Efficacy of a Multi-Strain Probiotic Formulation in Pediatric Populations: A Comprehensive Review of Clinical Studies
Abstract
:1. Introduction
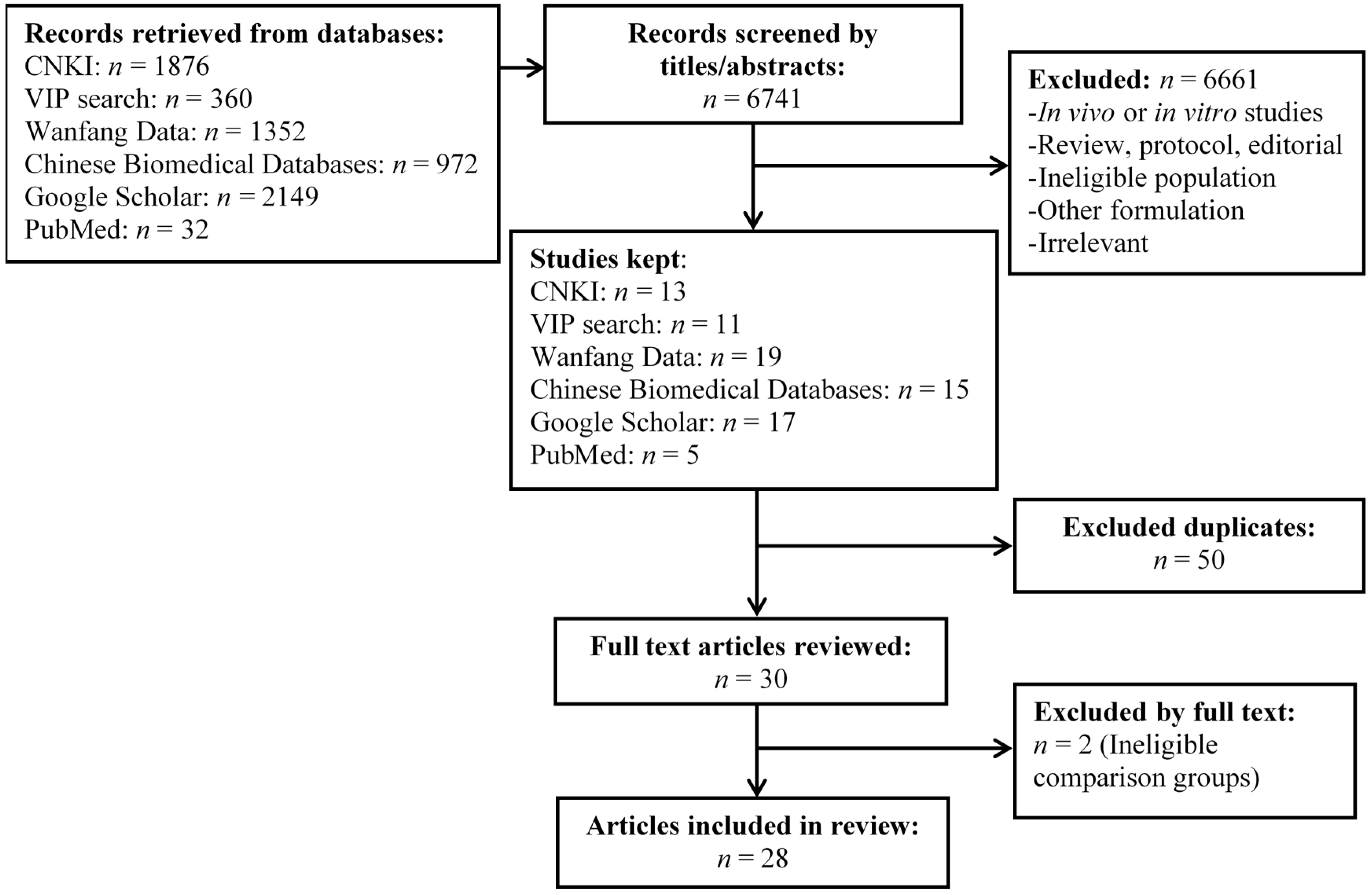
2. Materials and Methods
3. Results
3.1. Classification of Identified Clinical Studies
3.2. Studies on Diarrhea of Various Etiologies
3.3. Studies on Immune Competence and Inflammatory Markers
3.4. Studies on the Prevention of Common Infections
3.5. Studies in Other Indications
3.5.1. Oral Candidiasis (Thrush)
3.5.2. Eczema
3.5.3. Iron Deficiency Anemia
3.5.4. GI Function and Necrotizing Enterocolitis in Newborns
3.5.5. Jaundice in Newborns
4. Discussion and Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Heeney, D.D.; Gareau, M.G.; Marco, M.L. Intestinal Lactobacillus in health and disease, a driver or just along for the ride? Curr. Opin. Biotechnol. 2018, 49, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Arboleya, S.; Watkins, C.; Stanton, C.; Ross, R.P. Gut Bifidobacteria Populations in Human Health and Aging. Front. Microbiol. 2016, 7, 1204. [Google Scholar] [CrossRef] [Green Version]
- Gaufin, T.; Tobin, N.H.; Aldrovandi, G.M. The importance of the microbiome in pediatrics and pediatric infectious diseases. Curr. Opin. Pediatr. 2018, 30, 117–124. [Google Scholar] [CrossRef]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Strain-Specificity and Disease-Specificity of Probiotic Efficacy: A Systematic Review and Meta-Analysis. Front. Med. 2018, 5, 124. [Google Scholar] [CrossRef]
- Naser, S.M.; Hagen, K.E.; Vancanneyt, M.; Cleenwerck, I.; Swings, J.; Tompkins, T.A. Lactobacillus suntoryeus Cachat and Priest 2005 is a later synonym of Lactobacillus helveticus (Orla-Jensen 1919) Bergey et al. 1925 (Approved Lists 1980). Int. J. Syst. Evol. Microbiol. 2006, 56, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Canada Vigilance Adverse Reaction Online Database. Government of Canada. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/adverse-reaction-database.html (accessed on 21 May 2021).
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarzer, G. Meta: An R Package for Meta-Analysis. R News 2007, 7, 40–45. Available online: http://cran.r-project.org/doc/Rnews/Rnews_2007-3.pdf (accessed on 31 May 2021).
