Chemical Characterization of Particulate Matter in the Renaissance City of Ferrara
Abstract
1. Introduction
2. Materials and Methods
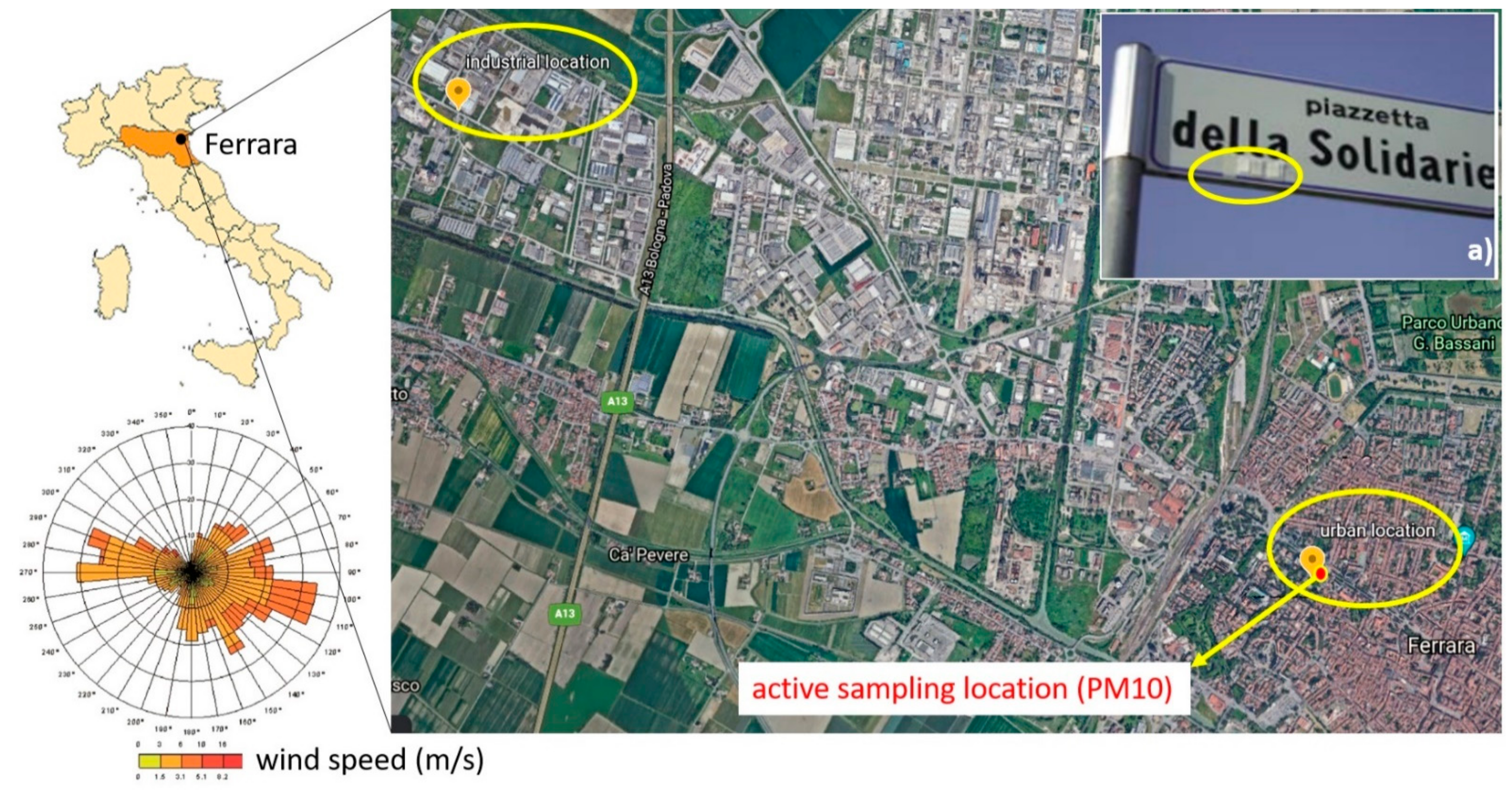
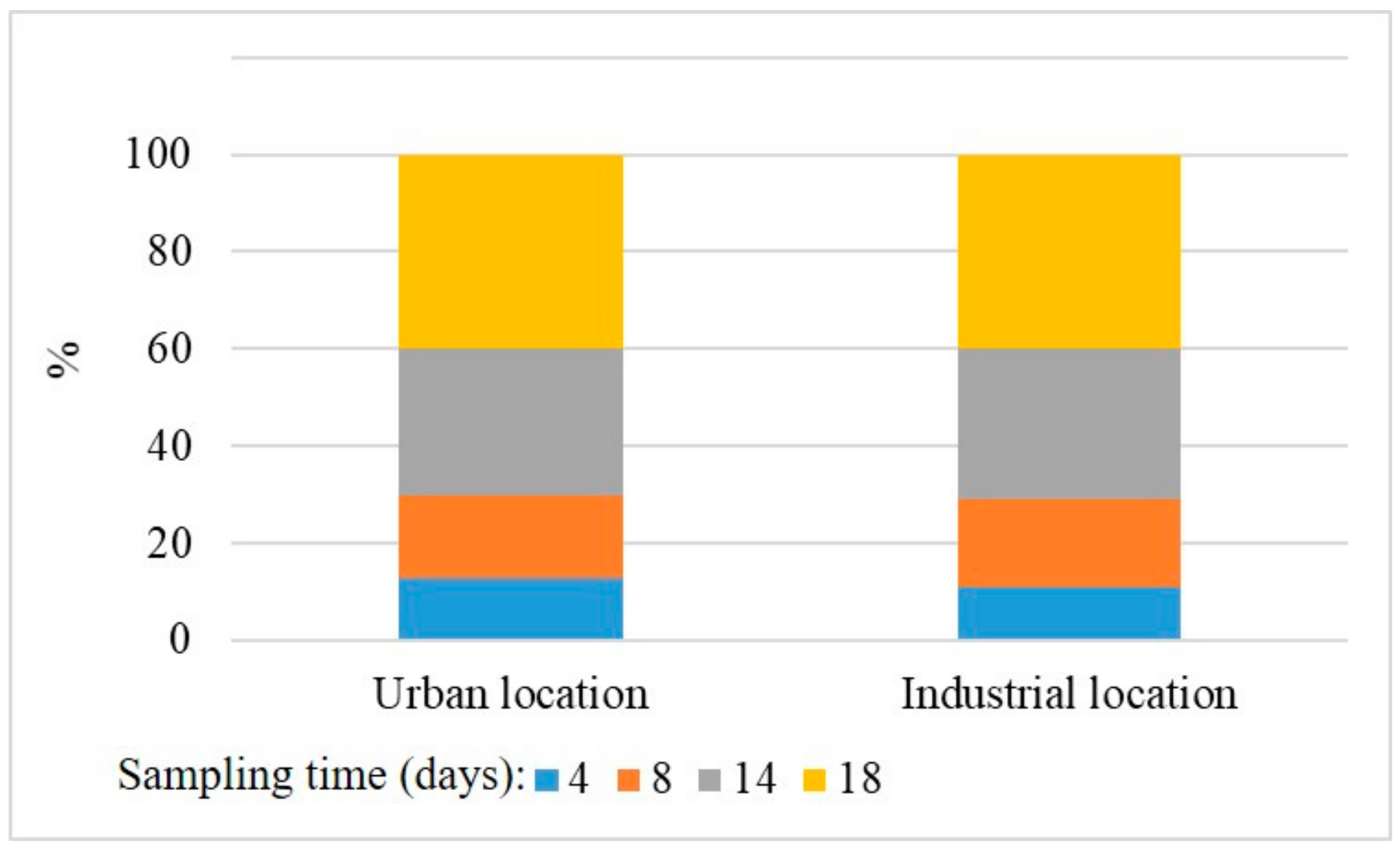
2.1. Study Area, Sampling and Meteorological Conditions
