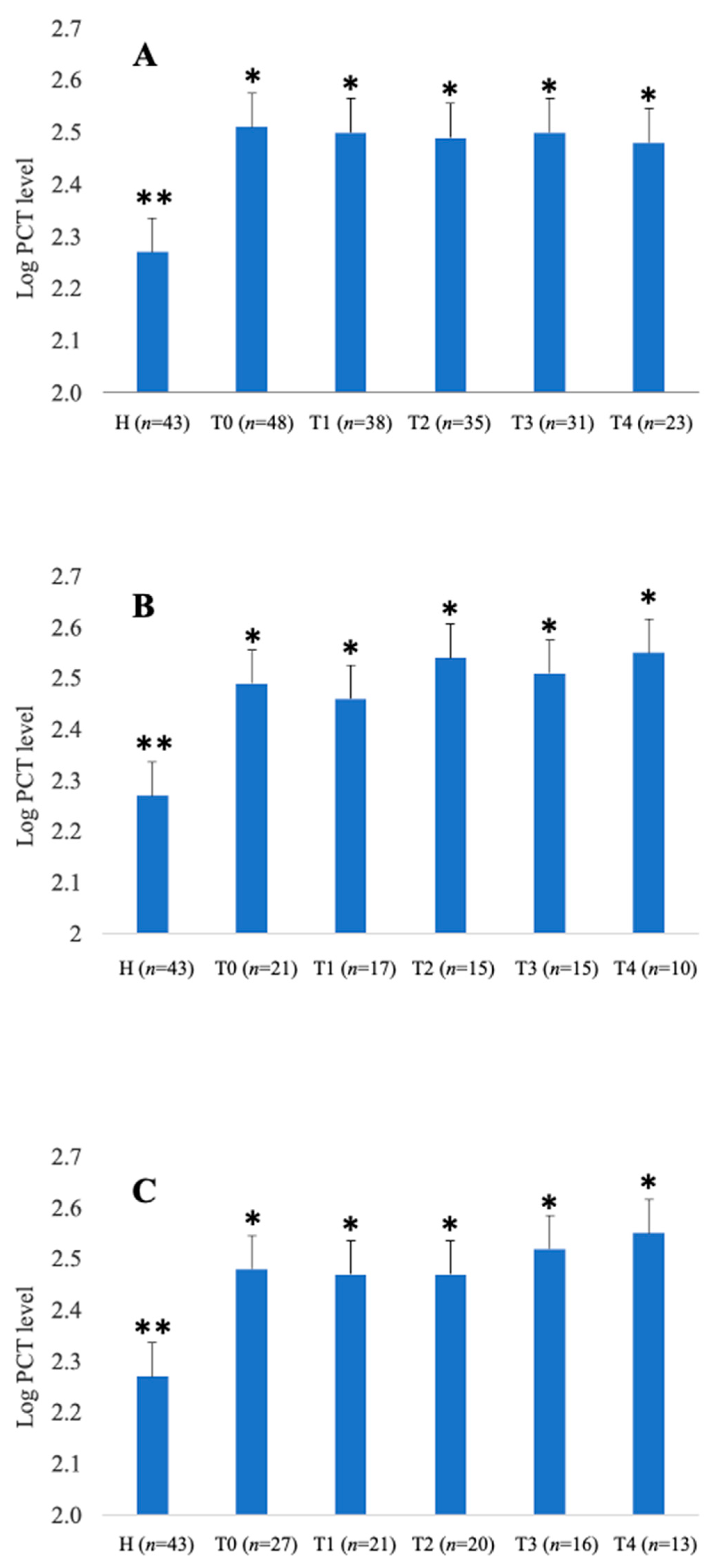
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Curtis, L.; Burford, J.H.; England, C.G.W.; Freeman, S.L. Risk factors for acute abdominal pain (colic) in the adult horse: A scoping review of risk factors, and a systematic review of the effect of management-related changes. PLoS ONE 2019, 14, e0219307. [Google Scholar] [CrossRef]
- Moore, J.N.; Vandenplas, M.L. Is the systemic inflammatory response syndrome or endotoxemia in horses with colic? Vet. Clin. Equine Pract. 2014, 30, 337–351. [Google Scholar] [CrossRef]
- Sheats, M.K. A Comparative Review of Equine SIRS, Sepsis, and Neutrophils. Front. Vet. Sci. 2019, 6, 69. [Google Scholar] [CrossRef]
- Roy, M.F.; Kwong, G.P.S.; Lambert, J.; Massie, S.; Lockhart, S. Prognostic Value and Development of a Scoring System in Horses with Systemic Inflammatory Response Syndrome. J. Vet. Intern. Med. 2017, 31, 582–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prkno, A.; Wacker, C.; Brunkhorst, F.M.; Schlattmann, P. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock—A systemic review and meta-analysis. Crit. Care 2013, 17, R29. [Google Scholar] [CrossRef] [Green Version]
- Afsar, I.; Sener, A.G. Is Procalcitonin a diagnostic and/or prognostic marker in sepsis? Infect. Dis. Clin. Pract. 2015, 23, 3–6. [Google Scholar] [CrossRef]
- Epstein, K.L.; Brainard, B.M.; Gomez-Ibanez, S.E.; Lopes, M.A.; Barton, M.H.; Moore, J.N. Thrombelastography in horses with acute gastrointestinal disease. J. Vet. Intern. Med. 2011, 25, 307–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, M.J.; Smith, E.R.; Turfle, P.G. Biomarkers in Veterinary Medicine. Annu. Rev. Anim. Biosci. 2017, 5, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Strimbu, K.; Tavel, J.A. What are Biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Riedel, S. Procalcitonin and the role of biomarkers in the diagnosis and management of sepsis. Diagn. Microbiol. Infect. Dis. 2012, 73, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Toribio, R.E.; Kohn, C.W.; Leone, G.W.; Capen, C.C.; Rosol, T.J. Molecular cloning and expression of equine calcitonin, calcitonin gene-related peptide-I and calcitonin gene-related peptide II. Mol. Cell. Endocrinol. 2003, 199, 119–128. [Google Scholar] [CrossRef]
- Giunti, M.; Peli, A.; Battilani, M.; Zacchini, S.; Militerno, G.; Otto, C.M. Evaluation of CALC-I gene (CALCA) expression in tissues of dogs with signs of the systemic inflammatory response syndrome. J. Vet. Emerg. Crit. Care 2010, 20, 523–557. [Google Scholar] [CrossRef]
- Bonelli, F.; Meucci, V.; Divers, T.; Radcliffe, R.; Jose-Cunilleras, E.; Corazza, M.; Guidi, G.; Tognetti, R.; Castagnetti, C.; Intorre, L.; et al. Evaluation of plasma Procalcitonin concentrations in healthy foals and foals affected by septic Systemic Inflammatory Response Syndrome. J. Equine Vet. Sci. 2015, 35, 645–649. [Google Scholar] [CrossRef]
- Bonelli, F.; Meucci, V.; Divers, T.J.; Jose-Cunilleras, E.; Corazza, M.; Tognetti, R.; Guidi, G.; Intorre, L.; Sgorbini, M. Plasma procalcitonin concentration in healthy horses and horses affected by systemic inflammatory response syndrome. J. Vet. Intern. Med. 2015, 29, 1689–1691. [Google Scholar] [CrossRef]