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. A Nonparametric “Trim and Fill” Method of Accounting for Publication Bias in Meta-Analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar] [CrossRef]
- Manzano, S.; De Andres, J.; Castro, I.; Rodriguez, J.M.; Jimenez, E.; Espinosa-Martos, I. Safety and tolerance of three probiotic strains in healthy infants: A multi-centre randomized, double-blind, placebo-controlled trial. Benef. Microbes 2017, 8, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Gong, C.; Ding, Y.; Ding, G.; Xu, X.; Deng, C.; Ze, X.; Malard, P.; Ben, X. Probiotics maintain intestinal secretory immunoglobulin A levels in healthy formula-fed infants: A randomised, double-blind, placebo-controlled study. Benef. Microbes 2019, 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gao, M. The Efficacy Analysis of the Combination of Smecta and BIOSTIME Probiotics in the Treatment of Non-Infectious Diarrhea in Children. Med. Front. 2013, 164. Available online: https://irp-cdn.multiscreensite.com/a17cd43e/files/uploaded/Gao-2013-The%20efficacy%20analysis%20of%20the%20combinat.pdf (accessed on 31 May 2021).
- Li, S. Efficacy analysis of Montmorillonite combined with Biostime probiotics in children with non-infectious diarrhea. Spec. Health Issue 2020, 10, 92. [Google Scholar]
- Liang, Y.; Wang, X.; Zhao, B. Efficacy analysis of Montmorillonite combined with Biostime probiotics in children with non-infectious diarrhea. Women Health Res. 2018, 121–123. [Google Scholar] [CrossRef]
- Liu, P.; Liang, Y.; Zhu, M. Observation on the efficacy of Montmorillonite Powder and Biostime Probiotics in Children with non-infectious diarrhea. Hebei Med. J. 2015, 37, 1846–1847. [Google Scholar]
- Luo, G. Smecta Combined with Biostime Probiotics in the Treatment of Non-Infectious Diarrhea in Children. China Rural Health 2013, 311–312. Available online: https://xueshu.baidu.com/usercenter/paper/show?paperid=1d1d04y0fm4r0ja0bd4h0c90vh477682&site=xueshu_se (accessed on 31 May 2021).
- Wang, F. The effectiveness analysis of combination of Smecta and Biostime probiotics in the treatment of infantile non-infectious diarrhea. Mod. Prev. Med. 2012, 39, 2726–2727. [Google Scholar]
- Wu, X. The efficacy analysis of the combination of Smecta and Biostime probiotics in the treatment of non-infectious diarrhea in children. Road Health 2013, 12, 180–181. [Google Scholar]
- Cui, X.; Wure, G. The Treatment of 62 Cases of Rotavirus Gastroenteritis by Biostime Probiotics. Chin. J. Synth. Med. 2003, 5, 53–54. [Google Scholar]
- Jin, Y. Clinical Efficacy Observation of Biostime Probiotics in the Treatment of Autumn Diarrhea. Chin. J Clin. Ration. Drug Use 2014, 7, 44–45. [Google Scholar]
- Li, X.; Chen, Z. Evaluation of the efficacy of Biostime on rotaviral infection in children. Med. Inf. 2008, 21, 893–895. [Google Scholar]
- Yang, J.; Tian, X.; Chen, Y.; Li, P.; Lin, Y.; Wang, Y.; Xu, Z.; Zhao, W. Clinical Observation of Different Feeding Methods in Adjuvant Treatment of Infantile Diarrhea. Occup. Health 2010, 26, 2807–2808. [Google Scholar]
- Jiang, X. Clinical Evaluation of Biostime in the Treatment of Children with Persistent Diarrhea. In Proceedings of the 6th Chinese National Conference Pediatric Microecology, Shenzhen, China, 6 November 2016; Available online: https://xueshu.baidu.com/usercenter/paper/show?paperid=de84a9406f735672673620e40b7869eb&site=xueshu_se&hitarticle=1 (accessed on 31 May 2021).
- Chen, L.; Ouyang, L.; Liao, W.; Zhang, W. Mucous membrane immunity enhanced by taking Biostime probiotics. Chin. J. Ecol. 2007, 19, 137–141. [Google Scholar]
- De Andres, J.; Manzano, S.; Garcia, C.; Rodriguez, J.M.; Espinosa-Martos, I.; Jimenez, E. Modulatory effect of three probiotic strains on infants’ gut microbial composition and immunological parameters on a placebo-controlled, double-blind, randomised study. Benef. Microbes 2018, 9, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Pantović, F. Serum immunoglobulin levels in children with respiratory infections who used a synbiotic dietary supplement. PONS Med. J. 2013, 10, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Qiu, W.; Zhang, F.; Sun, L.; Yu, J.; Wang, S.; Li, G.; Tong, X. The effect of blue light phototherapy combined with synbiotics on neonatal hyperbilirubinemia. Chin. J. Med. 2020, 55, 1014–1016. [Google Scholar]
