Texture Analysis of Fractional Water Content Images Acquired during PET/MRI: Initial Evidence for an Association with Total Lesion Glycolysis, Survival and Gene Mutation Profile in Primary Colorectal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
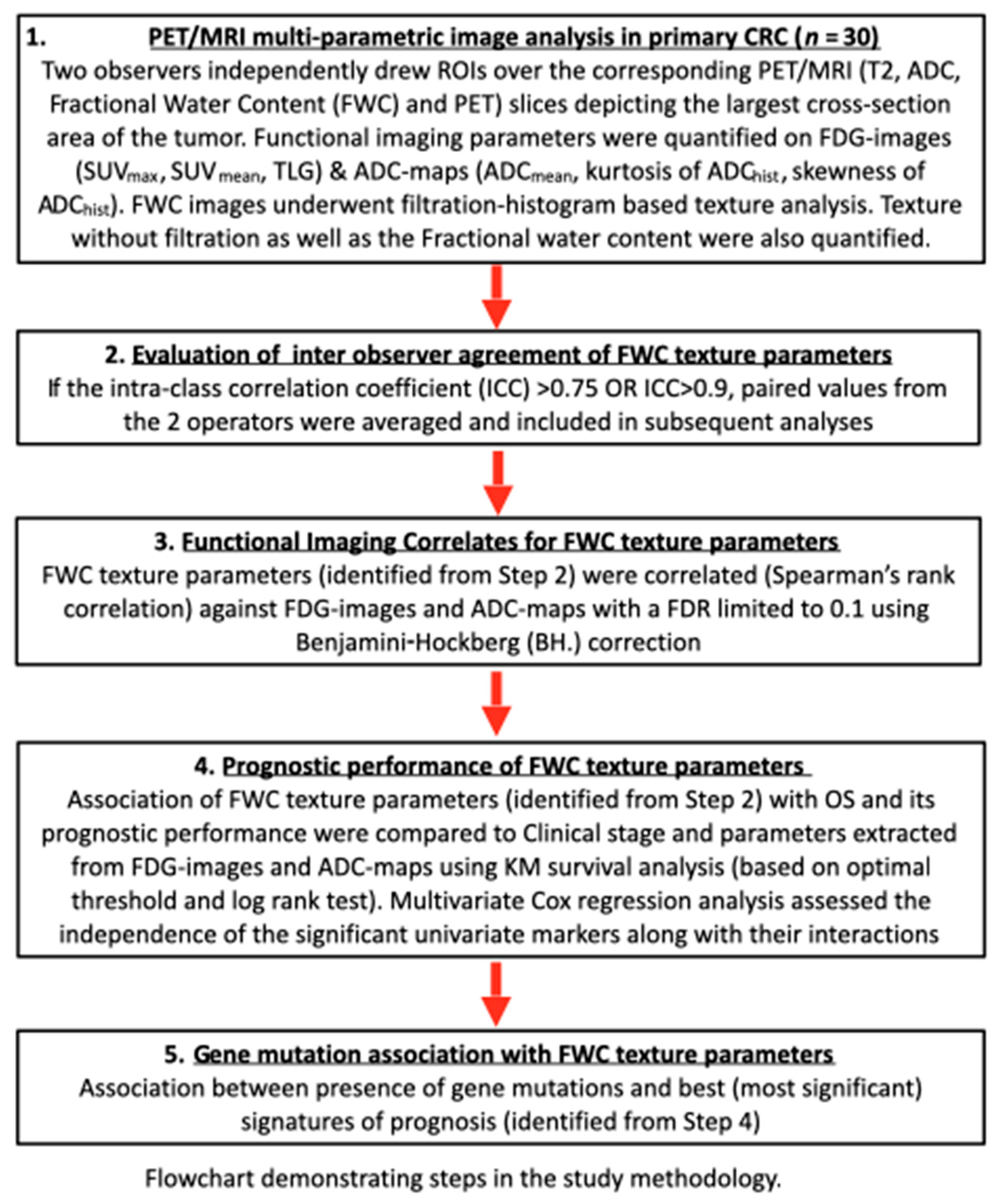
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. PET/MRI Image Acquisition
2.3.1. PET
2.3.2. MRI
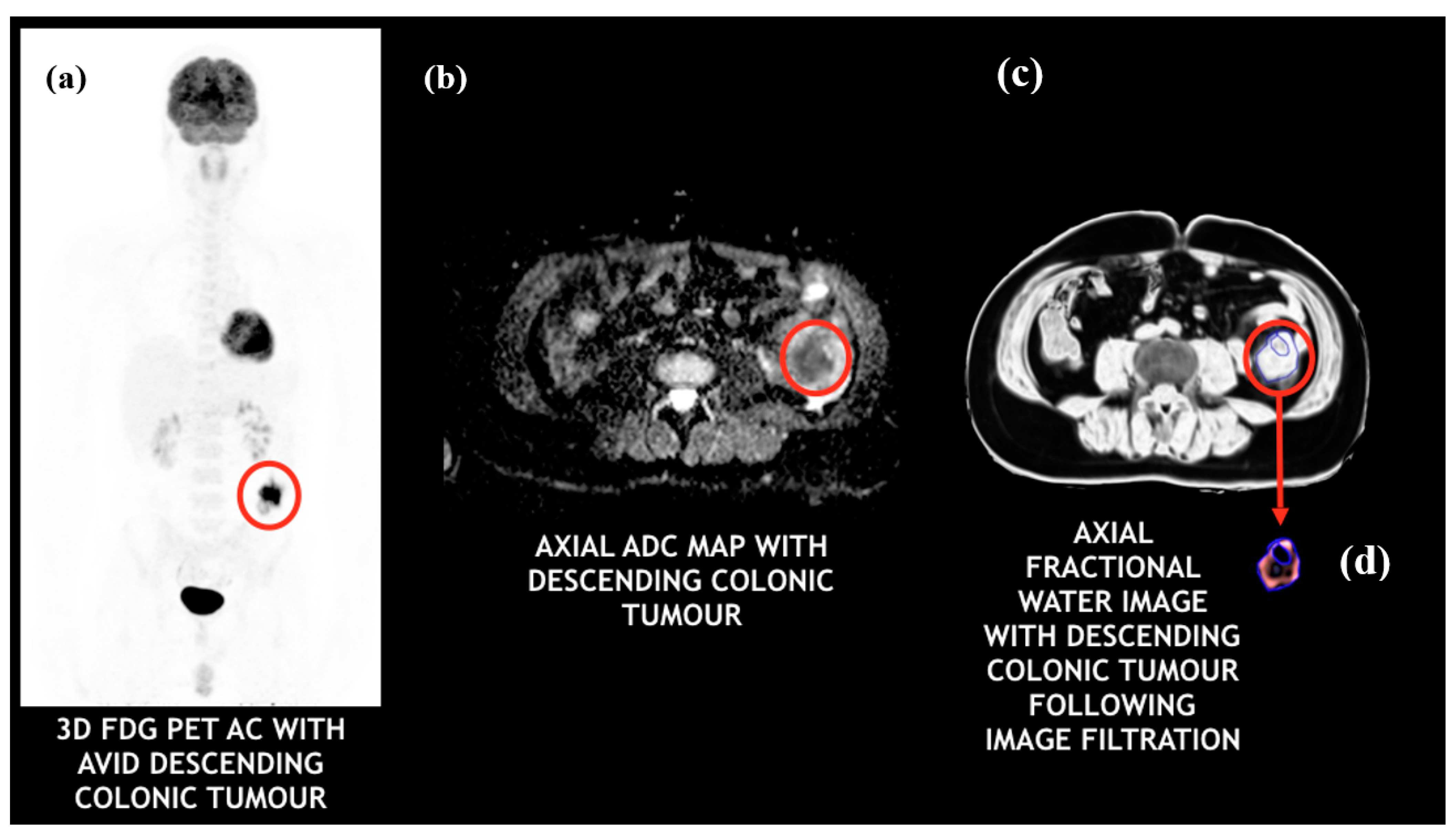
2.4. Multi-Parametric Image Analysis
2.5. Molecular Biology
2.6. Statistical Analysis
2.6.1. Inter-Operator Agreement of Texture Parameters
2.6.2. Functional Imaging Correlates for FWC Texture Parameters
2.6.3. Prognostic Performance of FWC Texture Parameters
2.6.4. Gene Mutation Association with FWC Texture Parameters
3. Results
3.1. Inter-Operator Agreement of FWC Texture Parameters
3.2. Functional Imaging Correlates for FWC Texture Parameters
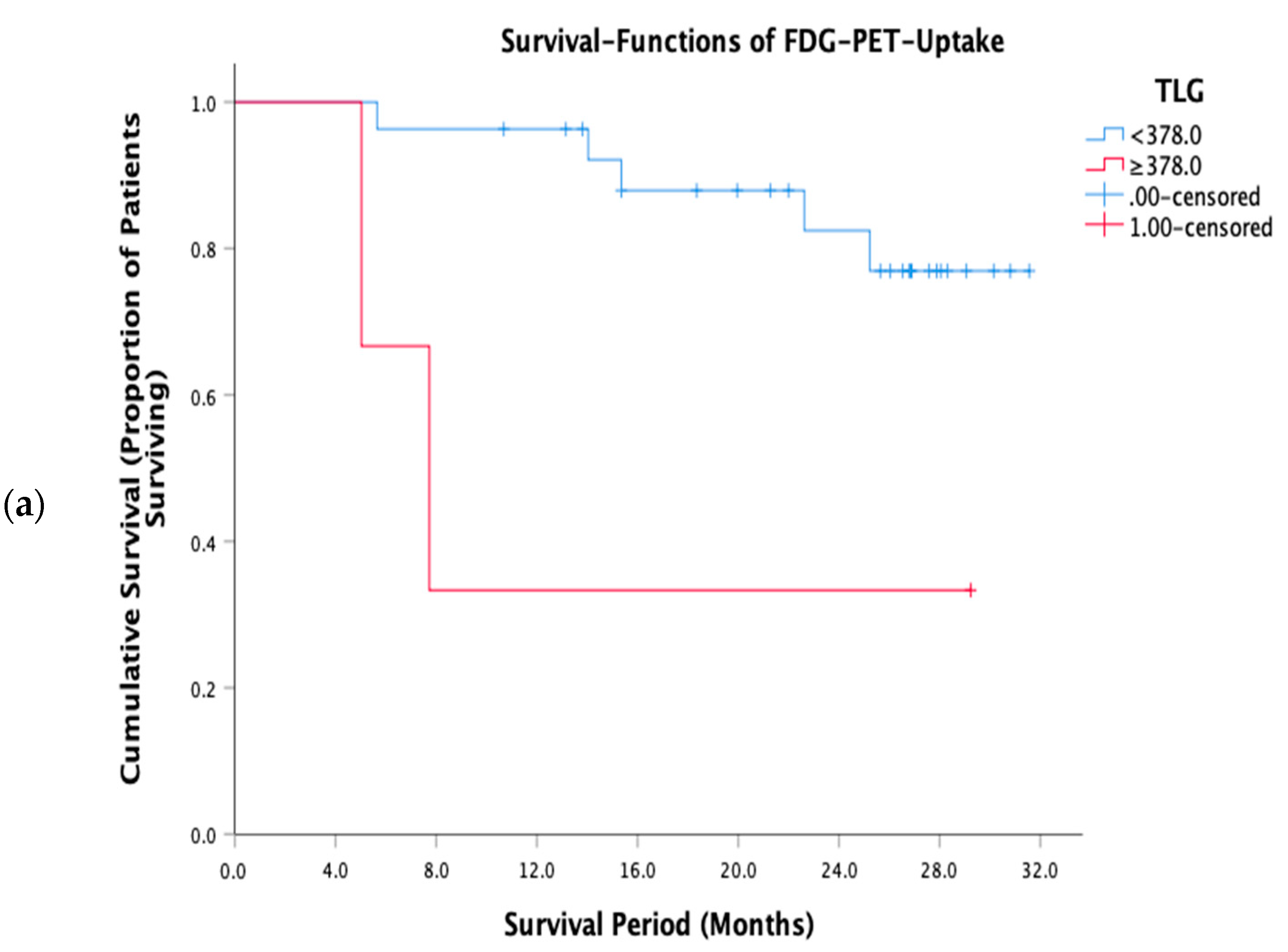
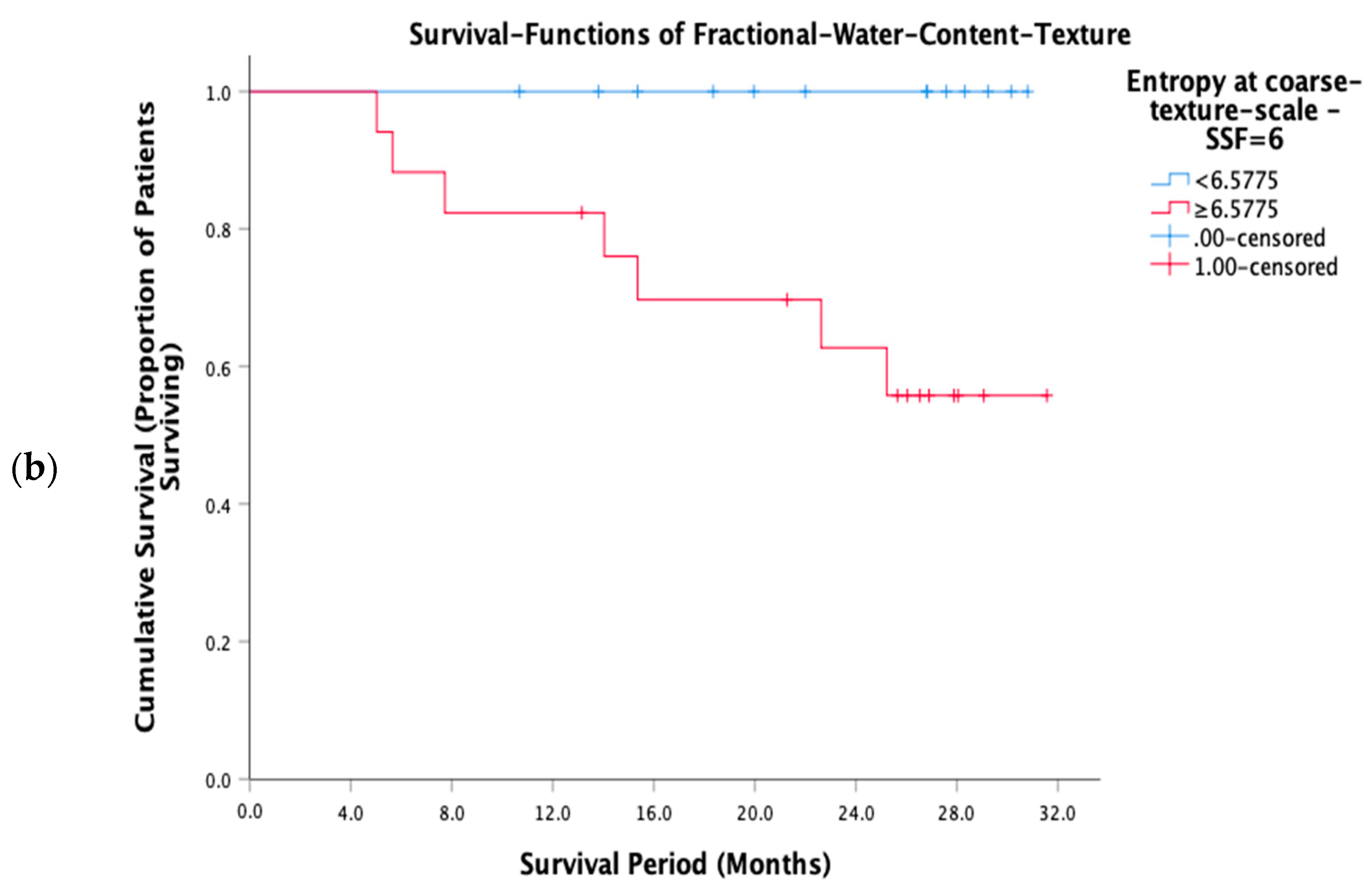
3.3. Prognostic Performance of FWC Texture Parameters
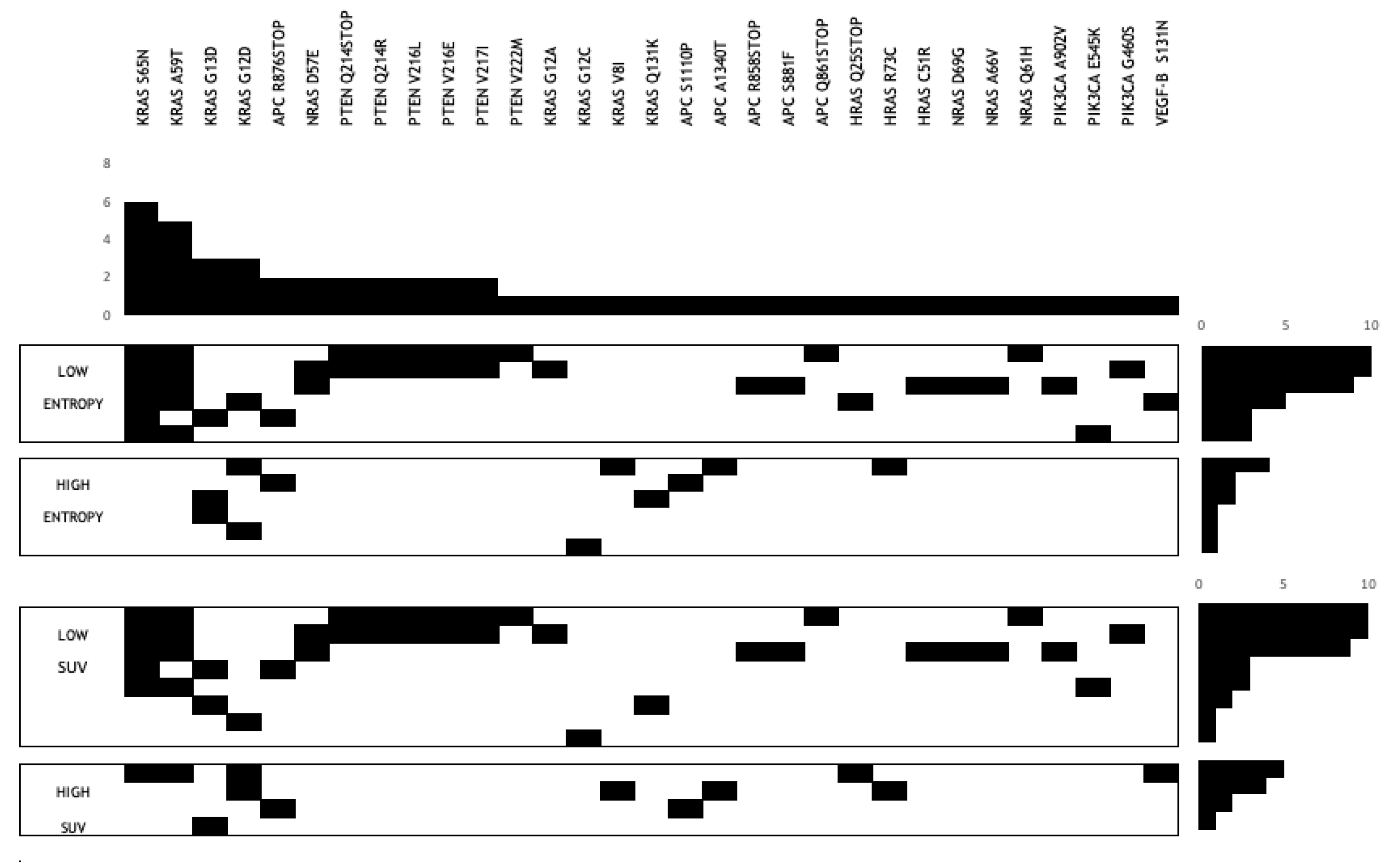
3.4. Gene Mutation Association with FWC Texture Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miles, K.A.; Voo, S.A.; Groves, A.M. Additional Clinical Value for PET/MRI in Oncology: Moving Beyond Simple Diagnosis. J. Nucl. Med. 2018, 59, 1028–1032. [Google Scholar] [CrossRef]
- Yin, Q.; Hung, S.-C.; Wang, L.; Lin, W.; Fielding, J.R.; Rathmell, W.K.; Khandani, A.H.; Woods, M.E.; Milowsky, M.I.; Brooks, S.A.; et al. Associations between Tumor Vascularity, Vascular Endothelial Growth Factor Expression and PET/MRI Radiomic Signatures in Primary Clear-Cell–Renal-Cell-Carcinoma: Proof-of-Concept Study. Sci. Rep. 2017, 7, 43356. [Google Scholar] [CrossRef]