- Teschner, D.; Rieger, M.; Koopmann, C.; Gehlen, H. Procalcitonin in horses with an acute colic. Pferdeheilkunde 2015, 31, 371–377. [Google Scholar] [CrossRef]
- Ercan, N.; Tuzcu, N.; Başbug, O.; Tuzcu, M.; Alim, A. Diagnostic value of serum procalcitonin, neopterin, and gamma interferon in neonatal calves with septicemic colibacillosis. J. Vet. Diagn. Investig. 2016, 28, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Bonelli, F.; Meucci, V.; Divers, T.J.; Wagner, B.; Intorre, L.; Sgorbini, M. Kinetics of plasma procalcitonin, soluble CD14, CCL2 and IL-10 after a sublethal infusion of lipopolysaccharide in horses. Vet. Immunol. Immunopathol. 2017, 184, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, F.; Meucci, V.; Divers, T.J.; Boccardo, A.; Pravettoni, D.; Meylan, M.; Belloli, A.G.; Sgorbini, M. Plasma procalcitonin concentration in healthy calves and those with septic systemic inflammatory response syndrome. Vet. J. 2018, 234, 61–65. [Google Scholar] [CrossRef]
- El-Deeb, W.; Fayez, M.; Elsohaby, I.; Mkrtchyan, H.V.; Alhaider, A. Changes in blood biomarkers in Arabian horses with Clostridium difficile-induced enterocolitis. Comp. Immunol. Microbiol. Infect. Dis. 2020, 73, 101525. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, W.; Elsohaby, I.; Fayez, M.; Mkrtchyan, H.V.; El-Etriby, D.; ElGioushy, M. Use of procalcitonin, neopterin, haptoglobin, serum amyloid A and proinflammatory cytokines in diagnosis and prognosis of bovine respiratory disease in feedlot calves under field conditions. Acta Trop. 2020, 204, 105336. [Google Scholar] [CrossRef] [PubMed]
- Kilcoyne, I.; Nieto, J.E.; Dechant, J.E. Diagnostic value of plasma and peritoneal fluid procalcitonin concentrations in horses with strangulating intestinal lesions. J. Am. Vet. Med. A 2020, 256, 927–933. [Google Scholar] [CrossRef]
- Harikrishna, J.; Mohan, A.; Chakravarthi, D.P.K.; Chaudhury, A.; Kumar, S.; Sarma, K.V.S. Serum procalcitonin as a biomarker of bloodstream infection & focal bacterial infection in febrile patients. Indian J. Med. Res. 2020, 151, 342–349. [Google Scholar]
- Barton, A.K.; Rieger, M.; Teschener, D.; Gehlen, H. Procalcitonin—A useful biomarker for pneumonia associated with Rhodococcus equi? Mod. Res. Inflamm. 2016, 5, 13–19. [Google Scholar] [CrossRef]
- Barton, A.K.; Peli, A.; Rieger, M.; Gehlen, H. Procalcitonin as a biomarker in equine chronic pneumopathies. BMC Vet. Res. 2016, 12, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basbug, O.; Yurdakul, I.; Yuksel, M. Evaluation of serum Amyloid A and procalcitonin in some inflammatory diseases of cattle. Kafkas Univ. Vet. Fakültesi Derg. 2020, 26, 397–402. [Google Scholar]
- Zak, A.; Siwinska, N.; Elzinga, S.; Barker, V.D.; Stefaniak, T.; Schanbacher, B.J.; Placed, N.J.; Niedzwiedz, A.; Adams, A.A. Effects of equine metabolic syndrome on inflammation and acute-phase markers in horses. Domest. Anim. Endocrinol. 2020, 72, 106448. [Google Scholar] [CrossRef] [PubMed]