2.2. Analytical Methods
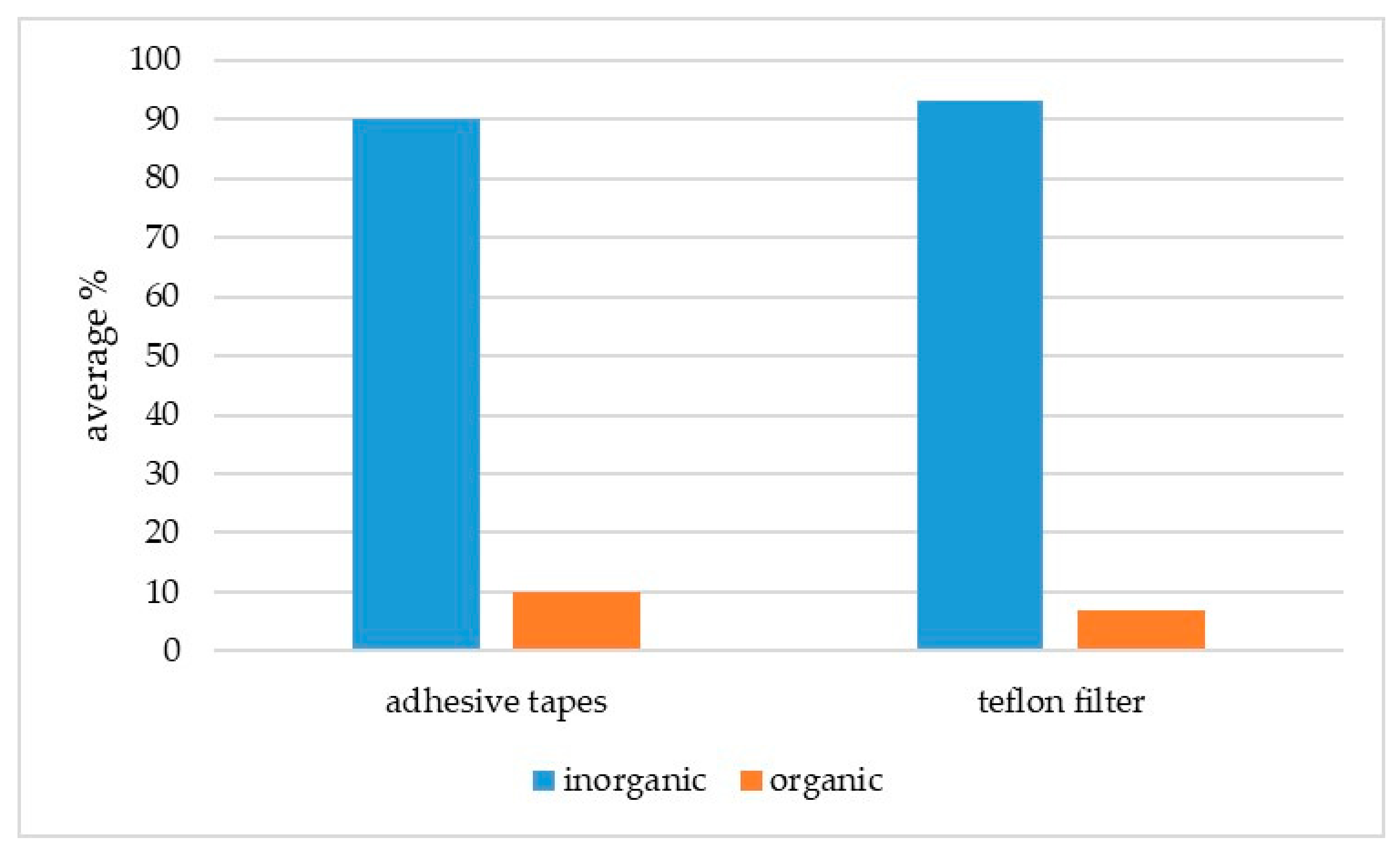
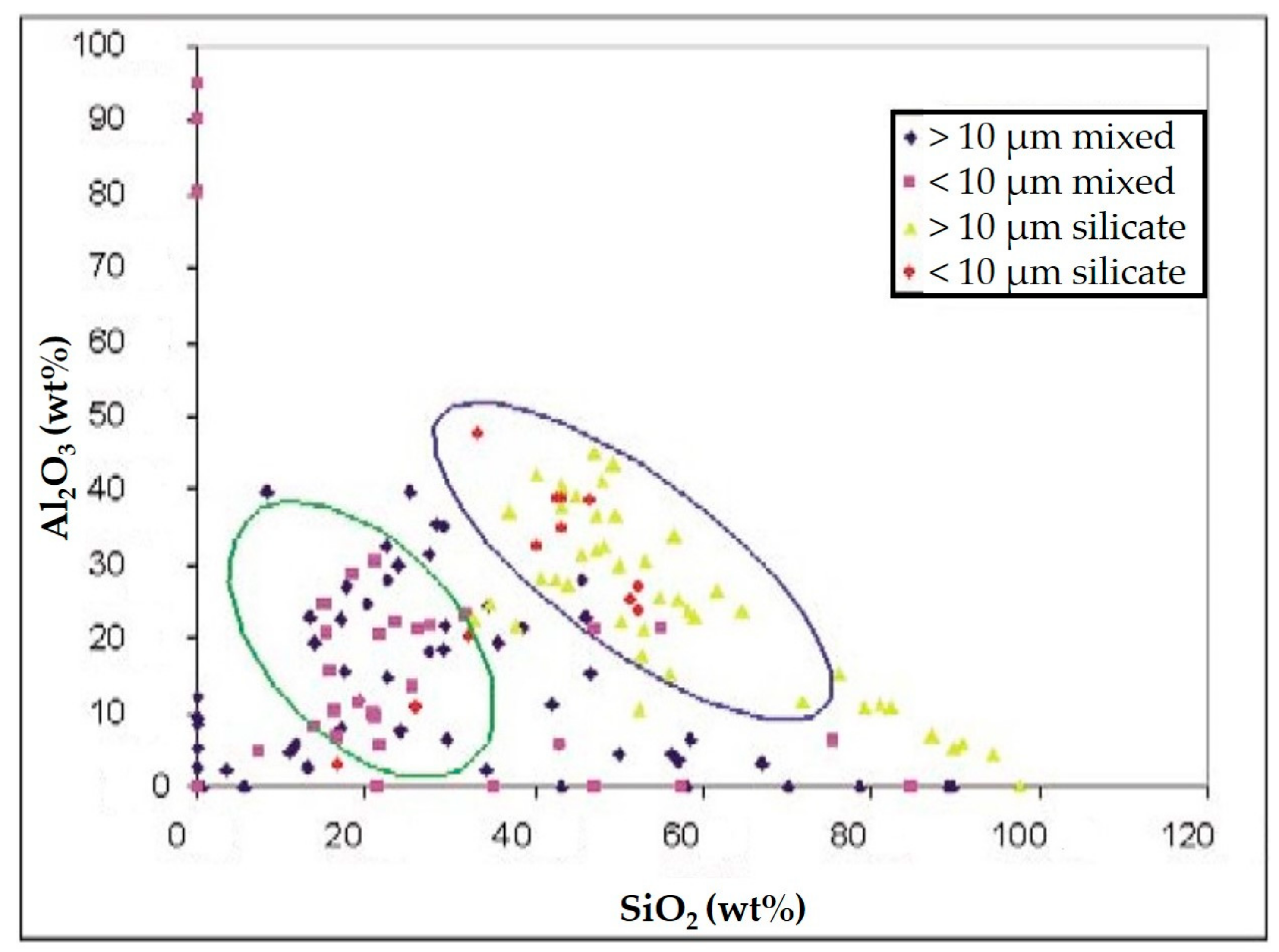
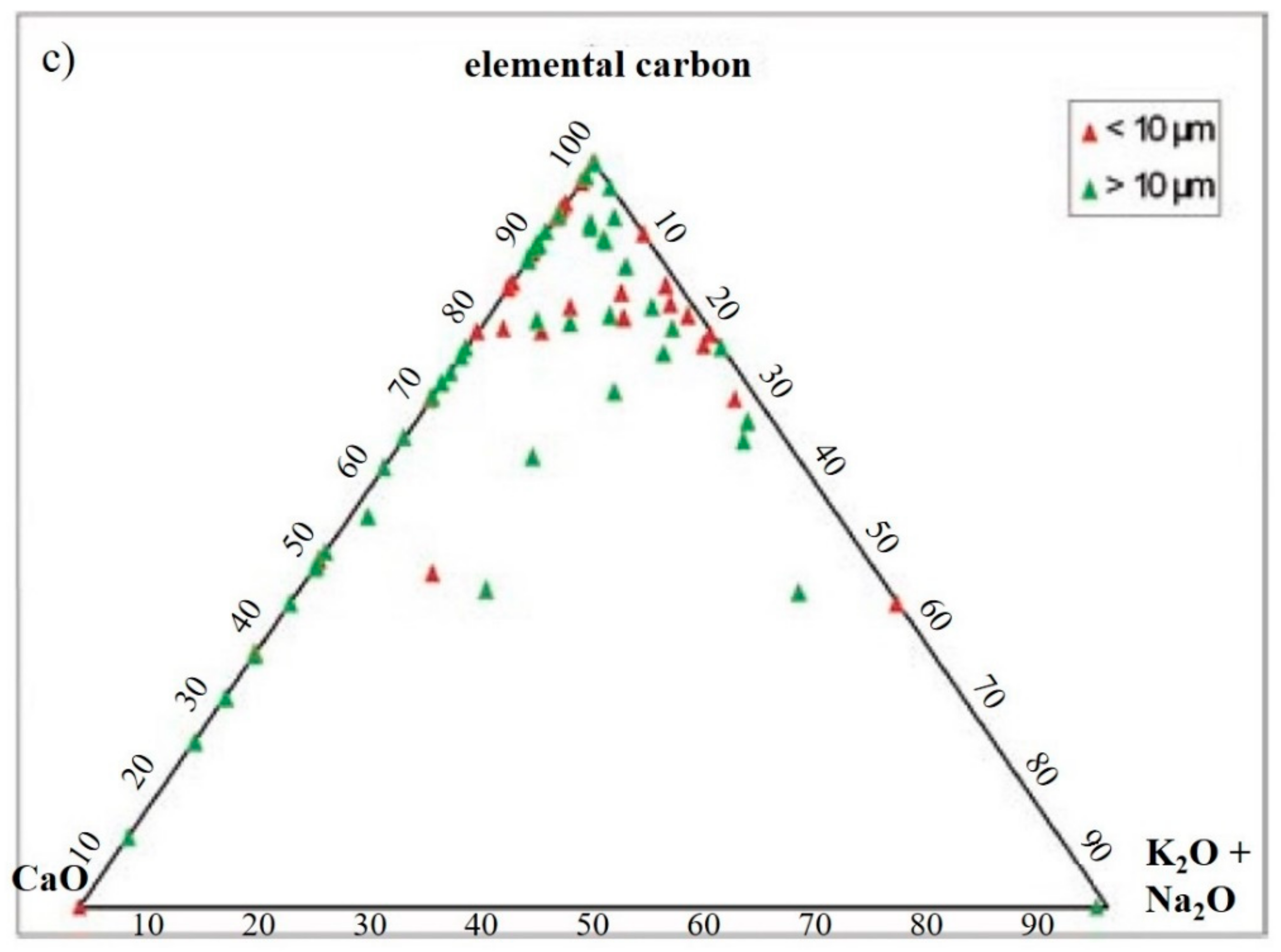
3. Results
- −
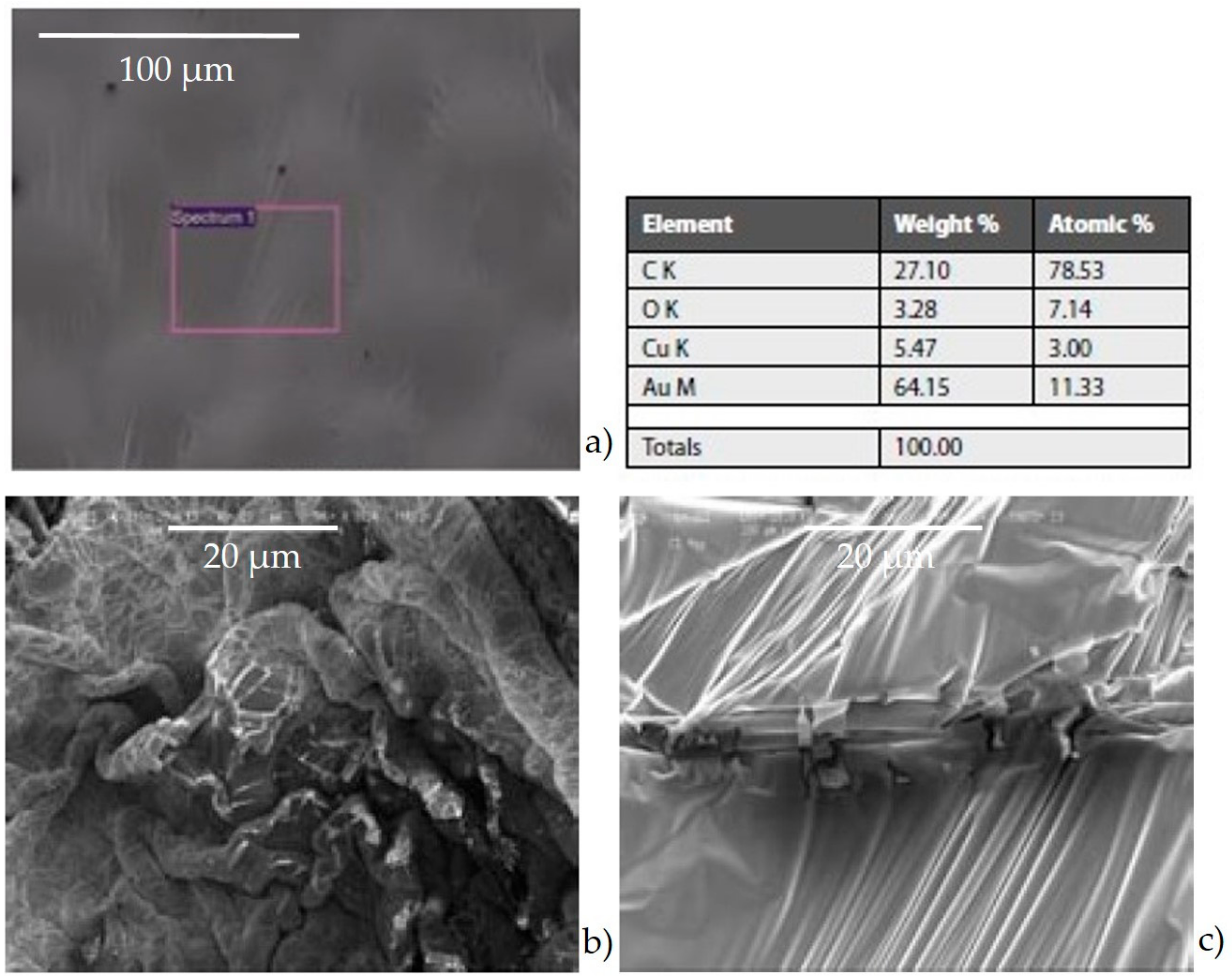
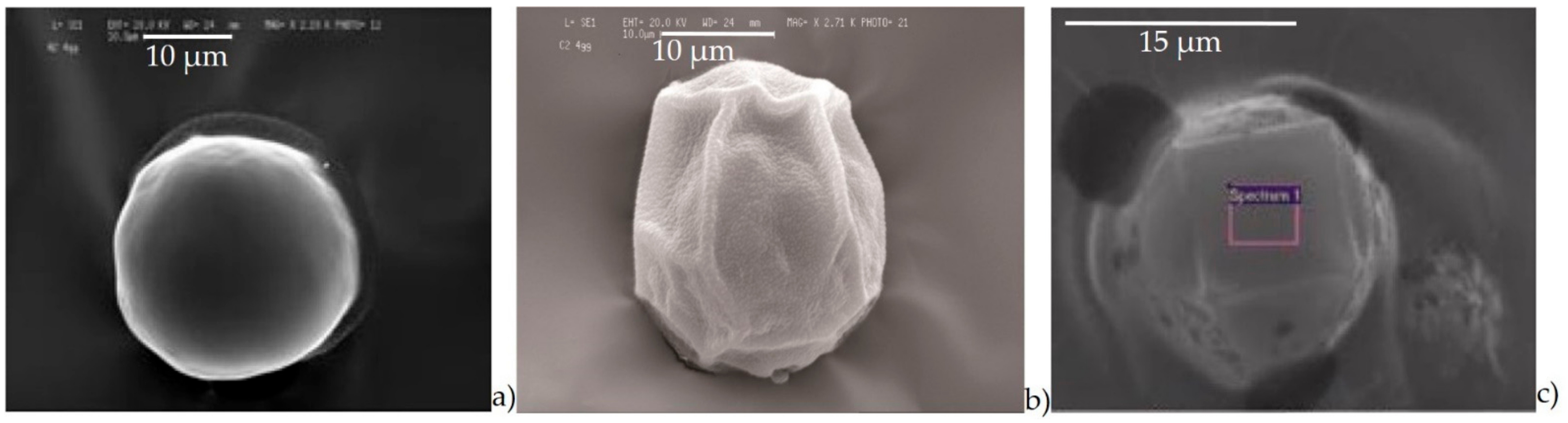
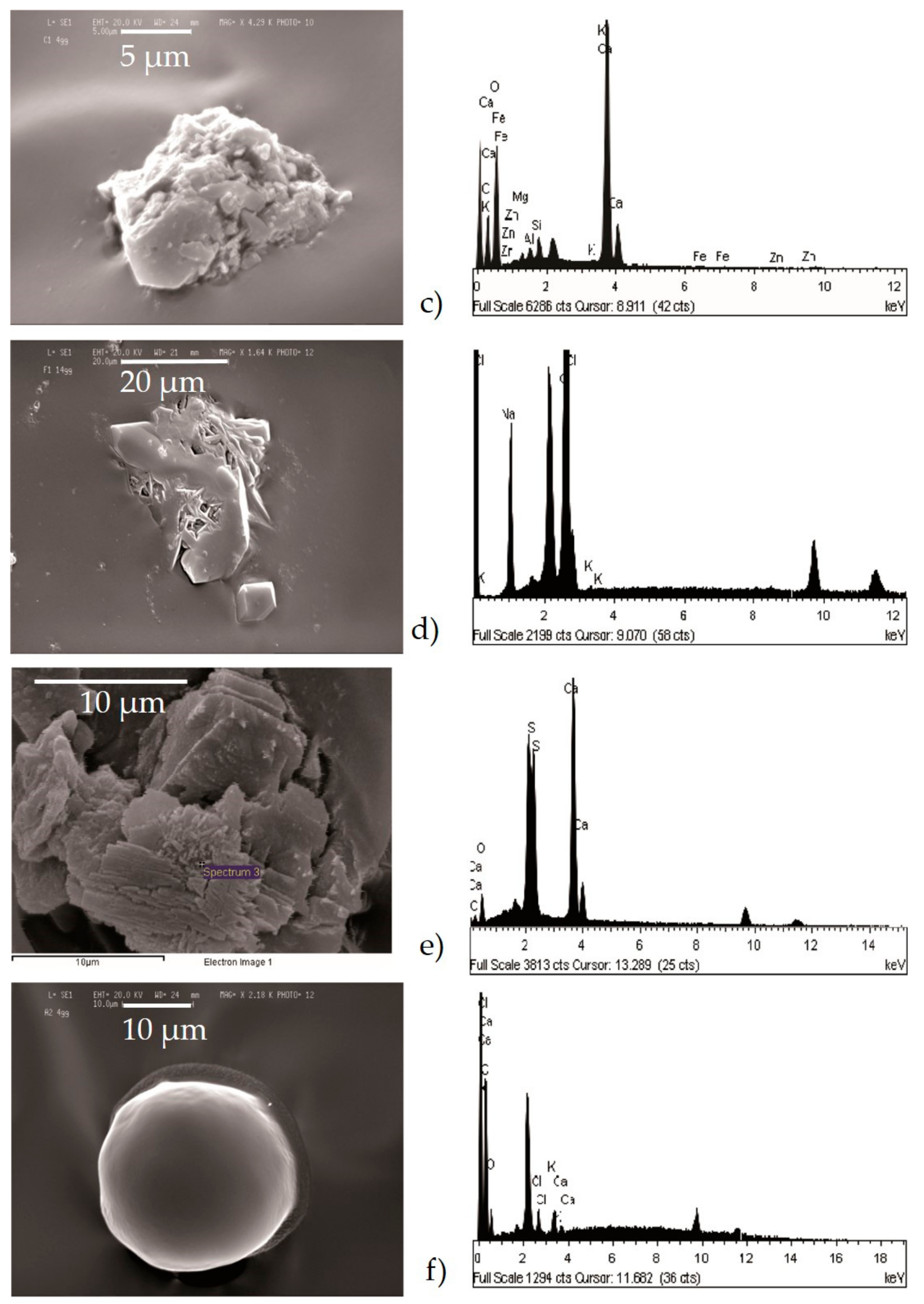
