Clock-Modulating Activities of the Anti-Arrhythmic Drug Moricizine
Abstract
:1. Introduction
2. Results
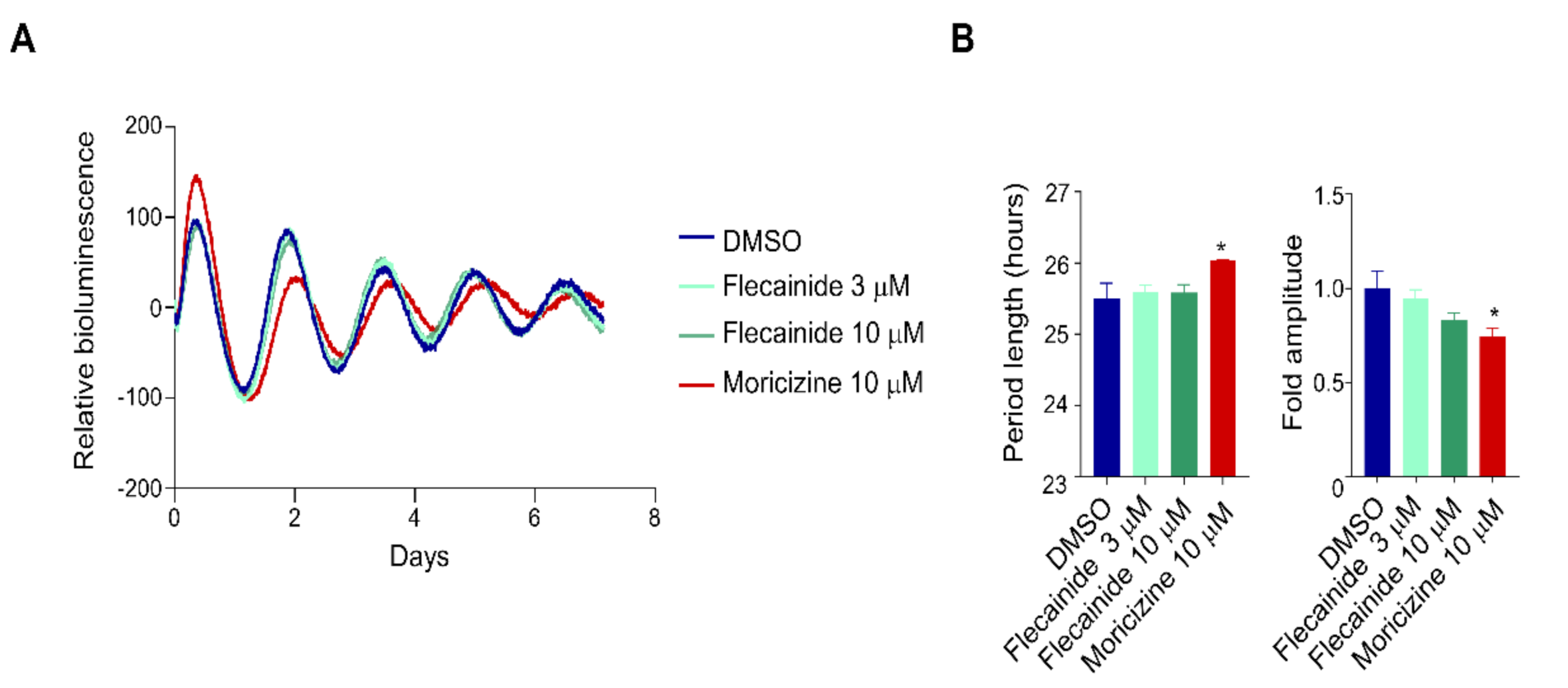
2.1. Clock-Altering Activities of Moricizine
2.2. Flecainide Does Not Affect Circadian Reporter Rhythms
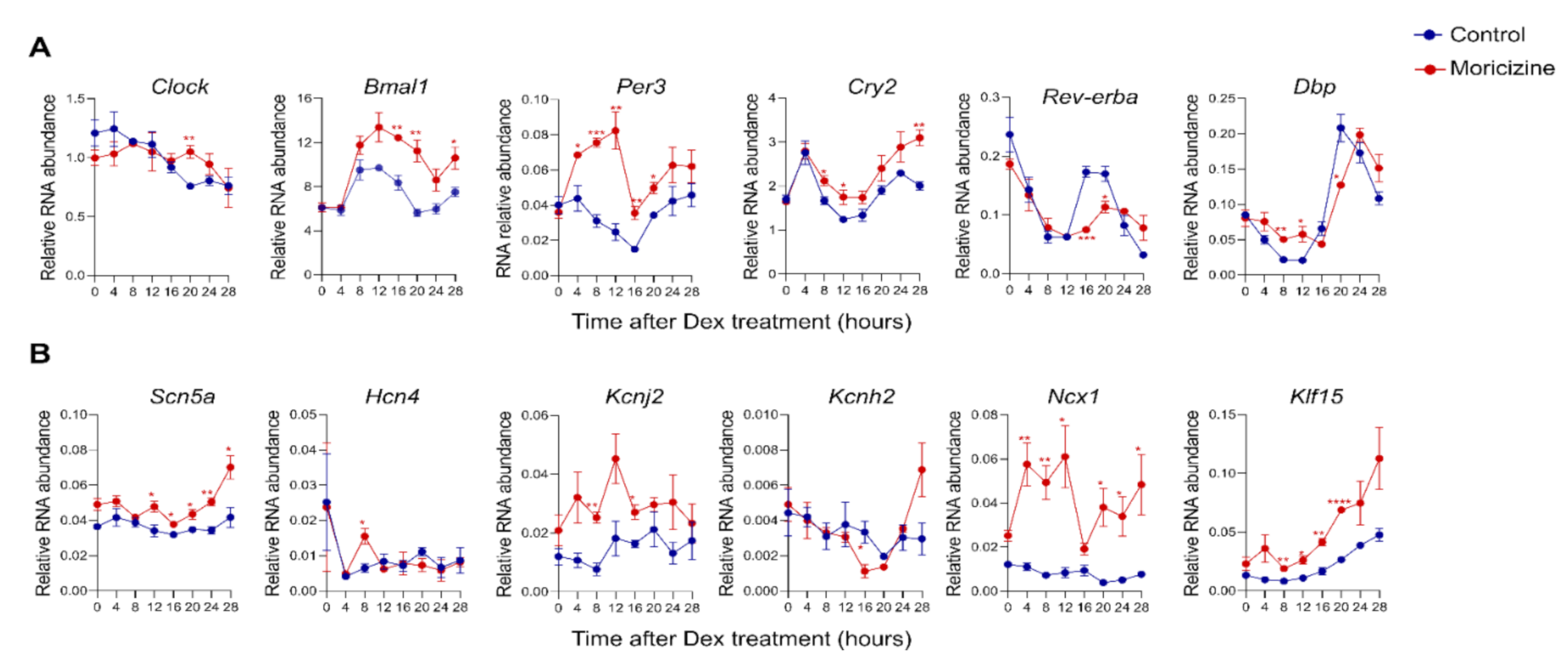
2.3. Moricizine Regulates Circadian and Cardiac Channel Gene Expression
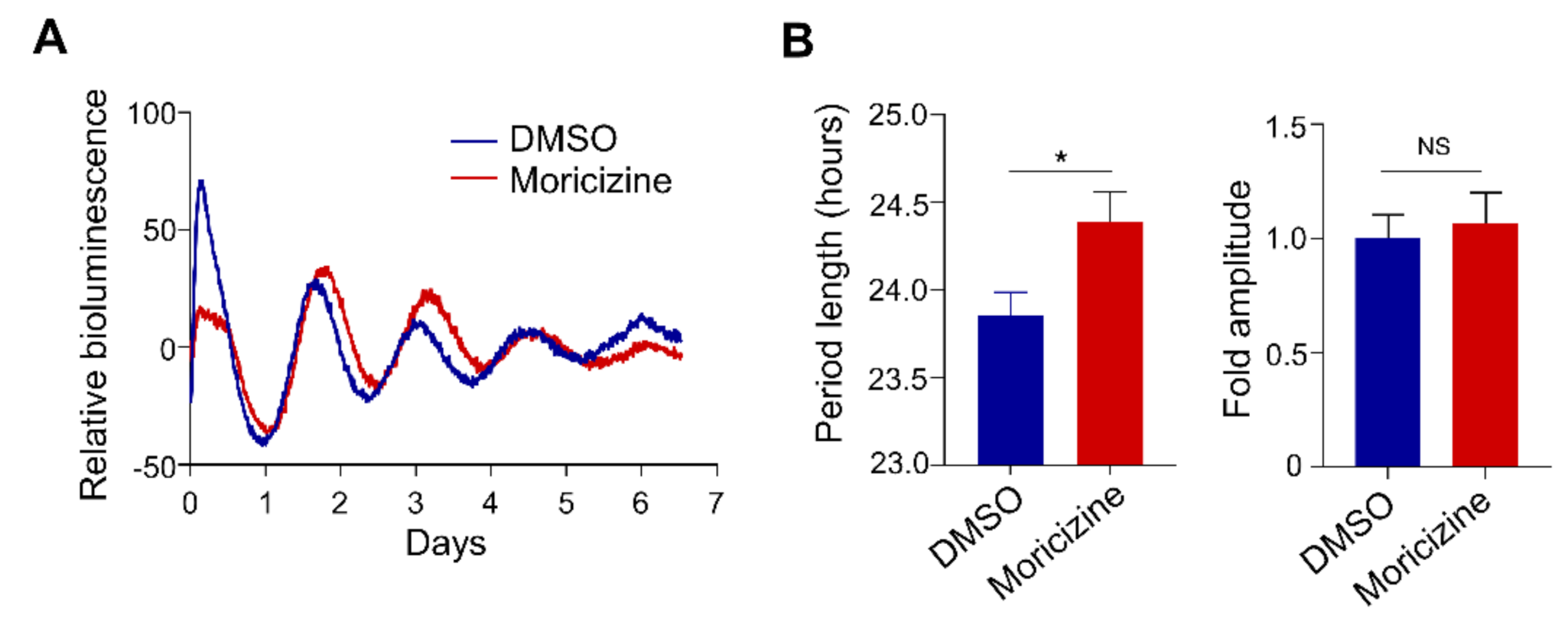
2.4. Moricizine Affects Tissue Clocks
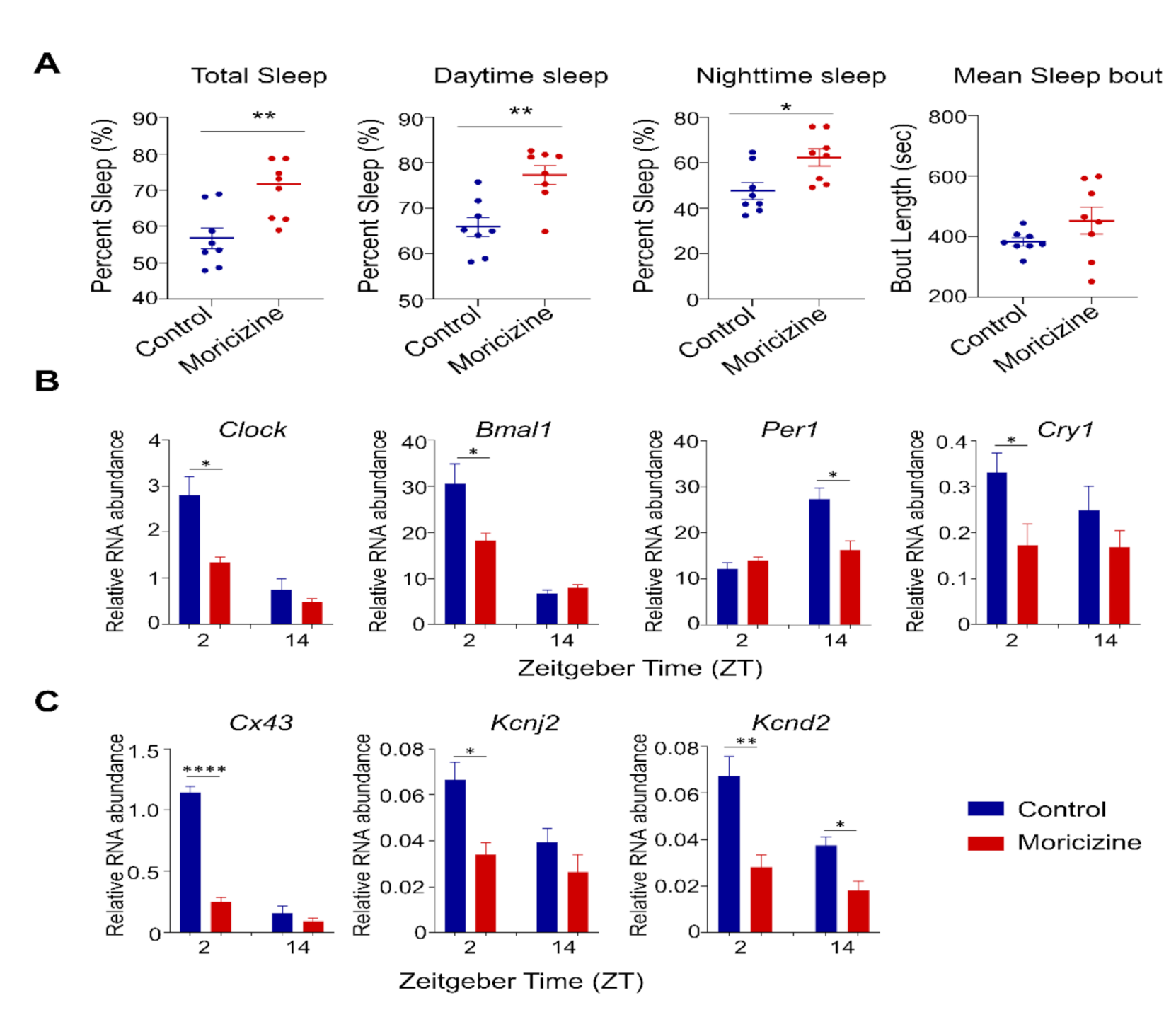
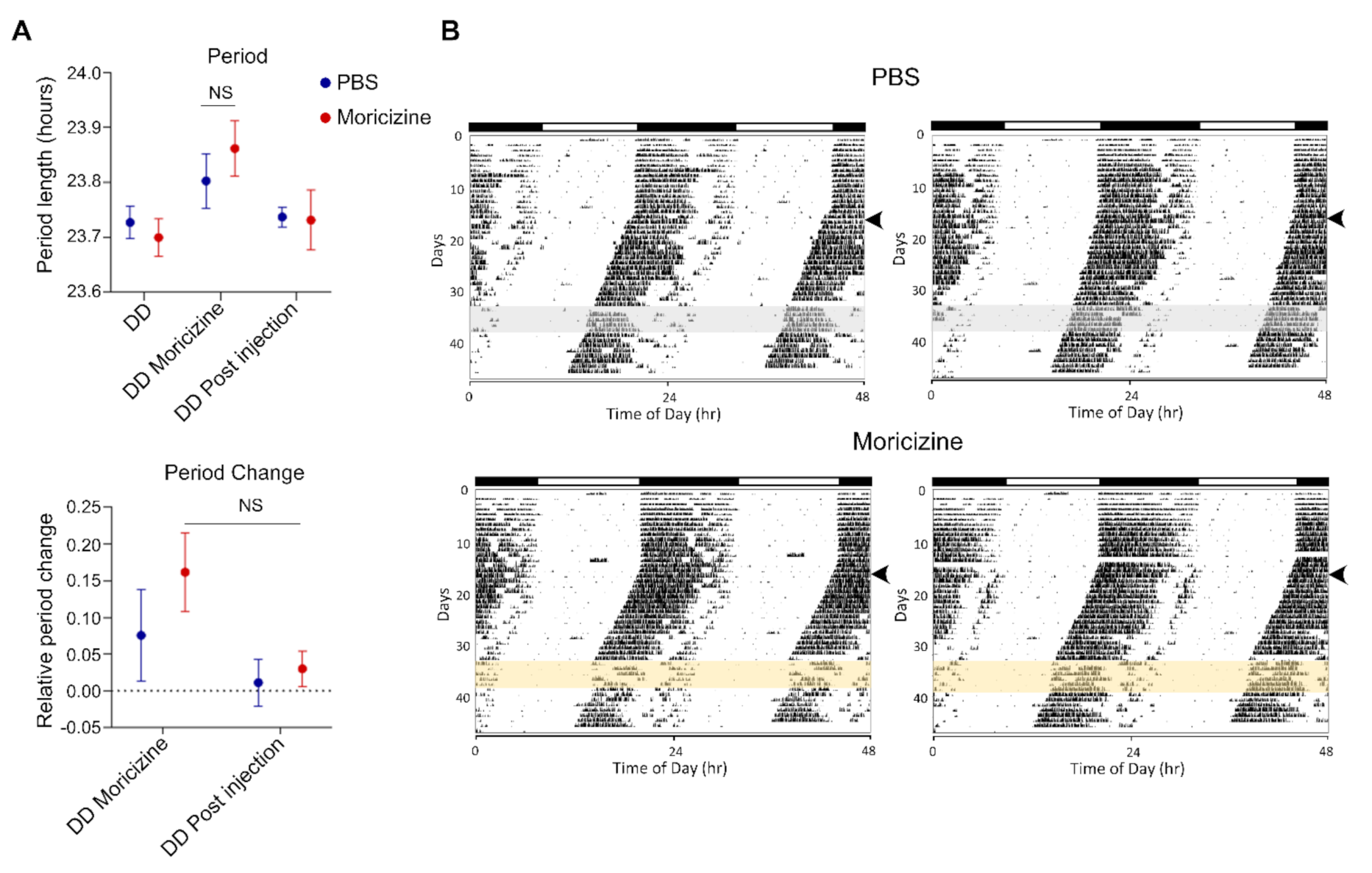
2.5. In Vivo Effects of Moricizine on Sleep and Circadian Activity
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Circadian Reporter Luminescence Monitoring
4.3. Real-Time RT-PCR Analysis
4.4. Atrium Ex Vivo Cultures for Real-Time Circadian Bioluminescence Monitoring
4.5. In Vivo Dose Consideration and Animals
4.6. Piezo Sleep Recording
4.7. Mouse Wheel-Running Behavioral Study
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bass, J.; Lazar, M.A. Circadian time signatures of fitness and disease. Science 2016, 354, 994–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mure, L.S.; Le, H.D.; Benegiamo, G.; Chang, M.W.; Rios, L.; Jillani, N.; Ngotho, M.; Kariuki, T.; Dkhissi-Benyahya, O.; Cooper, H.M.; et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 2018, 359. [Google Scholar] [CrossRef] [Green Version]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef] [Green Version]
- Paschos, G.K.; FitzGerald, G.A. Circadian clocks and vascular function. Circ. Res. 2010, 106, 833–841. [Google Scholar] [CrossRef]
- Durgan, D.J.; Young, M.E. The cardiomyocyte circadian clock: Emerging roles in health and disease. Circ. Res. 2010, 106, 647–658. [Google Scholar] [CrossRef] [Green Version]
- Tsimakouridze, E.V.; Alibhai, F.J.; Martino, T.A. Therapeutic applications of circadian rhythms for the cardiovascular system. Front. Pharmacol. 2015, 6, 77. [Google Scholar] [CrossRef] [Green Version]
- Thosar, S.S.; Shea, S.A. Circadian control of human cardiovascular function. Curr. Opin. Pharm. 2021, 57, 89–97. [Google Scholar] [CrossRef]
