Sustainable Pest Management Using Biodegradable Apitoxin-Loaded Calcium-Alginate Microspheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microsphere Formulation
2.1.1. Calibration Diagram for Apitoxin Determination
2.1.2. Preparation of Microspheres
2.1.3. Encapsulation Efficiency (EE%) and Loading Capacity (Lc)
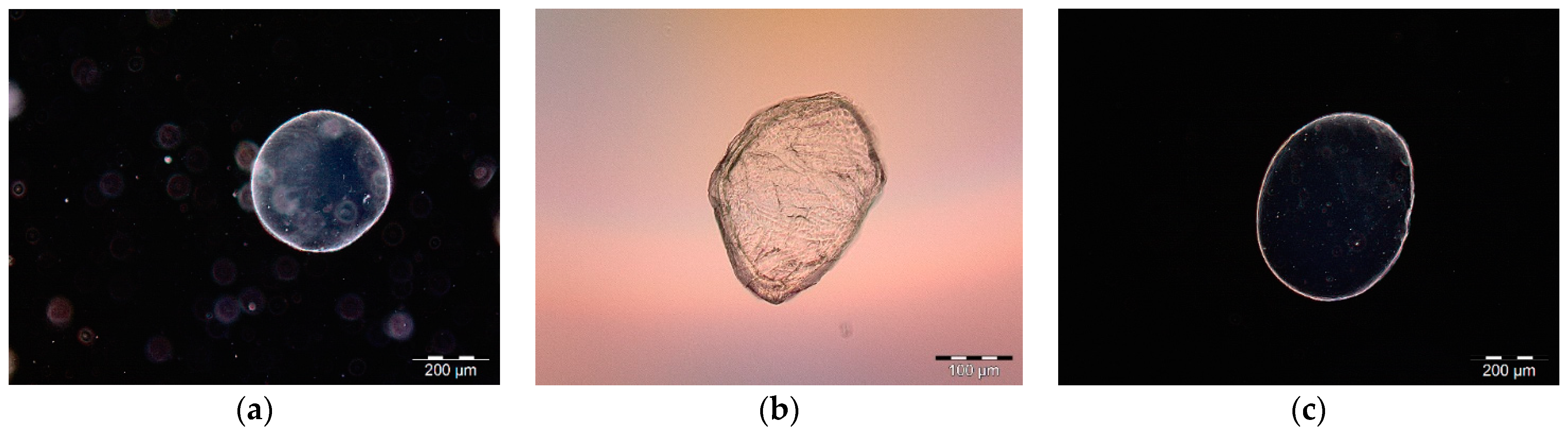
2.1.4. Microscopic Observations
2.1.5. Dry Matter Content (%) and Swelling Degree (Sw%)
2.1.6. In Vitro Release Profiles of Apitoxin from Microspheres
2.2. Efficacy Estimation of Encapsulated Apitoxin on Selected Insect Pests
2.2.1. Insects
2.2.2. Apitoxin Efficacy on Insects
2.3. Statistical Analysis and Efficacy Assessment
2.4. Economic Analysis of the Application of Apitoxin in Agriculture
3. Results and Discussion
3.1. Physico-Chemical Characterization of Microspheres
3.1.1. Encapsulation Efficiency (EE%) and Loading Capacity (Lc)
3.1.2. Morphological and Swelling Properties of Selected Microsphere Formulations
3.1.3. In Vitro Release of Apitoxin from Microsphere Formulations
3.2. The Efficiency of Apitoxin-Based Microspheres on Selected Inesct Pest Species
3.3. Economic Analysis of the Apitoxin-Based Microsphere Uses in Agricultural Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barzman, M.; Bàrberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P.; et al. Eight principles of integrated pest management. Agron. Sustain. Dev. 2015, 35, 1199–1215. [Google Scholar] [CrossRef]
- Callaghan, A. Mechanisms and detection of insecticide resistance: A review. Sci. Prog. 1991, 75, 423–438. [Google Scholar]
- Miller, G.T. Sustaining the Earth; Thompson Learning, Inc.: Pacific Grove, CA, USA, 2004. [Google Scholar]
- Štivičić, A.; Pajač Živković, I.; Lemic, D. Methods for determining resistance in entomological sciences. Fragm. Phytom. 2020, 34, 27–38. [Google Scholar]
- Javed, S.; Agurla, R. Molecular tools for detection of insecticide resistance. Int. J. Multidiscip. Adv. Res. Trends 2017, 4, 165–173. [Google Scholar]
- Zhang, L.; Yan, C.; Guo, Q.; Zhang, J.; Ruiz-Menjivar, J. The impact of agricultural chemical inputs on environment: Global evidence from informetrics analysis and visualization. Int. J. Low-Carbon Technol. 2018, 13, 338–352. [Google Scholar] [CrossRef] [Green Version]
- Jurić, S.; Đermić, E.; Topolovec-Pintarić, S.; Bedek, M.; Vinceković, M. Physicochemical properties and release characteristics of calcium alginate microspheres loaded with Trichoderma viride spores. J. Integr. Agric. 2019, 18, 2534–2548. [Google Scholar] [CrossRef]
- Pavela, R. Effectiveness of some botanical insecticides against Spodoptera littoralis Boisduvala (Lepidoptera: Noctudiae), Myzus persicae Sulzer (Hemiptera: Aphididae) and Tetranychus urticae Koch (Acari: Tetranychidae). Plant Prot. Sci. 2009, 45, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Manzoli, M.; Gobbi, N.; Palma, M. Insects as biological models to assay spider and scorpion venom toxicity. J. Venom. Anim. Toxins 2003, 9, 174–185. [Google Scholar] [CrossRef]
