Effects of Deacetylasperulosidic Acid on Atopic Dermatitis through Modulating Immune Balance and Skin Barrier Function in HaCaT, HMC-1, and EOL-1 Cells
Abstract
:1. Introduction
2. Results
2.1. HPLC-PDA Chromatogram of Noni and F. noni
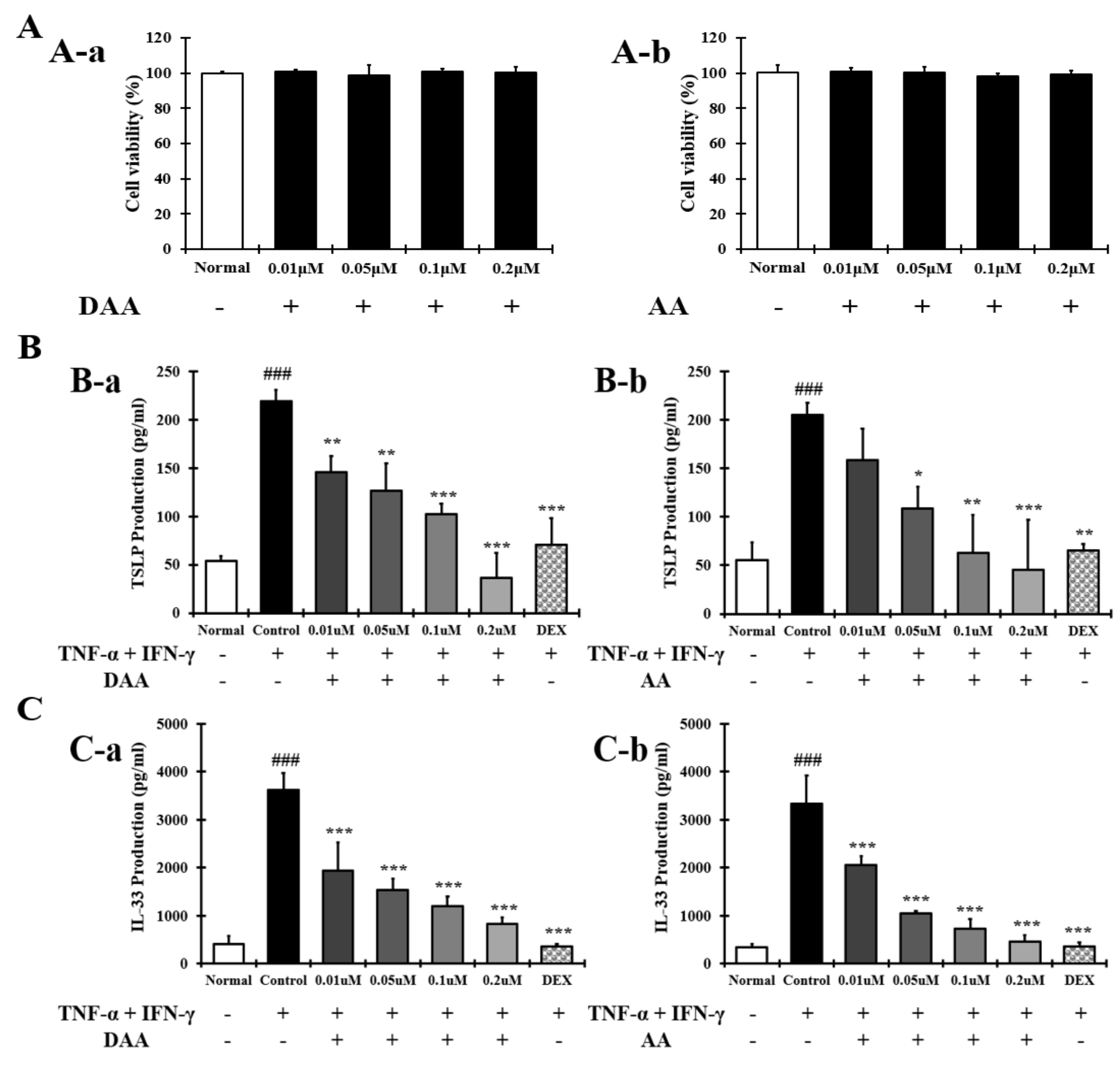
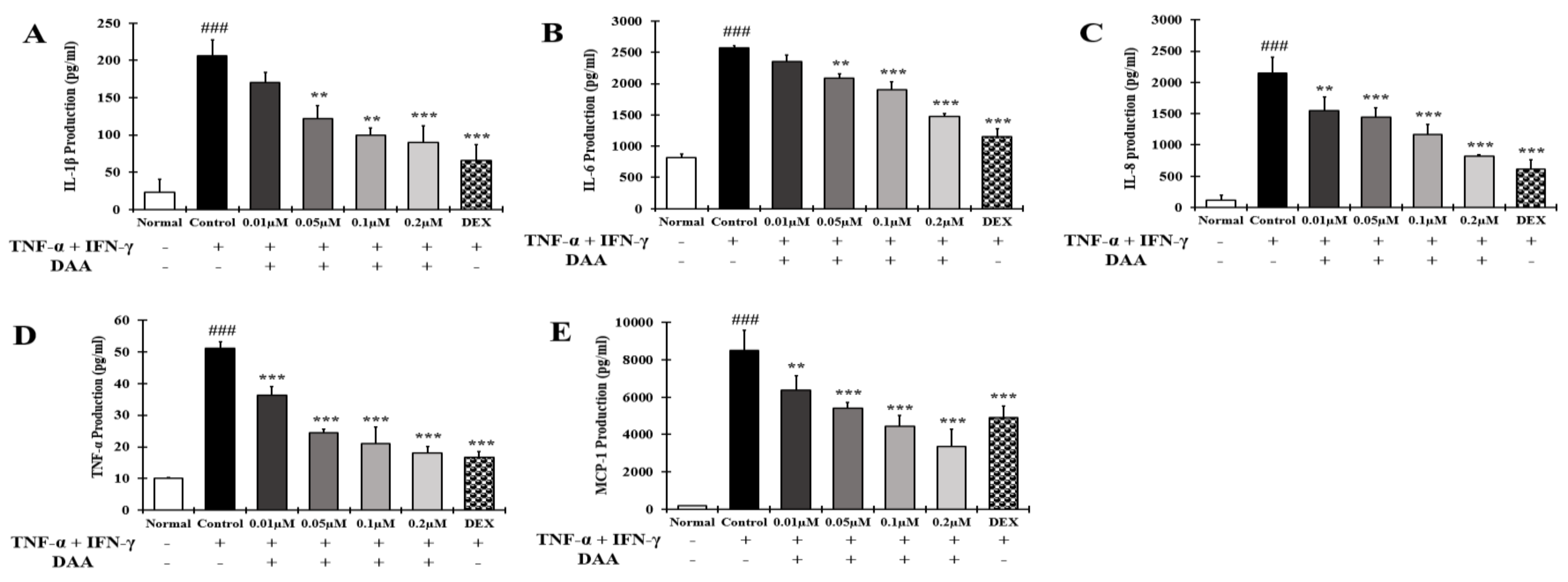
2.2. DAA and AA Inhibited the Secretion of AD-Related Inflammatory Cytokines and Chemokines Increased by TNF-α and IFN-γ in HaCaT Cells
2.3. DAA and AA Inhibited the Secretion of AD-Related Inflammatory Cytokines and Chemokines Increased by PMACI in HMC-1 Cells
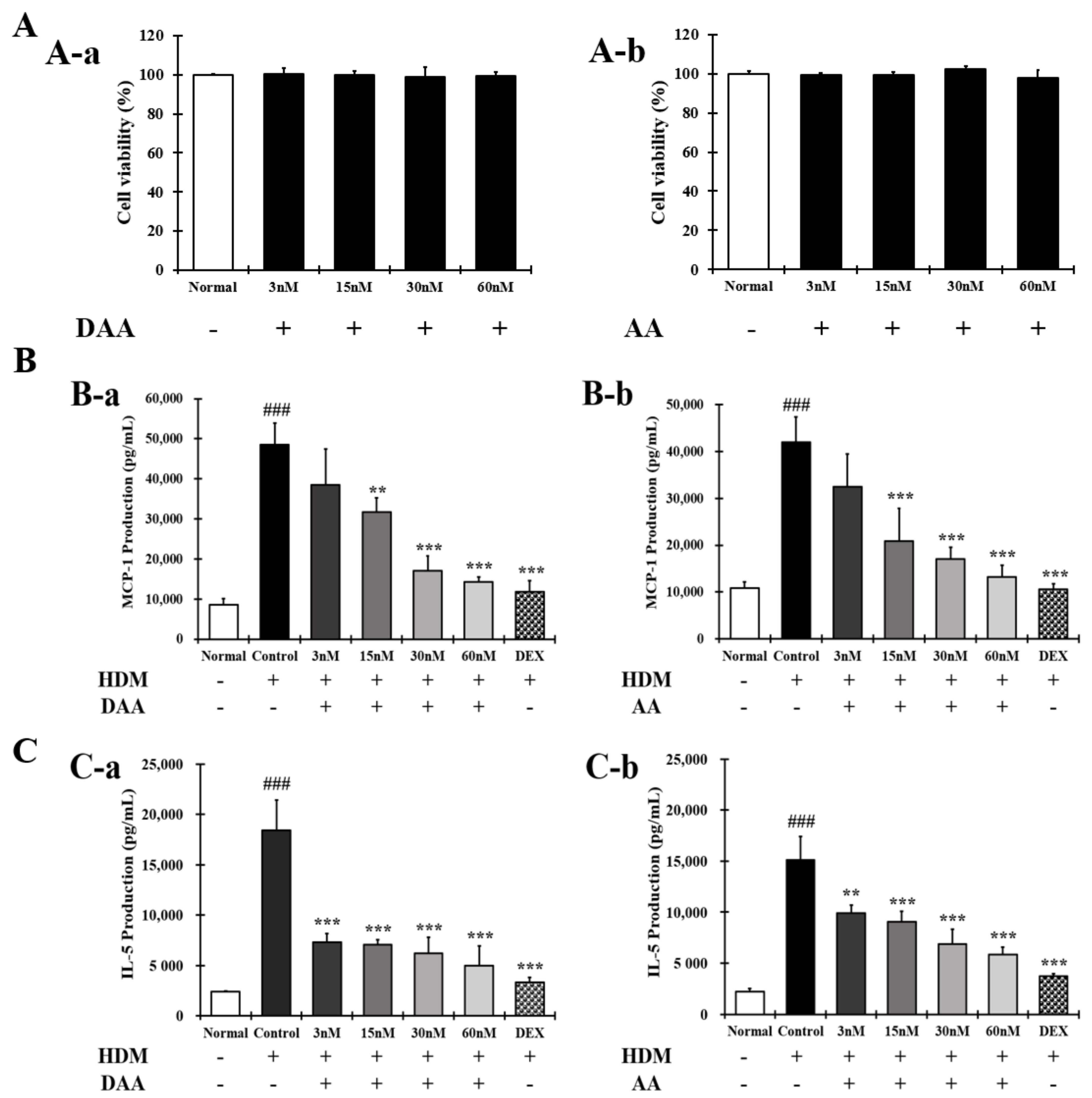
2.4. DAA and AA Inhibited the Secretion of AD-Related Inflammatory Cytokines and Chemokines Increased by House Dust Mite (HDM) in EOL-1 Cells
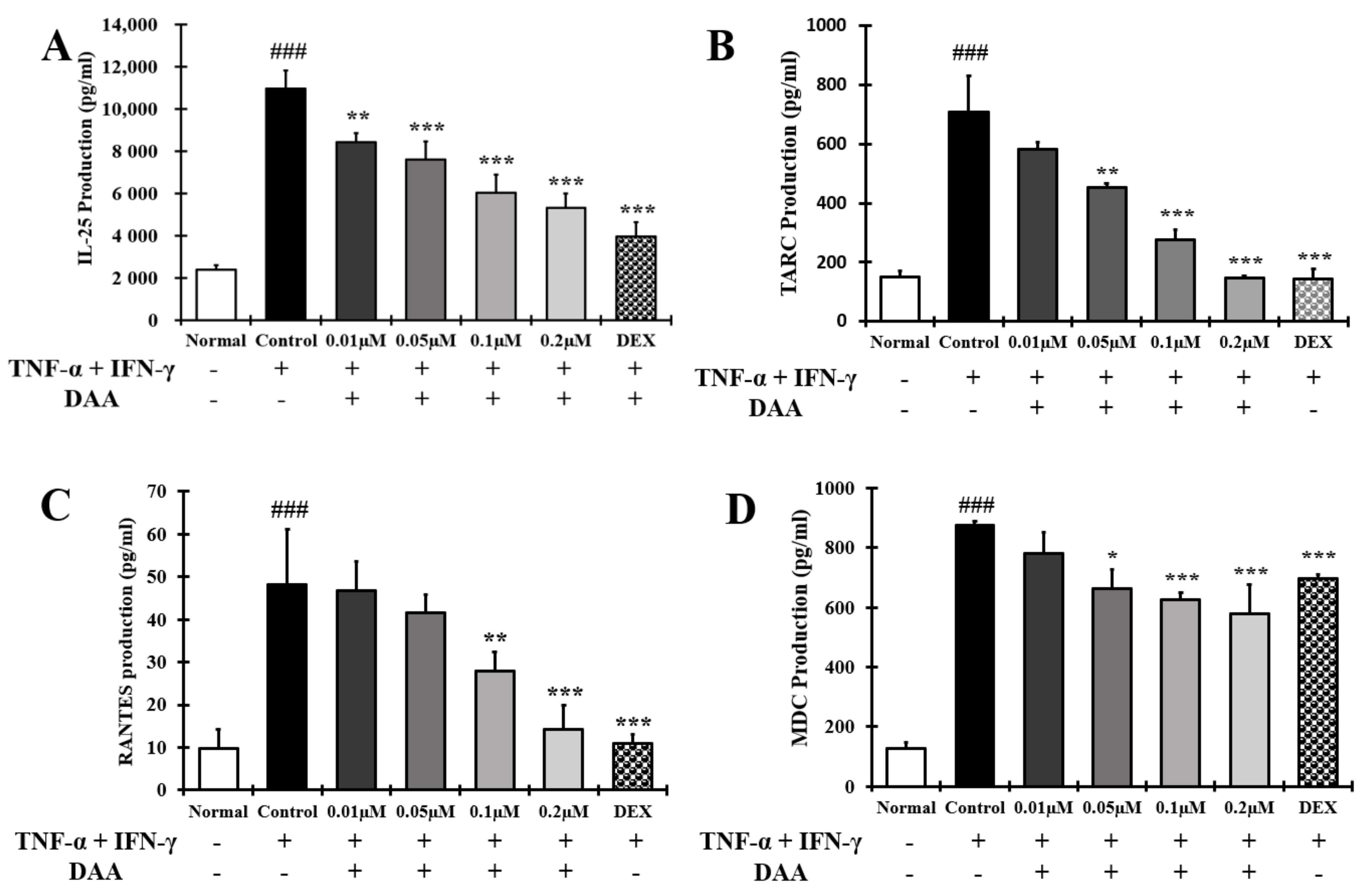
2.5. DAA Inhibited the Secretion of AD-Related Cytokines and Chemokines by TNF-α and IFN-γ Treatment in HaCaT Cells
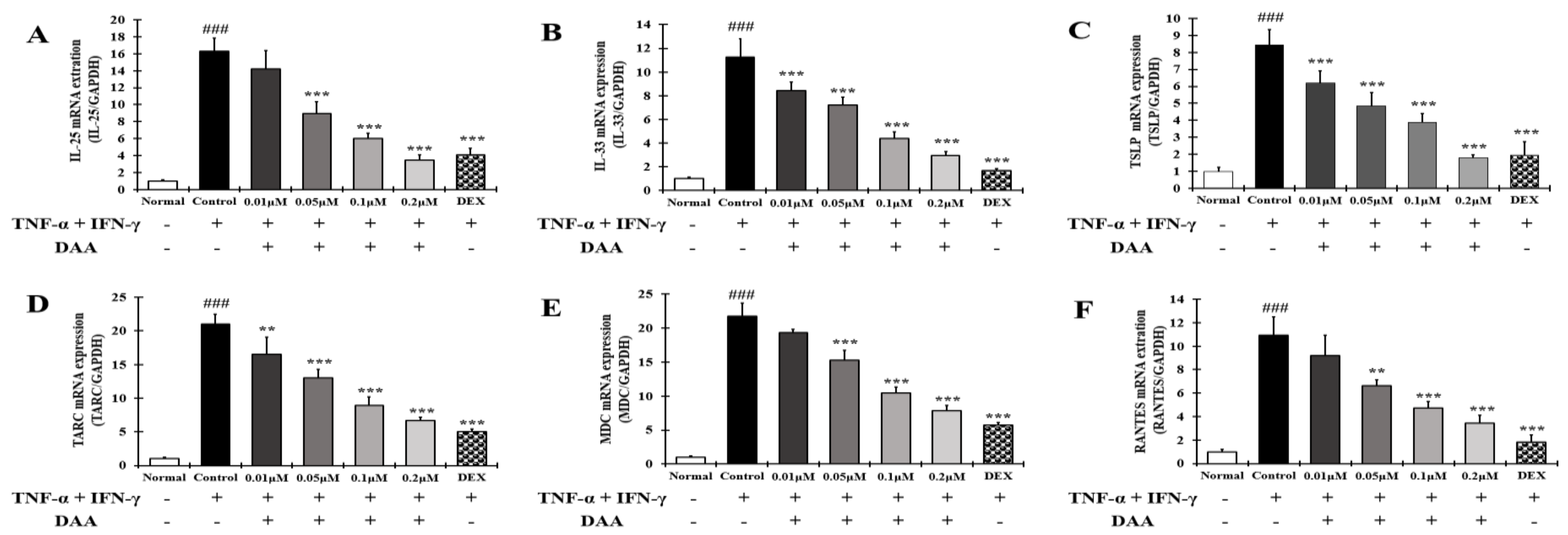
2.6. DAA Inhibited the Gene Expression Levels of AD-Related Cytokines and Chemokines Increased by TNF-α and IFN-γ in HaCaT Cells
2.7. DAA Inhibited AD-Related Cytokines and Chemokines Known to Activate Th2 Cells Secreted by HaCaT Cells Treated with TNF-α and IFN-γ
2.8. DAA Inhibited the Gene Expression Levels of AD-Related Cytokines and Chemokines Known to Increase Th2 Activity in HaCaT Cells Treated with TNF-α and IFN-γ
2.9. DAA Inhibited MAPK Phosphorylation and NF-κB Activation Increased by TNF-α and IFN-γ in HaCaT Cells
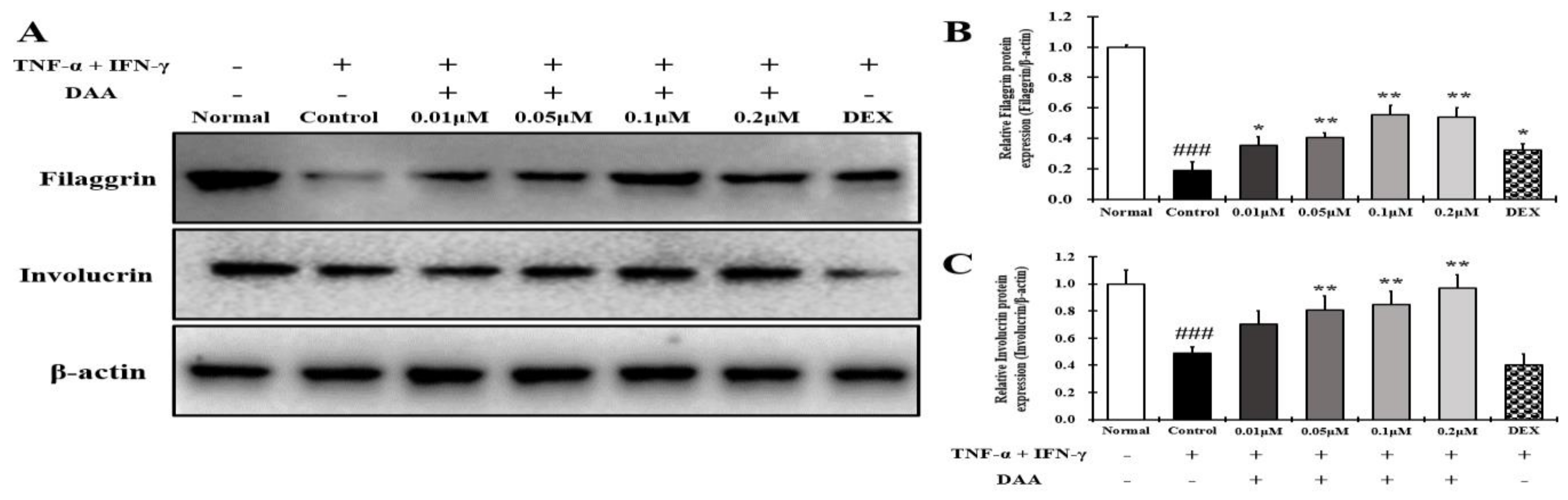
2.10. DAA Increased the Expression of Skin Barrier Proteins Reduced by TNF-α and IFN-γ in HaCaT Cells
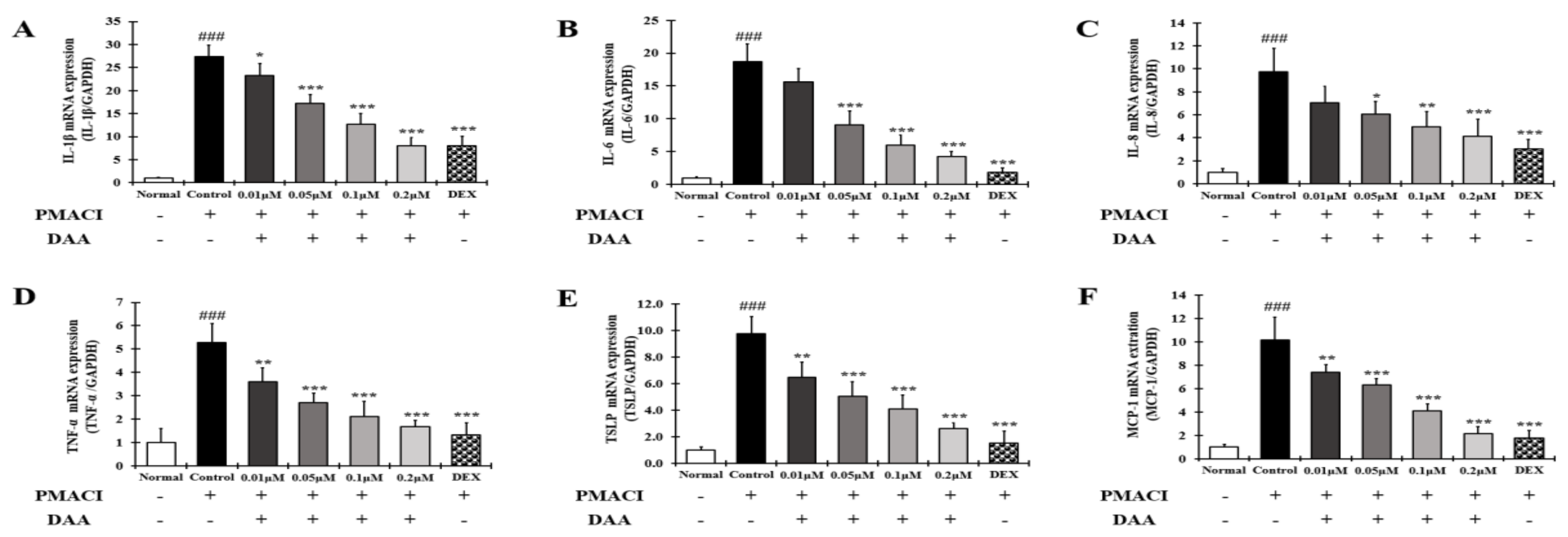
2.11. DAA Inhibited the Secreted Levels of AD-Related Cytokines and Chemokines Increased by PMACI in HMC-1 Cells
2.12. DAA Inhibited the Gene Expression Levels of AD-Related Cytokines and Chemokines Increased by PMACI in HMC-1 Cells
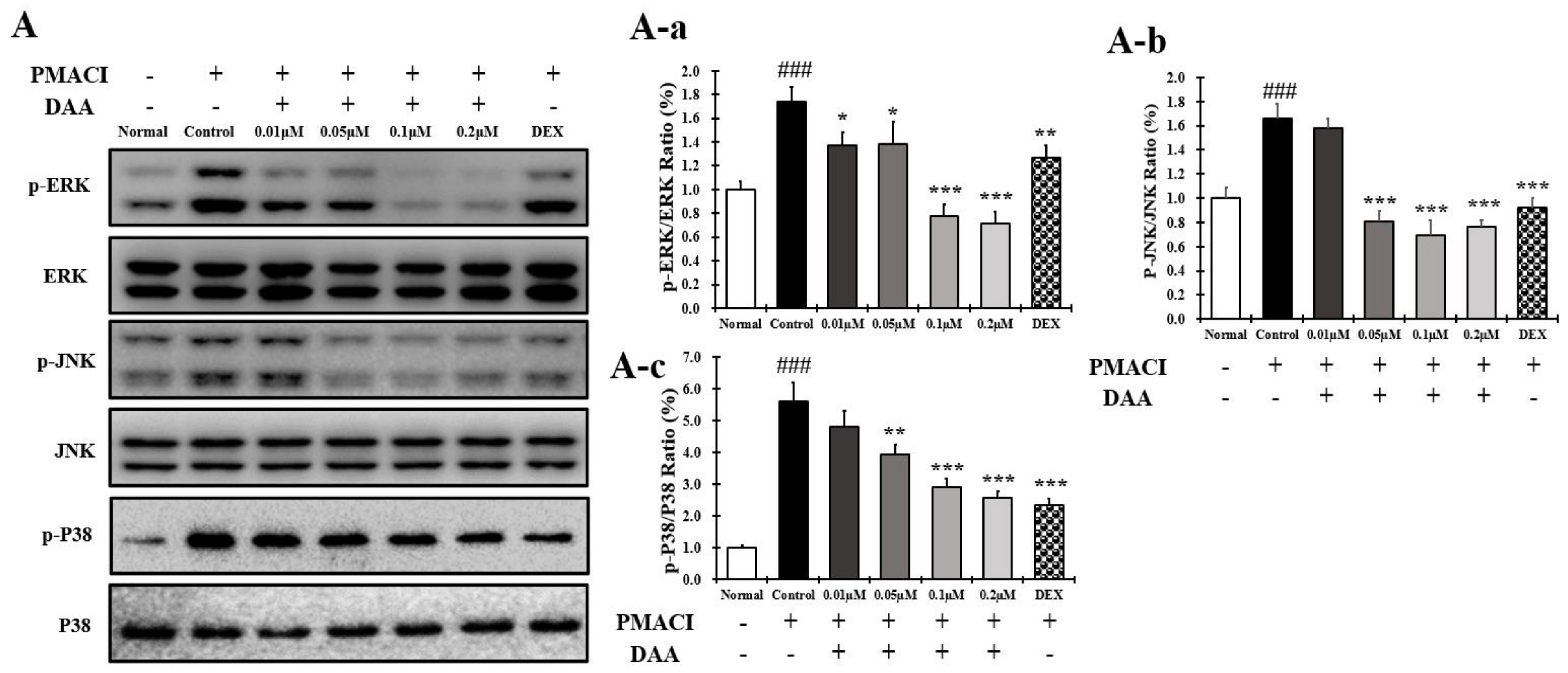
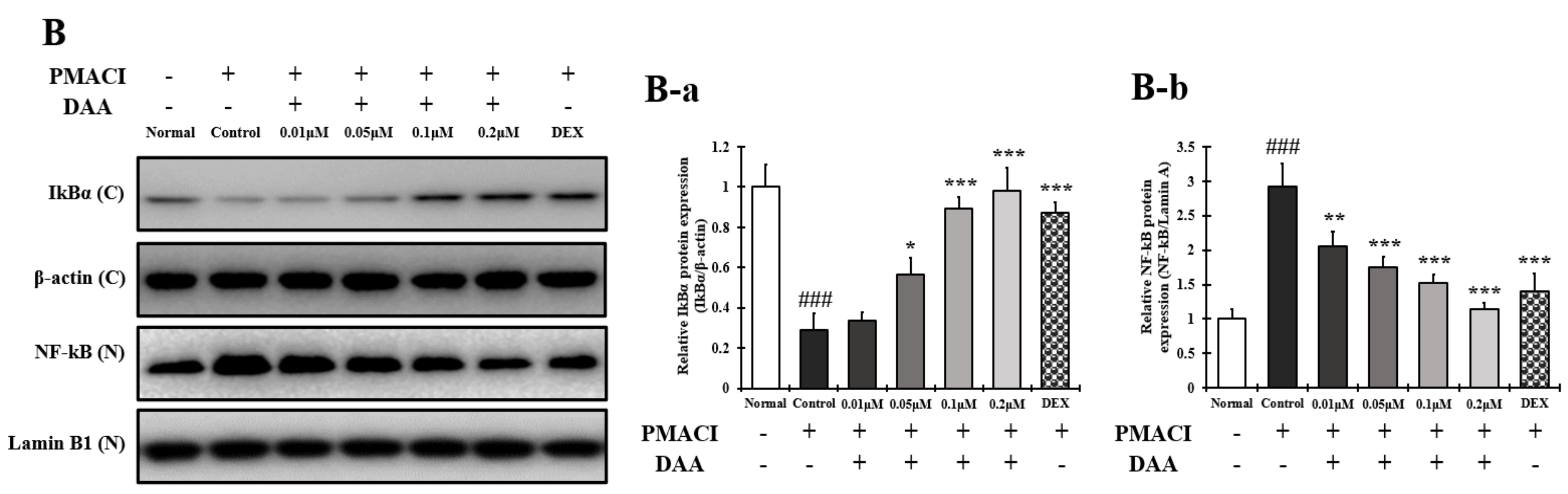
2.13. DAA Inhibited MAPK Phosphorylation and NF-κB Activation Increased by PMACI in HMC-1 Cells
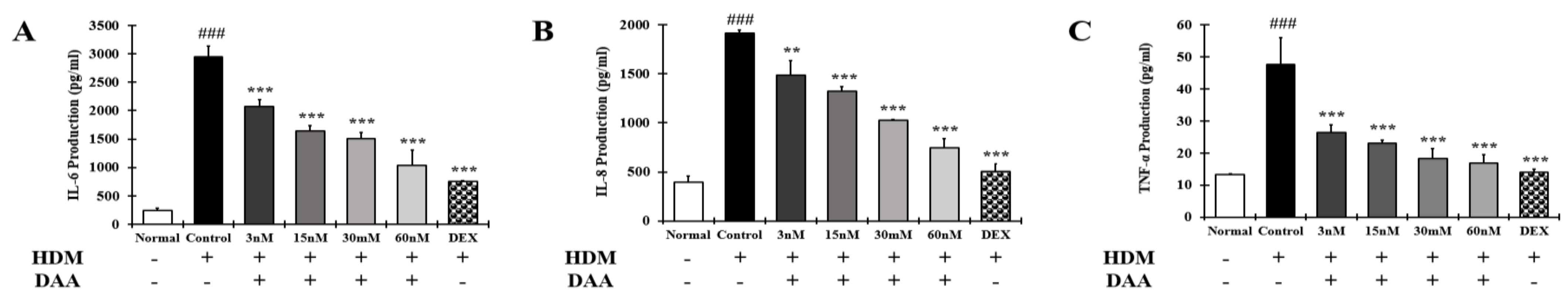
2.14. DAA Inhibited the Secretion of AD-Related Cytokines and Chemokines Increased by HDM in EOL-1 Cells
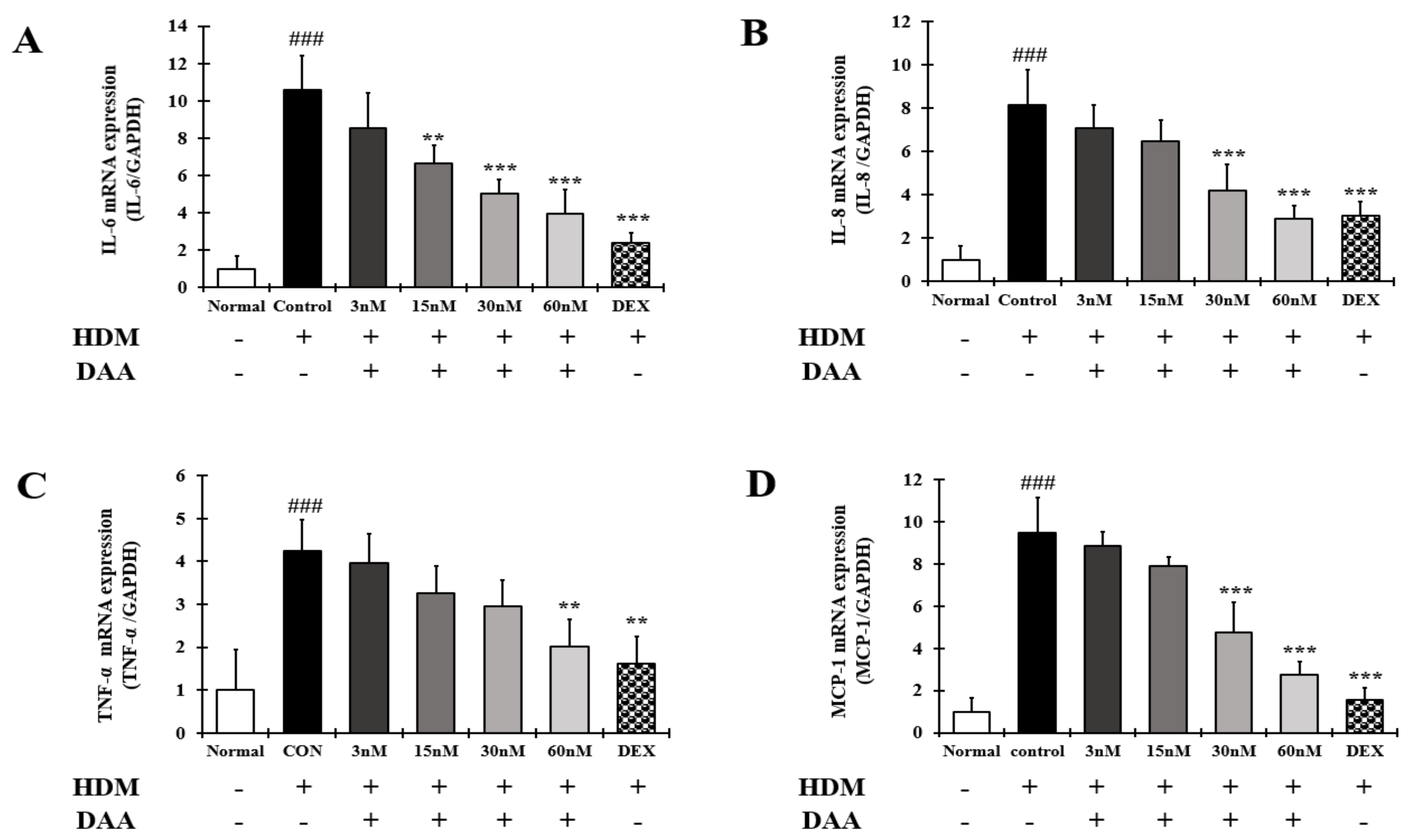
2.15. DAA Inhibited the Gene Expression of AD-Related Cytokines and Chemokines Increased by HDM in EOL-1 Cells
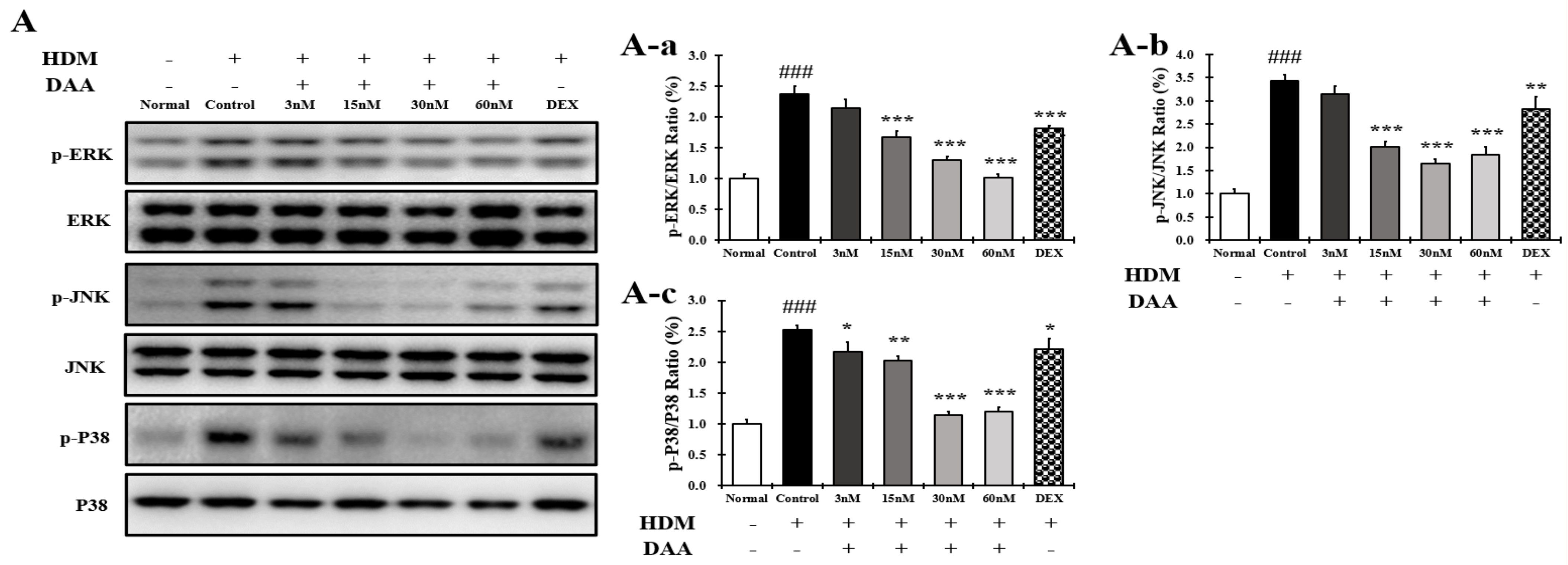
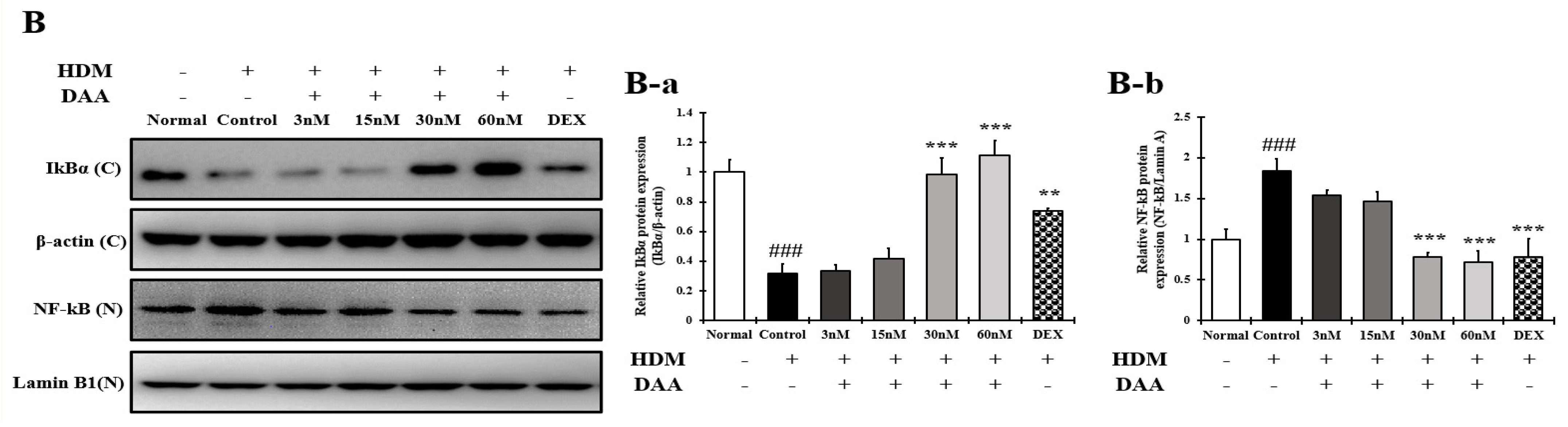
2.16. DAA Inhibited MAPK Phosphorylation and NF-κB Activation Increased by HDM in EOL-1 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of Fermented Noni Extract (F. noni)
4.3. Quantification of DAA and AA by HPLC-PDA Analysis
4.4. Cell Culture
4.5. Cell Viability
4.6. Cytokine and Chemokine Analyses
4.7. Histamine Analysis
4.8. RNA Extraction and Quantitative Real Time-Polymerase Chain Reaction (RT-qPCR)
