1. Introduction
Exposure to particulate matter and ozone severely affects public health worldwide. In recent years, fine particulate matter (or particulate matter with aerodynamic diameter ≤2.5 μm (PM
2.5)) was reported to exert harmful effects on several major systems and organs including the respiratory, cardiovascular, and immune systems. Studies have demonstrated an association of PM
2.5 with aggravation of asthma and chronic obstructive pulmonary disease (COPD), death due to cardiovascular disease, and lung cancer [
1,
2,
3,
4]. Among the systems and organs affected by PM
2.5, the skin is the external natural barrier and primary organ exposed to ambient pollutants and protects the human body by acting as an interface between the body and surrounding atmosphere. The effects of PM
2.5 on human skin have not been fully clarified. Many studies have indicated that air pollution may damage and exert deleterious effects on skin; for instance, an increase in the oxidative stress in skin can aggravate atopic dermatitis. Oxidative stress is a common mechanism underlying PM
2.5-induced skin damage. The possible adverse effects of PM
2.5 on skin have attracted the attention of clinical dermatologists and researchers. PM
2.5 could induce oxidative stress by increasing the number of reactive oxygen species, leading to DNA damage, lipid peroxidation, and protein carbonylation; these factors further resulted in endoplasmic reticulum stress, mitochondrial dysfunction, and autophagy, thus causing apoptotic HaCaT cell death and mouse skin injury [
5]. Similarly, inflammatory responses and oxidative stress caused by PM
0.3–2.5 in an in vitro model negatively affected the integrity and functions of skin [
6]. PM
2.5 can penetrate both disrupted and intact skin; it disrupts the skin barrier by reducing the numbers of cytokeratin, filaggrin, E-cadherin, and tight junction molecules [
7,
8]. In addition, studies on the respiratory system have indicated that PM
2.5 inhalation increased the levels of proinflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α), demonstrating that PM
2.5 is strongly correlated with inflammatory responses in the airway and in patients with COPD [
9,
10]. Moreover, exposure of the circulatory system to PM
2.5 can contribute to atherogenesis and acute cardiovascular and cerebrovascular events [
11]. Similarly, an in vitro study reported that the production of IL-6, TNF-α, and matrix metalloproteinase-1 in a keratinocyte and skin fibroblast coculture system was largely increased, whereas that of procollagen type I was significantly decreased after exposure to PM
2.5 [
12], indicating that PM
2.5 can negatively affect skin cells and lead to inflammatory responses in the epidermis and dermis. Most of the PM