- Fukuda, T.; Huang, M.; Janardhanan, A.; Schweitzer, M.E.; Huang, C. Correlation of bone marrow cellularity and metabolic activity in healthy volunteers with simultaneous PET/MR imaging. Skelet. Radiol. 2018, 48, 527–534. [Google Scholar] [CrossRef]
- Schraml, C.; Schmid, M.; Gatidis, S.; Schmidt, H.; La Fougère, C.; Nikolaou, K.; Schwenzer, N.F. Multiparametric analysis of bone marrow in cancer patients using simultaneous PET/MR imaging: Correlation of fat fraction, diffusivity, metabolic activity, and anthropometric data. J. Magn. Reson. Imaging 2015, 42, 1048–1056. [Google Scholar] [CrossRef]
- Tate, C.J.; Mollee, P.N.; A Miles, K. Combination bone marrow imaging using positron emission tomography (PET)-MRI in plasma cell dyscrasias: Correlation with prognostic laboratory values and clinicopathological diagnosis. BJR|Open 2019, 1, 20180020. [Google Scholar] [CrossRef] [PubMed]
- Kiricuta, I.C.; Simplăceanu, V. Tissue water content and nuclear magnetic resonance in normal and tumor tissues. Cancer Res. 1975, 35, 1164–1167. [Google Scholar] [PubMed]
- Ross, K.F.A.; Gordon, R.E. Water in malignant tissue, measured by cell refractometry and nuclear magnetic resonance. J. Microsc. 1982, 128, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Miles, K.A.; Ganeshan, B.; Hayball, M.P. CT texture analysis using the filtration-histogram method: What do the measurements mean? Cancer Imaging 2013, 13, 400–406. [Google Scholar] [CrossRef] [Green Version]
- Ganeshan, B.; Miles, K.A. Quantifying tumour heterogeneity with CT. Cancer Imaging 2013, 13, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Davnall, F.; Yip, C.S.P.; Ljungqvist, G.; Selmi, M.; Ng, F.; Sanghera, B.; Ganeshan, B.; Miles, K.A.; Cook, G.; Goh, V. Assessment of tumor heterogeneity: An emerging imaging tool