4.9. Protein Extraction and Western Blotting
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Camargo Lopes de Oliveira, L.; Pierotti, F.F.; Mallozi, M.; Rodrigues Cocco, R.; Rosario, N.; Rugue Genov, I.; Aberg, K.M.; Borres, M.P.; Sole, D. rBlo t 5 is a potential contributor to the severity of atopic dermatitis in a Brazilian population. Pediatr. Allergy Immunol. 2019, 30, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, K.; Shamoto, M.; Sakamoto, N.; Shinzato, M.; Osada, A.; Sugiura, M.; Hayakawa, R.; Kato, Y. It is true that, when Langerhans cells migrate from the skin to the lymph node, they are transported via lymph vessels. Dermatology 2003, 206, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Novak, N.; Bieber, T. The role of dendritic cell subtypes in the pathophysiology of atopic dermatitis. J. Am. Acad. Dermatol. 2005, 53, S171–S176. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Seong, G.S.; Choung, S.Y. Fermented Morinda citrifolia (Noni) Alleviates DNCB-Induced Atopic Dermatitis in NC/Nga Mice through Modulating Immune Balance and Skin Barrier Function. Nutrients 2020, 12, 249. [Google Scholar] [CrossRef] [Green Version]
- Mansouri, Y.; Guttman-Yassky, E. Immune Pathways in Atopic Dermatitis, and Definition of Biomarkers through Broad and Targeted Therapeutics. J. Clin. Med. 2015, 4, 858–873. [Google Scholar] [CrossRef] [Green Version]
- Werfel, T. The role of leukocytes, keratinocytes, and allergen-specific IgE in the development of atopic dermatitis. J. Investig. Dermatol. 2009, 129, 1878–1891. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, S.; Nomura, T.; Common, J.; Kabashima, K. Insights into atopic dermatitis gained from genetically defined mouse models. J. Allergy Clin. Immunol. 2019, 143, 13–25. [Google Scholar] [CrossRef]
- Pummi, K.; Malminen, M.; Aho, H.; Karvonen, S.L.; Peltonen, J.; Peltonen, S. Epidermal tight junctions: ZO-1 and occludin are expressed in mature, developing, and affected skin and in vitro differentiating keratinocytes. J. Investig. Dermatol. 2001, 117, 1050–1058. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.M.; Lee, K.Y.; An, H.J. Inhibitory Effects of Helianthus tuberosus Ethanol Extract on Dermatophagoides farina body-induced Atopic Dermatitis Mouse Model and Human Keratinocytes. Nutrients 2018, 10, 1657. [Google Scholar] [CrossRef] [Green Version]
- Cha, K.J.; Kashif, A.; Hong, M.H.; Kim, G.; Lee, J.S.; Kim, I.S. Poncirus Trifoliata (L.) Raf. Extract Inhibits the Development of Atopic Dermatitis-like Lesions in Human Keratinocytes and NC/Nga mice. Int. J. Med. Sci. 2019, 16, 1116–1122. [Google Scholar] [CrossRef] [Green Version]
- Bak, D.H.; Lee, E.; Lee, B.C.; Choi, M.J.; Kwon, T.R.; Hong, J.; Mun, S.K.; Lee, K.; Kim, S.; Na, J.; et al. Therapeutic potential of topically administered gamma-AlOOH on 2,4-dinitrochlorobenzene-induced atopic dermatitis-like lesions in Balb/c mice. Exp. Dermatol. 2019, 28, 169–176. [Google Scholar] [CrossRef]