- Smolensky, M.H.; Hermida, R.C.; Geng, Y.J. Chronotherapy of cardiac and vascular disease: Timing medications to circadian rhythms to optimize treatment effects and outcomes. Curr. Opin. Pharm. 2020, 57, 41–48. [Google Scholar] [CrossRef]
- Parra, V.; Rothermel, B.A. Calcineurin signaling in the heart: The importance of time and place. J. Mol. Cell Cardiol. 2017, 103, 121–136. [Google Scholar] [CrossRef] [Green Version]
- Kervezee, L.; Kosmadopoulos, A.; Boivin, D.B. Metabolic and cardiovascular consequences of shift work: The role of circadian disruption and sleep disturbances. Eur. J. Neurosci. 2020, 51, 396–412. [Google Scholar] [CrossRef] [Green Version]
- Schilperoort, M.; Rensen, P.C.N.; Kooijman, S. Time for Novel Strategies to Mitigate Cardiometabolic Risk in Shift Workers. Trends Endocrinol. Metab. 2020, 31, 952–964. [Google Scholar] [CrossRef]
- Ramsey, A.M.; Stowie, A.; Castanon-Cervantes, O.; Davidson, A.J. Environmental Circadian Disruption Increases Stroke Severity and Dysregulates Immune Response. J. Biol. Rhythm. 2020, 35, 368–376. [Google Scholar] [CrossRef]
- Curtis, A.M.; Cheng, Y.; Kapoor, S.; Reilly, D.; Price, T.S.; Fitzgerald, G.A. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc. Natl. Acad. Sci. USA 2007, 104, 3450–3455. [Google Scholar] [CrossRef] [Green Version]
- Anea, C.B.; Zhang, M.; Stepp, D.W.; Simkins, G.B.; Reed, G.; Fulton, D.J.; Rudic, R.D. Vascular disease in mice with a dysfunctional circadian clock. Circulation 2009, 119, 1510–1517. [Google Scholar] [CrossRef] [Green Version]
- Su, W.; Xie, Z.; Guo, Z.; Duncan, M.J.; Lutshumba, J.; Gong, M.C. Altered clock gene expression and vascular smooth muscle diurnal contractile variations in type 2 diabetic db/db mice. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H621–H633. [Google Scholar] [CrossRef] [Green Version]
- Delisle, B.P.; Stumpf, J.L.; Wayland, J.L.; Johnson, S.R.; Ono, M.; Hall, D.; Burgess, D.E.; Schroder, E.A. Circadian clocks regulate cardiac arrhythmia susceptibility, repolarization, and ion channels. Curr. Opin. Pharm. 2020, 57, 13–20. [Google Scholar] [CrossRef]