- Cazzola, M.; Pham-Thi, N.; Kerihuel, J.C.; Durand, H.; Bohbot, S. Efficacy of a synbiotic supplementation in the prevention of common winter diseases in children: A randomized, double-blind, placebo-controlled pilot study. Ther. Adv. Respir. Dis. 2010, 4, 271–278. [Google Scholar] [CrossRef]
- Stojković, A.; Simović, A.; Bogdanović, Z.; Banković, D.; Poskurica, M. Optimal time period to achieve the effects on synbiotic-controlled wheezing and respiratory infections in young children. Srp. Arh. Celok. Lek. 2016, 144, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Ding, S.; Guo, J.; Ma, S.; Kong, X.; Pan, L. Observation of Clinical effects of Biostime probiotics on thrush. Chin. J. Microecol. 2013, 25, 830–831. [Google Scholar]
- Li, F. Biostime probiotics combined with compound Huangbai solution in treatment of children eczema: An observation on the efficacy. China Pediatrics Integr. Tradit. West. Med. 2017, 9, 121–123. [Google Scholar]
- Wei, J. Evaluation of Synbiotics Probiotics Granules and Iron Dextran Oral Liquid in the Treatment of Pediatric IDA. J. Med. Theory Pract. 2018, 31, 566–567. [Google Scholar]
- Wu, W. Prospective study of iron dextran combined with synbiotic probiotics in the treatment of nutritional iron deficiency anemia in children. Matern. Child Health Care China 2014, 29, 3284–3286. [Google Scholar]
- Yang, Y. Efficacy analysis of the combined treatment of iron dextran oral liquid and Biostime probiotics in children with nutritional iron deficiency. Matern. Child Health Care China 2015, 30, 6390–6392. [Google Scholar]
- Huang, T.; Ouyang, F. Clinical Study on the Effect of Biostime Probiotics Granules on Gastrointestinal Function in Very Low Birth Weight Infants. Med. Inf. 2015, 28, 317–318. [Google Scholar]
- Gao, Y.; Peng, F.; Wang, J.; Zhu, W.; Wang, S.; Xu, J.; Liu, P.; Sun, Z. Study on the preventive effect of probiotics on neonatal jaundice and neonatal tolerance of probiotics. Matern. Child Health Care China 2015, 30, 6229–6231. [Google Scholar]
- Fang, H.; Duan, S.; Dong, Z.; Yu, A.; Liu, X.; Zhang, J.; Peng, W.; Xiao, D.; Zheng, Q.; Xie, L.; et al. The diagnosis and treatment strategy of diarrhea in China. Chin. J. Pract. Pediatr. 1998, 13, 381–384. [Google Scholar]
- Sohail, G.; Xu, X.; Christman, M.C.; Tompkins, T.A. Probiotic Medilac-S® for the induction of clinical remission in a Chinese population with ulcerative colitis: A systematic review and meta-analysis. World J. Clin. Cases 2018, 6, 961–984. [Google Scholar] [CrossRef] [PubMed]
- Corthesy, B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front. Immunol. 2013, 4, 185. [Google Scholar] [CrossRef] [Green Version]
- Mantis, N.J.; Rol, N.; Corthesy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal. Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef]
- Cacho, N.T.; Lawrence, R.M. Innate Immunity and Breast Milk. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, D.B.; Wilson, C.B. CHAPTER 4—Developmental Immunology and Role of Host Defenses in Fetal and Neonatal Susceptibility to Infection. In Infectious Diseases of the Fetus and Newborn, 7th ed.; Remington, J.S., Klein, J.O., Wilson, C.B., Nizet, V., Maldonado, Y.A., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2011; pp. 80–191. [Google Scholar] [CrossRef]
- Leong, K.W.; Ding, J.L. The unexplored roles of human serum IgA. DNA Cell Biol. 2014, 33, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Yel, L. Selective IgA deficiency. J. Clin. Immunol. 2010, 30, 10–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neonatology Group of Pediatrics Branch of Chinese Medical Association, Editorial Board of Chinese Journal of Pediatrics. Expert consensus on diagnosis and treatment of neonatal hyperbilirubinemia. Chin. Pediatrics J. 2014, 52, 745–748. [Google Scholar]