- Basler, K.; Brandner, J.M. Tight junctions in skin inflammation. Pflugers Arch. 2017, 469, 3–14. [Google Scholar] [CrossRef]
- Egawa, G.; Kabashima, K. Barrier dysfunction in the skin allergy. Allergol. Int. 2018, 67, 3–11. [Google Scholar] [CrossRef]
- Imaizumi, A.; Kawakami, T.; Murakami, F.; Soma, Y.; Mizoguchi, M. Effective treatment of pruritus in atopic dermatitis using H1 antihistamines (second-generation antihistamines): Changes in blood histamine and tryptase levels. J. Dermatol. Sci. 2003, 33, 23–29. [Google Scholar] [CrossRef]
- Waljee, A.K.; Rogers, M.A.; Lin, P.; Singal, A.G.; Stein, J.D.; Marks, R.M.; Ayanian, J.Z.; Nallamothu, B.K. Short term use of oral corticosteroids and related harms among adults in the United States: Population based cohort study. BMJ 2017, 357. [Google Scholar] [CrossRef] [Green Version]
- Kee, J.Y.; Jeon, Y.D.; Kim, D.S.; Han, Y.H.; Park, J.; Youn, D.H.; Kim, S.J.; Ahn, K.S.; Um, J.Y.; Hong, S.H. Korean Red Ginseng improves atopic dermatitis-like skin lesions by suppressing expression of proinflammatory cytokines and chemokines in vivo and in vitro. J. Ginseng Res. 2017, 41, 134–143. [Google Scholar] [CrossRef] [Green Version]
- Komine, M.; Kakinuma, T.; Kagami, S.; Hanakawa, Y.; Hashimoto, K.; Tamaki, K. Mechanism of thymus- and activation-regulated chemokine (TARC)/CCL17 production and its modulation by roxithromycin. J. Investig. Dermatol. 2005, 125, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Divekar, R.; Kita, H. Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.F.; Kim, D.H.; Yoon, Y.S.; Li, J.H.; Song, S.B.; Jin, D.; Huang, X.Z.; Teng, Y.C.; Lee, K.J. The adenylyl cyclase-cAMP system suppresses TARC/CCL17 and MDC/CCL22 production through p38 MAPK and NF-kappaB in HaCaT keratinocytes. Mol. Immunol. 2009, 46, 1925–1934. [Google Scholar] [CrossRef]
- Yang, H.J.; Kim, M.J.; Kang, S.; Moon, N.R.; Kim, D.S.; Lee, N.R.; Kim, K.S.; Park, S. Topical treatments of Saussurea costus root and Thuja orientalis L. synergistically alleviate atopic dermatitis-like skin lesions by inhibiting protease-activated receptor-2 and NF-kappaB signaling in HaCaT cells and Nc/Nga mice. J. Ethnopharmacol. 2017, 199, 97–105. [Google Scholar] [CrossRef]
- Kwon, D.-J.; Bae, Y.-S.; Ju, S.M.; Goh, A.R.; Youn, G.S.; Choi, S.Y.; Park, J.J.B. Casuarinin suppresses TARC/CCL17 and MDC/CCL22 production via blockade of NF-κB and STAT1 activation in HaCaT cells. Biochem. Biophys. Res. Commun. 2012, 417, 1254–1259. [Google Scholar] [CrossRef]
- Saeki, H.; Tamaki, K. Thymus and activation regulated chemokine (TARC)/CCL17 and skin diseases. J. Dermatol. Sci. 2006, 43, 75–84. [Google Scholar] [CrossRef]