for clinical practice? Insights Imaging 2012, 3, 573–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubner, M.G.; Smith, A.D.; Sandrasegaran, K.; Sahani, D.V.; Pickhardt, P.J. CT Texture Analysis: Definitions, Applications, Biologic Correlates, and Challenges. Radiographics 2017, 37, 1483–1503. [Google Scholar] [CrossRef] [PubMed]
- Parikh, J.; Selmi, M.; Charles-Edwards, G.; Glendenning, J.; Ganeshan, B.; Verma, H.; Mansi, J.; Harries, M.; Tutt, A.; Goh, V. Changes in Primary Breast Cancer Heterogeneity May Augment Midtreatment MR Imaging Assessment of Response to Neoadjuvant Chemotherapy. Radiology 2014, 272, 100–112. [Google Scholar] [CrossRef]
- De Cecco, C.N.; Ganeshan, B.; Ciolina, M.; Rengo, M.; Meinel, F.G.; Musio, D.; De Felice, F.; Raffetto, N.; Tombolini, V.; Laghi, A. Texture Analysis as Imaging Biomarker of Tumoral Response to Neoadjuvant Chemoradiotherapy in Rectal Cancer Patients Studied with 3-T Magnetic Resonance. Investig. Radiol. 2015, 50, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Graham, C.M.; Elci, O.; Griswold, M.E.; Zhang, X.; Khan, M.A.; Pitman, K.; Caudell, J.J.; Hamilton, R.D.; Ganeshan, B.; et al. Locally Advanced Squamous Cell Carcinoma of the Head and Neck: CT Texture and Histogram Analysis Allow Independent Prediction of Overall Survival in Patients Treated with Induction Chemotherapy. Radiology 2013, 269, 801–809. [Google Scholar] [CrossRef]
- Ng, F.; Ganeshan, B.; Kozarski, R.; Miles, K.A.; Goh, V. Assessment of Primary Colorectal Cancer Heterogeneity by Using Whole-Tumor Texture Analysis: Contrast-enhanced CT Texture as a Biomarker of 5-year Survival. Radiology 2013, 266, 177–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Win, T.; Miles, K.A.; Janes, S.M.; Ganeshan, B.; Shastry, M.; Endozo, R.; Meagher, M.; Shortman, R.I.; Wan, S.; Kayani, I.; et