The Impact of the Circadian Clock on Skin Physiology and Cancer Development
Abstract
1. Skin Cancers and Risk Factors
1.1. Melanoma and Non-Melanoma Skin Cancers
1.2. Skin Cancer Risk Factors
2. The Circadian Clock
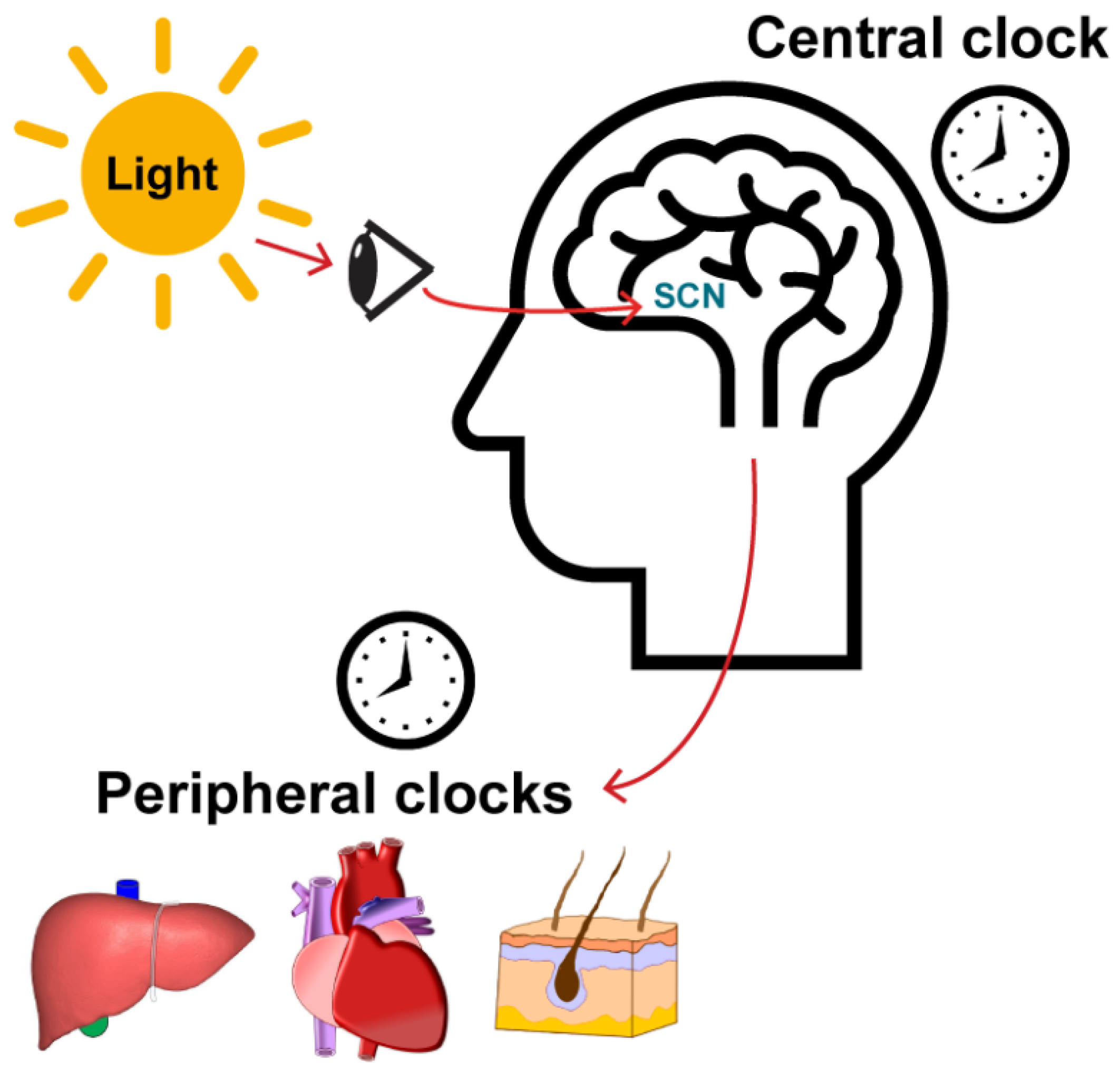
2.1. Central and Peripheral Clocks
2.2. Molecular Architecture of the Clock
2.3. Shiftwork and Clock Disruption
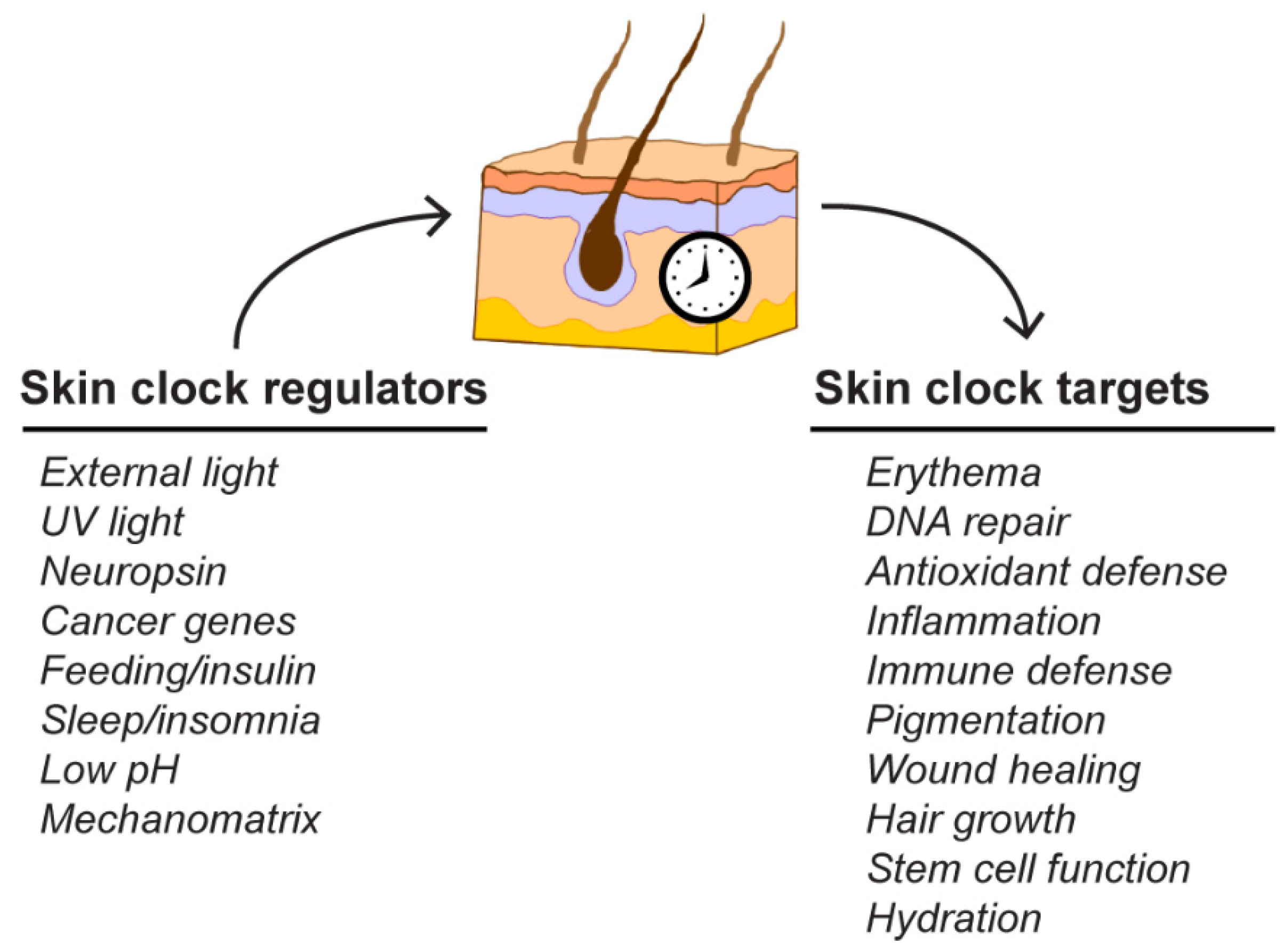
3. The Skin Circadian Clock
3.1. Identifying and Characterizing the Molecular Clock in Human Skin
3.2. Regulators of Circadian Clock Function in the Skin
3.3. Physiological Targets of the Skin Clock
4. Interplay between the Skin Circadian Clock, DNA Damage Responses, and Carcinogenesis
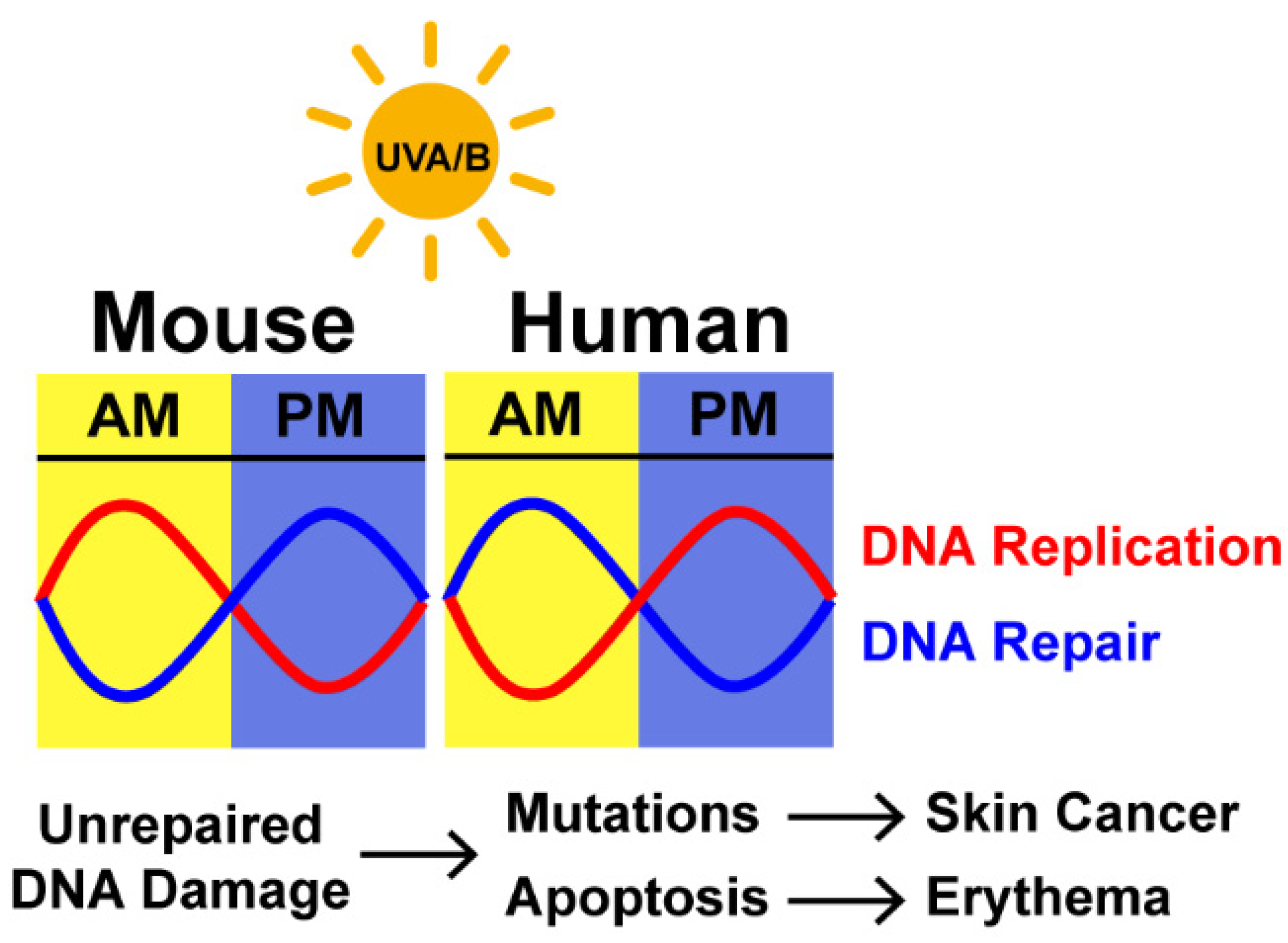
4.1. Induction of DNA Damage as Function of the Time of the Day
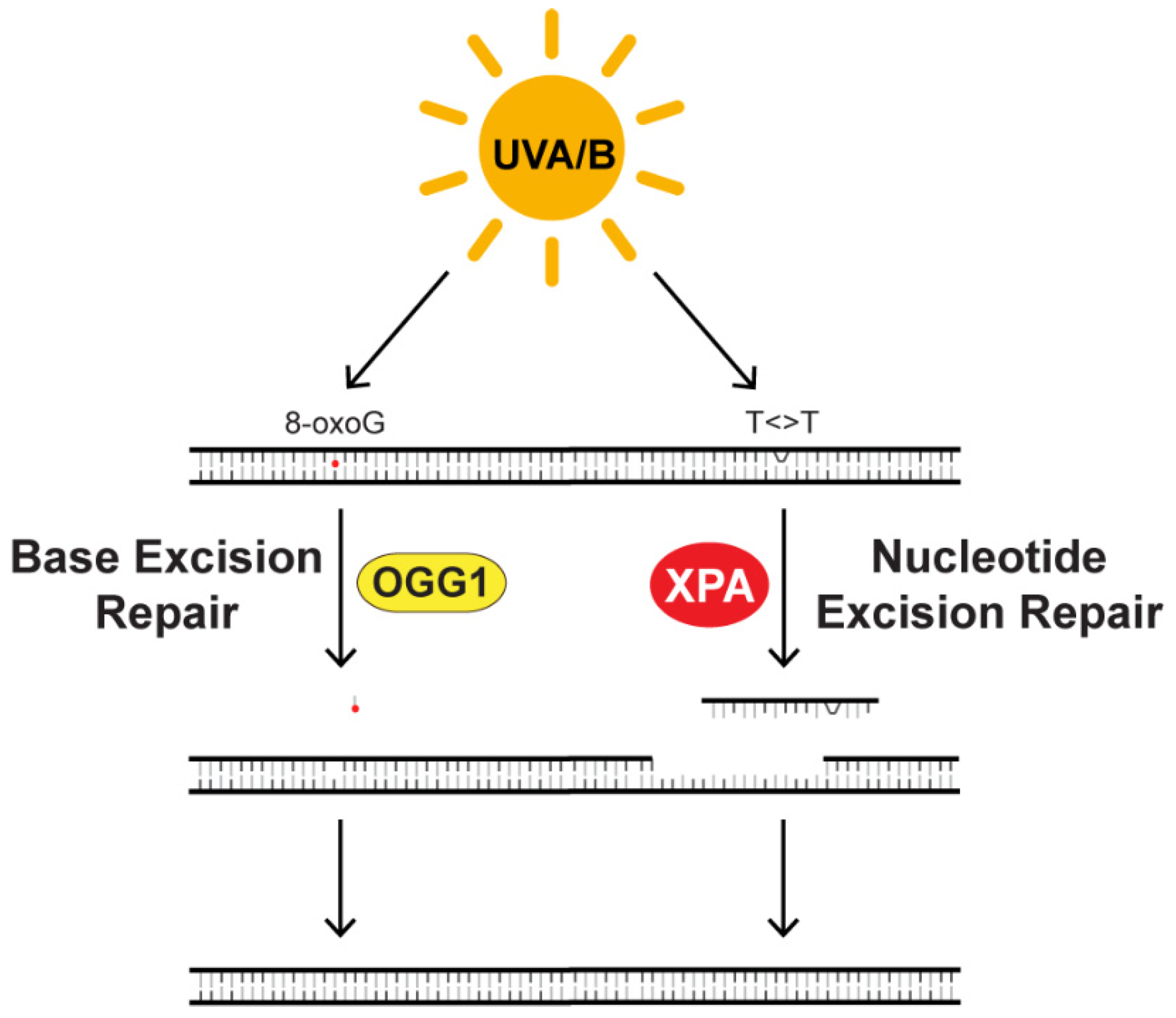
4.2. Circadian Regulation of DNA Repair, DNA Synthesis, and the DNA Damage Response
4.3. Circadian Regulation of UV Erythema and Skin Carcinogenesis
4.4. Circadian Regulation of Radiation-Induced Dermatitis
5. The Circadian Clock and Disease Treatment
5.1. Chronopharmacology and Chronotherapy
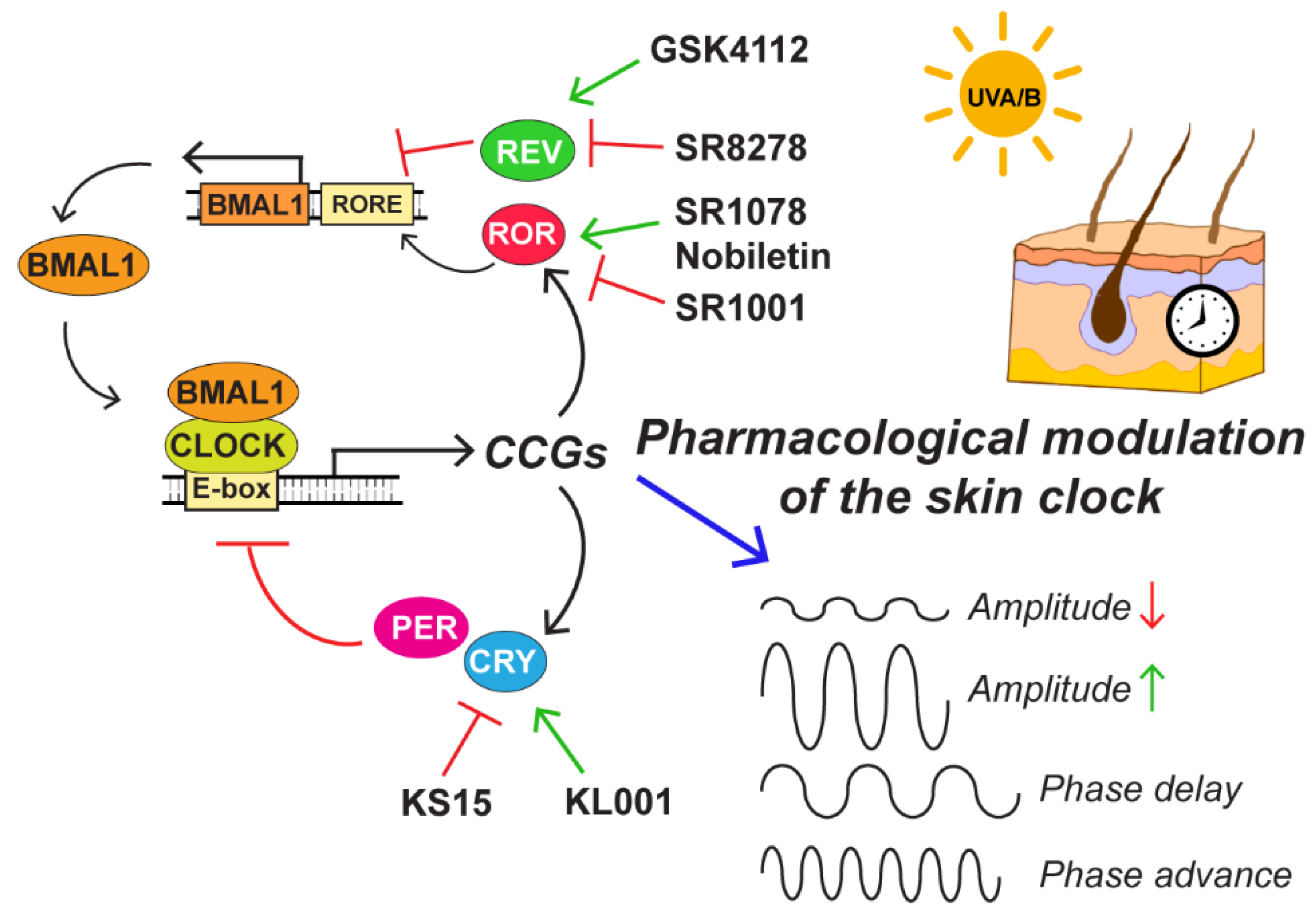
5.2. Pharmacological Modulation of the Skin Circadian Clock
6. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Apalla, Z.; Nashan, D.; Weller, R.B.; Castellsagué, X. Skin Cancer: Epidemiology, Disease Burden, Pathophysiology, Diagnosis, and Therapeutic Approaches. Dermatol. Ther. 2017, 7, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Guy, G.P., Jr.; Ekwueme, D.U.; Tangka, F.K.; Richardson, L.C. Melanoma treatment costs: A systematic review of the literature, 1990–2011. Am. J. Prev. Med. 2012, 43, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.A.; Kampp, J. Skin Cancer Epidemiology, Detection, and Management. Med. Clin. N. Am. 2015, 99, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Didona, D.; Paolino, G.; Bottoni, U.; Cantisani, C. Non Melanoma Skin Cancer Pathogenesis Overview. Biomedicines 2018, 6, 6. [Google Scholar] [CrossRef]
- Linares, M.A.; Zakaria, A.; Nizran, P. Skin Cancer. Prim. Care 2015, 42, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Rass, U.; Ahel, I.; West, S.C. Defective DNA repair and neurodegenerative disease. Cell 2007, 130, 991–1004. [Google Scholar] [CrossRef]
- Mack, T. The pathogenesis of melanoma induced by ultraviolet radiation. N. Engl. J. Med. 1999, 341, 766. [Google Scholar]
- Mudigonda, T.; Pearce, D.J.; Yentzer, B.A.; Williford, P.; Feldman, S.R. The economic impact of non-melanoma skin cancer: A review. J. Natl. Compr. Canc. Netw. 2010, 8, 888–896. [Google Scholar] [CrossRef]
- Brash, D.E. UV signature mutations. Photochem. Photobiol. 2015, 91, 15–26. [Google Scholar] [CrossRef]
- Modenese, A.; Korpinen, L.; Gobba, F. Solar Radiation Exposure and Outdoor Work: An Underestimated Occupational Risk. Int. J. Environ. Res. Public Health 2018, 15, 2063. [Google Scholar] [CrossRef]
- Hault, K.; Rönsch, H.; Beissert, S.; Knuschke, P.; Bauer, A. Knowledge of outdoor workers on the effects of natural UV radiation and methods of protection against exposure. J. Eur. Acad. Dermatol. Venereol. 2016, 30 (Suppl. 3), 34–37. [Google Scholar] [CrossRef]
- Doré, J.-F.; Chignol, M.-C. UV Driven Tanning Salons: Danger on Main Street. Adv. Exp. Med. Biol. 2017, 996, 335–346. [Google Scholar] [CrossRef]
- Nilsen, L.T.N.; Hannevik, M.; Veierød, M.B. Ultraviolet exposure from indoor tanning devices: A systematic review. Br. J. Dermatol. 2016, 174, 730–740. [Google Scholar] [CrossRef]
- Rizza, E.R.H.; DiGiovanna, J.J.; Khan, S.G.; Tamura, D.; Jeskey, J.D.; Kraemer, K.H. Xeroderma Pigmentosum: A Model for Human Premature Aging. J. Investig. Dermatol. 2021, 141, 976–984. [Google Scholar] [CrossRef]
- Gupta, V.; Sharma, V.K. Skin typing: Fitzpatrick grading and others. Clin. Dermatol. 2019, 37, 430–436. [Google Scholar] [CrossRef]