- Van den Nieuwboer, M.; Brummer, R.J.; Guarner, F.; Morelli, L.; Cabana, M.; Claassen, E. Safety of probiotics and synbiotics in children under 18 years of age. Benef. Microbes 2015, 6, 615–630. [Google Scholar] [CrossRef] [Green Version]
- Land, M.H.; Rouster-Stevens, K.; Woods, C.R.; Cannon, M.L.; Cnota, J.; Shetty, A.K. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 2005, 115, 178–181. [Google Scholar] [CrossRef]
- Salminen, M.K.; Rautelin, H.; Tynkkynen, S.; Poussa, T.; Saxelin, M.; Valtonen, V.; Järvinen, A. Lactobacillus bacteremia, clinical significance, and patient outcome, with special focus on probiotic L. rhamnosus GG. Clin. Infect. Dis. 2004, 38, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Thompson, S.G.; Spiegelhalter, D.J. A re-evaluation of random-effects meta-analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2009, 172, 137–159. [Google Scholar] [CrossRef] [Green Version]
- Culpepper, T.; Christman, M.C.; Nieves, C., Jr.; Specht, G.J.; Rowe, C.C.; Spaiser, S.J.; Ford, A.L.; Dahl, W.J.; Girard, S.A.; Langkamp-Henken, B. Bifidobacterium bifidum R0071 decreases stress-associated diarrhoea-related symptoms and self-reported stress: A secondary analysis of a randomised trial. Benef. Microbes 2016, 7, 327–336. [Google Scholar] [CrossRef]
- Langkamp-Henken, B.; Rowe, C.C.; Ford, A.L.; Christman, M.C.; Nieves, C., Jr.; Khouri, L.; Specht, G.J.; Girard, S.A.; Spaiser, S.J.; Dahl, W.J. Bifidobacterium bifidum R0071 results in a greater proportion of healthy days and a lower percentage of academically stressed students reporting a day of cold/flu: A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2015, 113, 426–434. [Google Scholar] [CrossRef] [Green Version]
- Macpherson, C.; Audy, J.; Mathieu, O.; Tompkins, T.A. Multistrain probiotic modulation of intestinal epithelial cells’ immune response to a double-stranded RNA ligand, poly(i·c). Appl. Environ. Microbiol. 2014, 80, 1692–1700. [Google Scholar] [CrossRef] [Green Version]
- MacPherson, C.W.; Shastri, P.; Mathieu, O.; Tompkins, T.A.; Burguière, P. Genome-Wide Immune Modulation of TLR3-Mediated Inflammation in Intestinal Epithelial Cells Differs between Single and Multi-Strain Probiotic Combination. PLoS ONE 2017, 12, e0169847. [Google Scholar] [CrossRef] [PubMed]
- Kuugbee, E.D.; Shang, X.; Gamallat, Y.; Bamba, D.; Awadasseid, A.; Suliman, M.A.; Zang, S.; Ma, Y.; Chiwala, G.; Xin, Y.; et al. Structural Change in Microbiota by a Probiotic Cocktail Enhances the Gut Barrier and Reduces Cancer via TLR2 Signaling in a Rat Model of Colon Cancer. Dig. Dis. Sci. 2016, 61, 2908–2920. [Google Scholar] [CrossRef]
- Cazzola, M.; Tompkins, T.A.; Matera, M.G. Immunomodulatory impact of a synbiotic in Th1 and Th2 models of infection. Ther. Adv. Respir. Dis. 2010, 4, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Yang, J.; Zhang, H.; Yu, J.; Yao, Z. Oral probiotic administration during pregnancy prevents autism-related behaviors in offspring induced by maternal immune activation via anti-inflammation in mice. Autism. Res. 2019, 12, 576–588. [Google Scholar] [CrossRef]
- Ng, Q.X.; Loke, W.; Venkatanarayanan, N.; Lim, D.Y.; Soh, A.Y.S.; Yeo, W.S. A Systematic Review of the Role of Prebiotics and Probiotics in Autism Spectrum Disorders. Medicina 2019, 55, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, S.; Zhu, H.; Zhang, Z.; Li, B.; Pierro, A.; Sherman, P.M. A103 Probiotic Formulations Differentially Affect Disease Outcome in a Mouse Model of Necrotizing Enterocolitis. J. Can. Assoc. Gastroenterol. 2020, 3, 119–120. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Butcher, J.; Stintzi, A.; Figeys, D. Advancing functional and translational microbiome research using meta-omics approaches. Microbiome 2019, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Polk, D.B. Probiotics and Probiotic-Derived Functional Factors—Mechanistic Insights Into Applications for Intestinal Homeostasis. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Paul, W.; Marta, C.; Tom, V.W. Resolving host-microbe interactions in the gut: The promise of in vitro models to complement in vivo research. Curr. Opin. Microbiol. 2018, 44, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Cassotta, M.; Forbes-Hernández, T.Y.; Calderón Iglesias, R.; Ruiz, R.; Elexpuru Zabaleta, M.; Giampieri, F.; Battino, M. Links between Nutrition, Infectious Diseases, and Microbiota: Emerging Technologies and Opportunities for Human-Focused Research. Nutrients 2020, 12, 1827. [Google Scholar] [CrossRef] [PubMed]