2.5-associated events that contribute to cellular dysfunction, inflammation, and extracellular matrix destruction may exert severe deleterious effects on skin.
Farnesol (Farn), a natural 15-carbon organic compound, possesses many biological and biomedical properties [
13,
14]. Farn can exert numerous therapeutic effects including antimicrobial, antiproliferative, antiallergic, and anti-inflammatory effects as well as induce apoptosis [
15,
16,
17]. In addition, studies have examined the effects of drugs or potential therapeutic compounds encapsulated by biocompatible and biodegradable liposomes. Liposomes can entrap both hydrophobic and hydrophilic drugs. Our previous studies have indicated that liposomal encapsulation could prolong and reinforce the effects of drugs by protecting them from degradation and could reduce the drug side effects [
14,
18,
19]. Furthermore, our previous studies have revealed the novel therapeutic effects of different Farn dosages on skin. Liposomal or pure Farn protected against ultraviolet B (UVB), increased collagen production, and improved skin smoothness while exerting anti-inflammatory and tissue-reparative effects on UVB-caused sunburn and third-degree burns in the epidermis in vitro and in the dermis in vivo. Farnesol has been found to promote wound healing by suppressing the production of proinflammatory cytokines such as interleukin (IL)-1 beta, IL-6, and tumor necrosis factor (TNF)-alpha [
14,
20,
21]. The present study investigated the deleterious effects of PM
2.5 on the epidermis and dermis and examined the anti-inflammatory and restoration-promoting effects of pure and liposomal Farn on various skin injuries caused by PM
2.5.
3. Discussion
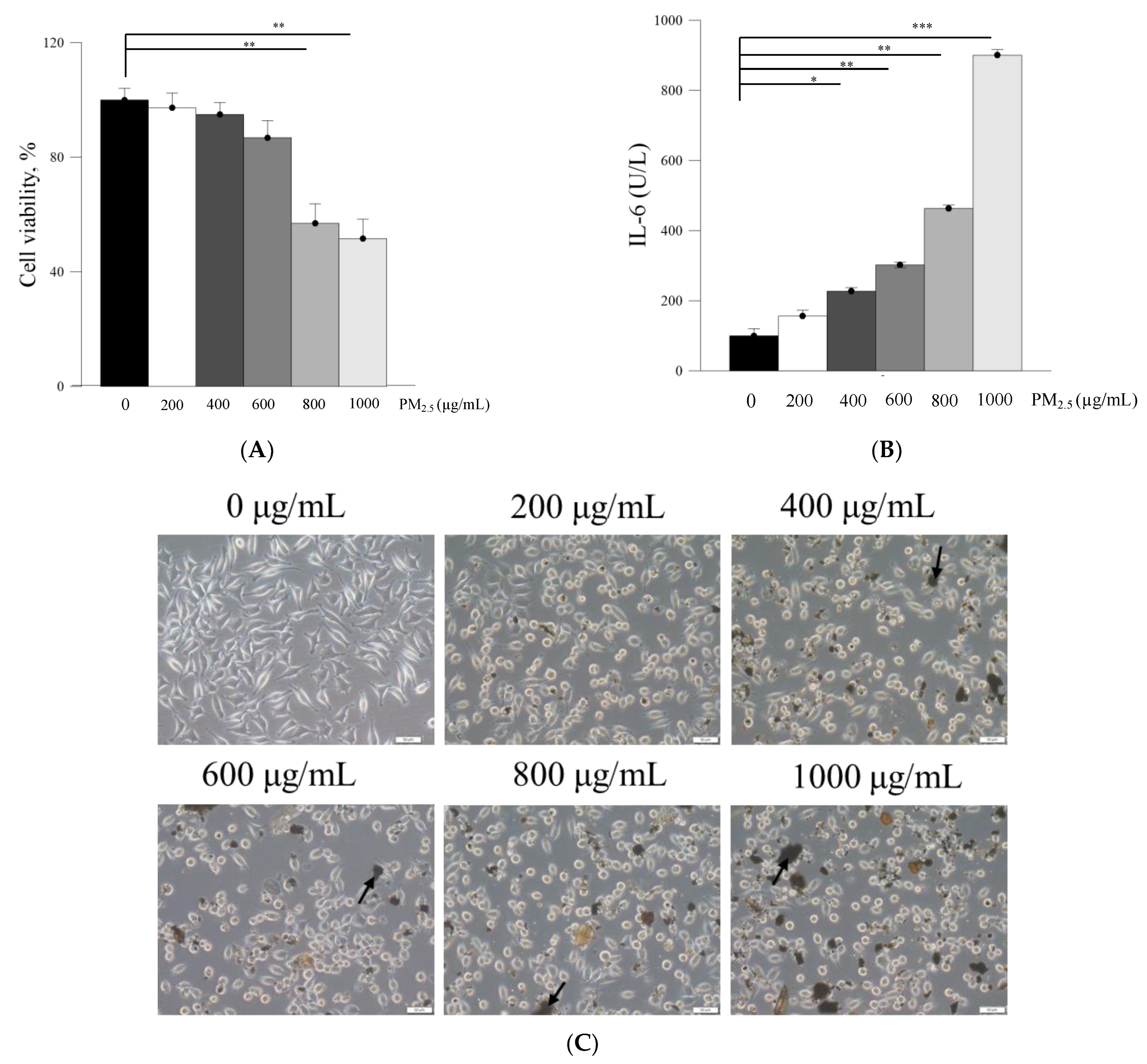
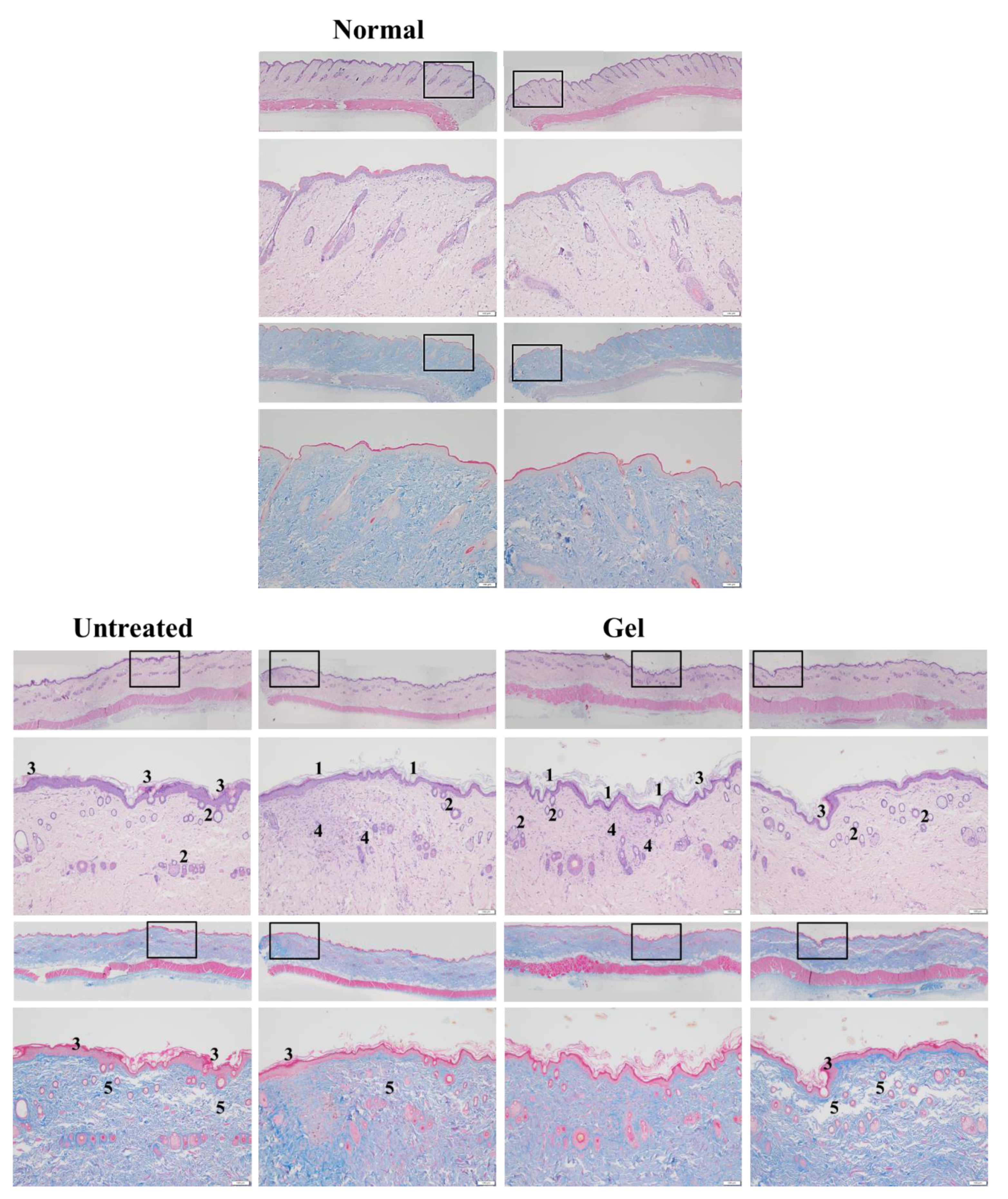
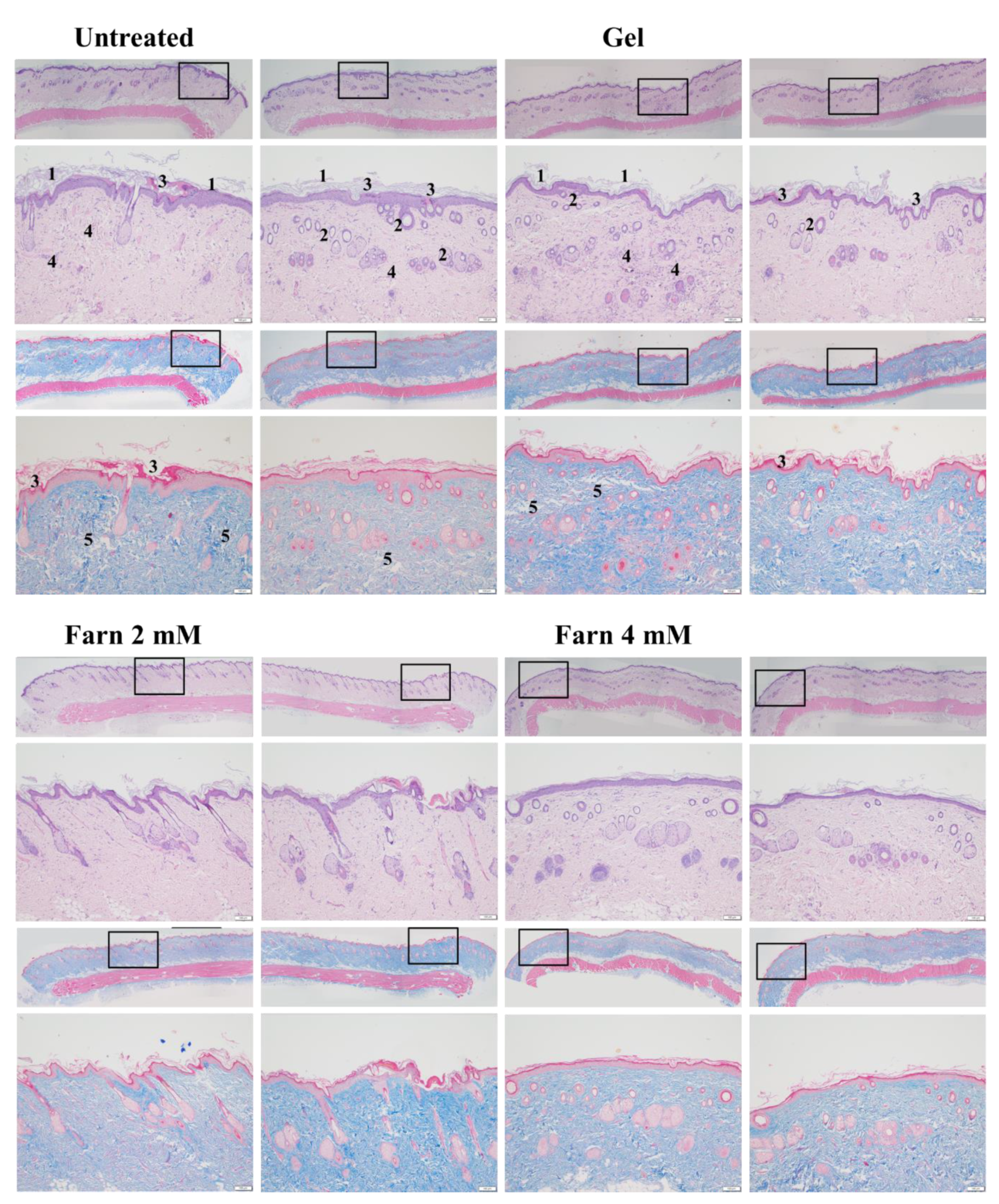
PM2.5, a crucial air pollutant, can damage several systems and organs in humans, leading to various diseases. In the current study, we observed that PM2.5 induced the production of IL-6 and decreased the viability of skin fibroblasts in vitro. The current in vitro results of PM2.5 on skin fibroblasts indicated that treatment with ≥200, ≥400, and ≥800 μg/mL PM2.5 disrupted the normal physiological activities of skin fibroblasts, resulted in the induction of inflammatory responses, and decreased the viability of skin fibroblasts, respectively. In addition, the sequential impairment of skin cells in vitro implied the likely deleterious effects of PM2.5 on skin in vivo. Subcutaneous injection of PM2.5 caused severe impairment in the epidermis and hair follicles as well as moderate injuries in the dermis. Pure Farn or Lipo-Farn in gelatin/HA/xanthan gel was topically applied to treat the adverse effects of the continuous subcutaneous injection of PM2.5 on the skin.