- LaBerge, G.S.; Duvall, E.; Grasmick, Z.; Haedicke, K.; Galan, A.; Leverett, J.; Baswan, S.; Yim, S.; Pawelek, J. Recent Advances in Studies of Skin Color and Skin Cancer. Yale J. Biol. Med. 2020, 93, 69–80. [Google Scholar] [PubMed]
- Premi, S.; Wallisch, S.; Mano, C.M.; Weiner, A.B.; Bacchiocchi, A.; Wakamatsu, K.; Bechara, E.J.; Halaban, R.; Douki, T.; Brash, D.E. Photochemistry. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science 2015, 347, 842–847. [Google Scholar] [CrossRef]
- DiGiovanna, J.J.; Kraemer, K.H. Shining a light on xeroderma pigmentosum. J. Investig. Dermatol. 2012, 132, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Norval, M.; Halliday, G.M. The consequences of UV-induced immunosuppression for human health. Photochem. Photobiol. 2011, 87, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Norval, M. Ultraviolet radiation-induced immunosuppression and its relevance for skin carcinogenesis. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2018, 17, 1872–1884. [Google Scholar] [CrossRef] [PubMed]
- Kubica, A.W.; Brewer, J.D. Melanoma in immunosuppressed patients. Mayo Clin. Proc. 2012, 87, 991–1003. [Google Scholar] [CrossRef]
- Euvrard, S.; Kanitakis, J.; Claudy, A. Skin cancers after organ transplantation. N. Engl. J. Med. 2003, 348, 1681–1691. [Google Scholar] [CrossRef]
- Herman, S.; Rogers, H.D.; Ratner, D. Immunosuppression and squamous cell carcinoma: A focus on solid organ transplant recipients. Skinmed 2007, 6, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. The Vitamin D Receptor as Tumor Suppressor in Skin. Adv. Exp. Med. Biol. 2020, 1268, 285–306. [Google Scholar] [CrossRef]
- El-Sharkawy, A.; Malki, A. Vitamin D Signaling in Inflammation and Cancer: Molecular Mechanisms and Therapeutic Implications. Molecules 2020, 25, 3219. [Google Scholar] [CrossRef]
- Wong, C.T.; Oh, D.H. Vitamin D Receptor Promotes Global Nucleotide Excision Repair by Facilitating XPC Dissociation from Damaged DNA. J. Investig. Dermatol. 2021. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Brożyna, A.A.; Zmijewski, M.A.; Janjetovic, Z.; Kim, T.-K.; Slominski, R.M.; Tuckey, R.C.; Mason, R.S.; Jetten, A.M.; Guroji, P.; et al. The Role of Classical and Novel Forms of Vitamin D in the Pathogenesis and Progression of Nonmelanoma Skin Cancers. Adv. Exp. Med. Biol. 2020, 1268, 257–283. [Google Scholar] [CrossRef] [PubMed]
- Smola, S. Human Papillomaviruses and Skin Cancer. Adv. Exp. Med. Biol. 2020, 1268, 195–209. [Google Scholar] [CrossRef]
- DeCaprio, J.A. Molecular Pathogenesis of Merkel Cell Carcinoma. Annu. Rev. Pathol. 2021, 16, 69–91. [Google Scholar] [CrossRef]
- Zhou, Q.; Xi, S. A review on arsenic carcinogenesis: Epidemiology, metabolism, genotoxicity and epigenetic changes. Regul. Toxicol. Pharmacol. 2018, 99, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Palma-Lara, I.; Martínez-Castillo, M.; Quintana-Pérez, J.C.; Arellano-Mendoza, M.G.; Tamay-Cach, F.; Valenzuela-Limón, O.L.; García-Montalvo, E.A.; Hernández-Zavala, A. Arsenic exposure: A public health problem leading to several cancers. Regul. Toxicol. Pharmacol. 2020, 110, 104539. [Google Scholar] [CrossRef]
- Sehgal, A. Physiology Flies with Time. Cell 2017, 171, 1232–1235. [Google Scholar] [CrossRef]
- Vitaterna, M.H.; King, D.P.; Chang, A.M.; Kornhauser, J.M.; Lowrey, P.L.; McDonald, J.D.; Dove, W.F.; Pinto, L.H.; Turek, F.W.; Takahashi, J.S. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 1994, 264, 719–725. [Google Scholar] [CrossRef]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. 2017, 18, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Vanselow, K.; Kramer, A. Role of phosphorylation in the mammalian circadian clock. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.Y.C.; Ptáček, L.J.; Fu, Y.-H. Genetic insights on sleep schedules: This time, it’s PERsonal. Trends Genet. 2012, 28, 598–605. [Google Scholar] [CrossRef]