| Indication | Number of Studies | N (Total) | N (Probiotic Arm) | Main Outcome Measures | References |
|---|---|---|---|---|---|
| Safety | |||||
| Healthy children/newborns | 2 | 340 | ≈50/strain [13] 1 66 [14] | Growth parameters, adverse events and serious adverse events, sleep and crying patterns, D-lactic acid | [13,14] |
| Gastrointestinal function | |||||
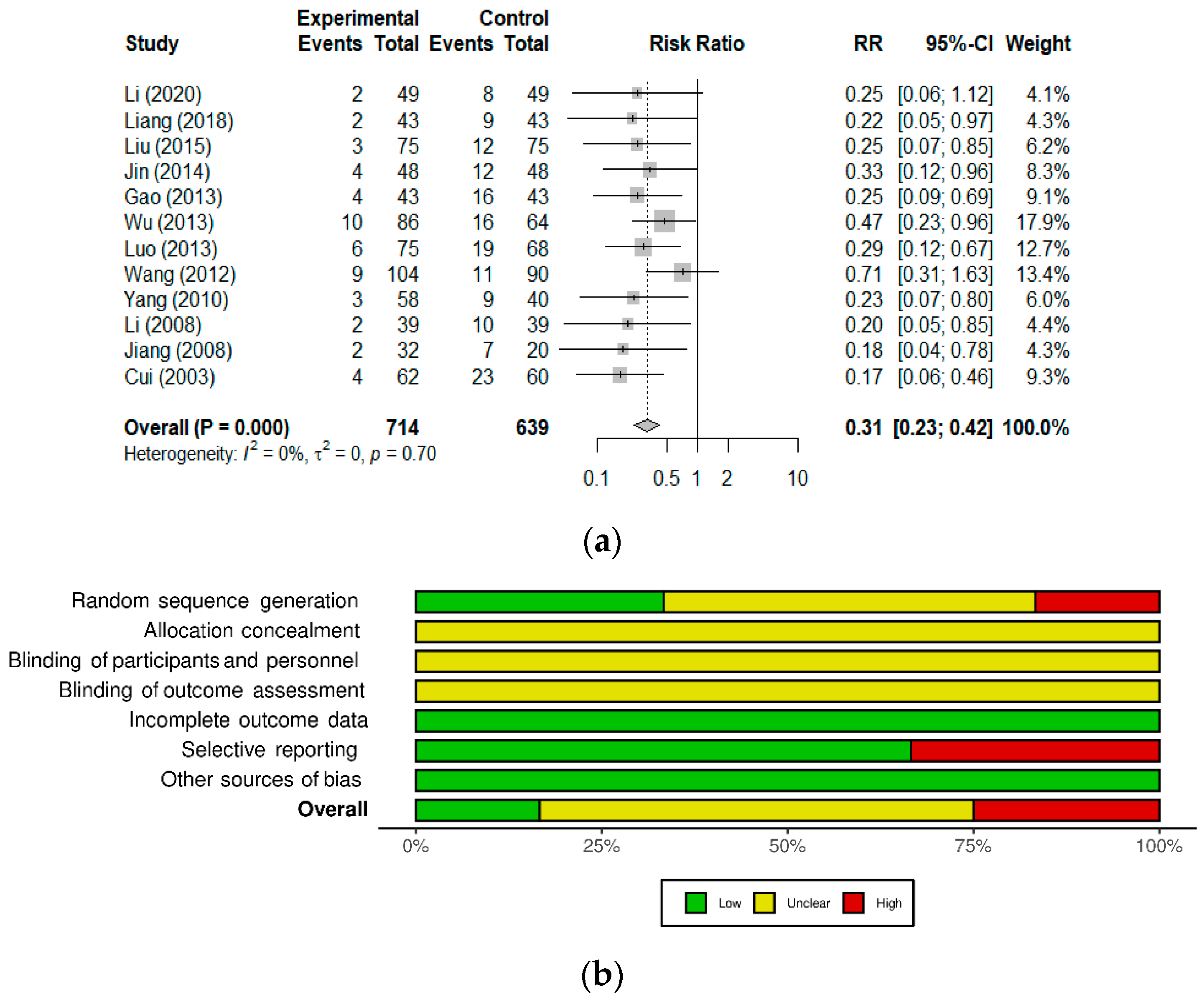
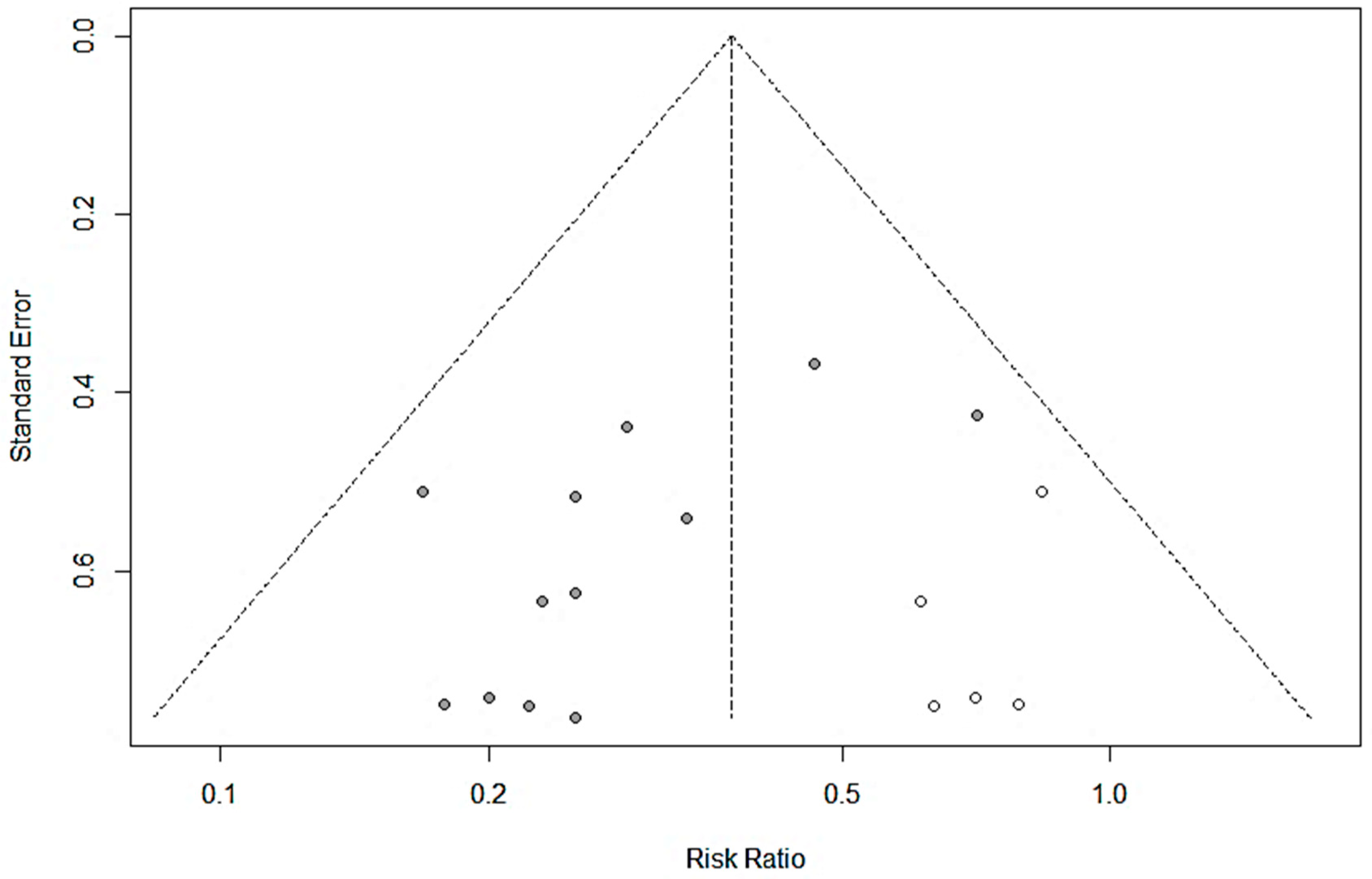
| Non-infectious diarrhea | 7 | 1001 | 521 | Time to symptom relief, effective rate | [15,16,17,18,19,20,21] |
| Rotavirus (RV)-induced diarrhea | 4 | 394 | 207 | Time to symptom relief, effective rate | [22,23,24,25] |
| Persistent diarrhea, undefined etiology | 1 | 52 | 32 | Time to symptom relief, effective rate | [26] |
| Immune system function and natural defenses | |||||
| Secretory IgA, cytokines, chemokines | 6 | 405 2 | 224 2 | Salivary, fecal or serum levels of secretory IgA, fecal or serum levels of cytokines/chemokines | [14,18,27,28,29,30] |
| Common infections 3 | 2 | 213 | 140 | Incidence of infections and related symptoms, adverse events | [31,32] |
| Other indications | |||||
| Oral candidiasis (thrush) | 1 | 70 | 35 | Effective rate, recurrence | [33] |
| Eczema | 1 | 76 | 38 | Eczema Area and Severity Index (EASI) score, effective rate | [34] |
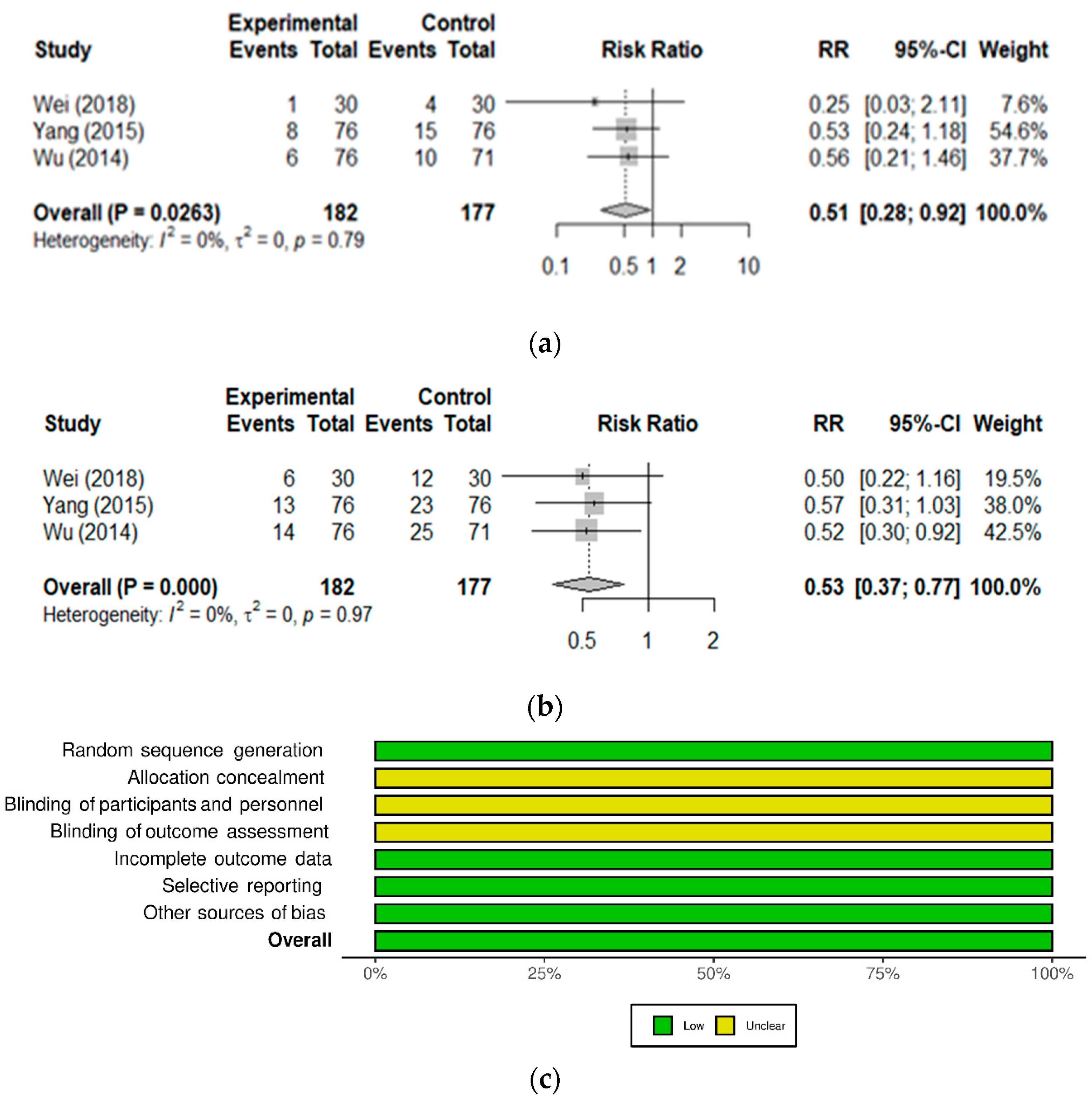
| Iron deficiency anemia | 3 | 364 | 182 | Anemia blood markers, effective rate, side effects | [35,36,37] |
| Necrotizing enterocolitis | 1 | 60 | 30 | Incidence, severity, mortality, food tolerance | [38] |
| Jaundice | 2 | 1064 | 532 | Incidence, severity (bilirubin levels) | [30,39] |
| Reference [Language] | Study Dates | Population | Study Design Arms, n | Probiotic Regimen | Results (vs. Control) | Adverse Events |
|---|---|---|---|---|---|---|
| Cui, 2003 [22] [Chinese] | September 2002–November 2002 | Children 6–24 months old RV-ag+ diarrhea Onset < 72 h | Randomized, controlled Ribavirin + Pro, n = 62 Ribavirin + Lacidophilin, n = 60 | <12 months: 1 sachet QD 12–24 months: 1 sachet BID Orally in warm water or milk Until resolution or up to 72 h | Shorter duration of diarrhea (39.3 ± 17.1 vs. 63.8 ± 22.9 h) Higher total effective rate (93.5% vs. 61.7%; p < 0.01) | n.r. |
| Li, 2008 [24] [Chinese] | June 2005–December 2007 | Children 0–60 months old RV-ag+ diarrhea | Randomized, controlled Ribavirin + Pro, n = 39 Ribavirin alone, n = 39 | 1 sachet BID Orally. 7 days | Higher total effective rate (94.9% vs. 74.3%; p < 0.05) | n.r. |
| Jiang, 2008 [26] [Chinese] | December 2006–June 2008 | Children 3–24 months old Persistent diarrhea | Randomized, active control Pro, n = 32 GoldenBifido, n = 20 | <6 months: 0.5 sachet BID 6–12 months: 1 sachet BID 12–24 months: 1–2 sachet BID Until resolution | Shorter duration of diarrhea (7.14 ± 0.78 vs. 12.6 ± 1.75 d; p < 0.001) Higher total effective rate (91% vs. 65%; p < 0.01) | n.r. |
| Yang, 2010 [25] [Chinese] | January 2008–October 2009 | Children 6–30 months old RV-ag+ diarrhea | Randomized, controlled CST + Pro (in formula), n = 58 CST (breastfed or formula), n = 40 | 1 sachet QD Orally In lactose-free formula Duration not stated | Shorter duration of diarrhea (2.8 ± 1.1 vs. 4.9 ± 2.6 d; p< 0.01) Shorter hospital stay (5.5 ± 1.7 vs. 8.5 ± 2.3 d; p < 0.01). Higher total effective rate (94.8% vs. 77.5%; p ≤ 0.05) | n.r. |
| Wang, 2012 [20] [Chinese] | May 2010–December 2010 | Children 3–36 months old Non-infectious diarrhea Onset < 72 h | Randomized, controlled Smecta® + Pro, n = 104 Smecta®, n = 90 | <12 months: 0.33 sachet TID 12–24 months: 0.5 sachet BID 24–36 months: 1 sachet BID Orally. 3 days | Higher markedly effective rate in three age groups (p > 0.05): < 12 months: 78.8% vs. 74.2% 12–24 months: 79.1% vs. 74/3% 24–36 months: 82.1% vs. 75% | None observed |