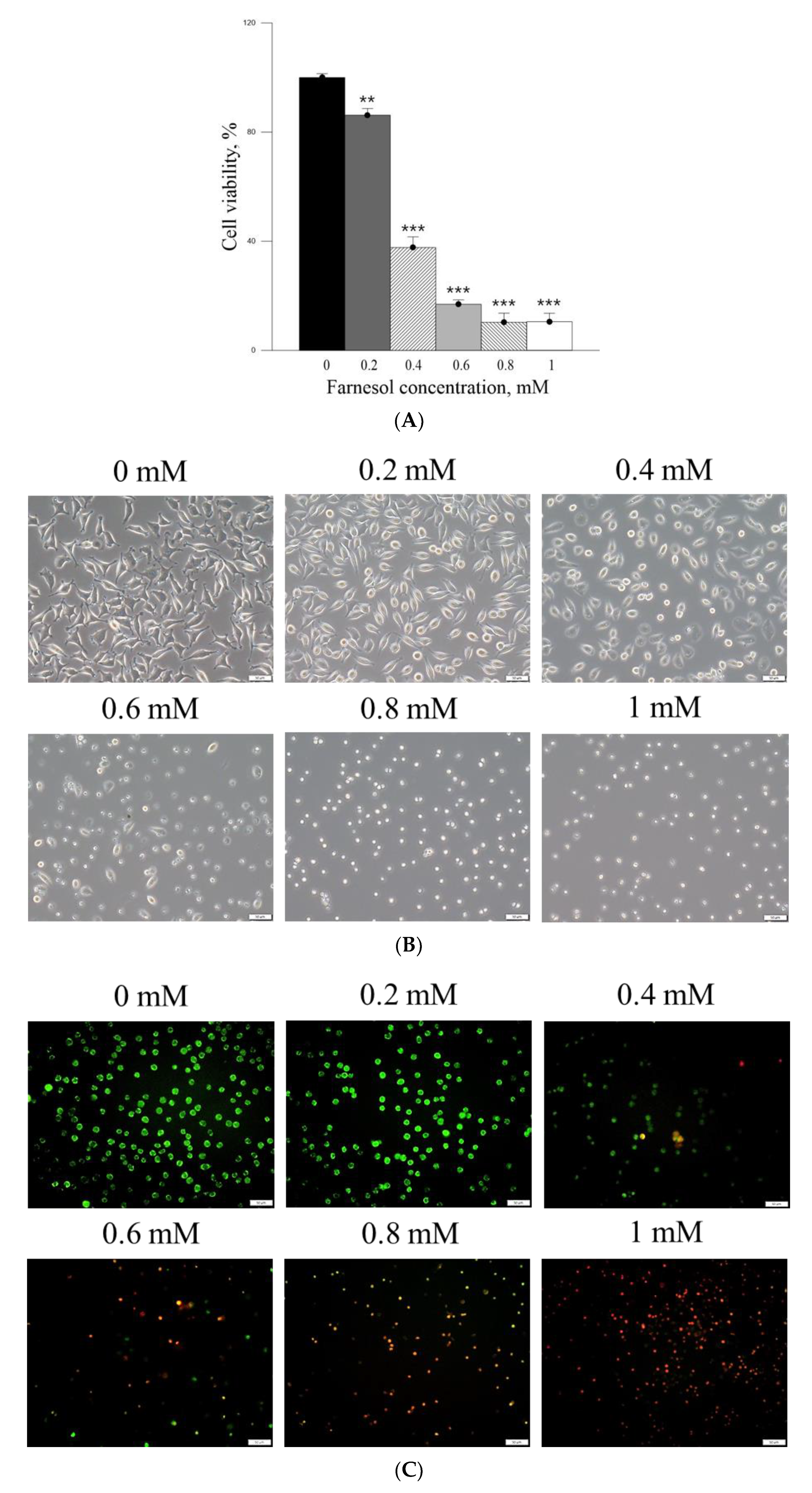
The cell viability-inhibitory data of pure Farn in the current study is similar to that of our previous findings [
21]. Furthermore, the cell morphology results revealed that treatment with 0.4 mM pure Farn led to loss of the normal spindle-like shape in approximately 50% of skin fibroblasts in vitro (
Figure 3B); this finding is in line with that of our previous study [
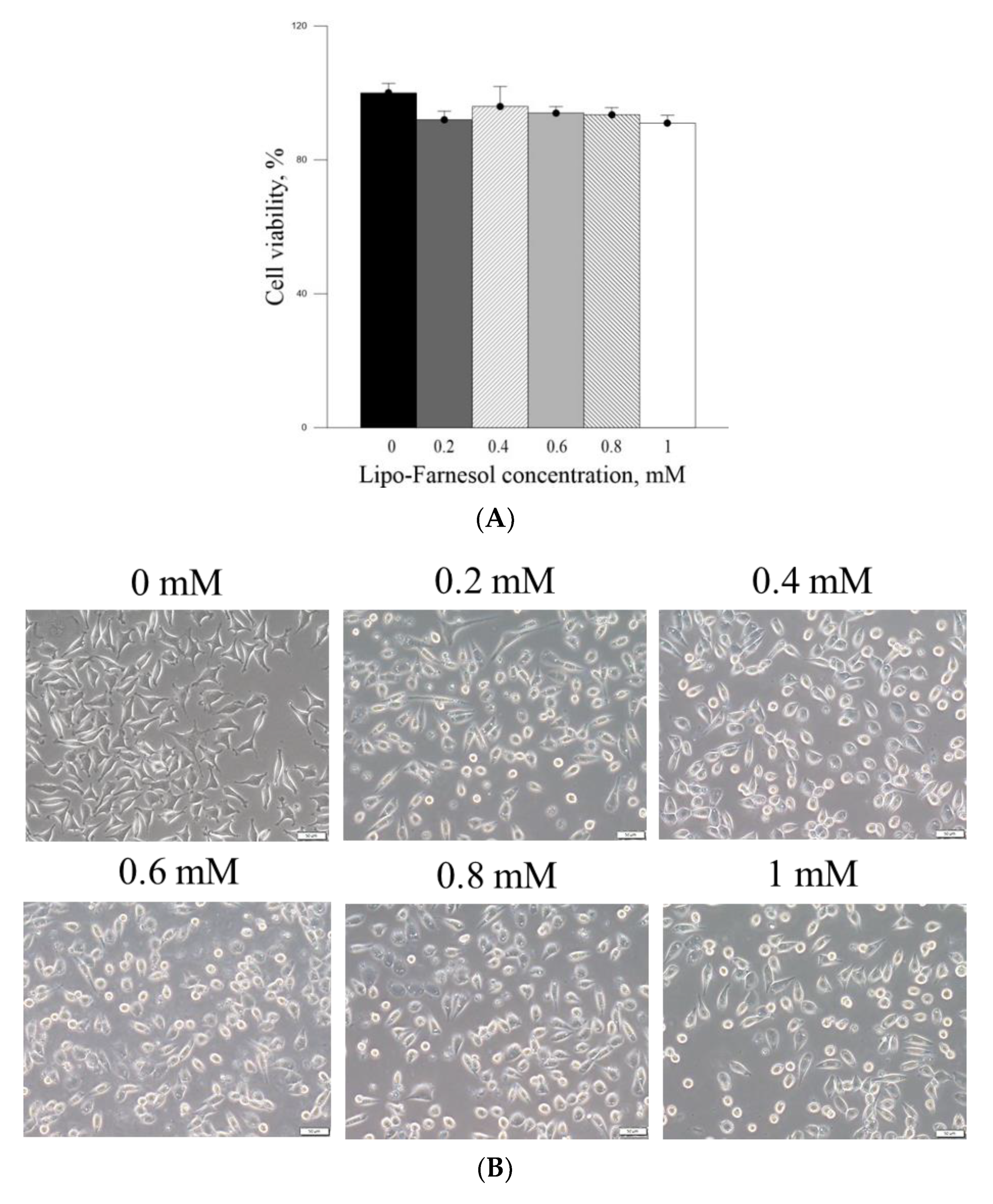
21]. These results indicated that pure Farn at a concentration of >0.4 mM exerted adverse effects on skin fibroblasts. The MTT assay revealed that liposome encapsulation largely reduced the inhibitory effect of Farn on the viability of skin fibroblasts (
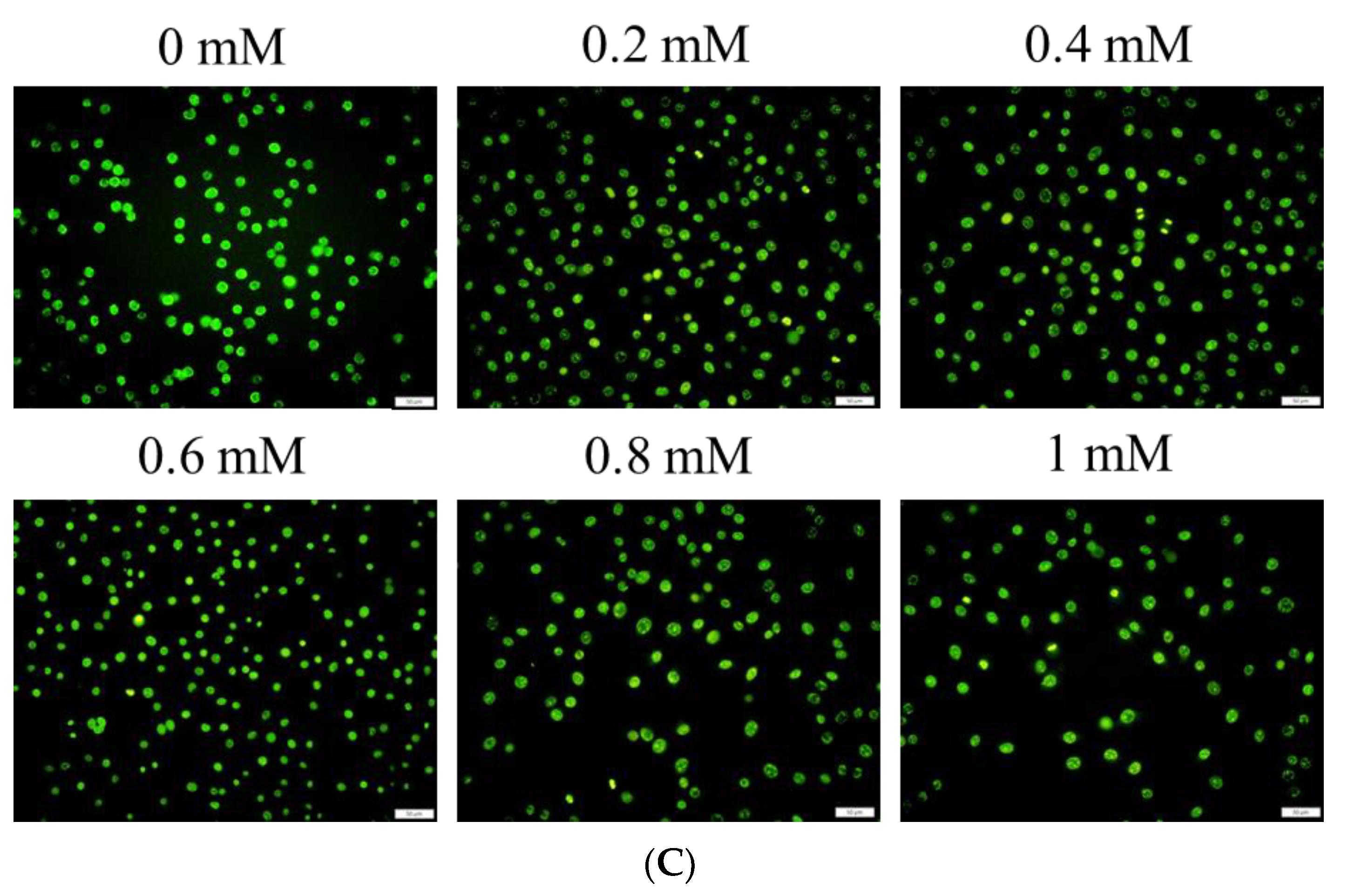
Figure 4A). Furthermore, the live–dead cell staining showed that the fluorescence staining of live cells had decreased by approximately 20% after treatment with 1.0 mM Lipo-Farn (
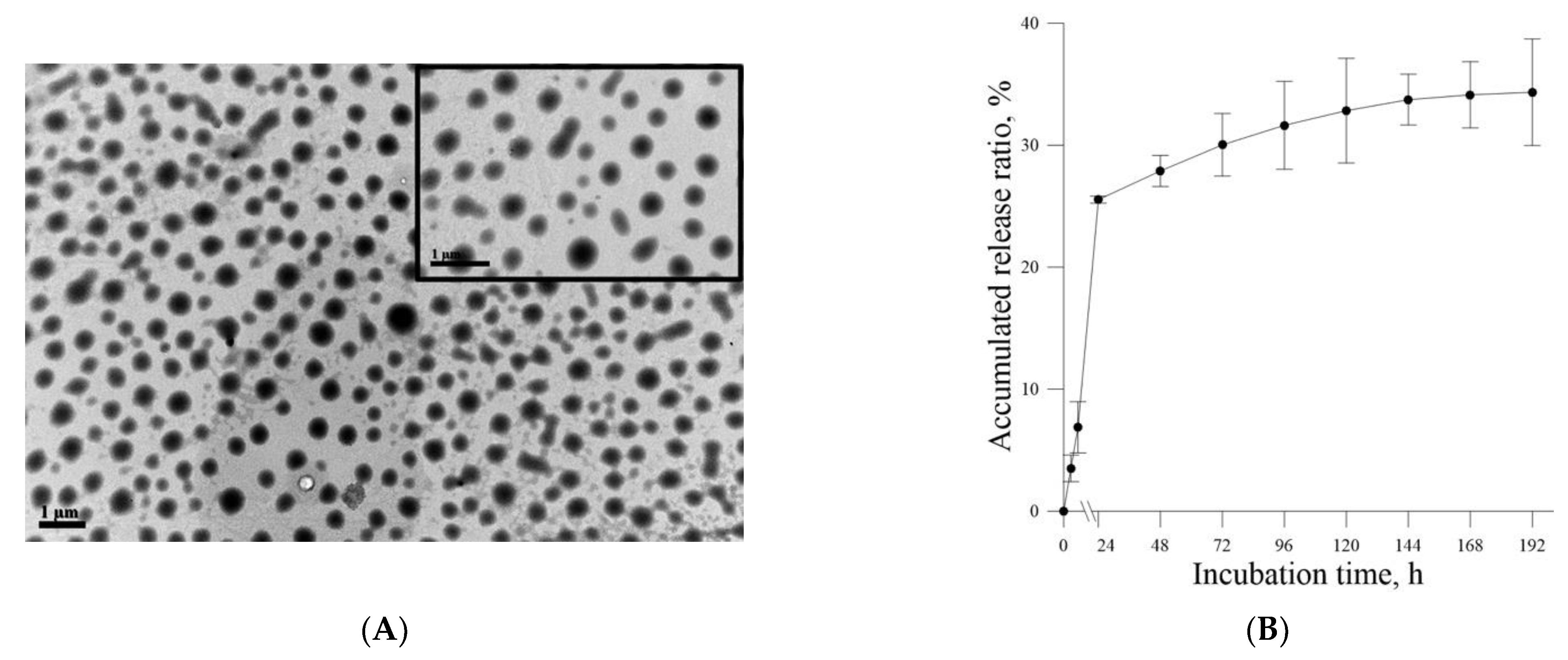
Figure 4B). The aforementioned results of the MTT assay and live–dead cell staining are consistent with those of our previous study and indicate that liposome encapsulation could reduce the cytotoxic effects of Farn on skin fibroblasts in vitro. The liposome encapsulation rate for Farn was 69%, and Lipo-Farn was determined to be 342 ± 90 nm in diameter (
Figure 1). Although approximately 30% of the Farn was released from Lipo-Farn after incubation for 192 h (
Figure 1), our in vivo results indicated that Lipo-Farn could still exert protective and therapeutic effects on PM