- Bernardi, J.; Aromolaran, K.A.; Zhu, H.; Aromolaran, A.S. Circadian Mechanisms: Cardiac Ion Channel Remodeling and Arrhythmias. Front. Physiol. 2020, 11, 611860. [Google Scholar] [CrossRef]
- Hayter, E.A.; Wehrens, S.M.T.; Van Dongen, H.P.A.; Stangherlin, A.; Gaddameedhi, S.; Crooks, E.; Barron, N.J.; Venetucci, L.A.; Neill, J.S.O.; Brown, T.M.; et al. Distinct circadian mechanisms govern cardiac rhythms and susceptibility to arrhythmia. Nat. Commun. 2021, 12, 2472. [Google Scholar] [CrossRef]
- Antzelevitch, C.; Burashnikov, A. Overview of Basic Mechanisms of Cardiac Arrhythmia. Card. Electrophysiol. Clin. 2011, 3, 23–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroder, E.A.; Lefta, M.; Zhang, X.; Bartos, D.C.; Feng, H.Z.; Zhao, Y.; Patwardhan, A.; Jin, J.P.; Esser, K.A.; Delisle, B.P. The cardiomyocyte molecular clock, regulation of Scn5a, and arrhythmia susceptibility. Am. J. Physiol. Cell Physiol. 2013, 304, C954–C965. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, D.; Haldar, S.M.; Wan, X.; McCauley, M.D.; Ripperger, J.A.; Hu, K.; Lu, Y.; Eapen, B.L.; Sharma, N.; Ficker, E.; et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature 2012, 483, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Podrid, P.J. Moricizine (ethmozine HCl)—A new antiarrhythmic drug: Is it unique? Am. J. Cardiol. 1991, 68, 1521–1525. [Google Scholar] [CrossRef]
- Clyne, C.A.; Estes, N.A., 3rd; Wang, P.J. Moricizine. N. Engl. J. Med. 1992, 327, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Mahler, S.A.; Borland, R.M. Clinical development of moricizine as an antiarrhythmic agent. Am. J. Cardiol. 1990, 65, 11D–14D, discussion 68D–71D. [Google Scholar] [CrossRef]
- Tsuji, Y.; Nishimura, M.; Osada, M.; Watanabe, Y. Membrane action of ethmozin on normoxic and hypoxic canine Purkinje fibers. J. Cardiovasc. Pharm. 1983, 5, 961–967. [Google Scholar] [CrossRef]