| Luo, 2013 [19] [Chinese] | April 2010–February 2011 | Children 4–36 months old Non-infectious diarrhea Onset < 72 h | Randomized, controlled Smecta® + Pro, n = 75 Smecta®, n = 68 | <12 months: 0.33 sachet TID 12–24 months: 0.5 sachet BID 24–36 months: 1 sachet BID Orally. 3 days | Higher effective rate (94.8% vs. 73%; p < 0.05) | n.r. |
| Gao, 2013 [15] [Chinese] | January 2011–January 2012 | Children 0–36 months old Non-infectious diarrhea | Randomized, controlled Smecta® + Pro, n = 43 Smecta®, n = 43 | <12 months: 0.33 sachet TID 12–36 months: 0.5 sachet TID Orally. 3 days | Higher total effective rate (90.7% vs. 62.8%; p < 0.05) | None observed |
| Wu, 2013 [21] [Chinese] | April 2011–December 2011 | Children 2–36 months old Non-infectious diarrhea Onset < 72 h | Randomized, controlled Smecta® + Pro, n = 84 Smecta®, n = 64 | <12 months: 0.33 sachet TID 12–24 months: 0.5 sachet BID 24–36 months: 1 sachet BID Orally. 3 days | Higher total effective rate (90.5% vs. 75%; p < 0.05) | n.r. |
| Jin, 2014 [23] [Chinese] | October 2011–June 2013 | Children 5–52 months old (mean 17.3 mo) RV-ag+ diarrhea. Onset < 72 h | Randomized, active control Smecta® + Ribavirin + Pro, n = 48 Smecta® + Ribavirin, n = 48 | 0.5–1 sachet BID Orally. In warm water. Duration not stated. Fluids provided as needed | Shorter time to symptom relief (diarrhea, 31.6 ± 5.2 h vs. 34.6 ± 4.1 h; p< 0.05) Higher total effective rate (91.7% vs. 75.0%; p < 0.05) | n.r. |
| Liu, 2015 [18] [Chinese] | May 2011–May 2014 | Children 3–38 months old Non-infectious diarrhea | Randomized, controlled Smecta® + Pro, n = 75 Smecta®, n = 75 | <12 months: 0.33 sachet TID 12–24 months: 0.5 sachet BID 24–36 months: 1 sachet BID Orally. 3 days | Higher markedly effective rate (72% vs. 45.33%; p < 0.05) Higher total effective rate (96% vs. 84%; p < 0.05) Reduced diarrhea frequency (p < 0.05), time to diarrhea relief (p < 0.05) and symptom disappearance (p < 0.05) in the probiotic group | n.r. |
| Liang, 2018 [17] [Chinese] | February 2015–May 2017 | Children 3–39 months old Non-infectious diarrhea | Randomized, active control Smecta® + Probiotic, n = 43 Smecta® alone, n = 43 | <12 months: 0.33 sachet TID 12–24 months: 0.5 sachet BID 24–36 months: 1 sachet BID Orally. 3 days | Higher total effective rate (95.35% vs. 79.07%; p < 0.05) | n.r. |
| Li, 2020 [16] [Chinese] | January 2019–February 2020 | Children 6–37 months old Non-infectious diarrhea | Randomized, controlled Smecta® + Pro, n = 49 Smecta®, n = 49 | <12 months: 0.33 sachet TID 12–24 months: 0.33 or 0.66 sachet TID 24–36 months: 1 sachet TID Orally. 3 days | Higher total effective rate (95.91% vs. 83.67%; p < 0.05) | n.r. |
| Reference [Language] | Study Dates | Population | Study Design Arms, n | Probiotic Regimen | Results (vs. Control) | Adverse Events |
|---|---|---|---|---|---|---|
| Chen, 2007 [27] [Chinese] | Not stated | Children 0–48 months old Healthy with low salivary sIgA | Randomized, controlled Probiotic, n = 20 No Intervention, n = 8 | 1 sachet BID Orally. For 14 days | Increase in salivary sIgA compared to baseline in probiotic but not in controls. No statistical analyses reported. | n.r. |
| Cazzola, 2010 [31] [English] | December 2006–March 2007 | Children 3–7 years old ≥ 3 infections (ENTI, URTI, GI illness) in past winter. | Randomized, double-blind, placebo-controlled Probiotic, n = 62 Placebo, n = 73 | 1 sachet QD Orally. For 3 months | Lower incidence of ENTI, URTI, or GI health events (51.6% vs. 68.5%; p = 0.044), representing a 25% reduction in RR. Less participants experienced school day losses for sickness (25.8% vs. 42.5%; p = 0.0443). | 2 SAEs; 1 abdominal pain in placebo and 1 otitis media in Probiotic. 24 AEs in 20 children (9 in placebo, 11 in Probiotic), most were expected respiratory or GI events. |