2.5-induced severe damage in the epidermis, hair follicles, and dermis. These findings are supported by those of previous studies reporting that nanoparticles approximately 300 nm in size penetrated deeper into the pilosebaceous unit than did nanoparticle elements [
22]. Cyproterone acetate—which is used to treat skin disorders such as acne, hirsutism, and alopecia—accumulated the most in hair follicles when administered in the form of a 300 nm nanostructured lipid carrier [
23]. The findings of previous studies and the current study have consistently indicated that nanoparticles approximately 300 nm in size exert great favorable therapeutic effects on skin injuries and disorders in vivo. The histopathological findings demonstrated that PM
2.5 caused severe injuries to pilosebaceous units in the untreated groups. Loss of the epithelium (epidermal erosion) was observed in the untreated groups of both categories, suggesting that PM
2.5 exerted highly deleterious effects on the epidermis. The impairment on hair follicles, inflammatory cells, and fibroblast infiltration as well as loose extracellular matrix and loss of extracellular collagen-like substance in the upper dermis indicated moderate damage caused by PM
2.5 in the dermis. These histopathological findings verified the inflammatory effects of PM
2.5 on the skin and are consistent with those of previous studies [
9,
10,
12]. Moreover, the groups injected with PM
2.5 and then treated with HPMC gel not containing either dosage form of Farn in both categories displayed histopathological features similar to those of the untreated groups, indicating that the basal gel did not possess either anti-inflammatory or skin-restorative actions against PM