- Peters, R.W.; Mitchell, L.B.; Brooks, M.M.; Echt, D.S.; Barker, A.H.; Capone, R.; Liebson, P.R.; Greene, H.L. Circadian pattern of arrhythmic death in patients receiving encainide, flecainide or moricizine in the Cardiac Arrhythmia Suppression Trial (CAST). J. Am. Coll. Cardiol. 1994, 23, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Balsalobre, A.; Marcacci, L.; Schibler, U. Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr. Biol. 2000, 10, 1291–1294. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, Y.; Minami, I.; Kadotani, H.; Nishida, E. Resetting of peripheral circadian clock by prostaglandin E2. EMBO Rep. 2005, 6, 256–261. [Google Scholar] [CrossRef] [Green Version]
- Hirota, T.; Lewis, W.G.; Liu, A.C.; Lee, J.W.; Schultz, P.G.; Kay, S.A. A chemical biology approach reveals period shortening of the mammalian circadian clock by specific inhibition of GSK-3beta. Proc. Natl. Acad. Sci. USA 2008, 105, 20746–20751. [Google Scholar] [CrossRef] [Green Version]
- Isojima, Y.; Nakajima, M.; Ukai, H.; Fujishima, H.; Yamada, R.G.; Masumoto, K.H.; Kiuchi, R.; Ishida, M.; Ukai-Tadenuma, M.; Minami, Y.; et al. CKIepsilon/delta-dependent phosphorylation is a temperature-insensitive, period-determining process in the mammalian circadian clock. Proc. Natl. Acad. Sci. USA 2009, 106, 15744–15749. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Yoo, S.H.; Takahashi, J.S. Development and Therapeutic Potential of Small-Molecule Modulators of Circadian Systems. Annu. Rev. Pharm. Toxicol. 2018, 58, 231–252. [Google Scholar] [CrossRef]
- Miller, S.; Hirota, T. Pharmacological Interventions to Circadian Clocks and Their Molecular Bases. J. Mol. Biol. 2020, 432, 3498–3514. [Google Scholar] [CrossRef]
- Wallach, T.; Kramer, A. Chemical chronobiology: Toward drugs manipulating time. FEBS Lett. 2015, 589, 1530–1538. [Google Scholar] [CrossRef] [Green Version]
- Tamai, T.K.; Nakane, Y.; Ota, W.; Kobayashi, A.; Ishiguro, M.; Kadofusa, N.; Ikegami, K.; Yagita, K.; Shigeyoshi, Y.; Sudo, M.; et al. Identification of circadian clock modulators from existing drugs. EMBO Mol. Med. 2018, 10. [Google Scholar] [CrossRef]