- Bass, J.T. The circadian clock system’s influence in health and disease. Genome Med. 2017, 9, 94. [Google Scholar] [CrossRef]
- Morgan, M.N.; Dvuchbabny, S.; Martinez, C.-A.; Kerr, B.; Cistulli, P.A.; Cook, K.M. The Cancer Clock Is (Not) Ticking: Links between Circadian Rhythms and Cancer. Clocks Sleep 2019, 1, 435–458. [Google Scholar] [CrossRef]
- Sancar, A.; Lindsey-Boltz, L.A.; Kang, T.H.; Reardon, J.T.; Lee, J.H.; Ozturk, N. Circadian clock control of the cellular response to DNA damage. FEBS Lett. 2010, 584, 2618–2625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef] [PubMed]
- Zanello, S.B.; Jackson, D.M.; Holick, M.F. Expression of the circadian clock genes clock and period1 in human skin. J. Investig. Dermatol. 2000, 115, 757–760. [Google Scholar] [CrossRef]
- Spörl, F.; Schellenberg, K.; Blatt, T.; Wenck, H.; Wittern, K.-P.; Schrader, A.; Kramer, A. A circadian clock in HaCaT keratinocytes. J. Investig. Dermatol. 2011, 131, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Sandu, C.; Dumas, M.; Malan, A.; Sambakhe, D.; Marteau, C.; Nizard, C.; Schnebert, S.; Perrier, E.; Challet, E.; Pevet, P.; et al. Human skin keratinocytes, melanocytes, and fibroblasts contain distinct circadian clock machineries. Cell. Mol. Life Sci. 2012, 69, 3329–3339. [Google Scholar] [CrossRef]
- Bjarnason, G.A.; Jordan, R.C.; Wood, P.A.; Li, Q.; Lincoln, D.W.; Sothern, R.B.; Hrushesky, W.J.; Ben-David, Y. Circadian expression of clock genes in human oral mucosa and skin: Association with specific cell-cycle phases. Am. J. Pathol. 2001, 158, 1793–1801. [Google Scholar] [CrossRef]
- Brown, S.A.; Fleury-Olela, F.; Nagoshi, E.; Hauser, C.; Juge, C.; Meier, C.A.; Chicheportiche, R.; Dayer, J.-M.; Albrecht, U.; Schibler, U. The period length of fibroblast circadian gene expression varies widely among human individuals. PLoS Biol. 2005, 3, e338. [Google Scholar] [CrossRef]
- Spörl, F.; Korge, S.; Jürchott, K.; Wunderskirchner, M.; Schellenberg, K.; Heins, S.; Specht, A.; Stoll, C.; Klemz, R.; Maier, B.; et al. Krüppel-like factor 9 is a circadian transcription factor in human epidermis that controls proliferation of keratinocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 10903–10908. [Google Scholar] [CrossRef] [PubMed]
- Akashi, M.; Soma, H.; Yamamoto, T.; Tsugitomi, A.; Yamashita, S.; Yamamoto, T.; Nishida, E.; Yasuda, A.; Liao, J.K.; Node, K. Noninvasive method for assessing the human circadian clock using hair follicle cells. Proc. Natl. Acad. Sci. USA 2010, 107, 15643–15648. [Google Scholar] [CrossRef]
- Watanabe, M.; Hida, A.; Kitamura, S.; Enomoto, M.; Ohsawa, Y.; Katayose, Y.; Nozaki, K.; Moriguchi, Y.; Aritake, S.; Higuchi, S.; et al. Rhythmic expression of circadian clock genes in human leukocytes and beard hair follicle cells. Biochem. Biophys. Res. Commun. 2012, 425, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Bracci, M.; Ciarapica, V.; Copertaro, A.; Barbaresi, M.; Manzella, N.; Tomasetti, M.; Gaetani, S.; Monaco, F.; Amati, M.; Valentino, M.; et al. Peripheral Skin Temperature and Circadian Biological Clock in Shift Nurses after a Day off. Int. J. Mol. Sci. 2016, 17, 623. [Google Scholar] [CrossRef]