| Pantovic, 2013 [29] [English] | Not stated | Children 6–42 months old low IgA levels hospitalized for URTI or ENTI | Open-label, uncontrolled before–after study Probiotic, n = 31 | 1 sachet QD Orally. For 6 months | Increase in serum IgA levels in 35% of the children after 3 months and 81% after 6 months (p < 0.05), normalized to normal range. Clinical improvement in URTI after 3 months, and no infections diagnosed between 3 and 6 months. | n.r. |
| Liu, 2015 [18] [Chinese] | May 2011–May 2014 | Children 3–38 months old Non-infectious diarrhea | Randomized, controlled Smecta® + Probiotic, n = 75 Smecta®, n = 75 | <12 months: 0.33 sachet TID 13–24 months: 0.5 sachet BID 24–36 months: 1 sachet BID Orally. For 3 days | Lower serum levels of pro-inflammatory cytokines IL-6 and IL-17 (p < 0.05). Higher levels of salivary sIgA (p < 0.05). | n.r. |
| Stojkovic, 2016 [32] [English] | Not stated. | Children <5 years Hospitalized during the past year for respiratory diseases | Open label, before–after Probiotic, n = 78 Divided into 3 groups based on medical history: G1: URTI + wheezing, n = 50 G2: URTI w/o wheezing, n = 17 G3: Wheezing w/o URTI, n = 11 | 1 sachet QD Orally. For 9 months | Decrease in URTI and wheezing after 3 months (p < 0.01), reaching 0% after 6 months. No recurrence of URTI or wheezing (0%) in all groups) at 9 months. Increase in serum IgA from 3 months onwards (p < 0.01). Increase in serum IgG from 3 months onwards (p < 0.01). Decrease in serum IgE at 9 months (p < 0.01). | None observed. |
| Manzano, 2017 [13]; De Andres, 2018 [28] [English] | August 2014–December 2016 | Children 3–12 months old Healthy | Randomized, double-blind, placebo-controlled L. helveticus Rosell®-52, n = 52 B. infantis Rosell®-33, n = 53 B. bifidum Rosell®-71, n = 51 Placebo, n = 52 | 3 × 109 CFU of each single strain QD Orally. For 8 weeks | Increased IL10/IL12 ratio (anti-inflammatory) in B. infantis (p < 0.01). Increased TNFα/IL10 ratio (pro-inflammatory) in L. helveticus and placebo (p < 0.01). Placebo group showed a microbiota composition related to the weaning process, while the probiotics groups were similar to 4-month-old un-weaned infants. | No difference between groups for the number and severity of adverse events (mild) nor in behavioral and anthropometric parameters. No SAEs observed. |
| Xiao, 2019 [14] [English] | December 2014–November 2015 | Children 3.5–6 months old Healthy | Randomized, placebo-controlled Probiotic, n = 66 Placebo, n = 66 | 1 sachet QD Orally, in formula. For 4 weeks | Maintained higher fecal sIgA levels at the end of the four-week treatment period (p < 0.05). | All AEs reported were minor and more frequent in the placebo group. Probiotic: 37 AEs; 21 respiratory, 12 GI, 4 dermatological. Placebo: 69 AEs; 38 respiratory, 15 GI, 16 dermatological. No effect on growth rate. |
| Qiu, 2020 [30] [Chinese] | September 2017–May 2018 | Full-term neonates with hyperbilirubinemia | Randomized, controlled Blue light photo- therapy + Probiotic, n = 32 Blue light photo- therapy, n = 32 | 1 sachet BID, for 5 days | Reduction in IL-6 vs. controls (p < 0.05). Reduction in IL-6 and IL-8 at day 3 and 5 vs. baseline (p < 0.05). Increase in IL-10 at day 5 vs. baseline (p < 0.05). Increase in serum catalase levels at day 5 vs. baseline (p < 0.05). | n.r. |
| Reference [Language] | Study Dates | Population | Study Design Arms, n | Probiotic Regimen | Results (vs. Control) | Adverse Events |
|---|---|---|---|---|---|---|
| Xi, 2013 [33] [Chinese] (Thrush) | January 2011–December 2012 | Children 1–26 months old oral candidiasis infection | Randomized, controlled 2% NaHCO₃ + nystatin + Pro, n = 35 2% NaHCO₃ + nystatin, n = 35 | 1 sachet BID Orally. For 14 days | Higher total effective rate (94.3% vs. 77.1%, p ≤ 0.05). Lower recurrence rate (2.9% vs. 17.1%, p ≤ 0.05). | None observed. |
| Li, 2017 [34] [Chinese] (Eczema) | August 2014–January 2016 | Children 1–24 months old infantile eczema | Randomized, controlled Topical treatment + Probiotic, n = 38 Topical treatment, n = 38 | 1 sachet BID, for 2 weeks | Lower EASI scores at 2 weeks after treatment (p < 0.05). Higher total effective rate (92.1% vs. 65.7%, p < 0.01). | None observed. |