- Chen, Z.; Yoo, S.H.; Park, Y.S.; Kim, K.H.; Wei, S.; Buhr, E.; Ye, Z.Y.; Pan, H.L.; Takahashi, J.S. Identification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screening. Proc. Natl. Acad. Sci. USA 2012, 109, 101–106. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Nohara, K.; Park, N.; Park, Y.-S.; Guillory, B.; Zhao, Z.; Garcia, J.M.; Koike, N.; Lee, C.C.; Takahashi, J.S. The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab. 2016, 23, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Burish, M.J.; Han, C.; Mawatari, K.; Wirianto, M.; Kim, E.; Ono, K.; Parakramaweera, R.; Chen, Z.; Yoo, S.H. The first-line cluster headache medication verapamil alters the circadian period and elicits sex-specific sleep changes in mice. Chronobiol. Int. 2021, 1–12. [Google Scholar] [CrossRef]
- Izumo, M.; Sato, T.R.; Straume, M.; Johnson, C.H. Quantitative analyses of circadian gene expression in mammalian cell cultures. PLoS Comput. Biol. 2006, 2, e136. [Google Scholar] [CrossRef]
- Yoo, S.H.; Yamazaki, S.; Lowrey, P.L.; Shimomura, K.; Ko, C.H.; Buhr, E.D.; Siepka, S.M.; Hong, H.K.; Oh, W.J.; Yoo, O.J.; et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. USA 2004, 101, 5339–5346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Veen, D.R.; Shao, J.; Xi, Y.; Li, L.; Duffield, G.E. Cardiac atrial circadian rhythms in PERIOD2::LUCIFERASE and per1:luc mice: Amplitude and phase responses to glucocorticoid signaling and medium treatment. PLoS ONE 2012, 7, e47692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donohue, K.D.; Medonza, D.C.; Crane, E.R.; O’Hara, B.F. Assessment of a non-invasive high-throughput classifier for behaviours associated with sleep and wake in mice. Biomed. Eng. Online 2008, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, A.C.; Welsh, D.K.; Ko, C.H.; Tran, H.G.; Zhang, E.E.; Priest, A.A.; Buhr, E.D.; Singer, O.; Meeker, K.; Verma, I.M.; et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell 2007, 129, 605–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, E.E.; Liu, A.C.; Hirota, T.; Miraglia, L.J.; Welch, G.; Pongsawakul, P.Y.; Liu, X.; Atwood, A.; Huss, J.W., 3rd; Janes, J.; et al. A genome-wide RNAi screen for modifiers of the circadian clock in human cells. Cell 2009, 139, 199–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levi, F.; Schibler, U. Circadian rhythms: Mechanisms and therapeutic implications. Annu. Rev. Pharm. Toxicol. 2007, 47, 593–628. [Google Scholar] [CrossRef] [PubMed]