- Rieger, M.; Kochleus, C.; Teschner, D.; Rascher, D.; Barton, A.K.; Geerlof, A. A new ELISA for the quantification of equine procalcitonin in plasma as potential inflammation biomarker in horses. Anal. Bioanal. Chem. 2014, 406, 5507–5512. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Nix, D.; Wilson, M.F.; Aljada, A.; Love, J.; Assicot, M.; Bohuon, C. Procalcitonin increase after endotoxin injection in normal subjects. J. Clin. Endocrinol. Metab. 1994, 79, 1605–1608. [Google Scholar] [PubMed]
- Brunkhorst, F.M.; Heinza, U.; Forycki, Z.F. Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med. 1998, 24, 888–892. [Google Scholar] [CrossRef]
- Rhodes, A.A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock. Crit. Care Med. 2017, 45, 486–552. [Google Scholar] [CrossRef]
- Huang, H.B.; Peng, J.M.; Weng, L.; Wang, C.Y.; Jiang, W.; Du, B. Procalcitonin-guided antibiotic therapy in intensive care unit patients: A systematic review and meta-analysis. Ann. Intensive Care 2017, 7, 114. [Google Scholar] [CrossRef] [PubMed]
| Colic Diagnosis | Total Colic 48/91 (52.7%) | |
|---|---|---|
| obstructive non-strangulating colic | 34/48 (70.8%) | 22/34 treated medically 12/24 treated surgically |
| obstructive strangulating colic | 14/48 (29.2%) | 14/14 treated surgically |
| SIRS-negative colic 27/48 (56%) | ||
| obstructive non-strangulating colic | 19/27 (70.4%) | 13/19 medically 6/19 surgically |
| obstructive strangulating colic | 8/27 (29.6%) | 8/8 surgically |
| SIRS-positive colic 21/48 (43.7%) | ||
| obstructive non-strangulating colic | 15/21 (71.4%) | 9/15 medically 6/15 surgically |
| obstructive strangulating colic | 6/21 (28.6%) | 6/6 surgically |
| Colic Diagnosis | Discharged 44/48 (91.7%) | Euthanized 4/48 (8.3%) | ||
|---|---|---|---|---|
| SIRS-Negative 26/44 (59.1%) | SIRS-Positive 18/44 (40.9%) | SIRS-Negative 1/4 (25%) | SIRS-Positive 3/4 (74%) | |
| obstructive non-strangulating colic | 33/44 (75%) | 1/4 (25%) | ||
| obstructive strangulating colic | 11/44 (25%) | 3/4 (75%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nocera, I.; Bonelli, F.; Vitale, V.; Meucci, V.; Conte, G.; Jose-Cunilleras, E.; Gracia-Calvo, L.A.; Sgorbini, M. Evaluation of Plasmatic Procalcitonin in Healthy, and in Systemic Inflammatory Response Syndrome (SIRS) Negative or Positive Colic Horses. Animals 2021, 11, 2015. https://doi.org/10.3390/ani11072015
Nocera I, Bonelli F, Vitale V, Meucci V, Conte G, Jose-Cunilleras E, Gracia-Calvo LA, Sgorbini M. Evaluation of Plasmatic Procalcitonin in Healthy, and in Systemic Inflammatory Response Syndrome (SIRS) Negative or Positive Colic Horses. Animals. 2021; 11(7):2015. https://doi.org/10.3390/ani11072015
Chicago/Turabian StyleNocera, Irene, Francesca Bonelli, Valentina Vitale, Valentina Meucci, Giuseppe Conte, Eduard Jose-Cunilleras, Luis Alfonso Gracia-Calvo, and Micaela Sgorbini. 2021. "Evaluation of Plasmatic Procalcitonin in Healthy, and in Systemic Inflammatory Response Syndrome (SIRS) Negative or Positive Colic Horses" Animals 11, no. 7: 2015. https://doi.org/10.3390/ani11072015
APA StyleNocera, I., Bonelli, F., Vitale, V., Meucci, V., Conte, G., Jose-Cunilleras, E., Gracia-Calvo, L. A., & Sgorbini, M. (2021). Evaluation of Plasmatic Procalcitonin in Healthy, and in Systemic Inflammatory Response Syndrome (SIRS) Negative or Positive Colic Horses. Animals, 11(7), 2015. https://doi.org/10.3390/ani11072015