- Hattammaru, M.; Tahara, Y.; Kikuchi, T.; Okajima, K.; Konishi, K.; Nakajima, S.; Sato, K.; Otsuka, K.; Sakura, H.; Shibata, S.; et al. The effect of night shift work on the expression of clock genes in beard hair follicle cells. Sleep Med. 2019, 56, 164–170. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Matsumura, R.; Matsuzaki, T.; Nakamura, W.; Node, K.; Akashi, M. A simple method using ex vivo culture of hair follicle tissue to investigate intrinsic circadian characteristics in humans. Sci. Rep. 2017, 7, 6824. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, Z.; Lovig, C.; Kommedal, S.; Keszthelyi, R.; Szekeres, G.; Battyáni, Z.; Csernus, V.; Nagy, A.D. Altered expression patterns of clock gene mRNAs and clock proteins in human skin tumors. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2013, 34, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Ruben, M.D.; Schmidt, R.E.; Francey, L.J.; Smith, D.F.; Anafi, R.C.; Hughey, J.J.; Tasseff, R.; Sherrill, J.D.; Oblong, J.E.; et al. Population-level rhythms in human skin with implications for circadian medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 12313–12318. [Google Scholar] [CrossRef]
- Kimball, A.B.; Alora-Palli, M.B.; Tamura, M.; Mullins, L.A.; Soh, C.; Binder, R.L.; Houston, N.A.; Conley, E.D.; Tung, J.Y.; Annunziata, N.E.; et al. Age-induced and photoinduced changes in gene expression profiles in facial skin of Caucasian females across 6 decades of age. J. Am. Acad. Dermatol. 2018, 78, 29–39.e7. [Google Scholar] [CrossRef]
- Wu, G.; Ruben, M.D.; Francey, L.J.; Smith, D.F.; Sherrill, J.D.; Oblong, J.E.; Mills, K.J.; Hogenesch, J.B. A population-based gene expression signature of molecular clock phase from a single epidermal sample. Genome Med. 2020, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Ruben, M.D.; Smith, D.F.; FitzGerald, G.A.; Hogenesch, J.B. Dosing time matters. Science 2019, 365, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Valekunja, U.K.; Stangherlin, A.; Howell, S.A.; Snijders, A.P.; Damodaran, G.; Reddy, A.B. Circadian rhythms in the absence of the clock gene Bmal1. Science 2020, 367, 800–806. [Google Scholar] [CrossRef]
- Fan, S.M.-Y.; Chang, Y.-T.; Chen, C.-L.; Wang, W.-H.; Pan, M.-K.; Chen, W.-P.; Huang, W.-Y.; Xu, Z.; Huang, H.-E.; Chen, T.; et al. External light activates hair follicle stem cells through eyes via an ipRGC-SCN-sympathetic neural pathway. Proc. Natl. Acad. Sci. USA 2018, 115, E6880–E6889. [Google Scholar] [CrossRef]
- Rüger, M.; Gordijn, M.C.M.; Beersma, D.G.M.; de Vries, B.; Daan, S. Acute and phase-shifting effects of ocular and extraocular light in human circadian physiology. J. Biol. Rhythm. 2003, 18, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Buhr, E.D.; Vemaraju, S.; Diaz, N.; Lang, R.A.; Van Gelder, R.N. Neuropsin (OPN5) Mediates Local Light-Dependent Induction of Circadian Clock Genes and Circadian Photoentrainment in Exposed Murine Skin. Curr. Biol. 2019, 29, 3478–3487.e4. [Google Scholar] [CrossRef] [PubMed]
- Nikkola, V.; Miettinen, M.E.; Karisola, P.; Grönroos, M.; Ylianttila, L.; Alenius, H.; Snellman, E.; Partonen, T. Ultraviolet B radiation modifies circadian time in epidermal skin and in subcutaneous adipose tissue. Photodermatol. Photoimmunol. Photomed. 2019, 35, 157–163. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, L.; Chen, X.; Li, Q.; Li, J. Association between circadian disruption and diseases: A narrative review. Life Sci. 2020, 262, 118512. [Google Scholar] [CrossRef]
- Lippert, J.; Halfter, H.; Heidbreder, A.; Röhr, D.; Gess, B.; Boentert, M.; Osada, N.; Young, P. Altered dynamics in the circadian oscillation of clock genes in dermal fibroblasts of patients suffering from idiopathic hypersomnia. PLoS ONE 2014, 9, e85255. [Google Scholar] [CrossRef]
- Materna, L.; Halfter, H.; Heidbreder, A.; Boentert, M.; Lippert, J.; Koch, R.; Young, P. Idiopathic Hypersomnia Patients Revealed Longer Circadian Period Length in Peripheral Skin Fibroblasts. Front. Neurol. 2018, 9, 424. [Google Scholar] [CrossRef]