2.5 caused impairment on the rat skin.
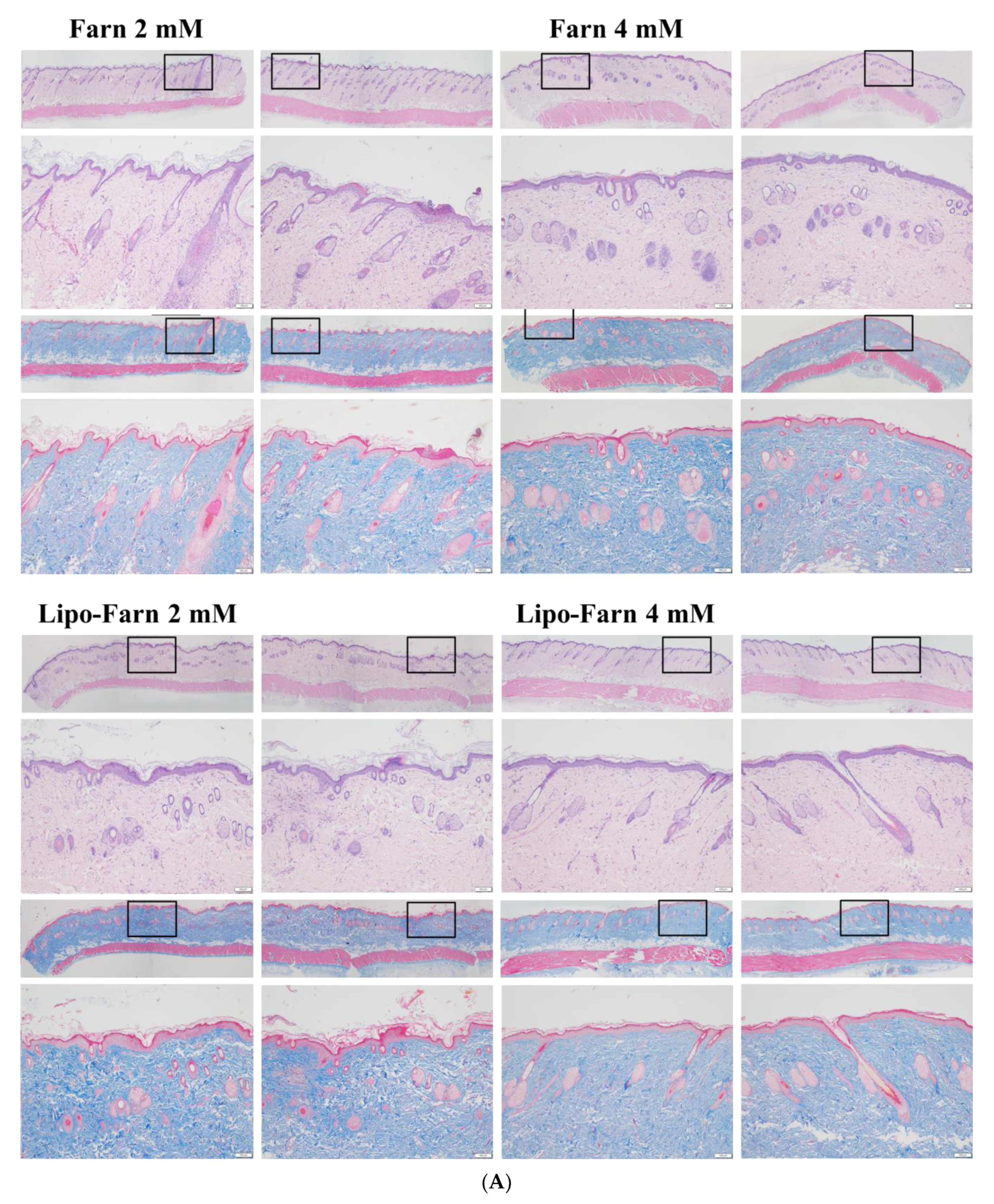
Our histopathological data showed that treatment with 4 mM Lipo-Farn exerted the strongest reparative, regenerative, and anti-inflammatory effects on the epidermis, dermis, and hair follicles of rat skin in both experimental categories. Treatment with pure 4 mM Farn did not exert a similar reparative effect on injured hair follicles. These findings indicated that liposomal encapsulation of Farn is crucial and beneficial for the repair of hair follicles, likely because it increases the transdermal penetration capacity through pilosebaceous units. That is, 4 mM Lipo-Farn could exert the strongest regenerative and anti-inflammatory effects on hair follicles damaged by PM2.5 in the epidermis and dermis in this present study.
Compared with results in the untreated and 4 mM Lipo-Farn-treated groups, the histopathological findings of the rats received 2 mM Lipo-Farn in both experimental categories suggested that a 2 mM dose of Lipo-Farn was insufficient for the repair of injured hair follicles in the epidermis. Moreover, 2 mM pure Farn did not exert reparative effects on the damage caused by PM2.5 in the epidermis and could not alleviate inflammatory responses; a few pustule-like areas were also observed in the groups treated with 2 mM pure Farn. These findings indicated that 2 mM Lipo-Farn or pure Farn could not restore the injured epidermis or alleviate PM2.5-induced inflammation, although it enhanced the arrangement and abundance of collagen-like substance in the dermis.