- Imai, T.; Chantry, D.; Raport, C.J.; Wood, C.L.; Nishimura, M.; Godiska, R.; Yoshie, O.; Gray, P.W. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J. Biol. Chem. 1998, 273, 1764–1768. [Google Scholar] [CrossRef] [Green Version]
- Kakinuma, T.; Nakamura, K.; Wakugawa, M.; Mitsui, H.; Tada, Y.; Saeki, H.; Torii, H.; Asahina, A.; Onai, N.; Matsushima, K.; et al. Thymus and activation-regulated chemokine in atopic dermatitis: Serum thymus and activation-regulated chemokine level is closely related with disease activity. J. Allergy Clin. Immunol. 2001, 107, 535–541. [Google Scholar] [CrossRef]
- Hashimoto, S.; Nakamura, K.; Oyama, N.; Kaneko, F.; Tsunemi, Y.; Saeki, H.; Tamaki, K. Macrophage-derived chemokine (MDC)/CCL22 produced by monocyte derived dendritic cells reflects the disease activity in patients with atopic dermatitis. J. Dermatol. Sci. 2006, 44, 93–99. [Google Scholar] [CrossRef]
- Shimada, Y.; Takehara, K.; Sato, S. Both Th2 and Th1 chemokines (TARC/CCL17, MDC/CCL22, and Mig/CXCL9) are elevated in sera from patients with atopic dermatitis. J. Dermatol. Sci. 2004, 34, 201–208. [Google Scholar] [CrossRef]
- Giustizieri, M.L.; Mascia, F.; Frezzolini, A.; De Pita, O.; Chinni, L.M.; Giannetti, A.; Girolomoni, G.; Pastore, S. Keratinocytes from patients with atopic dermatitis and psoriasis show a distinct chemokine production profile in response to T cell-derived cytokines. J. Allergy Clin. Immunol. 2001, 107, 871–877. [Google Scholar] [CrossRef]
- Kim, B.E.; Leung, D.Y. Epidermal barrier in atopic dermatitis. Allergy Asthma Immunol. Res. 2012, 4, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Czarnowicki, T.; Krueger, J.G.; Guttman-Yassky, E. Skin barrier and immune dysregulation in atopic dermatitis: An evolving story with important clinical implications. J. Allergy Clin. Immunol. Pract. 2014, 2, 371–379; quiz 380–391. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Alysandratos, K.D.; Angelidou, A.; Delivanis, D.A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast cells and inflammation. Biochim. Biophys. Acta 2012, 1822, 21–33. [Google Scholar] [CrossRef] [Green Version]
- De Benedetto, A.; Kubo, A.; Beck, L.A. Skin barrier disruption: A requirement for allergen sensitization? J. Investig. Dermatol. 2012, 132, 949–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawakami, T.; Ando, T.; Kimura, M.; Wilson, B.S.; Kawakami, Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 2009, 21, 666–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.T.; Goodarzi, H.; Chen, H.Y. IgE, mast cells, and eosinophils in atopic dermatitis. Clin. Rev. Allergy Immunol. 2011, 41, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Takatsu, K. Interleukin-5 and IL-5 receptor in health and diseases. Proc. Jpn Acad. Ser. B Phys. Biol. Sci. 2011, 87, 463–485. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.S.; Song, G.Y.; Kim, D.H.; Cho, S.H.; Yun, C.Y.; Lee, J.S. Effect of (E)-2-(3,4-dimethoxyphenyl)-4-oxo-4H-chromen-7-yl-3-(3,4-dimethoxyphenyl) acrylate on the development of atopic dermatitis-like lesions. Life Sci. 2012, 91, 338–344. [Google Scholar] [CrossRef]