- Wang, H.; van Spyk, E.; Liu, Q.; Geyfman, M.; Salmans, M.L.; Kumar, V.; Ihler, A.; Li, N.; Takahashi, J.S.; Andersen, B. Time-Restricted Feeding Shifts the Skin Circadian Clock and Alters UVB-Induced DNA Damage. Cell Rep. 2017, 20, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Kajimoto, J.; Matsumura, R.; Node, K.; Akashi, M. Potential role of the pancreatic hormone insulin in resetting human peripheral clocks. Genes Cells 2018, 23, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Relógio, A.; Thomas, P.; Medina-Pérez, P.; Reischl, S.; Bervoets, S.; Gloc, E.; Riemer, P.; Mang-Fatehi, S.; Maier, B.; Schäfer, R.; et al. Ras-mediated deregulation of the circadian clock in cancer. PLoS Genet. 2014, 10, e1004338. [Google Scholar] [CrossRef]
- Zagni, C.; Almeida, L.O.; Balan, T.; Martins, M.T.; Rosselli-Murai, L.K.; Papagerakis, P.; Castilho, R.M.; Squarize, C.H. PTEN Mediates Activation of Core Clock Protein BMAL1 and Accumulation of Epidermal Stem Cells. Stem Cell Rep. 2017, 9, 304–314. [Google Scholar] [CrossRef]
- De Assis, L.V.M.; Moraes, M.N.; Magalhães-Marques, K.K.; Kinker, G.S.; da Silveira Cruz-Machado, S.; De Lauro Castrucci, A.M. Non-Metastatic Cutaneous Melanoma Induces Chronodisruption in Central and Peripheral Circadian Clocks. Int. J. Mol. Sci. 2018, 19, 1065. [Google Scholar] [CrossRef]
- Kowalska, E.; Ripperger, J.A.; Hoegger, D.C.; Bruegger, P.; Buch, T.; Birchler, T.; Mueller, A.; Albrecht, U.; Contaldo, C.; Brown, S.A. NONO couples the circadian clock to the cell cycle. Proc. Natl. Acad. Sci. USA 2013, 110, 1592–1599. [Google Scholar] [CrossRef]
- Janich, P.; Pascual, G.; Merlos-Suárez, A.; Batlle, E.; Ripperger, J.; Albrecht, U.; Cheng, H.-Y.M.; Obrietan, K.; Di Croce, L.; Benitah, S.A. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature 2011, 480, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Janich, P.; Toufighi, K.; Solanas, G.; Luis, N.M.; Minkwitz, S.; Serrano, L.; Lehner, B.; Benitah, S.A. Human epidermal stem cell function is regulated by circadian oscillations. Cell Stem Cell 2013, 13, 745–753. [Google Scholar] [CrossRef]
- Silveira, E.J.D.; Nascimento Filho, C.H.V.; Yujra, V.Q.; Webber, L.P.; Castilho, R.M.; Squarize, C.H. BMAL1 Modulates Epidermal Healing in a Process Involving the Antioxidative Defense Mechanism. Int. J. Mol. Sci. 2020, 21, 901. [Google Scholar] [CrossRef]
- Sasaki, H.; Hokugo, A.; Wang, L.; Morinaga, K.; Ngo, J.T.; Okawa, H.; Nishimura, I. Neuronal PAS Domain 2 (Npas2)-Deficient Fibroblasts Accelerate Skin Wound Healing and Dermal Collagen Reconstruction. Anat. Rec. 2020, 303, 1630–1641. [Google Scholar] [CrossRef]
- Deshayes, N.; Genty, G.; Berthelot, F.; Paris, M. Human long-term deregulated circadian rhythm alters regenerative properties of skin and hair precursor cells. Eur. J. Dermatol. 2018, 28, 467–475. [Google Scholar] [CrossRef]
- Hoyle, N.P.; Seinkmane, E.; Putker, M.; Feeney, K.A.; Krogager, T.P.; Chesham, J.E.; Bray, L.K.; Thomas, J.M.; Dunn, K.; Blaikley, J.; et al. Circadian actin dynamics drive rhythmic fibroblast mobilization during wound healing. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- Lin, K.K.; Kumar, V.; Geyfman, M.; Chudova, D.; Ihler, A.T.; Smyth, P.; Paus, R.; Takahashi, J.S.; Andersen, B. Circadian clock genes contribute to the regulation of hair follicle cycling. PLoS Genet. 2009, 5, e1000573. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Vollmers, C.; de la Cruz, D.; Chaix, A.; Ramos, R.; Panda, S.; Chuong, C.-M. Local circadian clock gates cell cycle progression of transient amplifying cells during regenerative hair cycling. Proc. Natl. Acad. Sci. USA 2013, 110, E2106–E2115. [Google Scholar] [CrossRef] [PubMed]