| Wu, 2014 [36] [Chinese] (IDA) | June 2010–April 2013 | Children 6–60 months old Nutritional iron deficiency anemia | Randomized, controlled Iron dextran, n = 71 Iron dextran + Probiotic, n = 76 | 1 sachet QD, for 8 weeks | Higher total effective rate (76.3% vs. 60.6 %; χ2 = 4.236, p = 0.040). | Decreased cases of side effects of Iron dextran (13.2% vs. 25.4%; p = 0.06). |
| Yang, 2015 [37] [Chinese] (IDA) | February 2011–December 2013 | Children 6–60 months old Nutritional iron deficiency anemia | Randomized, controlled Iron dextran, n = 76 Iron dextran + Probiotic, n = 76 | 1 sachet BID, for 8 weeks | Higher markedly effective rate (75% vs. 55.3%; χ2 = 6.453, p = 0.011). | Decreased cases of side effects of iron dextran (17.1% vs. 30.3%; p = 0.06). |
| Wei, 2018 [35] [Chinese] (IDA) | January 2015–December 2016 | Children 7–72 months old Nutritional iron deficiency anemia | Randomized, Controlled Iron dextran, n = 30 Iron dextran + Probiotic, n = 30 | 1 sachet BID, for 8 weeks All received also Smecta® and antibiotics | Total effective rate 10% higher in probiotic vs. control (96.7% vs. 86.7%; p > 0.1). | Decreased cases of side effects of iron dextran (20% vs. 40%; p = 0.09). |
| Huang and Ouyang, 2015 [38] [Chinese] (GI function; NEC) | August 2011–August 2013 | Premature newborns (1000–1500 g; mean 34 weeks gestational age) | Randomized, placebo-controlled Placebo, n = 30 Probiotic, n = 30 | 0.5 sachet BID, for 2 weeks | Reduced feeding intolerance vs. control (36.7% vs. 70%; p = 0.0104). Nonsignificant reduction in NEC incidence in probiotic (2/30; 6.7%) vs. control (5/30; 16.7%). Nonsignificant reduction in duration of hospital stay in probiotic (40.1 ± 15.6 d) vs. control (47.3 ± 16.7 d). | One death (3.3%) in the probiotic group, 2 deaths in the control group (6.7%). Parenteral nutrition-associated cholestasis: 1 case (3.3%) in probiotic vs. 3 cases (10%) in control (p = 0.2755). |
| Gao, 2015 [39] [Chinese] (Jaundice) | December 2013–December 2013 | Healthy full-term neonates gestational age 37–42 weeks (mean 40 weeks), birth weight 2500–4000 g | Randomized, placebo-controlled Placebo, n = 500 Probiotic, n = 500 | 1 sachet BID, for 7 days after birth | Similar time of onset of jaundice between groups (p > 0.05), with significantly lower incidence in probiotic (9%) vs. placebo (17%); p < 0.001. Similar daily percutaneous bilirubin levels until day 5 (p > 0.05), with a significant reduction in bilirubin levels in probiotic on treatment days 6 and 7 vs. placebo (p < 0.05). | Similar between groups (erythema, vomiting, diarrhea; all p > 0.05). |
| Qiu, 2020 [30] [Chinese] (Jaundice) | September 2017–May 2018 | Healthy full-term neonates gestational age 37–41 weeks, aged ≤3 days, with neonatal hyperbilirubinemia developing within 24 h of birth | Randomized, controlled Blue light phototherapy, n = 32 Blue light phototherapy + Probiotic, n = 32 | 1 sachet BID, for 5 days | Significant decrease in total and unconjugated bilirubin levels in probiotic vs. controls at day 3 and day 5 (p < 0.05). Significant increase in catalase levels vs. baseline at day 5 (p < 0.05) in the probiotic group, but no change in controls. | n.r. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tremblay, A.; Xu, X.; Colee, J.; Tompkins, T.A. Efficacy of a Multi-Strain Probiotic Formulation in Pediatric Populations: A Comprehensive Review of Clinical Studies. Nutrients 2021, 13, 1908. https://doi.org/10.3390/nu13061908
Tremblay A, Xu X, Colee J, Tompkins TA. Efficacy of a Multi-Strain Probiotic Formulation in Pediatric Populations: A Comprehensive Review of Clinical Studies. Nutrients. 2021; 13(6):1908. https://doi.org/10.3390/nu13061908
Chicago/Turabian StyleTremblay, Annie, Xiaoyu Xu, James Colee, and Thomas A. Tompkins. 2021. "Efficacy of a Multi-Strain Probiotic Formulation in Pediatric Populations: A Comprehensive Review of Clinical Studies" Nutrients 13, no. 6: 1908. https://doi.org/10.3390/nu13061908
APA StyleTremblay, A., Xu, X., Colee, J., & Tompkins, T. A. (2021). Efficacy of a Multi-Strain Probiotic Formulation in Pediatric Populations: A Comprehensive Review of Clinical Studies. Nutrients, 13(6), 1908. https://doi.org/10.3390/nu13061908