- Moustaka, K.; Maleskou, E.; Lambrianidou, A.; Papadopoulos, S.; Lekka, M.E.; Trangas, T.; Kitsiouli, E. Docosahexaenoic Acid Inhibits Proliferation of EoL-1 Leukemia Cells and Induces Cell Cycle Arrest and Cell Differentiation. Nutrients 2019, 11, 574. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.S.; Kim, M.J.; Shin, D.H.; Son, K.H.; Park, H.Y.; Lee, J.S. Arazyme inhibits cytokine expression and upregulates skin barrier protein expression. Mol. Med. Rep. 2013, 8, 551–556. [Google Scholar] [CrossRef] [Green Version]
- Kinghorn, A.D.; Chai, H.B.; Sung, C.K.; Keller, W.J. The classical drug discovery approach to defining bioactive constituents of botanicals. Fitoterapia 2011, 82, 71–79. [Google Scholar] [CrossRef]
- Dussossoy, E.; Brat, P.; Bony, E.; Boudard, F.; Poucheret, P.; Mertz, C.; Giaimis, J.; Michel, A. Characterization, anti-oxidative and anti-inflammatory effects of Costa Rican noni juice (Morinda citrifolia L.). J. Ethnopharmacol. 2011, 133, 108–115. [Google Scholar] [CrossRef]
- Potterat, O.; Felten, R.V.; Dalsgaard, P.W.; Hamburger, M. Identification of TLC markers and quantification by HPLC-MS of various constituents in noni fruit powder and commercial noni-derived products. J. Agric. Food Chem. 2007, 55, 7489–7494. [Google Scholar] [CrossRef]
- Pandy, V.; Bonam, S.R.; Raya, A.R.; Nadendla, R.R. Morinda citrifolia Linn.(Noni) fruit, antioxidant effects, and neuroprotection. In Oxidative Stress and Dietary Antioxidants in Neurological Diseases; Elsevier: Amsterdam, The Netherlands, 2020; pp. 291–307. [Google Scholar]
- Lee, J.W.; Wu, Q.; Jang, Y.P.; Choung, S.Y. Pinus densiflora bark extract ameliorates 2,4-dinitrochlorobenzene-induced atopic dermatitis in NC/Nga mice by regulating Th1/Th2 balance and skin barrier function. Phytother. Res. 2018, 32, 1135–1143. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choi, M.J.; Bak, D.H.; Lee, B.C.; Ko, E.J.; Ahn, G.R.; Ahn, S.W.; Kim, M.J.; Na, J.; Kim, B.J. Topical administration of EGF suppresses immune response and protects skin barrier in DNCB-induced atopic dermatitis in NC/Nga mice. Sci. Rep. 2018, 8, 11895. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.Y.; Peng, L.; Jensen, C.J.; Deng, S.; West, B.J. Noni juice reduces lipid peroxidation-derived DNA adducts in heavy smokers. Food Sci. Nutr 2013, 1, 141–149. [Google Scholar] [CrossRef]
- He, J.; Lu, X.; Wei, T.; Dong, Y.; Cai, Z.; Tang, L.; Liu, M. Asperuloside and Asperulosidic Acid Exert an Anti-Inflammatory Effect via Suppression of the NF-kappaB and MAPK Signaling Pathways in LPS-Induced RAW 264.7 Macrophages. Int. J. Mol. Sci. 2018, 19, 2027. [Google Scholar] [CrossRef] [Green Version]