- Zmrzljak, U.P.; Rozman, D. Circadian regulation of the hepatic endobiotic and xenobitoic detoxification pathways: The time matters. Chem. Res. Toxicol. 2012, 25, 811–824. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Chen, Z. Molecular Targets for Small-Molecule Modulators of Circadian Clocks. Curr. Drug Metab. 2016, 17, 503–512. [Google Scholar] [CrossRef] [Green Version]
- Nohara, K.; Yoo, S.H.; Chen, Z.J. Manipulating the circadian and sleep cycles to protect against metabolic disease. Front. Endocrinol. 2015, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Cederroth, C.R.; Albrecht, U.; Bass, J.; Brown, S.A.; Dyhrfjeld-Johnsen, J.; Gachon, F.; Green, C.B.; Hastings, M.H.; Helfrich-Forster, C.; Hogenesch, J.B.; et al. Medicine in the Fourth Dimension. Cell Metab. 2019, 30, 238–250. [Google Scholar] [CrossRef]
- Hermida, R.C.; Crespo, J.J.; Otero, A.; Dominguez-Sardina, M.; Moya, A.; Rios, M.T.; Castineira, M.C.; Callejas, P.A.; Pousa, L.; Sineiro, E.; et al. Asleep blood pressure: Significant prognostic marker of vascular risk and therapeutic target for prevention. Eur. Heart J. 2018, 39, 4159–4171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montaigne, D.; Marechal, X.; Modine, T.; Coisne, A.; Mouton, S.; Fayad, G.; Ninni, S.; Klein, C.; Ortmans, S.; Seunes, C.; et al. Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbalpha antagonism: A single-centre propensity-matched cohort study and a randomised study. Lancet 2018, 391, 59–69. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, R.; Tien, C.L.; Chan, R.E.; Sugi, K.; Fu, C.; Griffin, A.C.; Shen, Y.; Burris, T.P.; Liao, X.; et al. REV-ERBalpha ameliorates heart failure through transcription repression. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reitz, C.J.; Alibhai, F.J.; Khatua, T.N.; Rasouli, M.; Bridle, B.W.; Burris, T.P.; Martino, T.A. SR9009 administered for one day after myocardial ischemia-reperfusion prevents heart failure in mice by targeting the cardiac inflammasome. Commun. Biol. 2019, 2, 353. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.; Le, H.D.; Melkani, G.C.; Panda, S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science 2015, 347, 1265–1269. [Google Scholar] [CrossRef] [Green Version]
- Gabel, K.; Cienfuegos, S.; Kalam, F.; Ezpeleta, M.; Varady, K.A. Time-Restricted Eating to Improve Cardiovascular Health. Curr. Atheroscler. Rep. 2021, 23, 22. [Google Scholar] [CrossRef]
- Nohara, K.; Nemkov, T.; D’Alessandro, A.; Yoo, S.H.; Chen, Z. Coordinate Regulation of Cholesterol and Bile Acid Metabolism by the Clock Modifier Nobiletin in Metabolically Challenged Old Mice. Int. J. Mol. Sci. 2019, 20, 4281. [Google Scholar] [CrossRef] [Green Version]
- Nohara, K.; Mallampalli, V.; Nemkov, T.; Wirianto, M.; Yang, J.; Ye, Y.; Sun, Y.; Han, L.; Esser, K.A.; Mileykovskaya, E.; et al. Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat. Commun. 2019, 10, 3923. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.H.; Kojima, S.; Shimomura, K.; Koike, N.; Buhr, E.D.; Furukawa, T.; Ko, C.H.; Gloston, G.; Ayoub, C.; Nohara, K.; et al. Period2 3′-UTR and microRNA-24 regulate circadian rhythms by repressing PERIOD2 protein accumulation. Proc. Natl. Acad. Sci. USA 2017, 114, E8855–E8864. [Google Scholar] [CrossRef] [Green Version]
- Nohara, K.; Kim, E.; Wirianto, M.; Mileykovskaya, E.; Dowhan, W.; Chen, Z.; Yoo, S.-H. Cardiolipin Synthesis in Skeletal Muscle Is Rhythmic and Modifiable by Age and Diet. Oxidative Med. Cell. Longev. 2020, 2020, 5304768. [Google Scholar] [CrossRef]
- Wirianto, M.; Yang, J.; Kim, E.; Gao, S.; Paudel, K.R.; Choi, J.M.; Choe, J.; Gloston, G.F.; Ademoji, P.; Parakramaweera, R.; et al. The GSK-3beta-FBXL21 Axis Contributes to Circadian TCAP Degradation and Skeletal Muscle Function. Cell Rep. 2020, 32, 108140. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]

| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Clock | CCTTCAGCAGTCAGTCCATAAAC | AGACATCGCTGGCTGTGTTAA |