- Levanić, M. Kemijska Karakterizacija Pčelinjeg Otrova Primjenom Infracrvene (FTIR-ATR) Spektroskopije. Master’s Thesis, University of Zagreb, Faculty of Agriculture, Zagreb, Croatia, 2019. [Google Scholar]
- Peiren, N.; de Graaf, D.C.; Vanrobaeys, F.; Danneels, E.L.; Devreese, B.; Van Beeumen, J.; Jacobs, F.J. Proteomic analysis of the honey bee worker venom gland focusing on the mechanisms of protection against tissue damage. Toxicon 2008, 52, 72–83. [Google Scholar] [CrossRef]
- Bellik, Y. Bee Venom: Its potential use in alternative medicine. Anti-Infect. Agents 2015, 13, 3–16. [Google Scholar] [CrossRef]
- Liu, X.; Chen, D.; Xie, L.; Zhang, R. Effect of honey bee venom on proliferation of K1735M2 mouse melanoma cells in-vitro and growth of murine B16 melanomas in-vivo. J. Pharm. Pharmacol. 2002, 54, 1083–1089. [Google Scholar] [CrossRef]
- Oršolić, N. Potentiation of Bleomycin lethality in HeLa and V79 cells by bee venom. Arch. Ind. Hyg. Toxicol. 2009, 60, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Oršolić, N. Bee venom in cancer therapy. Cancer Metast. Rev. 2012, 3, 173–194. [Google Scholar] [CrossRef]
- Hossen, M.S.; Shapla, U.M. Impact of Bee Venom Enzymes on Diseases and Immune Responses. Molecules 2016, 22, 25. [Google Scholar] [CrossRef]
- Quistad, G.B.; Skinner, W.S.; Schooley, D.A. Venoms of social Hymenoptera-toxicity to the lepidopteran. Manduca sexta. Insect Biochem. 1988, 18, 511–514. [Google Scholar] [CrossRef]
- Lima, P.R.; Brochetto-Braga, M.R. Hymenoptera venom review focusing on Apis mellifera. J. Venom. Anim. Toxins 2003, 9, 149–162. [Google Scholar] [CrossRef]
- Bogdanov, S. Bee venom: Composition, health, medicine: A review. Bee Prod. Sci. 2017, 24, 1–20. [Google Scholar]
- Banks, B.; Shipolini, R. Chemistry and pharmacology of honey-bee venom. In Venoms of the Hymenoptera; Piek, T., Ed.; Academic Press: London, UK, 1986; pp. 330–416. [Google Scholar]
- Kim, H.; Lee, G.; Park, S.; Chung, H.S.; Lee, H.; Kim, J.Y.; Nam, S.; Kim, S.K.; Bae, H. Bee venom mitigates cisplatin-induced nephrotoxicity by regulating CD4+ CD5+ Foxp3+ Regulatory T cells in mice. Evid. Based Complementary Altern. Med. 2013, 10, 879845. [Google Scholar]
- Park, S.; Baek, H.; Jung, K.H.; Lee, G.; Lee, H.; Kang, G.H.; Lee, G.; Bae, H. Bee venom phospholipase a2 suppresses allergic airway inflammation in an ovalbumin-induced asthma model through the induction of regulatory t cells. Immun. Inflamm. Dis. 2014, 3, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Sobral, F.; Sampaio, A.; Queiroz, M.J.; Calhelha, R.C.; Vilas-Boas, M.; Ferreira, I.C. Chemical characterization, antioxidant, anti-inflammatory and cytotoxic properties of bee venom collected in Northeast Portugal. Food Chem. Toxicol. 2016, 94, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Ross, D.C.; Crim, J.W.; Brown, M.R.; Herzong, G.A.; Lea, A.O. Toxic and antifeeding actions of melittin in the corn earworm, Heliothis zea (Boddie): Comparisons to bee venom and insecticides chlorpyriphos and cyromazine. Toxicon 1987, 25, 307–313. [Google Scholar] [CrossRef]