- Kee, J.Y.; Hong, S.H. Ginsenoside Rg3 suppresses mast cell-mediated allergic inflammation via mitogen-activated protein kinase signaling pathway. J. Ginseng Res. 2019, 43, 282–290. [Google Scholar] [CrossRef]
- Passante, E.; Frankish, N. The RBL-2H3 cell line: Its provenance and suitability as a model for the mast cell. Inflamm. Res. 2009, 58, 737–745. [Google Scholar] [CrossRef]
- Saito, H.; Bourinbaiar, A.; Ginsburg, M.; Minato, K.; Ceresi, E.; Yamada, K.; Machover, D.; Breard, J.; Mathe, G. Establishment and characterization of a new human eosinophilic leukemia cell line. Blood 1985, 66, 1233–12403. [Google Scholar] [CrossRef] [Green Version]
- Galli, S.J.; Kalesnikoff, J.; Grimbaldeston, M.A.; Piliponsky, A.M.; Williams, C.M.; Tsai, M. Mast cells as “tunable” effector and immunoregulatory cells: Recent advances. Annu. Rev. Immunol. 2005, 23, 749–786. [Google Scholar] [CrossRef]
- Choi, E.J.; Iwasa, M.; Han, K.I.; Kim, W.J.; Tang, Y.; Hwang, Y.J.; Chae, J.R.; Han, W.C.; Shin, Y.S.; Kim, E.K. Heat-Killed Enterococcus faecalis EF-2001 Ameliorates Atopic Dermatitis in a Murine Model. Nutrients 2016, 8, 146. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Pan, W.H.; Wang, X.R.; Liu, Y.; Chen, M.; Xu, X.G.; Liao, W.Q.; Hu, J.H. Tryptase and protease-activated receptor-2 stimulate scratching behavior in a murine model of ovalbumin-induced atopic-like dermatitis. Int. Immunopharmacol. 2015, 28, 507–512. [Google Scholar] [CrossRef]
- Gould, H.J.; Sutton, B.J.; Beavil, A.J.; Beavil, R.L.; McCloskey, N.; Coker, H.A.; Fear, D.; Smurthwaite, L. The biology of IGE and the basis of allergic disease. Annu. Rev. Immunol. 2003, 21, 579–628. [Google Scholar] [CrossRef]
- Kim, H.H.; Kim, S.W.; Kim, D.S.; Oh, H.M.; Rho, M.C.; Kim, S.H. Vigna angularis inhibits mast cell-mediated allergic inflammation. Int. J. Mol. Med. 2013, 32, 736–742. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yang, M.; Deng, J.; Wang, K.; Shi, J.; Sun, Y. Elevated Levels of Activated and Pathogenic Eosinophils Characterize Moderate-Severe House Dust Mite Allergic Rhinitis. J. Immunol. Res. 2020, 2020, 8085615. [Google Scholar] [CrossRef]
- Gu, L.; Tseng, S.; Horner, R.M.; Tam, C.; Loda, M.; Rollins, B.J. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature 2000, 404, 407–411. [Google Scholar] [CrossRef]
- Huang, C.D.; Wang, C.H.; Liu, C.Y.; Lin, S.M.; Chou, C.L.; Liu, W.T.; Lin, H.C.; Kuo, H.P. Eosinophils from asthmatics release IL-5 in an autocrine fashion to prevent apoptosis through upregulation of Bcl-2 expression. J. Asthma 2005, 42, 395–403. [Google Scholar] [CrossRef]
- Furue, M.; Ulzii, D.; Vu, Y.H.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T. Atopic Dermatitis and Type 2 Immune Deviation. Curr. Treat. Opt. Allergy 2019, 6, 200–210. [Google Scholar] [CrossRef]