Our histopathological findings revealed that 2 mM pure Farn exerted mild-to-moderate protective effects against the continuous injection of PM2.5; however, the reparative effects of 2 mM pure Farn were not observed in the post-PM2.5 injective experimental category, indicating that only higher doses of Farn may exert reparative and regenerative effects on both the dermis and epidermis. The findings in 4 mM pure Farn-treated groups in either experimental category sensibly confirmed this proposal. Our data indicated that 4 mM pure Farn exerted moderate restorative and anti-inflammatory effects on PM2.5-induced skin injury in the group that received post-PM2.5 injection and the group that received continuous injection of PM2.5 despite lack of restorative effects on hair follicles/pilosebaceous units. The significant hair-follicle-restorative effects exerted by 4 mM Lipo-Farn can be attributed to the greater solubility of Lipo-Farn than of pure Farn; higher solubility meant that more Farn could penetrate the skin barrier and be sustained in the hair follicles of the rats. The histopathological findings and wound healing scores in our study indicate that liposome encapsulation is crucial and necessary if Farn is to fully exert its anti-inflammatory, reparative, and regenerative effects on hair follicles in the dermis and epidermis in vivo.
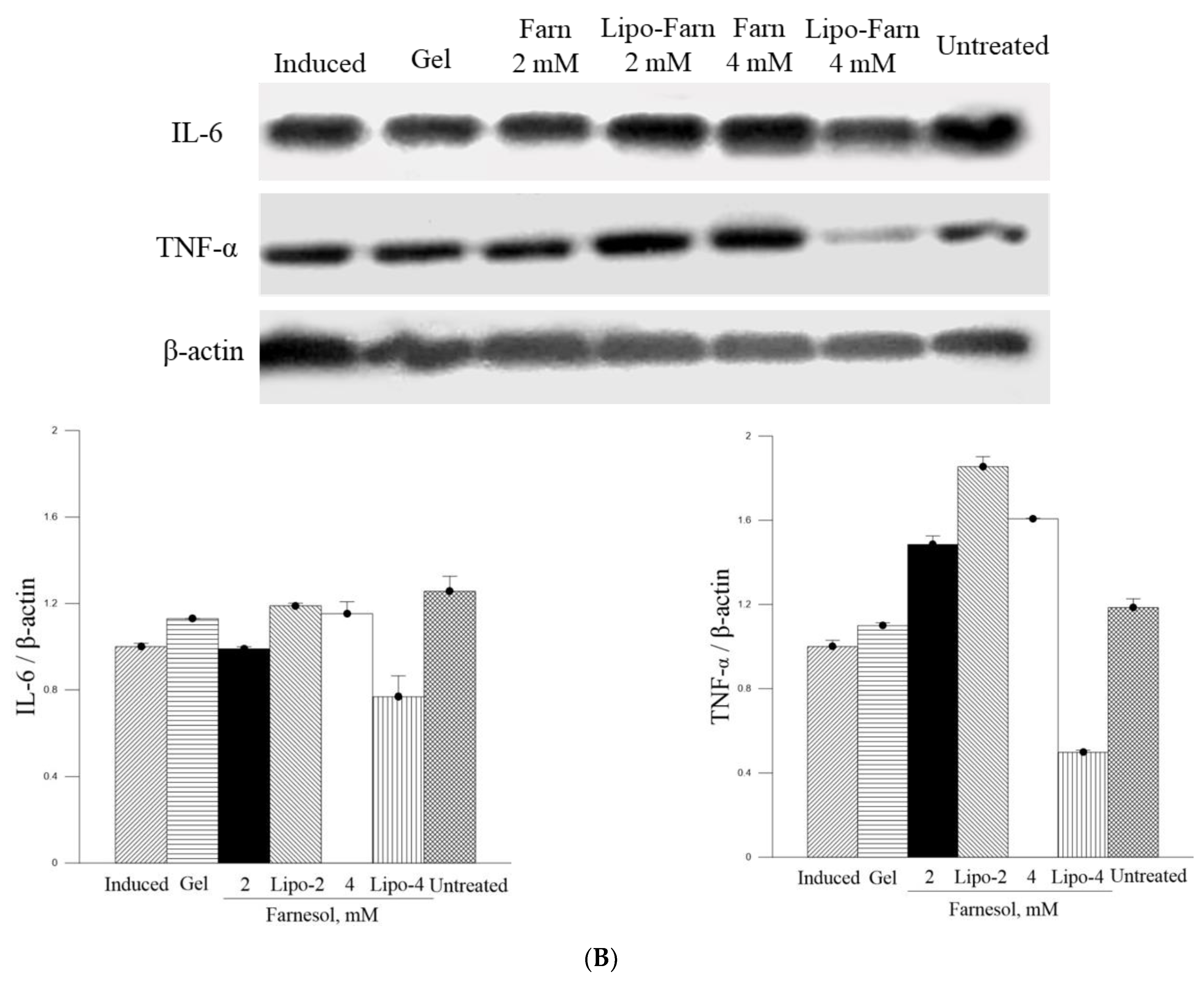
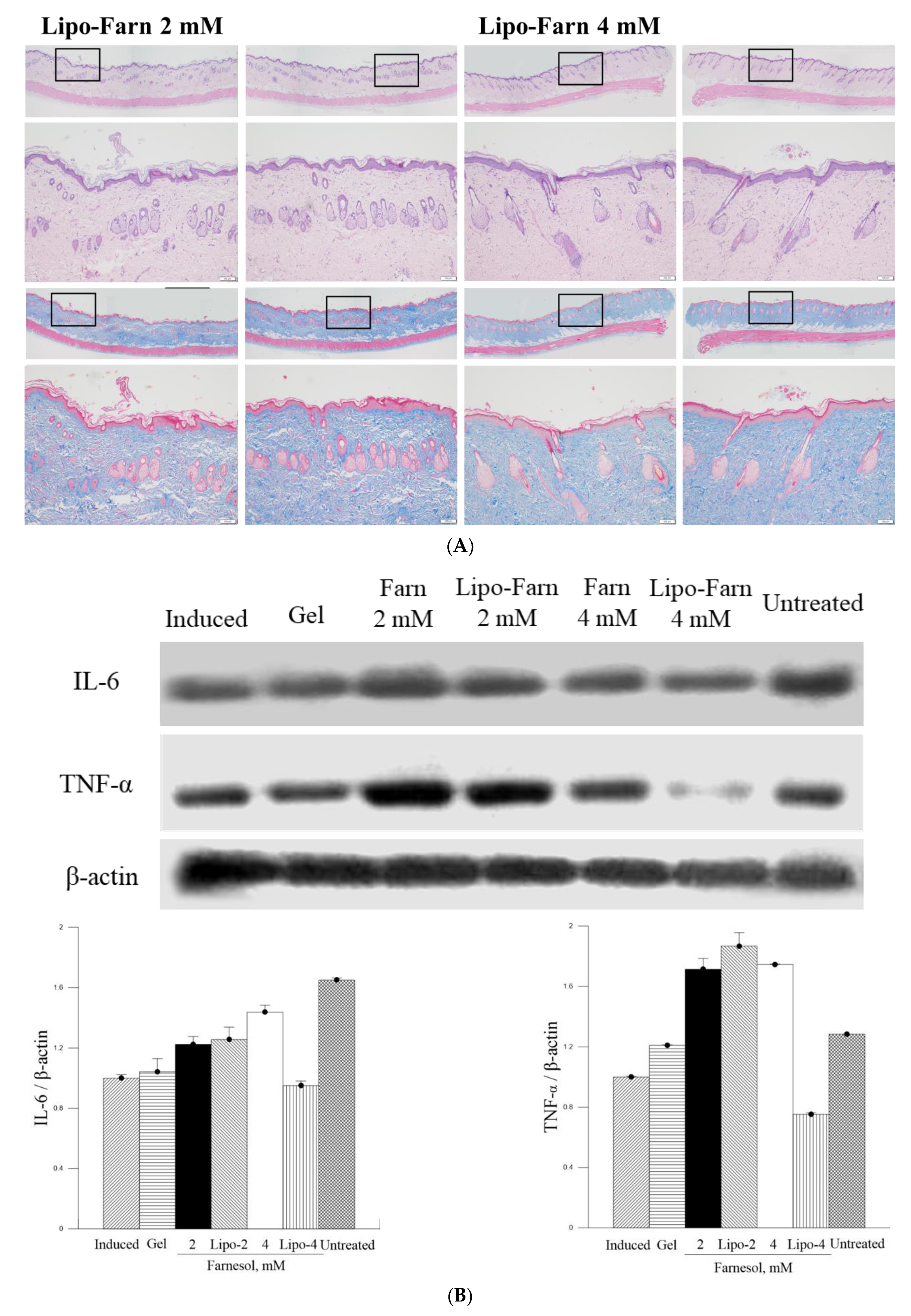
The strongest inhibitory effect of 4 mM Lipo-Farn on IL-6 and TNF-α in both experimental categories exhibited by Western blot was coherent with the histopathological data and wound-healing scores. Furthermore, the groups treated with 2 mM pure Farn and Lipo-Farn and 4 mM pure Farn displayed a higher level of TNF-α than did the untreated groups, implying that TNF-α plays crucial roles in the regulation of inflammation and wound healing in skin. The TNF-α level in wound tissues was higher than the normal basal level, suggesting that TNF-α may be correlated with the wound-healing process [
24]. Moreover, compared with the control mice, the mice treated with the anti-TNF-α monoclonal antibody on day 3 had delayed wound closure, greater distances between the edges of the panniculus carnosus, and decreased inflammatory cell and fibroblast density, again indicating that TNF-α may be essential for would healing in skin [
24]. TNF-α was reported to play a crucial positive role in the early process of skin wound healing, demonstrating that TNF-α administration exerted a beneficial effect on this response [
24]. In addition to directly regulating inflammatory cytokine production, TNF-α may further regulate the skin’s wound-healing process [
25]. Thus, these findings suggested that 4 mM Lipo-Farn not only strongly suppressed proinflammatory responses mediated by TNF-α but also directly promoted epidermal and dermal repair, which was shown in our previous studies [
14,
20,
21]. Because the groups treated with 2 mM pure Farn and Lipo-Farn and 4 mM pure Farn exhibited steady upregulation of TNF-α (
Figure 5 and
Figure 6), TNF-α could partially exert beneficial effects on wound healing in PM
2.5-induced skin injury.