| Bmal1 | CCACCTCAGAGCCATTGATACA | GAGCAGGTTTAGTTCCACTTTGTCT |
| Per1 | CCCAGCTTTACCTGCAGAAG | ATGGTCGAA AGG AAGCCTCT |
| Per2 | TGTGCGATGATGATTCGTGA | GGTGAAGGTACGTTTGGTTTGC |
| Per3 | GTGATTGTTCACGCGTCT GT | CACTGCCATCTCGAGTTCAA |
| Cry1 | TGA GGC AAG CAG ACT GAA TAT TG | CCT CTG TAC CGG GAA AGC TG |
| Cry2 | CTG GCG AGA AGG TAG AGT GG | GACGCAGAATTA GCCTTTGC |
| Rev-erba | CATGGTGCTACTGTGTAAGGTGTGT | CACAGGCGTGCACTCCATAG |
| Rev-erbb | TGAACGCAGGAGGTGTGATTG | GAGGACTGGAAGCTATTCTCAGA |
| Dbp | CTGGCCCGAGTCTTTTTGC | CCAGGTCCACGTATTCCACG |
| Scn5a | GCAGAAGGTGAAGTTCGTGG | TGAAGACCAAGTTTCCGACC |
| Hcn4 | CGACAGCGCATCCATGACTA | GCTGGAAGACCTCGAAACGC |
| Kcnj2 | TGAAGTTGCCCTAACAAGCA | AAAGTAAGTATGACAAAGACGGAA |
| Cacna1c | CCCTTCTTGTGCTCTTCGTC | TATGCCCTCCTGGTTGTAGC |
| Kcnh2 | GATCGCCTTCTACCGGAAA | CATTCTTCACGGGTACCACA |
| Ncx1 | GAGGTTGGAGCAGTTGGAAG | ACCAGACGAAATCCCATTGA |
| Klf15 | TGTGGGCCAGAAGTTTCC | AAGTGCATTTGTGCATTTTGAG |
| Cx43 | GAGAGCCCGAACTCTCCTTT | TGGGCACCTCTCTTTCACTT |
| KChIP2 | AACTATCCACGGTGTGCCAC | GGACATTCGTTCTTGAAGCCT |
| Kcnd2 | GCAAGCGGAATGGGCTAC | TGGTTTTCTCCAGGCAGTG |
| Gapdh | CAAGGTCATCCATGACAACTTTG | GGCCATCCACAGTCTTCTGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, C.; Wirianto, M.; Kim, E.; Burish, M.J.; Yoo, S.-H.; Chen, Z. Clock-Modulating Activities of the Anti-Arrhythmic Drug Moricizine. Clocks & Sleep 2021, 3, 351-365. https://doi.org/10.3390/clockssleep3030022
Han C, Wirianto M, Kim E, Burish MJ, Yoo S-H, Chen Z. Clock-Modulating Activities of the Anti-Arrhythmic Drug Moricizine. Clocks & Sleep. 2021; 3(3):351-365. https://doi.org/10.3390/clockssleep3030022
Chicago/Turabian StyleHan, Chorong, Marvin Wirianto, Eunju Kim, Mark J. Burish, Seung-Hee Yoo, and Zheng Chen. 2021. "Clock-Modulating Activities of the Anti-Arrhythmic Drug Moricizine" Clocks & Sleep 3, no. 3: 351-365. https://doi.org/10.3390/clockssleep3030022
APA StyleHan, C., Wirianto, M., Kim, E., Burish, M. J., Yoo, S.-H., & Chen, Z. (2021). Clock-Modulating Activities of the Anti-Arrhythmic Drug Moricizine. Clocks & Sleep, 3(3), 351-365. https://doi.org/10.3390/clockssleep3030022