al. Tumor Heterogeneity and Permeability as Measured on the CT Component of PET/CT Predict Survival in Patients with Non–Small Cell Lung Cancer. Clin. Cancer Res. 2013, 19, 3591–3599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yip, C.; Landau, D.; Kozarski, R.; Ganeshan, B.; Thomas, R.; Michaelidou, A.; Goh, V. Primary Esophageal Cancer: Heterogeneity as Potential Prognostic Biomarker in Patients Treated with Definitive Chemotherapy and Radiation Therapy. Radiology 2014, 270, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Alessandrino, F.; Gujrathi, R.; Nassar, A.H.; Alzaghal, A.; Ravi, A.; McGregor, B.; Sonpavde, G.; Shinagare, A.B. Predictive Role of Computed Tomography Texture Analysis in Patients with Metastatic Urothelial Cancer Treated with Programmed Death-1 and Programmed Death-ligand 1 Inhibitors. Eur. Urol. Oncol. 2020, 3, 680–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durot, C.; Mulé, S.; Soyer, P.; Marchal, A.; Grange, F.; Hoeffel, C. Metastatic melanoma: Pretreatment contrast-enhanced CT texture parameters as predictive biomarkers of survival in patients treated with pembrolizumab. Eur. Radiol. 2019, 29, 3183–3191. [Google Scholar] [CrossRef] [PubMed]
- Ravanelli, M.; Agazzi, G.M.; Milanese, G.; Roca, E.; Silva, M.; Tiseo, M.; Rondi, P.; Baggi, A.; Ganeshan, B.; Muri, M.; et al. Prognostic and predictive value of histogram analysis in patients with non-small cell lung cancer refractory to platinum treated by nivolumab: A multicentre retrospective study. Eur. J. Radiol. 2019, 118, 251–256. [Google Scholar] [CrossRef]