The in vitro experiments revealed that PM
2.5 dose-dependently increased IL-6 production in L929 murine skin fibroblasts. Moreover, the amount of IL-6 detected through Western blot analysis was consistent with histopathologic results demonstrating acute inflammatory responses induced by PM
2.5 despite the presence of some nonsignificant differences between the groups. The untreated groups in both experimental categories exhibited the highest IL-6 level, whereas only the treatment involving 4 mM Lipo-Farn in both the experimental categories exerted the strongest inhibitory effects on IL-6. Together with our in vitro IL-6 data, these findings indicate that IL-6 mainly plays a proinflammatory role in injured skin. Moreover, IL-6 was demonstrated to be essential in acute inflammation and for the appropriate start of the subsequent wound-healing process [
26]. A decreased IL-6 level reflected alleviation of inflammation in the skin. This is in accordance with the previous finding that the IL-6 level was significantly decreased during the remodeling phase in skin’s normal wound-healing process [
27]. Moreover, our results support the finding that IL-6 serves as a switch that exerts reparative effects during the inflammatory progress [
28]. Compared with the untreated groups and the group treated with 4 mM Lipo-Farn, the group treated with pure Farn and Lipo-Farn had a moderate IL-6 level (
Figure 5 and
Figure 6); this finding is consistent with the histopathological analysis findings and wound-healing scores obtained in the current study. Moreover, these results are in accordance with those of our previous studies indicating that Farn regulates the IL-6 level in third-degree burns and UVB-caused sunburn in murine skin [
14,
21].
4. Materials and Methods
4.1. Materials
Farn was purchased from Sigma (St. Louis, MO, USA). HPMC (hydroxypropyl Methylcellulose), HA (hyaluronic acid), cholesterol, lectin, distearoylphosphatidylcholine (DSPC; molecular weight: 790.15 Da), chloroform, and methanol were also procured from Sigma (St. Louis, MO, USA). All reagents used in this study were of reagent grade.
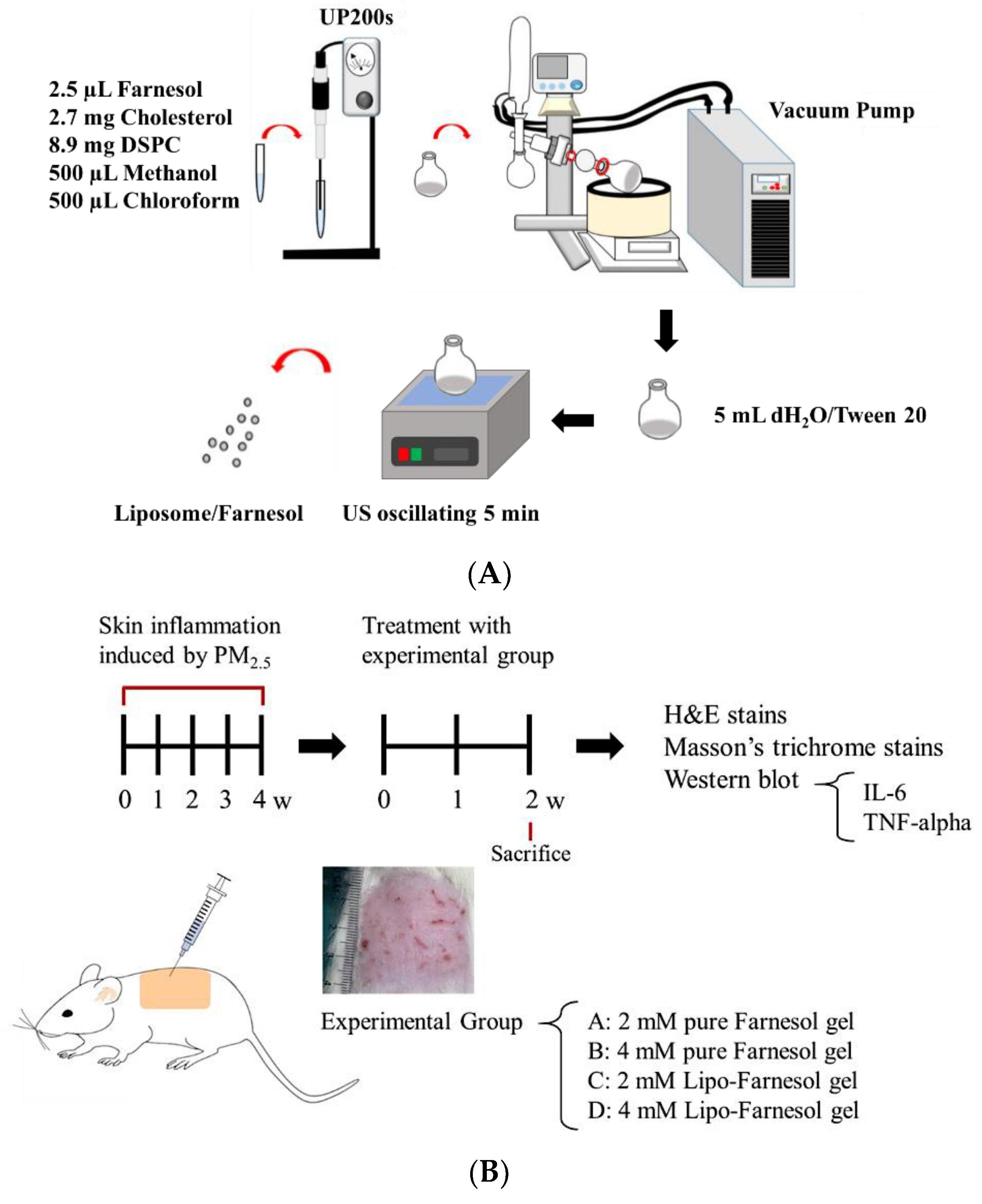
4.2. Production and Characterization of Liposome-Encapsulated Farn
Liposome-encapsulated farnesol (Lipo-Farn) was prepared using the evaporation–sonication method as described in our previous study. Briefly, a mixture of DSPC (8.9 mg), cholesterol (2.7 mg), and Farn (2.5 μL) was used as phospholipids for liposomes. DSPC, cholesterol, lecithin, and Farn were dissolved in methanol:chloroform (1:1,
v/
v) and then placed in a flask. The mixture in the flask was homogenized for 2 min (UP 200S, Hielscher, Teltow, Germany) and dried using a rotary evaporator (N-1300, EYELA, Tokyo, Japan) to form a thin film. The produced film was rehydrated with deionized water (5 mL) and sonicated for 5 min (
Figure 7A).