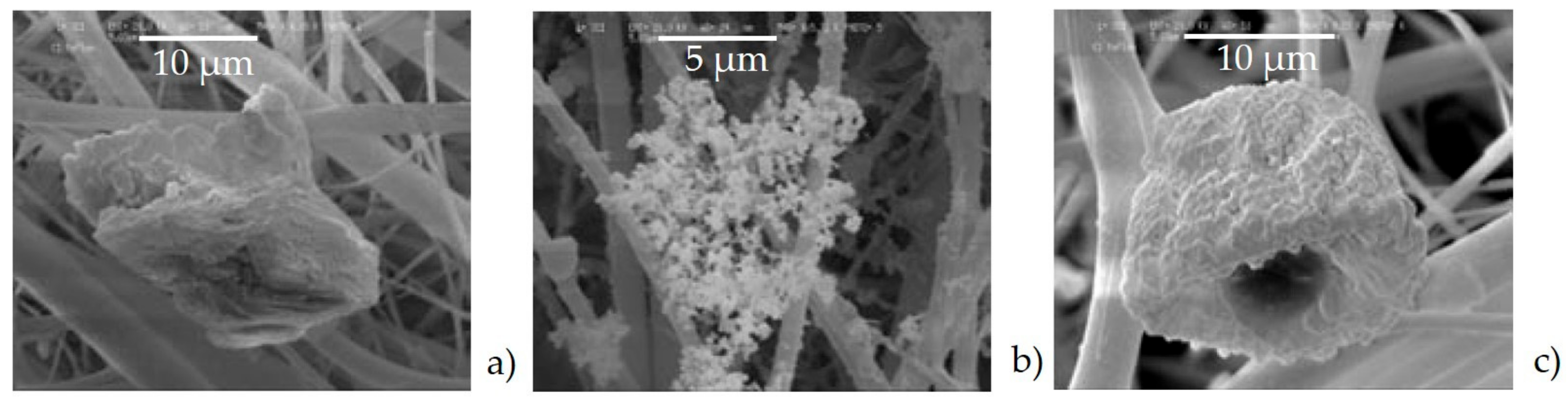
- Agglomerates (Figure 6a) were composed by different kinds of small particles that joined together to form a single particle. The spectrum showed the prevalence of silicates with the high peak of Si, Al, Fe, as well as Mg. The aggregates on the adhesive tapes appeared as a single monodisperse particle: they were not surrounded by other particles, such as those with a small size. This suggested that aggregation occurred directly in the atmosphere and not after being captured by sampling.