- Ravanelli, M.; Agazzi, G.M.; Ganeshan, B.; Roca, E.; Tononcelli, E.; Bettoni, V.; Caprioli, A.; Borghesi, A.; Berruti, A.; Maroldi, R.; et al. CT texture analysis as predictive factor in metastatic lung adenocarcinoma treated with tyrosine kinase inhibitors (TKIs). Eur. J. Radiol. 2018, 109, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Tamboli, P.; Vikram, R.; Rao, A. Imaging-genomic pipeline for identifying gene mutations using three-dimensional intra-tumor heterogeneity features. J. Med. Imaging 2015, 2, 41009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocak, B.; Durmaz, E.S.; Ates, E.; Ulusan, M.B. Radiogenomics in Clear Cell Renal Cell Carcinoma: Machine Learning–Based High-Dimensional Quantitative CT Texture Analysis in PredictingPBRM1Mutation Status. Am. J. Roentgenol. 2019, 212, W55–W63. [Google Scholar] [CrossRef]
- Miles, K.A.; Ganeshan, B.; Rodriguez-Justo, M.; Goh, V.J.; Ziauddin, Z.; Engledow, A.; Meagher, M.; Endozo, R.; Taylor, S.A.; Halligan, S.; et al. Multifunctional Imaging Signature for V-KI-RAS2 Kirsten Rat Sarcoma Viral Oncogene Homolog (KRAS) Mutations in Colorectal Cancer. J. Nucl. Med. 2014, 55, 386–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, G.J.; Ganeshan, B.; Miles, K.A.; Campbell, D.H.; Cheung, P.Y.; Frank, S.; Korn, R.L. Noninvasive Image Texture Analysis Differentiates K-ras Mutation from Pan-Wildtype NSCLC and Is Prognostic. PLoS ONE 2014, 9, e100244. [Google Scholar] [CrossRef]
- Digumarthy, S.R.; Padole, A.M.; Gullo, R.L.; Sequist, L.V.; Kalra, M.K. Can CT radiomic analysis in NSCLC predict histology and EGFR mutation status? Medicine 2019, 98, e13963. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.A.; Ganeshan, B.; Barnes, A.; Bisdas, S.; Jaunmuktane, Z.; Brandner, S.; Endozo, R.; Groves, A.; Thust, S.C. Filtration-histogram based magnetic resonance texture analysis (MRTA) for glioma IDH and 1p19q genotyping. Eur. J. Radiol. 2019, 113, 116–123. [Google Scholar] [CrossRef]