- Mahgoub, M.O.; Lau, W.H.; Omar, D.B.; El Naim, A.M. Evaluation the Toxicity of Honey Bee Venom on Achroia grisella Developmental Stages. World J. Agric. Res. 2018, 6, 5–9. [Google Scholar]
- Hider, R.C. Honey bee venom: A rich source of pharmacologically active peptides. Endeavor 1988, 12, 60–65. [Google Scholar] [CrossRef]
- Hoffman, D.R. Hymenoptera venom proteins. Nat. Toxins 1996, 2, 169–186. [Google Scholar]
- Glinski, Z.; Jarosz, M. Infection and immunity in the honey bee Apis mellifera. Apiacata 2001, 36, 12–24. [Google Scholar]
- European Commision. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions; The European Green Deal COM/2019/640 final; European Commision: Brussels, Belgium, 2019. [Google Scholar]
- Ferrándiz, M.; Capablanca, L.; García, D.; Bonet, M.Á. Application of Antimicrobial Microcapsules on Agrotextiles. J. Agric. Chem. Environ. 2017, 6, 62–82. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of probiotic living cells: From laboratory scale to industrial applications. J. Food Eng. 2011, 104, 467–483. [Google Scholar] [CrossRef]
- Boras, A. Mikroinkapsulacija soja Lactobacillus Sakei MRS_296 S Potencijalom Primjene kao Starter Kkultura. Master’s Thesis, University of Zagreb, Faculty of Agriculture, Zagreb, Croatia, 2019. [Google Scholar]
- Gallo, M.; Corbo, M.R. Microencapsulation as a New Approach to Protect Active Compounds in Food. Application of Alternative Food-Preservation Technologies to Enhance Food Safety and Stability; Bentham Science Publisher: Sharjah, United Arab Emirates, 2010; pp. 188–195. [Google Scholar]
- Teixeira da Silva, P.; Martins Fries, L.L.; Ragagnin de Menezes, C.; Tasch Holkem, A.; Schwan, C.L.; Wigmann, É.F.; De Oliveira Bastos, J.; De Bona da Silva, C. Microencapsulation: Concepts, mechanisms, methods and some applications in food technology. Ciência Rural 2014, 44, 1304–1311. [Google Scholar] [CrossRef]
- Usmiati, S.; Richana, N.; Mangunwidjaja, D.; Noor, E.; Prangdimurti, E. The Using of Ionic Gelation Method Based on Polysaccharides for Encapsulating the Macromolecules. Int. Conf. Food Secur. Nutr. 2014, 67, 79–84. [Google Scholar]
- Vinceković, M.; Jalšenjak, N.; Topolovec-Pintarić, S.; Đermić, E.; Bujan, M.; Jurić, S. Encapsulation of Biological and Chemical Agents for Plant Nutrition and Protection: Chitosan/Alginate Microcapsules Loaded with Copper Cations and Trichoderma viride. J. Agric. Food Chem. 2016, 64, 8073–8083. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, Y.; Yang, F.; Cui, H.; Wu, D. Development of a chlorantraniliprole microcapsule formulation with a high loading content and controlled-release property. J. Agric. Food Chem. 2017, 66, 6561–6568. [Google Scholar] [CrossRef] [PubMed]
- Oxley, J. Bioencapsulation Innovations. In Microencapsulation: Guide to Industrial Applications; Bioencapsulation Research Group: Sucé sur Erdre, France, 2015; Volume 41, pp. 16–17. [Google Scholar]
- Knowles, A. Recent developments of safer formulations of agrochemicals. Environmentalist 2008, 28, 35–44. [Google Scholar] [CrossRef]