- Li, C.; Maillet, I.; Mackowiak, C.; Viala, C.; Di Padova, F.; Li, M.; Togbe, D.; Quesniaux, V.; Lai, Y.; Ryffel, B. Experimental atopic dermatitis depends on IL-33R signaling via MyD88 in dendritic cells. Cell Death Dis. 2017, 8, e27350. [Google Scholar] [CrossRef]
- Marschall, P.; Wei, R.; Segaud, J.; Yao, W.; Hener, P.; German, B.F.; Meyer, P.; Hugel, C.; Ada Da Silva, G.; Braun, R.; et al. Dual function of Langerhans cells in skin TSLP-promoted TFH differentiation in mouse atopic dermatitis. J. Allergy Clin. Immunol. 2021, 147, 1778–1794. [Google Scholar] [CrossRef]
- Zaniboni, M.C.; Samorano, L.P.; Orfali, R.L.; Aoki, V. Skin barrier in atopic dermatitis: Beyond filaggrin. An. Bras. Dermatol. 2016, 91, 472–478. [Google Scholar] [CrossRef]
- Cabanillas, B.; Novak, N. Atopic dermatitis and filaggrin. Curr. Opin. Immunol. 2016, 42, 1–8. [Google Scholar] [CrossRef]
- Kim, B.E.; Leung, D.Y.; Boguniewicz, M.; Howell, M.D. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin. Immunol. 2008, 126, 332–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, M.J.; Zhou, B. A new itch to scratch for TSLP. Trends Immunol. 2014, 35, 49–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proksch, E.; Folster-Holst, R.; Brautigam, M.; Sepehrmanesh, M.; Pfeiffer, S.; Jensen, J.M. Role of the epidermal barrier in atopic dermatitis. J. Dtsch Dermatol. Ges. 2009, 7, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Fulkerson, P.C.; Rothenberg, M.E. Targeting eosinophils in allergy, inflammation and beyond. Nat. Rev. Drug Discov. 2013, 12, 117–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, H.F.; Dyer, K.D.; Foster, P.S. Eosinophils: Changing perspectives in health and disease. Nat. Rev. Immunol. 2013, 13, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.O.; ETAC Study Group. Early Treatment of the Atopic Child. A double-blinded, randomized, placebo-controlled trial of cetirizine in preventing the onset of asthma in children with atopic dermatitis: 18 months’ treatment and 18 months’ posttreatment follow-up. J. Allergy Clin. Immunol. 2001, 108, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Bochner, B.S.; Gleich, G.J. What targeting eosinophils has taught us about their role in diseases. J. Allergy Clin. Immunol. 2010, 126, 16–25; quiz 26–27. [Google Scholar] [CrossRef] [Green Version]
- Moon, P.D.; Kim, H.M. Thymic stromal lymphopoietin is expressed and produced by caspase-1/NF-kappaB pathway in mast cells. Cytokine 2011, 54, 239–243. [Google Scholar] [CrossRef]
- Wong, C.K.; Ng, S.S.; Lun, S.W.; Cao, J.; Lam, C.W. Signalling mechanisms regulating the activation of human eosinophils by mast-cell-derived chymase: Implications for mast cell-eosinophil interaction in allergic inflammation. Immunology 2009, 126, 579–587. [Google Scholar] [CrossRef]