- Xu, F.; Ma, X.; Wang, Y.; Tian, Y.; Tang, W.; Wang, M.; Wei, R.; Zhao, X. CT texture analysis can be a potential tool to differentiate gastrointestinal stromal tumors without KIT exon 11 mutation. Eur. J. Radiol. 2018, 107, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018 GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, B.; Lee, J.M.; Song, Y.S.; Woo, S.; Hur, B.Y.; Jeon, J.H.; Paeng, J.C. Added Value of Integrated Whole-Body PET/MRI for Evaluation of Colorectal Cancer: Comparison with Contrast-Enhanced MDCT. Am. J. Roentgenol. 2016, 206. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, J.M. Whole-body PET/MRI for colorectal cancer staging: Is it the way forward? J. Magn. Reson. Imaging 2017, 45, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Vallières, M.; Freeman, C.R.; Skamene, S.; El Naqa, I. A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys. Med. Biol. 2015, 60, 5471–5496. [Google Scholar] [CrossRef]
- Antunes, J.; Viswanath, S.; Rusu, M.; Valls, L.; Hoimes, C.; Avril, N.; Madabhushi, A. Radiomics Analysis on FLT-PET/MRI for Characterization of Early Treatment Response in Renal Cell Carcinoma: A Proof-of-Concept Study. Transl. Oncol. 2016, 9, 155–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganeshan, B.; Goh, V.; Mandeville, H.C.; Ng, Q.S.; Hoskin, P.J.; Miles, K.A. Non–Small Cell Lung Cancer: Histopathologic Correlates for Texture Parameters at CT. Radiology 2013, 266, 326–336. [Google Scholar] [CrossRef] [PubMed]
- QIAamp DNA FFPE Tissue Handbook. Available online: https://www.qiagen.com/gb/resources/resourcedetail?id=7d3df4c2-b522-4f6d-b990-0ac3a71799b6&lang=en (accessed on 23 April 2021).