The entrapment efficiency (EE) of Farn in liposomes was determined as follows: 1 mL of the solution containing the prepared Lipo-Farn was centrifuged at 10,000 rpm for 10 min, and the amount of nonencapsulated Farn in the supernatant was measured through high-performance liquid chromatography (HPLC, Agilent 1100 series, Sana Clara, CA, USA). The EE was calculated using the following formula:
The in vitro release of Farn from liposomes was assessed. A total of 1 mL of Lipo-Farn was added to a 1.5 mL microcentrifuge tube. Subsequently, the tube was placed on a shaker with the temperature and shaking rate set at 37 °C and 40 rpm, respectively. At predetermined time points, the sample was centrifuged at 14,000 rpm for 60 min. The amount of nonencapsulated Farn in the supernatant was determined through HPLC. The in vitro release rate of Farn from liposomes was calculated as follows:
4.3. In Vitro Cell Viability Tests for Lipo-Farn, Farn, and PM2.5
L929 mouse fibroblasts were suspended at a density of 7 × 103 cells/mL and seeded onto 96-well plates. Various concentrations of Lipo-Farn, Farn, or PM2.5 were added to each well in triplicate. The cell viability of the L929 fibroblasts was examined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. After 24 h exposure to treatment, 20 μL of MTT solution (5 mg/mL) were added to each well, and the cells were incubated for an additional 3 h. The formed formazan precipitate was dissolved in 200 μL of dimethyl sulfoxide, the solution was vigorously mixed to dissolve the dye, and absorbance was measured at 570 nm by using a multiplate reader (Thermo Scientific, Waltham, MA, USA).
Cell viability was examined by performing a live–dead cell assay (Invitrogen, Carlsbad, CA, USA). Briefly, 1 mL of phosphate-buffered saline (PBS) containing 2.5 μL/mL of 4 μM ethidium homodimer-1 (EthD-1) assay solution and 1 μL/mL of 2 μM calcein AM solution was prepared. This assay solution (100 μL) was added to the culture, and the mixture was placed in 37 °C and 5% CO2 for 15 min. The sample was observed using a fluorescence microscope at excitation wavelengths 494 nm (green, calcein) and 528 nm (red, EthD-1; Olympus IX71, Tokyo, Japan).
4.4. Determination of PM2.5-induced Inflammatory Response
L929 fibroblasts (2 × 105 cells/well) were seeded in a 6-well plate and incubated for 24 h. Subsequently, the cells were treated with various PM2.5 concentrations (200–1000 μg/mL). After incubation for 24 h, the medium was collected and centrifuged at 2000× g for 10 min to obtain a cell-free supernatant. This supernatant was subsequently used in the enzyme-linked immunosorbent assay (ELISA) for IL-6. IL-6 was quantified using the Elabscience mouse IL-6 ELISA kit (Minneapolis, MN, USA), and absorbance was measured at 450 nm following the manufacturer’s protocol.
4.5. In Vivo Animal Experiments
4.5.1. Establishment of a PM2.5-Induced Skin Inflammation Model in Rats
Animal experiments conducted in this study were approved by the Institutional Animal Care and Use Committee of I-Shou University, Taiwan (approval no.: IACUC-ISU107-022, approval date: 28 December 2018). A total of 19 six-week-old Sprague–Dawley female rats (approximately 250 g/each) were used to establish the skin inflammation model. The dorsal skin of the rats was treated with four doses of 100 μL of PM2.5 solution (200 μg/mL) per week for 4 weeks. The rats were randomized into the experimental group (with treatment; n = 16) and control group (without any treatment; n = 3). All the rats were provided access to normal food and water postoperatively. The experimental rats were randomly divided into four experimental groups: Group A (the rats were treated with 2 mM pure Farn gel), Group B (the rats were treated with 4 mM pure Farn gel), Group C (the rats were treated with 2 mM Lipo-Farn gel), and Group D (the rats were treated with 4 mM Lipo-Farn gel). In addition to the untreated control group, the rats in the negative control group received pure Farn gel treatment after the induction of skin inflammation. The gel was prepared by dissolving HPMC (2%) in 90 °C distilled water. The solution was cooled to room temperature, and HA (0.5%) and xanthan gum (0.5%) were added and mixed in through stirring (pure gel). Subsequently, pure Farn (2 or 4 mM) and Lipo-Farn (2 or 4 mM) were added and stirred at 100 rpm for 10 min to prepare Farn gel.
In vivo animal experiments were performed up to the sixth week (
Figure 7B). The first 4-week period was designated for the development of PM
2.5-induced skin inflammation, followed by treatment with pure Farn gel and gel containing pure Farn or Lipo-Farn (three treatments/week) for 2 weeks. Subsequently, the rats were sacrificed, and their skin samples were harvested to examine skin repair by performing histopathological analysis. To simulate the real effect of PM
2.5 on skin, we performed another animal study in which a continuous subcutaneous injection of PM
2.5 was administered during Farn treatment. The subcutaneous injection of PM
2.5 was administered under anesthesia using Zoletil (tiletamine with zolazepam, 40 mg/kg, intraperitoneal [i.p.], 50 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.). During the in vivo animal study period, clinical signs of pain, salivation, and abnormal behavior were carefully monitored.
4.5.2. Histopathological Analysis
After a 2-week treatment with pure Farn, Lipo-Farn, or control, the skin of the rats was harvested and fixed in 10% neutral-buffered formalin. The skin samples were then dehydrated in graded ethanol solutions, cleared in xylene, embedded in paraffin blocks, and cut into 5 μm thick sections. Hematoxylin and eosin (H & E) staining was performed for histopathological examination of the skins. Furthermore, Masson trichrome staining was conducted to assess changes in the collagen content of the skin of the rats treated with Farn or Lipo-Farn. ImageJ software (Version 1.50; National Institutes of Health, Bethesda, MD, USA) was used to measure the collagen content in each group. The color settings employed in ImageJ remained constant throughout the analysis of Masson’s trichrome-stained areas in every sample. The samples were evaluated at 100× magnification, and the calculation was repeated in three microscopic fields.
4.5.3. Western Blot Analysis
To examine the inflammation-alleviating and protective effects of Farn or Lipo-Farn on the rat skin treated with PM2.5, the rat skin specimens were harvested 2 weeks after treatment. The samples were placed in precooled (4 °C) PBS solution. The samples were then ground until fully homogenized and added to ice-cold lysis buffer; the tissue homogenate was lysed on ice for 0.5–1 h. After 1 h, the tissue homogenate was centrifuged at 13,000 rpm at 4 °C for 15 min. The proteins were separated through 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and subsequently transferred and blotted onto a nitrocellulose membrane. Nonspecific binding sites on the membrane were blocked using 5% skim milk powder in Tris buffered saline containing 0.05% Tween 20 for 1 h at room temperature. The membrane was then incubated with primary antimouse IL-6, TNF-α, and β-actin antibodies (Santa Cruz, CA, USA) overnight at 4 °C. The ratio of the IL-6 and TNF-α protein levels in the experimental groups to those in the control group was measured through semiquantitative intensity analysis (normalized by the respective β-actin and background) by using ImageJ.
4.6. Statistical Analysis
All values are expressed as the mean ± standard error of the mean. One-way analysis of variance was used to identify significant differences between the experimental and control groups. A p value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 20.0.