- Kumar Das, S.; Nakka David, S.R.; Rajabalaya, R.; Mukhopadhyay Kumar, H.; Halder, T.; Palanisamy, M.; Khanam, J.; Nanda, A. Microencapsulation techniques and its practice. J. Pharm. Sci. Technol. 2011, 2, 1–23. [Google Scholar]
- Bashir, O.; Claverie, J.P.; Lemoyne, P.; Vincent, C. Controlled-release of Bacillus thurigiensis formulations encapsulated in light-resistant colloidosomal microcapsules for the management of lepidopteran pests of Brassica crops. Peer J. 2016, 4, 2524. [Google Scholar] [CrossRef] [Green Version]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, Nano-Guard for pesticides: A new window for safe application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef] [PubMed]
- Haramija, F. Primjena Biopolimernih Kapsula u Konvencionalnom Uzgoju Salate. Master’s Thesis, University of Zagreb, Faculty of Agriculture, Zagreb, Croatia, 2019. [Google Scholar]
- Steinbrenner, U.; Bratz, M. Bioencapsulation Innovations. In Challenges for Microencapsulated Formulations in Agriculture; Bioencapsulation Research Group: Sucé sur Erdre, France, 2015; pp. 14–15. [Google Scholar]
- Jurić, S.; Tanuwidjaja, I.; Mrkonjić Fuka, M.; Vlahoviček-Kahlina, K.; Marijan, M.; Boras, A.; Kolić, N.U.; Vinceković, M. Encapsulation of two fermentation agents, Lactobacillus sakei and calcium ions in microspheres. Colloids Surf. B Biointerfaces 2021, 197, 111387. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.-M.; Liu, X.; He, X.; Wang, W.; Ma, X.-J. Chemical Method of Breaking the Cell-Loaded Sodium Alginate/Chitosan Microcapsules. Gaodeng Xuexiao Huaxue Xuebao/Chem. J. Chin. Univ 2004, 55, 25. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Gylling Data Management Inc. ARM 2019® GDM Software; Revision 2019.4; August 5,2019 (B=25105); Gylling Data Management Inc.: Brookings, SD, USA, 2019. [Google Scholar]
- Mong Thu, T.T.; Krasaekoopt, W. Encapsulation of protease from Aspergillus oryzae and lipase from Thermomyces lanuginoseus using alginate and different copolymer types. Agric. Nat. Resour. 2016, 50, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Aguilera, D.A.; Spinozzi Di Sante, L.; Pettignano, A.; Riccioli, R.; Roeske, J.; Albergati, L.; Corti, V.; Fochi, M.; Bernardi, L.; Quignard, F.; et al. Adsorption of a Chiral Amine on Alginate Gel Beads and Evaluation of its Efficiency as Heterogeneous Enantioselective Catalyst. Eur. J. Org. Chem. 2019, 2019, 3842–3849. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Thirawong, N.; Korkerd, K. Swelling, erosion and release behavior of alginate-based matrix tablets. Eur. J. Pharm. Biopharm. 2007, 66, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.J.; Gupta, R.K.; Min, C.; Siber, G.R.; Langer, R. Biodegradable microspheres as controlled-release tetanus toxoid delivery systems. Vaccine 1994, 12, 299–306. [Google Scholar] [CrossRef]
- Sánchez, A.; Villamayor, B.; Guo, Y.; McIver, J.; Alonso, M.J. Formulation strategies for the stabilization of tetanus toxoid in poly(lactide-co-glycolide) microspheres. Int. J. Pharm. 1999, 185, 255–266. [Google Scholar] [CrossRef]