- −
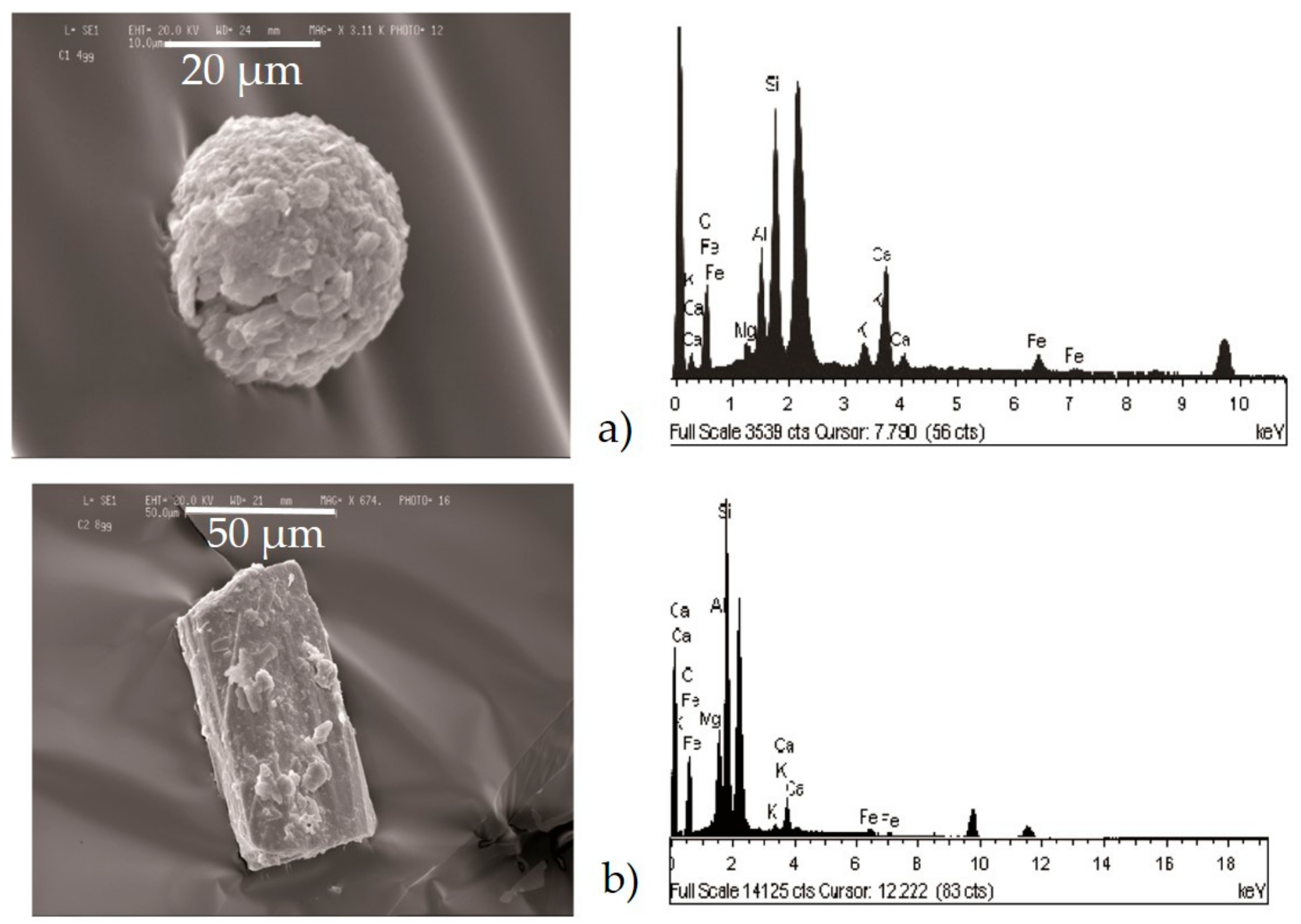
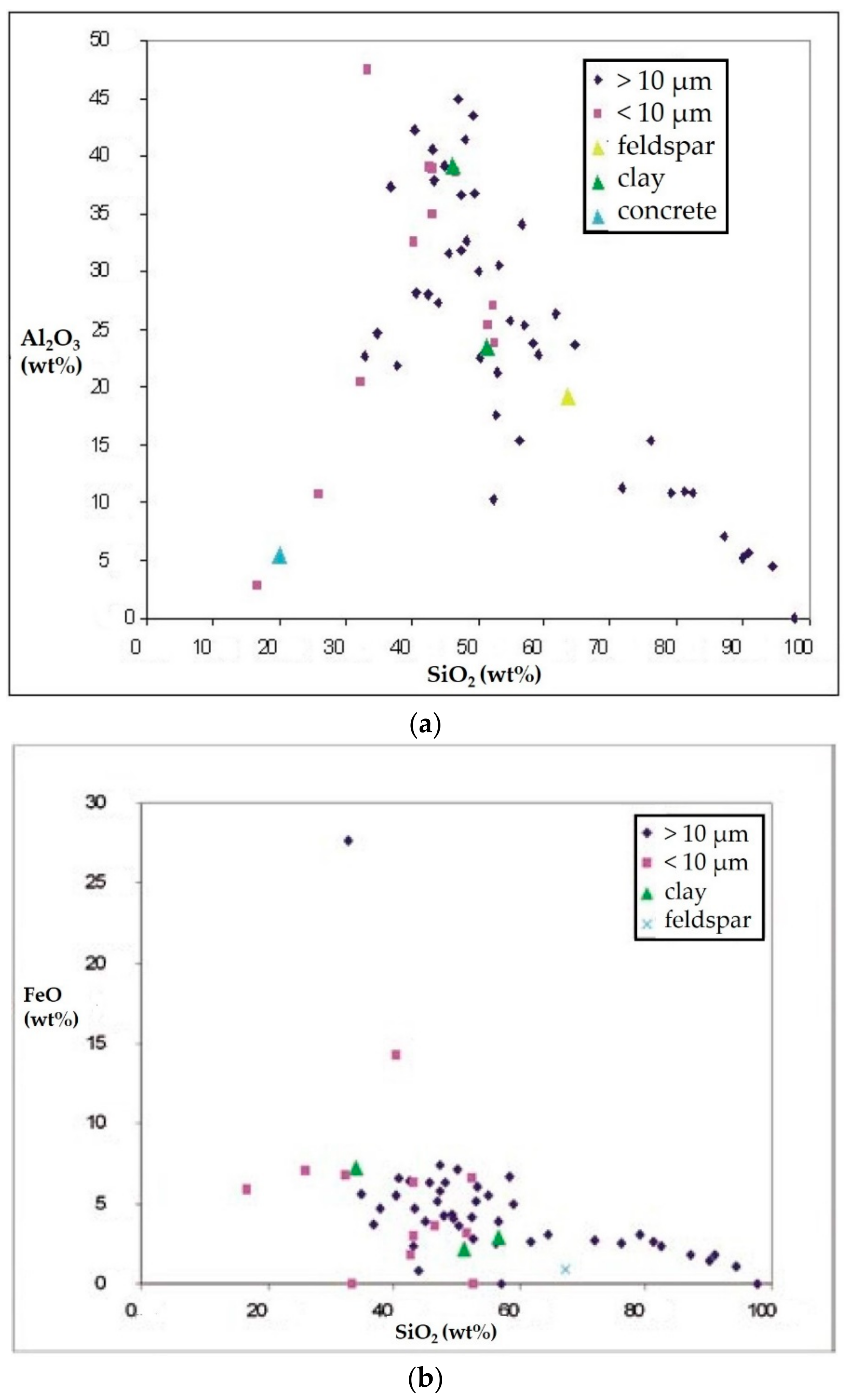
- Silicates (Figure 6b) with variable shapes, from rounded to elongated or irregular globular. The spectrum showed high peaks of Si, followed by Al, Fe, Mg, Ca, K, and sometimes small peaks of Na, Ti, and Zn. The natural ones originated form airborne soil dust from windblown soil dust and re-suspension of dust from roads. The anthropic ones could be assumed as fly ash particles originating from different kinds of combustion processes (coal-fired plants, traffic, and urban sources).