- Xiong, X.; Huang, C.; Wang, F.; Dong, J.; Zhang, D.; Jiang, J.; Feng, Y.; Wu, B.; Xie, T.; Cheng, L. Qingxue jiedu formulation ameliorated DNFB-induced atopic dermatitis by inhibiting STAT3/MAPK/NF-kappaB signaling pathways. J. Ethnopharmacol. 2020, 270, 113773. [Google Scholar] [CrossRef]
- Tan, Q.; Yang, H.; Liu, E.; Wang, H. P38/ERK MAPK signaling pathways are involved in the regulation of filaggrin and involucrin by IL17. Mol. Med. Rep. 2017, 16, 8863–8867. [Google Scholar] [CrossRef] [Green Version]
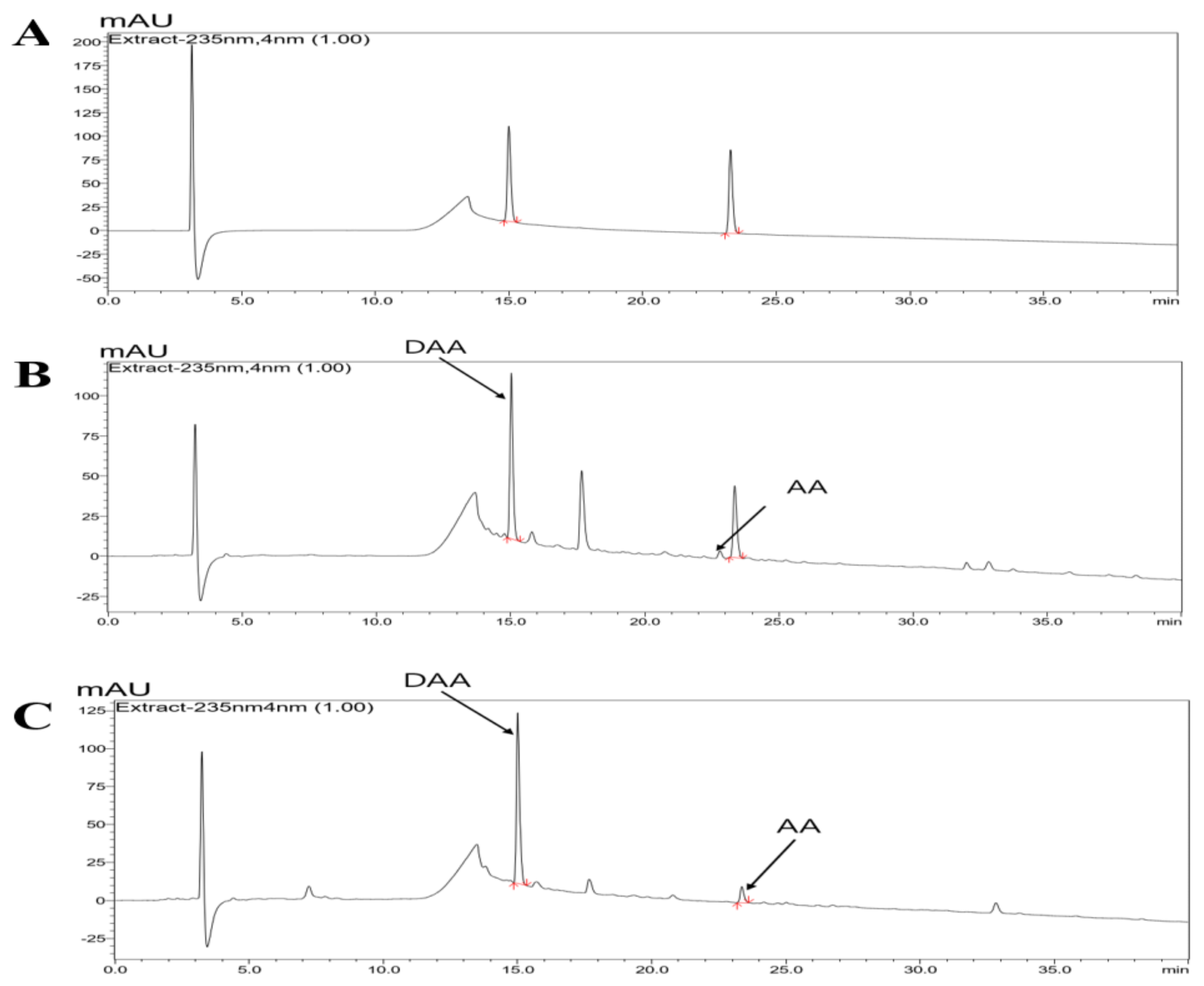




| Content of DAA and AA in the Noni/F. noni Extract Powder (mg/g) | ||
|---|---|---|
| Sample | DAA | AA |
| Noni | 10.37 ± 0.94 | 0.98 ± 0.08 |
| F. noni | 13.11 ± 0.25 | 1.40 ± 0.08 |
| Gene | Forward | Reverse |
|---|---|---|
| IL-1β (h) | AAA CAG ATG AAG GTG CTC CTT | TGG AGA ACA CCA CTT GTT GC |
| IL-6 (h) | AAA TTC GGT ACA TCC TCG ACG GCA | AGT GCC TCT TTG CTG CTT TCA CAC |
| IL-8 (h) | AAG CTG GCC GTG GCT CTC TTG | AGC CCT CTT CAA AAA CTT CTC |
| IL-25 (h) | CGA CCC AGA TTA GGT GAG GA | TCC ATC TTC ACT GGC CCT AC |
| IL-33 (h) | ACA GAA TAC TGA AAA ATG AAG CC | CTT CTC CAG TGG TAG CAT TTG |
| TNF-α (h) | AGG ACG AAC ATC CAA CCT TC | TTT GAG CCA GAA GAG GTT GA |
| TSLP (h) | TAG AGT GGG ACC AAA AGT ACC G | GGG ATT GAA GGT TAG GCT CTG G |
| MCP-1 (h) | GTC TCT GCC GCC CTT CTG T | TTG CAT CTG ATG GCA GTA GCT |
| TARC (h) | CTT CTC TGC AGC ACA TCC | AAG ACC TCT CAA GGC TTT G |
| MDC (h) | AGG ACA GAG CAT GGA TCG CCT ACA GA | AAT GGC AGG GAG GTA GGG CTC CTG A |
| RANTES (h) | CCG CGG CAG CCC TCG CTG TCA TCC | CAT CTC CAA AGA GTT GAT GTA CTC C |
| GAPDH (h) | TCG ACA GTC AGC CGC ATC TTC TTT | ACC AAA TCC GTT GAC TCC GAC CTT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.S.; Seong, G.S.; Kim, Y.D.; Choung, S.Y. Effects of Deacetylasperulosidic Acid on Atopic Dermatitis through Modulating Immune Balance and Skin Barrier Function in HaCaT, HMC-1, and EOL-1 Cells. Molecules 2021, 26, 3298. https://doi.org/10.3390/molecules26113298
Oh JS, Seong GS, Kim YD, Choung SY. Effects of Deacetylasperulosidic Acid on Atopic Dermatitis through Modulating Immune Balance and Skin Barrier Function in HaCaT, HMC-1, and EOL-1 Cells. Molecules. 2021; 26(11):3298. https://doi.org/10.3390/molecules26113298
Chicago/Turabian StyleOh, Jin Su, Geum Su Seong, Yong Deok Kim, and Se Young Choung. 2021. "Effects of Deacetylasperulosidic Acid on Atopic Dermatitis through Modulating Immune Balance and Skin Barrier Function in HaCaT, HMC-1, and EOL-1 Cells" Molecules 26, no. 11: 3298. https://doi.org/10.3390/molecules26113298
APA StyleOh, J. S., Seong, G. S., Kim, Y. D., & Choung, S. Y. (2021). Effects of Deacetylasperulosidic Acid on Atopic Dermatitis through Modulating Immune Balance and Skin Barrier Function in HaCaT, HMC-1, and EOL-1 Cells. Molecules, 26(11), 3298. https://doi.org/10.3390/molecules26113298