- Higuchi, T. Drug Release. In Modeling in Biopharmaceutics, Pharmacokinetics, and Pharmacodynamics; Macheras, P., Iliadis, A., Eds.; Springer: New York, NY, USA, 2006; pp. 57–88. [Google Scholar]
- Lupo, B.; Maestro, A.; Porras, M.; Gutiérrez, J.M.; González, C. Preparation of alginate microspheres by emulsification/internal gelation to encapsulate cocoa polyphenols. Food Hydrocoll. 2014, 38, 56–65. [Google Scholar] [CrossRef]
- Vinokurov, K.; Taranushenko, Y.; Krishnan, N. Protease, amylase, and protease-inhibitor activities in the gut of six cockroach species. J. Insect Physiol. 2007, 53, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Grayson, J. Digestive Tract pH of Six Species of Coleoptera; Virgina Agricultural Experminet Station: Blacksburg, VA, USA, 1958. [Google Scholar]
- Nassar, M.I. The potential of natural venom of Apis mellifera for the control of grain weevil adults (Sitophilus granarius Coleoptera Curculionidae). Int. J. Entomol. Res. 2013, 1, 25–31. [Google Scholar]
- Alyokhin, A.; Baker, M.; Mota-Sanchez, D.; Dively, G.; Grafius, E. Colorado Potato Beetle Resistance to Insecticides. Am. J. Pot. Res. 2008, 85, 395–413. [Google Scholar] [CrossRef]
- Bažok, R.; Čačija, M.; Lemić, D.; Virić Gašparić, H.; Drmić, Z. Rezistentnost štetnika na insekticide. Glas. Biljn. Zaštite 2017, 17, 429–438. [Google Scholar]
- Gluckselig, B. Rezistentnost Populacija Krumpirove Zlatice na Organofosforne Insekticide, Piretroide i Neonikotenoide. Master’s Thesis, University of Zagreb, Faculty of Agriculture, Zagreb, Croatia, 2019. [Google Scholar]
- Stewart, J.G.; Kennedy, G.G.; Sturz, A.V. Incidence of insecticide resistance in populations of Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae), on Prince Edward Island. Can. Entomol. 1997, 129, 21–26. [Google Scholar] [CrossRef]
- Stankovic, S.; Zabel, A.; Kostic, M.; Manojlovic, B.; Rajkovic, S. Colorado potato beetle [Leptinotarsa decemlineata (Say)] resistance to organophosphates and carbamates in Serbia. J. Pest Sci. 2004, 77, 11–15. [Google Scholar] [CrossRef]
- Mota-Sanchez, D.; Hollingworth, R.M.; Grafius, E.J.; Moyer, D.D. Resistance and cross-resistance to neonicotinoid insecticides and spinosad in the Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae). Pest Manag. Sci. 2006, 62, 30–37. [Google Scholar] [CrossRef]
- Benkovskaya, G.V.; Udalov, M.B.; Nikolenko, A.G.; Leontieva, T.L. Temporal and toxicological dynamics in the cover spot patterns of the Colorado potato beetle in South Ural. Resist. Pest Manag. 2006, 15, 13–15. [Google Scholar]
- Maceljski, M. Poljoprivredna Entomologija; Zrinski d.d.: Čakovec, Croatia, 2002. [Google Scholar]
- Kljajić, P.; Perić, I. Susceptibility to contact insecticides of granary weevil Sitophilus granarius (L.) (Coleoptera: Curculionidae) originating from different locations in the former Yugoslavia. J. Stored. Prod. Res. 2006, 42, 149–161. [Google Scholar] [CrossRef]
- White, N.D.G.; Leesch, J.G. Chemical control. In Integrated Management of Insects in Stored Products; Subramanyam, B., Hagstrum, D.W., Eds.; Marcel Dekker: New York, NY, USA; Basel, Switzerland; Hong Kong, China, 1996; pp. 287–330. [Google Scholar]