- Watabe, Y.; Tomioka, M.; Watabe, A.; Aihara, M.; Shimba, S.; Inoue, H. The clock gene brain and muscle Arnt-like protein-1 (BMAL1) is involved in hair growth. Arch. Dermatol. Res. 2013, 305, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Al-Nuaimi, Y.; Hardman, J.A.; Bíró, T.; Haslam, I.S.; Philpott, M.P.; Tóth, B.I.; Farjo, N.; Farjo, B.; Baier, G.; Watson, R.E.B.; et al. A meeting of two chronobiological systems: Circadian proteins Period1 and BMAL1 modulate the human hair cycle clock. J. Investig. Dermatol. 2014, 134, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, N.; Itcho, K.; Hamamura, K.; Ikeda, E.; Ikeyama, H.; Furuichi, Y.; Watanabe, M.; Koyanagi, S.; Ohdo, S. 24-hour rhythm of aquaporin-3 function in the epidermis is regulated by molecular clocks. J. Investig. Dermatol. 2014, 134, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Hardman, J.A.; Tobin, D.J.; Haslam, I.S.; Farjo, N.; Farjo, B.; Al-Nuaimi, Y.; Grimaldi, B.; Paus, R. The peripheral clock regulates human pigmentation. J. Investig. Dermatol. 2015, 135, 1053–1064. [Google Scholar] [CrossRef]
- Bilska, B.; Zegar, A.; Slominski, A.T.; Kleszczyński, K.; Cichy, J.; Pyza, E. Expression of antimicrobial peptide genes oscillates along day/night rhythm protecting mice skin from bacteria. Exp. Dermatol. 2020. [Google Scholar] [CrossRef]
- Matsuzawa, T.; Nakamura, Y.; Ogawa, Y.; Ishimaru, K.; Goshima, F.; Shimada, S.; Nakao, A.; Kawamura, T. Differential Day-Night Outcome to HSV-2 Cutaneous Infection. J. Investig. Dermatol. 2018, 138, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, E.N.; Marshall, M.E.; Jin, S.; Venkatesh, S.; Dragan, M.; Tsoi, L.C.; Gudjonsson, J.E.; Nie, Q.; Takahashi, J.S.; Andersen, B. Circadian control of interferon-sensitive gene expression in murine skin. Proc. Natl. Acad. Sci. USA 2020, 117, 5761–5771. [Google Scholar] [CrossRef]
- Dubrovsky, Y.V.; Samsa, W.E.; Kondratov, R.V. Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging 2010, 2, 936–944. [Google Scholar] [CrossRef]
- Hiramoto, K.; Orita, K.; Yamate, Y.; Kasahara, E.; Yokoyama, S.; Sato, E.F. The Clock Genes Are Involved in The Deterioration of Atopic Dermatitis after Day-and-Night Reversed Physical Stress in NC/Nga Mice. Open Biochem. J. 2018, 12, 87–102. [Google Scholar] [CrossRef]
- Dakup, P.P.; Porter, K.I.; Gaddameedhi, S. The circadian clock protects against acute radiation-induced dermatitis. Toxicol. Appl. Pharmacol. 2020, 399, 115040. [Google Scholar] [CrossRef] [PubMed]
- Ando, N.; Nakamura, Y.; Aoki, R.; Ishimaru, K.; Ogawa, H.; Okumura, K.; Shibata, S.; Shimada, S.; Nakao, A. Circadian Gene Clock Regulates Psoriasis-Like Skin Inflammation in Mice. J. Investig. Dermatol. 2015, 135, 3001–3008. [Google Scholar] [CrossRef]
- Takita, E.; Yokota, S.; Tahara, Y.; Hirao, A.; Aoki, N.; Nakamura, Y.; Nakao, A.; Shibata, S. Biological clock dysfunction exacerbates contact hypersensitivity in mice. Br. J. Dermatol. 2013, 168, 39–46. [Google Scholar] [CrossRef]
- Cadet, J.; Grand, A.; Douki, T. Solar UV radiation-induced DNA Bipyrimidine photoproducts: Formation and mechanistic insights. Top. Curr. Chem. 2015, 356, 249–275. [Google Scholar] [CrossRef]
- Mouret, S.; Baudouin, C.; Charveron, M.; Favier, A.; Cadet, J.; Douki, T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc. Natl. Acad. Sci. USA 2006, 103, 13765–13770. [Google Scholar] [CrossRef] [PubMed]
- Douki, T.; Reynaud-Angelin, A.; Cadet, J.; Sage, E. Bipyrimidine photoproducts rather than oxidative lesions are the main type of DNA damage involved in the genotoxic effect of solar UVA radiation. Biochemistry 2003, 42, 9221–9226. [Google Scholar] [CrossRef]
- Geyfman, M.; Kumar, V.; Liu, Q.; Ruiz, R.; Gordon, W.; Espitia, F.; Cam, E.; Millar, S.E.; Smyth, P.; Ihler, A.; et al. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc. Natl. Acad. Sci. USA 2012, 109, 11758–11763. [Google Scholar] [CrossRef]
- Klungland, A.; Bjelland, S. Oxidative damage to purines in DNA: Role of mammalian Ogg1. DNA Repair 2007, 6, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Manzella, N.; Bracci, M.; Strafella, E.; Staffolani, S.; Ciarapica, V.; Copertaro, A.; Rapisarda, V.; Ledda, C.; Amati, M.; Valentino, M.; et al. Circadian Modulation of 8-Oxoguanine DNA Damage Repair. Sci. Rep. 2015, 5, 13752. [Google Scholar] [CrossRef] [PubMed]