- Agilent High Sensitivity d1000 Screentape. Available online: https://www.agilent.com/cs/library/usermanuals/Public/ScreenTape_HSD1000_QG.pdf (accessed on 23 April 2021).
- Ion PGM HI-Q View OT2 Kit. Available online: https://www.thermofisher.com/order/catalog/product/A29900?SID=srch-srp-A29900#/A29900?SID=srch-srp-A29900 (accessed on 23 April 2021).
- Ion PGM HI-Q View Sequencing Kit. Available online: https://www.thermofisher.com/order/catalog/product/A30044?SID=srch-srp-A30044#/A30044?SID=srch-srp-A30044 (accessed on 23 April 2021).
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumura, N.; Oguro, S.; Okuda, S.; Jinzaki, M.; Matsumoto, M.; Nakamura, M.; Nagura, T. Quantitative assessment of fatty infiltration and muscle volume of the rotator cuff muscles using 3-dimensional 2-point Dixon magnetic resonance imaging. J. Shoulder Elb. Surg. 2017, 26, e309–e318. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, S.; Nozaki, T.; Tasaki, A.; Yamakawa, A.; Kaneko, Y.; Hara, T.; Yoshioka, H. Reliability of MR Quantification of Rotator Cuff Muscle Fatty Degeneration Using a 2-point Dixon Technique in Comparison with the Goutallier Classification. Acad. Radiol. 2017, 24, 1343–1351. [Google Scholar] [CrossRef]
- Ogawa, S.; Itabashi, M.; Kondo, C.; Momose, M.; Sakai, S.; Kameoka, S. Prognostic Value of Total Lesion Glycolysis Measured by 18F-FDG-PET/CT in Patients with Colorectal Cancer. Anticancer. Res. 2015, 35, 3495–3500. [Google Scholar]
- Riviere, P.; Goodman, A.M.; Okamura, R.; Barkauskas, D.A.; Whitchurch, T.J.; Lee, S.; Khalid, N.; Collier, R.; Mareboina, M.; Frampton, G.M.; et al. High Tumor Mutational Burden Correlates with Longer Survival in Immunotherapy-Naïve Patients with Diverse Cancers. Mol. Cancer Ther. 2020, 19, 2139–2145. [Google Scholar] [CrossRef]
- Holland, A.J.; Cleveland, D.W. Boveri revisited: Chromosomal instability, aneuploidy and tumorigenesis. Nat. Rev. Mol. Cell Biol. 2009, 10, 478–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, E.; D’Souza, D.; Nelson, A.C. Therapeutic Response of Metastatic Colorectal Cancer Harboring aKRASMissense Mutation After Combination Chemotherapy with the EGFR Inhibitor Panitumumab. J. Natl. Compr. Cancer Netw. 2017, 15, 427–432. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6295659/ (accessed on 20 October 2020). [CrossRef] [PubMed] [Green Version]
- Ganeshan, B.; Abaleke, S.; Young, R.C.; Chatwin, C.R.; Miles, K.A. Texture analysis of non-small cell lung cancer on unenhanced computed tomography: Initial evidence for a relationship with tumour glucose metabolism and stage. Cancer Imaging 2010, 10, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, B.; Skogen, K.; Pressney, I.; Coutroubis, D.; Miles, K. Tumour heterogeneity in oesophageal cancer assessed by CT texture analysis: Preliminary evidence of an association with tumour metabolism, stage, and survival. Clin. Radiol. 2012, 67, 157–164. [Google Scholar] [CrossRef]
- Dercle, L.; Ammari, S.; Bateson, M.; Durand, P.B.; Haspinger, E.; Massard, C.; Jaudet, C.; Varga, A.; Deutsch, E.; Soria, J.-C.; et al. Limits of radiomic-based entropy as a surrogate of tumor heterogeneity: ROI-area, acquisition protocol and tissue site exert substantial influence. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Just, N. Improving tumour heterogeneity MRI assessment with histograms. Br. J. Cancer 2014, 111, 2205–2213. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Liu, Y.; Xu, L.; Li, Z.; Lv, H.; Dong, N.; Li, W.; Yang, Z.; Wang, Z.; Jin, E. Application of texture analysis based on apparent diffusion coefficient maps in discriminating different stages of rectal cancer. J. Magn. Reson. Imaging 2016, 45, 1798–1808. [Google Scholar] [CrossRef]
- Taron, J.; Schraml, C.; Pfannenberg, C.; Reimold, M.; Schwenzer, N.; Nikolaou, K.; Martirosian, P.; Seith, F. Simultaneous multislice diffusion-weighted imaging in whole-body positron emission tomography/magnetic resonance imaging for multiparametric examination in oncological patients. Eur. Radiol. 2018, 28, 3372–3383. [Google Scholar] [CrossRef]
- Latifoltojar, A.; Hall-Craggs, M.; Bainbridge, A.; Rabin, N.; Popat, R.; Rismani, A.; D’Sa, S.; Dikaios, N.; Sokolska, M.; Antonelli, M.; et al. Whole-body MRI quantitative biomarkers are associated significantly with treatment response in patients with newly diagnosed symptomatic multiple myeloma following bortezomib induction. Eur. Radiol. 2017, 27, 5325–5336. [Google Scholar] [CrossRef] [PubMed]