- Tomlin, C. The Pesticide Manual; British Crop Protection Council and The Royal Society of Chemistry: Cambridge, UK, 2000. [Google Scholar]
- Hagstrum, D.W.; Subramanyam, B. Stored-Product Insect Resource; AACC International: St. Paul, MN, USA, 2009. [Google Scholar]
- Kavallieratos, N.G.; Papanikolaou, N.E.; Kazani, A.N.; Boukouvala, M.C.; Malesios, C. Using multilevel models to explore the impact of abiotic and biotic conditions on the efficacy of pirimiphos-methyl against Tenebrio molitor L. Environ. Sci. Pollut. Res. 2021, 28, 17200–17207. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, C.G.; Kavallieratos, N.G.; Boukouvala, M.C.; Mavroforos, M.E.; Kontodimas, D.C. Efficacy of alpha-cypermethrin and thiamethoxam against Trogoderma granarium Everts (Coleoptera: Dermestidae) and Tenebrio molitor L. (Coleoptera: Tenebrionidae) on concrete. J. Stored. Prod. Res. 2015, 62, 101–107. [Google Scholar] [CrossRef]
- Meyling, N.V.; Arthur, S.; Pedersen, K.E.; Dhakal, S.; Cedergreen, N.; Fredensborg, B.L. Implications of sequence and timing of exposure for synergy between the pyrethroid insecticide alpha-cypermethrin and the entomopathogenic fungus Beauveria bassiana. Pest Manag. Sci. 2018, 74, 2488–2495. [Google Scholar] [CrossRef] [PubMed]
- Serrinha, V.; Correia, S.D.; Marques, G. Productivity and Economic Analysis of a New Intensive Collector in the Portuguese Market with Implication of Open Innovation Perspective. J. Open Innov. Technol. Mark. Complex. 2019, 5, 71. [Google Scholar] [CrossRef] [Green Version]
- Čačija, M.; Drmić, Z.; Kadoić Balaško, M.; Skendžić, S.; Lemić, D.; O’Keffee, J.; Jurada, I.; Bažok, R. Kombinacije Insekticida u Subletalnim Dozama-Antirezistentna Strategija u Suzbijanju Krumpirove Zlatice; Hrvatsko društvo biljne zaštite: Zagreb, Croatia, 2020; pp. 31–32. [Google Scholar]
| Species | Developmental Stage Tested | Investigated Action | No. of Tested Individuals |
|---|---|---|---|
| Leptinotarsa decemlineata | Larvae | contact, digestive | 320 |
| Sitophilus granarius | Adults | contact, digestive | 320 |
| Tenebrio molitor | Larvae | contact, digestive | 320 |
| Variants (Amount of Apitoxin in Microspheres (%) | Apitoxin Based Microspheres Per Repetition (mg) |
Dose of Apitoxin Per Repetition (mg) |
|---|---|---|
| 0.2% | 53.36 | 0.11 |
| 0.4% | 53.36 | 0.21 |
| 0.6% | 53.36 | 0.32 |
| Control * | - | - |
| Apitoxin Content (%w/v) | Lc Dry (mg/g) | Lc Wet (mg/g) | EE (%) |
|---|---|---|---|
| 0.2% | 102.46 ± 3.07 a* | 2.96 ± 0.08 a | 73.87 ± 1.50 a |
| 0.4% | 108.69 ± 8.82 a | 5.22 ± 0.23 b | 74.13 ± 0.92 a |
| 0.5% | 219.11 ± 4.22 b | 6.33 ± 0.11 b | 76.53 ± 1.63 a |
| 0.6% | 274.84 ± 6.14 c | 7.94 ± 0.16 c | 73.76 ± 2.00 a |
| MS Composition | Wet MS Diameter (μm) | Dry MS Diameter (μm) | MS Diameter after Swelling (μm) | Swelling Degree (%) |
|---|---|---|---|---|
| 1.5% alginate/0.5% apitoxin | 301.58 ± 40.09 a* | 226.95 ± 29.06 a | 436.04 ± 37.79 a | 136.77 ± 4.17 a |
| 1.5% alginate (control) | 294.13 ± 46.05 a | 178.44 ± 36.71 b | 320.32 ± 19.78 b | 33.90 ± 1.25 b |