- −
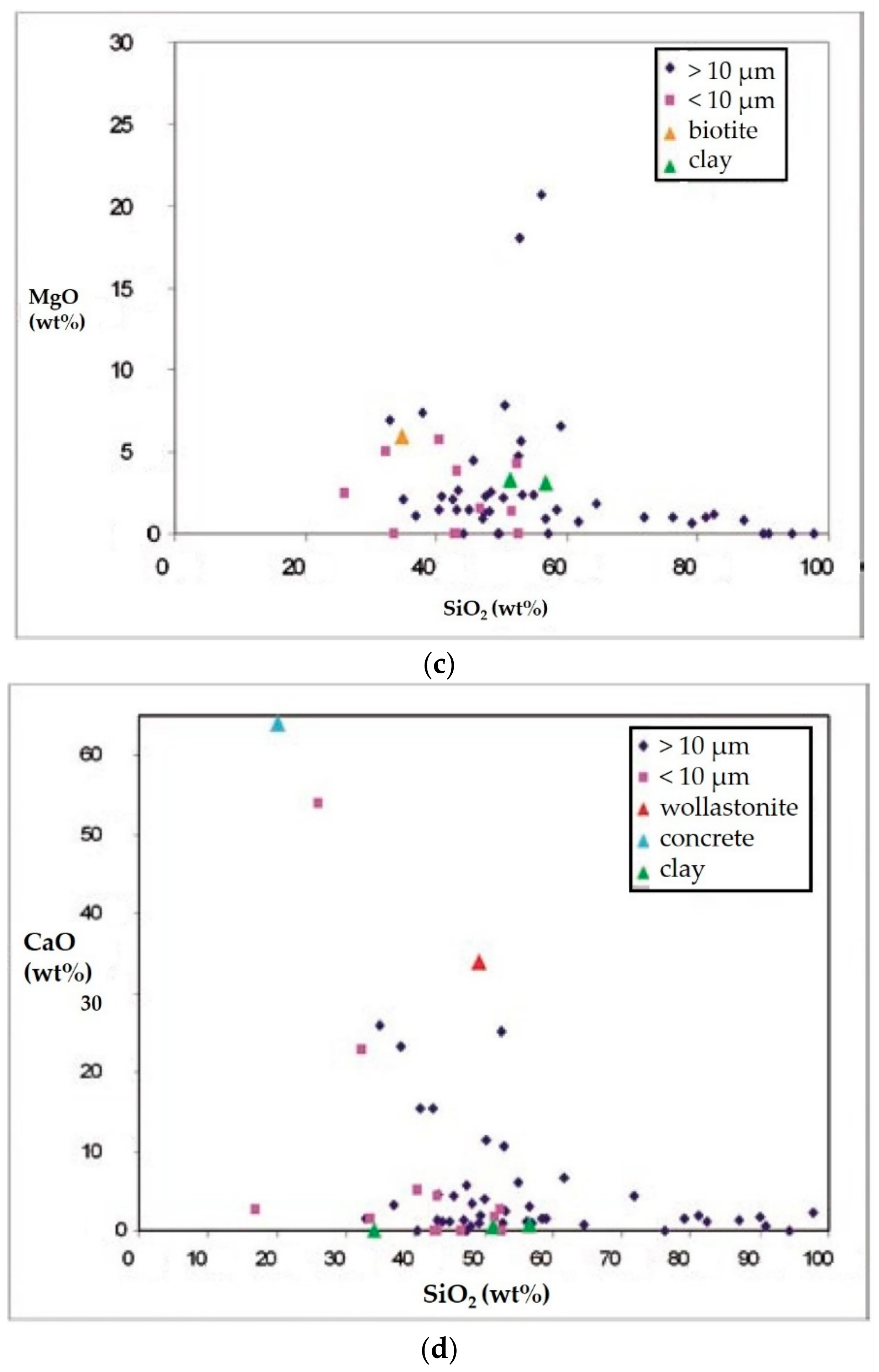
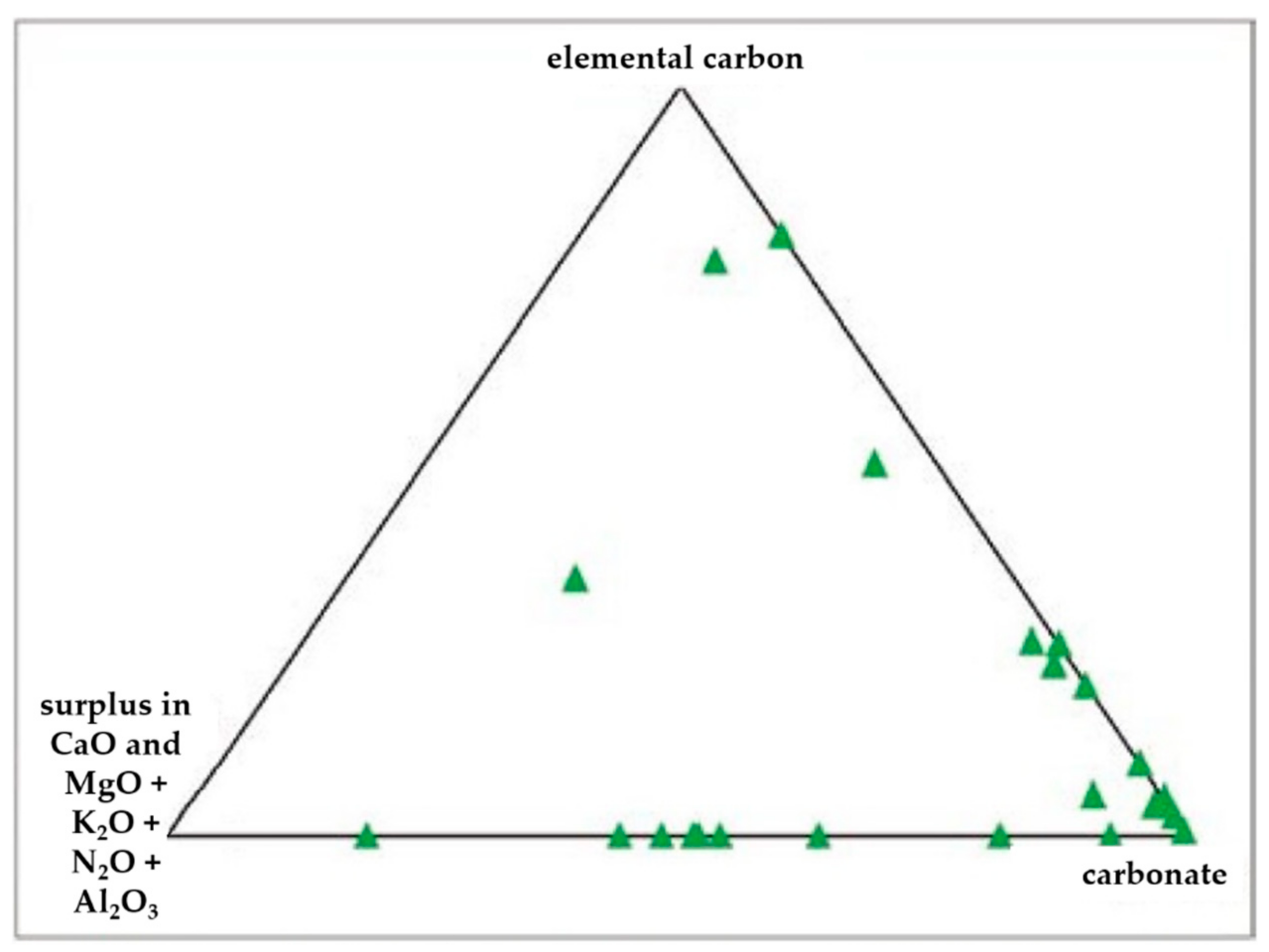
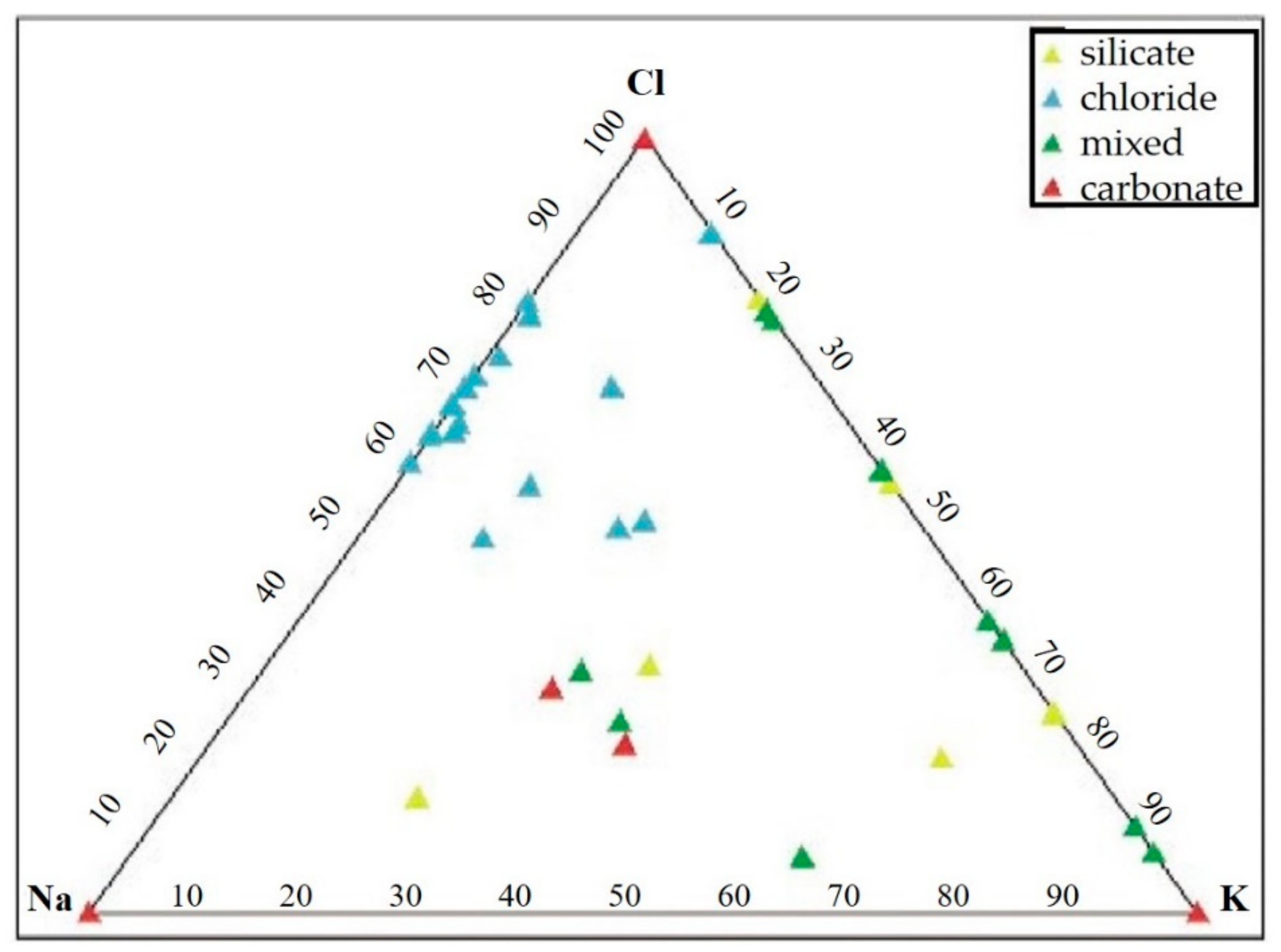
- Carbonates (Figure 6c) showed high peaks in Ca and Mg with impurities of Al, Fe, Cl, alkali (Na, K), and Si. These particles were probably of limestone and/or dolomitic origin. Limestone was distributed as crustal minerals and was widely used in building manufacturing and in metallurgic processes, and dolomite was generally used in metallurgical, chemical, and glass industry, in fertilizers, in ceramics, and as filler in paper and plastic industry. The content of Al, Fe, and Si could be related to impurities present both in dolomites and in limestone. The presence of narrow quantities of Cl and alkali could be explained with the presence of airborne halite particles in the atmosphere.
- −
- Chlorides (Figure 6d) showed a high value of Cl and Na, and K was also present. Generally, these kinds of particles are natural particles originating from marine aerosol; however, knowing the sampling area, which is rich in industrial activity, they were probably related to the fertilizer industry.
- −
- Sulphates (Figure 6e) were less abundant than others and were characterized by high peaks of S and Ca. They could be related to the presence of gypsum particles.
- −
- C-particles (Figure 6f) presented a large size and a rounded shape typical of traffic particles coming from the combustion of oils. The spectrum showed high peaks of C and O and very small Ca, Cl, and alkali peaks, which was probably related to the impurities present in the atmosphere.
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Council Directive 1999/30/EC of 22 April 1999 Relating to Limit Values for Sulphur Dioxide, Nitrogen Dioxide and Oxides of Nitrogen, Particulate Matter and Lead in Ambient Air. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:1999:163:0041:0060:EN:PDF (accessed on 15 April 2021).