- Bray, T.J.; Chouhan, M.D.; Punwani, S.; Bainbridge, A.; A Hall-Craggs, M. Fat fraction mapping using magnetic resonance imaging: Insight into pathophysiology. Br. J. Radiol. 2017, 91, 20170344. [Google Scholar] [CrossRef] [Green Version]
- Latifoltojar, A.; Hall-Craggs, M.; Rabin, N.; Popat, R.; Bainbridge, A.; Dikaios, N.; Sokolska, M.; Rismani, A.; D’Sa, S.; Punwani, S.; et al. Whole body magnetic resonance imaging in newly diagnosed multiple myeloma: Early changes in lesional signal fat fraction predict disease response. Br. J. Haematol. 2017, 176, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Yasaka, K.; Akai, H.; Mackin, D.; Court, L.; Moros, E.; Ohtomo, K.; Kiryu, S. Precision of quantitative computed tomography texture analysis using image filtering. Medicine 2017, 96, e6993. [Google Scholar] [CrossRef]
- Miles, K.A.; Ganeshan, B.; Griffiths, M.R.; Young, R.C.D.; Chatwin, C.R. Colorectal Cancer: Texture Analysis of Portal Phase Hepatic CT Images as a Potential Marker of Survival. Radiology 2009, 250, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.; Kozarski, R.; Ganeshan, B.; Goh, V. Assessment of tumor heterogeneity by CT texture analysis: Can the largest cross-sectional area be used as an alternative to whole tumor analysis? Eur. J. Radiol. 2013, 82, 342–348. [Google Scholar] [CrossRef]
- Altman, D.G.; McShane, L.M.; Sauerbrei, W.; Taube, S.E. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): Explanation and Elaboration. PLoS Med. 2012, 9, e1001216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Secco, G.B.; Fardelli, R.; Gianquinto, D.; Bonfante, P.; Baldi, E.; Ravera, G.; Derchi, L.; Ferraris, R. Efficacy and cost of risk-adapted follow-up in patients after colorectal cancer surgery: A prospective, randomized and controlled trial. Eur. J. Surg. Oncol. (EJSO) 2002, 28, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Coukos, G. Neoadjuvant immune-checkpoint blockade in resectable colon cancer. Nat. Med. 2020, 26, 473–474. [Google Scholar] [CrossRef]

| Parameter | Threshold (Direction Indicates Poor Prognosis) | Patients above/below Threshold | p-Value |
|---|---|---|---|
| Texture | |||
| Entropy SSF = 2 | ≥6.19 | 19/11 | 0.033 |
| Entropy SSF = 3 | ≥6.44 | 18/12 | 0.024 |
| Entropy SSF = 4 | ≥6.52 | 18/12 | 0.024 |
| Entropy SSF = 5 | ≥6.53 | 18/12 | 0.024 |
| Entropy SSF = 6 | ≥6.58 | 17/13 | 0.017 |
| FDG uptake | |||
| SUVmean | ≥10.1 | 9/21 | 0.047 |
| TLG | ≥378 | 3/27 | 0.016 |
| ADC maps | |||
| Skewness | ≥0.61 | 3/27 | 0.023 |
| Parameter Included in the Model | HR | 95% CI | p-Value |
| Entropy SSF = 6 * TLG | 44.7 | 4.0–505.5 | 0.002 |
| Parameters Not Included in the Model | Score | p-Value | |
| Entropy SSF = 6 | 4.1 | 0.042 | |
| TLG | 0.3 | 0.613 | |
| Skewness of ADC maps | 0.03 | 0.867 | |
| TLG * Skewness of ADC maps | 1.7 | 0.190 | |
| Entropy SSF = 6 * Skewness of ADC maps | 0.03 | 0.867 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganeshan, B.; Miles, K.; Afaq, A.; Punwani, S.; Rodriguez, M.; Wan, S.; Walls, D.; Hoy, L.; Khan, S.; Endozo, R.; et al. Texture Analysis of Fractional Water Content Images Acquired during PET/MRI: Initial Evidence for an Association with Total Lesion Glycolysis, Survival and Gene Mutation Profile in Primary Colorectal Cancer. Cancers 2021, 13, 2715. https://doi.org/10.3390/cancers13112715
Ganeshan B, Miles K, Afaq A, Punwani S, Rodriguez M, Wan S, Walls D, Hoy L, Khan S, Endozo R, et al. Texture Analysis of Fractional Water Content Images Acquired during PET/MRI: Initial Evidence for an Association with Total Lesion Glycolysis, Survival and Gene Mutation Profile in Primary Colorectal Cancer. Cancers. 2021; 13(11):2715. https://doi.org/10.3390/cancers13112715
Chicago/Turabian StyleGaneshan, Balaji, Kenneth Miles, Asim Afaq, Shonit Punwani, Manuel Rodriguez, Simon Wan, Darren Walls, Luke Hoy, Saif Khan, Raymond Endozo, and et al. 2021. "Texture Analysis of Fractional Water Content Images Acquired during PET/MRI: Initial Evidence for an Association with Total Lesion Glycolysis, Survival and Gene Mutation Profile in Primary Colorectal Cancer" Cancers 13, no. 11: 2715. https://doi.org/10.3390/cancers13112715