- Sancar, A. Mechanisms of DNA Repair by Photolyase and Excision Nuclease (Nobel Lecture). Angew. Chem. 2016, 55, 8502–8527. [Google Scholar] [CrossRef]
- Kang, T.H.; Lindsey-Boltz, L.A.; Reardon, J.T.; Sancar, A. Circadian control of XPA and excision repair of cisplatin-DNA damage by cryptochrome and HERC2 ubiquitin ligase. Proc. Natl. Acad. Sci. USA 2010, 107, 4890–4895. [Google Scholar] [CrossRef]
- Gaddameedhi, S.; Selby, C.P.; Kaufmann, W.K.; Smart, R.C.; Sancar, A. Control of skin cancer by the circadian rhythm. Proc. Natl. Acad. Sci. USA 2011, 108, 18790–18795. [Google Scholar] [CrossRef]
- Riddle, J.C.; Hsie, A.W. An effect of cell-cycle position on ultraviolet-light-induced mutagenesis in Chinese hamster ovary cells. Mutat. Res. 1978, 52, 409–420. [Google Scholar] [CrossRef]
- Grosovsky, A.J.; Little, J.B. Mutagenesis and lethality following S phase irradiation of xeroderma pigmentosum and normal human diploid fibroblasts with ultraviolet light. Carcinogenesis 1983, 4, 1389–1393. [Google Scholar] [CrossRef]
- Kaufmann, W.K.; Wilson, S.J. G1 arrest and cell-cycle-dependent clastogenesis in UV-irradiated human fibroblasts. Mutat. Res. 1994, 314, 67–76. [Google Scholar] [CrossRef]
- Brown, W.R. A review and mathematical analysis of circadian rhythms in cell proliferation in mouse, rat, and human epidermis. J. Investig. Dermatol. 1991, 97, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Gaddameedhi, S.; Selby, C.P.; Kemp, M.G.; Ye, R.; Sancar, A. The circadian clock controls sunburn apoptosis and erythema in mouse skin. J. Investig. Dermatol. 2015, 135, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Nikkola, V.; Grönroos, M.; Huotari-Orava, R.; Kautiainen, H.; Ylianttila, L.; Karppinen, T.; Partonen, T.; Snellman, E. Circadian Time Effects on NB-UVB-Induced Erythema in Human Skin In Vivo. J. Investig. Dermatol. 2018, 138, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Suggs, A.; Ahsanuddin, S.; Tarrillion, M.; Selph, J.; Lam, M.; Baron, E. 2016 Arte Poster Competition First Place Winner: Circadian Rhythm and UV-Induced Skin Damage: An In Vivo Study. J. Drugs Dermatol. 2016, 15, 1124–1130. [Google Scholar]
- McQuestion, M. Evidence-based skin care management in radiation therapy: Clinical update. Semin. Oncol. Nurs. 2011, 27, e1–e17. [Google Scholar] [CrossRef]
- Yoshioka, D.; Ando, H.; Ushijima, K.; Kumazaki, M.; Fujimura, A. Chronotherapy of maxacalcitol on skin inflammation induced by topical 12-O-tetradecanoylphorbol-13-acetate in mice. Chronobiol. Int. 2018, 35, 1269–1280. [Google Scholar] [CrossRef]
- Vaughn, A.R.; Clark, A.K.; Sivamani, R.K.; Shi, V.Y. Circadian rhythm in atopic dermatitis-Pathophysiology and implications for chronotherapy. Pediatr. Dermatol. 2018, 35, 152–157. [Google Scholar] [CrossRef]
- Ruan, W.; Yuan, X.; Eltzschig, H.K. Circadian rhythm as a therapeutic target. Nat. Rev. Drug Discov. 2021, 20, 287–307. [Google Scholar] [CrossRef]
- Gloston, G.F.; Yoo, S.H.; Chen, Z.J. Clock-Enhancing Small Molecules and Potential Applications in Chronic Diseases and Aging. Front. Neurol. 2017, 8, 100. [Google Scholar] [CrossRef]
- Chen, Z.; Yoo, S.H.; Takahashi, J.S. Development and Therapeutic Potential of Small-Molecule Modulators of Circadian Systems. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 231–252. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Sato, T.; Akimoto, N.; Yano, M.; Ito, A. Prevention of UVB-induced photoinflammation and photoaging by a polymethoxy flavonoid, nobiletin, in human keratinocytes in vivo and in vitro. Biochem. Pharmacol. 2004, 68, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Nakamura, Y.; Torikai, K.; Tanaka, T.; Koshiba, T.; Koshimizu, K.; Kuwahara, S.; Takahashi, Y.; Ogawa, K.; Yano, M.; et al. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res. 2000, 60, 5059–5066. [Google Scholar] [PubMed]