| Microspheres Formulations (Ca-Alginate/Apitoxin) | Release Medium | K (min−1) | n | R2 |
|---|---|---|---|---|
| wet | deionized water | 0.0091 | 0.4807 | 0.9996 |
| dry | 0.0018 | 0.5654 | 0.9984 | |
| dry | sodium-citrate buffer | 0.5710 | 0.2127 | 0.9901 |
| Treatment | Dose of Apitoxin Per Repetition (mg) | Days after the Treatment | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Leptinotarsa decemlineata | ||||
| contact | 0.11 | 0 ± 0 b | 22.81 ± 7.02 b | 43.86 ± 7.02 b |
| 0.21 | 3.3 ± 3.3 ab | 40.35 ± 3.51 ab | 61.4 ± 3.51 ab | |
| 0.32 | 3.3 ± 3.3 ab | 26.32 ±6.08 b | 47.37 ± 12.15 b | |
| digestive | 0.11 | 16.7 ± 6.7 ab | 47.37 ± 6.08 ab | 96.49 ± 3.51 a |
| 0.21 | 26.7 ± 3.3 a | 57.89 ± 6.08 a | 75.44 ± 3.51 ab | |
| 0.32 | 23.3 ± 8.8 ab | 47.37 ± 6.08 ab | 78.95 ± 12.16 ab | |
| HSD p = 0.05 ** | 25.33 | 28.11 | 35.35 | |
| Sitophilus granarius | ||||
| contact | 0.11 | 2.5 ± 2.5 | 0.6 ± 4.6 b* | 12.2 ± 2 b |
| 0.21 | 5.0 ± 5 | 9.1 ± 6.8 ab | 16.8 ± 3.6 b | |
| 0.32 | 15.0 ± 2.9 | 22.4 ± 1.7 a | 40.0 ± 0 a | |
| digestive | 0.11 | 0 ± 0 | 10.0 ± 0 b | 14.6 ± 2.3 b |
| 0.21 | 0 ± 0 | 12.2 ± 2 b | 20.0 ± 0 b | |
| 0.32 | 5.0 ± 2.9 | 24.8 ± 1.9 a | 47.5 ± 1.4 a | |
| HSD p = 0.05 ** | 13.0 | 10.4 | 11.6 | |
| Tenebrio molitor | ||||
| 0.11 | 10.0 ± 21.2 | 18.2 ± 2.2 | 18.2 ± 2.2 ab* | |
| contact | 0.21 | 10.0 ± 5.8 | 10.1 ± 0.9 | 19.3 ± 0.5 ab |
| 0.32 | 5.0 ± 2.9 | 21.6 ± 0.5 | 34.8 ± 0.2 a | |
| 0.11 | 0 ± 0 | 0 ± 0 | 0 ± 0 b | |
| digestive | 0.21 | 0 ± 0 | 0 ± 0 | 1.3 ± 0.6 b |
| 0.32 | 0 ± 0 | 0 ± 0 | 0 ± 0 b | |
| HSD p = 0.05 ** | ns | ns | 31.4 | |
| Agricultural Production | Type of Preparation | Recommended Dosage ** | Price Per Hectare (€/ha) |
|---|---|---|---|
| Arable crops | Chemical | 150 mL/ha | 22 * |
| Biological | 150 mL/ha | 32 | |
| Microspheres with apitoxin | 16 g/ha | 318 ¥ | |
| Vegetables | Chemical | 300 mL/ha | 32 |
| Biological | 600 g/ha | 34 | |
| Microspheres with apitoxin | 16 g/ha | 318 ¥ | |
| Orchards | Chemical | 100 mL/100l | 69 |
| Biological | 1000 g/ha | 90 | |
| Microspheres with apitoxin | 16 g/ha | 318 ¥ | |
| Ecological | Bioinsecticide | 50 g/m2 | 26,513 |
| Booster | 10 L/ha | 1753 | |
| Microorganism | 1 kg/ha | 131 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemic, D.; Orešković, M.; Mikac, K.M.; Marijan, M.; Jurić, S.; Vlahoviček-Kahlina, K.; Vinceković, M. Sustainable Pest Management Using Biodegradable Apitoxin-Loaded Calcium-Alginate Microspheres. Sustainability 2021, 13, 6167. https://doi.org/10.3390/su13116167
Lemic D, Orešković M, Mikac KM, Marijan M, Jurić S, Vlahoviček-Kahlina K, Vinceković M. Sustainable Pest Management Using Biodegradable Apitoxin-Loaded Calcium-Alginate Microspheres. Sustainability. 2021; 13(11):6167. https://doi.org/10.3390/su13116167
Chicago/Turabian StyleLemic, Darija, Matej Orešković, Katarina M. Mikac, Marijan Marijan, Slaven Jurić, Kristina Vlahoviček-Kahlina, and Marko Vinceković. 2021. "Sustainable Pest Management Using Biodegradable Apitoxin-Loaded Calcium-Alginate Microspheres" Sustainability 13, no. 11: 6167. https://doi.org/10.3390/su13116167