- Yang, G.; Li, S.; Yang, Y.; Yuan, L.; Wang, P.; Zhao, H.; Ho, C.-T.; Lin, C.-C. Nobiletin and 5-Hydroxy-6,7,8,3’,4’-pentamethoxyflavone Ameliorate 12- O-Tetradecanoylphorbol-13-acetate-Induced Psoriasis-Like Mouse Skin Lesions by Regulating the Expression of Ki-67 and Proliferating Cell Nuclear Antigen and the Differentiation of CD4. J. Agric. Food Chem. 2018, 66, 8299–8306. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lin, C.; Jiang, C.; Huang, Z.; Gao, W.; Lin, D. Nobiletin enhances the survival of random pattern skin flaps: Involvement of enhancing angiogenesis and inhibiting oxidative stress. Int. Immunopharmacol. 2020, 78, 106010. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Choo, M.-K.; Park, J.M.; Fisher, D.E. Topical ROR Inverse Agonists Suppress Inflammation in Mouse Models of Atopic Dermatitis and Acute Irritant Dermatitis. J. Investig. Dermatol. 2017, 137, 2523–2531. [Google Scholar] [CrossRef] [PubMed]
- Buscone, S.; Mardaryev, A.N.; Westgate, G.E.; Uzunbajakava, N.E.; Botchkareva, N.V. Cryptochrome 1 is modulated by blue light in human keratinocytes and exerts positive impact on human hair growth. Exp. Dermatol. 2021, 30, 271–277. [Google Scholar] [CrossRef]
- Jang, J.; Chung, S.; Choi, Y.; Lim, H.Y.; Son, Y.; Chun, S.K.; Son, G.H.; Kim, K.; Suh, Y.G.; Jung, J.W. The cryptochrome inhibitor KS15 enhances E-box-mediated transcription by disrupting the feedback action of a circadian transcription-repressor complex. Life Sci. 2018, 200, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.K.; Chung, S.; Kim, H.D.; Lee, J.H.; Jang, J.; Kim, J.; Kim, D.; Son, G.H.; Oh, Y.J.; Suh, Y.G.; et al. A synthetic cryptochrome inhibitor induces anti-proliferative effects and increases chemosensitivity in human breast cancer cells. Biochem. Biophys. Res. Commun. 2015, 467, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Kojetin, D.; Wang, Y.; Kamenecka, T.M.; Burris, T.P. Identification of SR8278, a synthetic antagonist of the nuclear heme receptor REV-ERB. ACS Chem. Biol. 2011, 6, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kumar, N.; Nuhant, P.; Cameron, M.D.; Istrate, M.A.; Roush, W.R.; Griffin, P.R.; Burris, T.P. Identification of SR1078, a synthetic agonist for the orphan nuclear receptors RORα and RORγ. ACS Chem. Biol. 2010, 5, 1029–1034. [Google Scholar] [CrossRef]
- Luze, H.; Nischwitz, S.P.; Zalaudek, I.; Müllegger, R.; Kamolz, L.P. DNA repair enzymes in sunscreens and their impact on photoageing-A systematic review. Photodermatol. Photoimmunol. Photomed. 2020, 36, 424–432. [Google Scholar] [CrossRef]
- Yarosh, D.B.; Rosenthal, A.; Moy, R. Six critical questions for DNA repair enzymes in skincare products: A review in dialog. Clin. Cosmet. Investig. Dermatol. 2019, 12, 617–624. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lubov, J.E.; Cvammen, W.; Kemp, M.G. The Impact of the Circadian Clock on Skin Physiology and Cancer Development. Int. J. Mol. Sci. 2021, 22, 6112. https://doi.org/10.3390/ijms22116112
Lubov JE, Cvammen W, Kemp MG. The Impact of the Circadian Clock on Skin Physiology and Cancer Development. International Journal of Molecular Sciences. 2021; 22(11):6112. https://doi.org/10.3390/ijms22116112
Chicago/Turabian StyleLubov, Janet E., William Cvammen, and Michael G. Kemp. 2021. "The Impact of the Circadian Clock on Skin Physiology and Cancer Development" International Journal of Molecular Sciences 22, no. 11: 6112. https://doi.org/10.3390/ijms22116112
APA StyleLubov, J. E., Cvammen, W., & Kemp, M. G. (2021). The Impact of the Circadian Clock on Skin Physiology and Cancer Development. International Journal of Molecular Sciences, 22(11), 6112. https://doi.org/10.3390/ijms22116112