- Polica, S.; Sahu, S.K.; Pandit, G.G. Chemical characterization of atmospheric particulate matter and their source apportionment at an emerging industrial coastal city, Visakhapatnam, India. Atmos. Pollut. Res. 2016, 7, 725–733. [Google Scholar] [CrossRef]
- Rushingabigwi, G.; Nsengiyumva, P.; Sibomana, L.; Twizere, C.; Kalisa, W. Analysis of the atmospheric dust in Africa: The breathable dust’s fine particulate matter PM2.5 in correlation with carbon monoxide. Atmos. Environ. 2020, 224, 117319. [Google Scholar] [CrossRef]
- Zhang, C.; Zou, Z.; Chang, Y.; Zhang, Y.; Wang, X.; Yang, X. Source assessment of atmospheric fine particulate matter in a Chinese megacity: Insights from long-term, high-time resolution chemical composition measurements from Shanghai flagship monitoring supersite. Chemosphere 2020, 251, 126598. [Google Scholar] [CrossRef] [PubMed]
- De Rooij, M.M.T.; Smit, L.A.M.; Erbrink, H.J.; Hagenaars, T.J.; Hoek, G.; Ogink, N.W.M.; Winkel, A.; Heederik, D.J.J.; Wouters, I.M. Endotoxin and particulate matter emitted by livestock farms and respiratory health effects in neighboring residents. Environ. Int. 2019, 132, 105009. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, H.; Pan, W.; Xue, Q.; Fu, J.; Liu, G.; Zheng, M.; Zhang, A. A novel computational solution to the health risk assessment of air pollution via joint toxicity prediction: A case study on selected PAH binary mixtures in particulate matters. Ecotoxicol. Environ. Saf. 2019, 170, 427–435. [Google Scholar] [CrossRef]
- Francová, A.; Chrastný, V.; Vítková, M.; Šillerová, H.; Komárek, M. Health risk assessment of metal(loid)s in soil and particulate matter from industrialized regions: A multidisciplinary approach. Environ. Pollut. 2020, 260, 114057. [Google Scholar] [CrossRef]
- Qin, N.; Liang, P.; Wu, C.; Wang, G.; Xu, Q.; Xiong, X.; Wang, T.; Zolfo, M.; Segata, N.; Qin, H.; et al. Longitudinal survey of microbiome associated with particulate matter in a megacity. Genome Biol. 2020, 21, 55. [Google Scholar] [CrossRef]
- Ramírez, O.; Sánchez de la Campa, A.M.; Sánchez-Rodas, D.; De la Rosa, J.D. Hazardous trace elements in thoracic fraction of airborne particulate matter: Assessment of temporal variations, sources, and health risks in a megacity. Sci. Total Environ. 2020, 710, 136344. [Google Scholar] [CrossRef]
- Fermo, P.; Comite, V.; Ciantelli, C.; Sardella, A.; Bonazza, A. A multi-analytical approach to study the chemical composition of total suspended particulate matter (TSP) to assess the impact on urban monumental heritage in Florence. Sci. Total Environ. 2020, 740, 140055. [Google Scholar] [CrossRef]
- Samara, C.; Melfos, V.; Kouras, A.; Karali, E.; Zacharopoulou, G.; Kyranoudi, M.; Papadopoulou, L.; Pavlidou, E. Morphological and geochemical characterization of the particulate deposits and the black crust from the Triumphal Arch of Galerius in Thessaloniki, Greece: Implications for deterioration assessment. Sci. Total Environ. 2020, 734, 139455. [Google Scholar] [CrossRef]
- Kampa, M.; Castanas, E. Human Health Effects of Air Pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- Kim, H.W.; Ahn, E.K.; Jee, B.K.; Yoon, H.K.; Lee, K.H.; Lim, Y. Nanoparticulate-Induced Toxicity and Related Mechanism in Vitro and in Vivo. J. Nanopart. Res. 2009, 11, 55–65. [Google Scholar] [CrossRef]
- Pacyna, J.M.; Pacyna, E.G. An Assessment of Global and Regional Emissions of Trace Metals to the Atmosphere from Anthropogenic Sources Worldwide. Environ. Res. 2001, 9, 269–298. [Google Scholar] [CrossRef]
- Askariyeh, M.H.; Venugopal, M.; Khreis, H.; Birt, A.; Zietsman, J. Near-Road Traffic-Related Air Pollution: Resuspended PM2.5 from Highways and Arterials. Int. J. Environ. Res. Public Health 2020, 17, 2851. [Google Scholar] [CrossRef]
- Candeias, C.; Vicente, E.; Tomé, M.; Rocha, F.; Ávila, P.; Célia, A. Geochemical, Mineralogical and Morphological Characterisation of Road Dust and Associated Health Risks. Int. J. Environ. Res. Public Health 2020, 17, 1563. [Google Scholar] [CrossRef]
- Lucarelli, F.; Calzolai, G.; Chiari, M.; Giardi, F.; Czelusniak, C.; Nava, S. Hourly Elemental Composition and Source Identification by Positive Matrix Factorization (PMF) of Fine and Coarse Particulate Matter in the High Polluted Industrial Area of Taranto (Italy). Atmosphere 2020, 11, 419. [Google Scholar] [CrossRef]
- Zeng, Q.; Tao, J.; Chen, L.; Zhu, H.; Zhu, S.; Wang, Y. Estimating Ground-Level Particulate Matter in Five Regions of China Using Aerosol Optical Depth. Remote Sens. 2020, 12, 881. [Google Scholar] [CrossRef]
- Dourado, T.A.; Gemeiner, H.; Gomes, A.C.F.; Almeida, E.; da Silva, A.C.; Valadão, N.; Menegário, A.A.; Govone, J.S.; Gastmans, D. Elemental Composition of Particulate Matter in the Southeastern Brazilian Ceramic Pole by Synchrotron Radiation X-ray Fluorescence Technique (SR-XRF). J. Braz. Chem. Soc. 2020, 31, 1203–1215. [Google Scholar] [CrossRef]
- Feng, S.X.; Pan, S.Q.; Wang, Y.R. Research on the Application of Porous Ceramic Waste Gas Purification Device in Ceramic Kiln. Mater. Sci. Forum 2020, 980, 410–418. [Google Scholar] [CrossRef]
- Liu, Z.; Bode, V.; Hadayati, P.; Onay, H.; Sudhölter, E.J.R. Understanding the stability mechanism of silica nanoparticles: The effect of cations and EOR chemicals. Fuel 2020, 280, 118650. [Google Scholar] [CrossRef]
- Conca, E.; Malandrino, M.; Giacomino, A.; Inaudi, P.; Buoso, S.; Bande, S.; Sacco, M.; Abollino, O. Contribution of the Incinerator to the Inorganic Composition of the PM10 Collected in Turin. Atmosphere 2020, 11, 400. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Gentilini, P.; Diella, G.; Lopuzzo, M.; Ridolfi, R. Biomonitoring of Metals in Children Living in an Urban Area and Close to Waste Incinerators. Int. J. Environ. Res. Public Health 2020, 17, 1919. [Google Scholar] [CrossRef]
- Viskup, R.; Wolf, C.; Baumgartner, W. Qualitative and Quantitative Characterisation of Major Elements in Particulate Matter from In-use Diesel Engine Passenger Vehicles by LIBS. Energies 2020, 13, 368. [Google Scholar] [CrossRef]
- Giechaskiel, B. Particle Number Emissions of a Diesel Vehicle during and between Regeneration Events. Catalysts 2020, 10, 587. [Google Scholar] [CrossRef]
- Park, J.; Choi, I.; Oh, J.; Lee, C. Nitrogen Oxides and Particulate Matter from Marine Diesel Oil (MDO), Emulsified MDO, and Dimethyl Ether Fuels in Auxiliary Marine Engines. J. Mar. Sci. Eng. 2020, 8, 322. [Google Scholar] [CrossRef]
- Winkler, A.; Contardo, T.; Vannini, A.; Sorbo, S.; Basile, A.; Loppi, S. Magnetic Emissions from Brake Wear are the Major Source of Airborne Particulate Matter Bioaccumulated by Lichens Exposed in Milan (Italy). Appl. Sci. 2020, 10, 2073. [Google Scholar] [CrossRef]
- Bralewska, K.; Rakowska, J. Concentrations of Particulate Matter and PM-Bound Polycyclic Aromatic Hydrocarbons Released during Combustion of Various Types of Materials and Possible Toxicological Potential of the Emissions: The Results of Preliminary Studies. Int. J. Environ. Res. Public Health 2020, 17, 3202. [Google Scholar] [CrossRef]
- Dirks, K.N.; Chester, A.; Salmond, J.A.; Talbot, N.; Thornley, S.; Davy, P. Arsenic in Hair as a Marker of Exposure to Smoke from the Burning of Treated Wood in Domestic Wood Burners. Int J. Environ. Res. Public Health 2020, 17, 3944. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, M.; Katsivela, E.; Kopanakis, I.; Raisi, L.; Mihalopoulos, N.; Panagiaris, G. Characterization of airborne particulate matter and microbes inside cultural heritage collections. J. Cult. Herit. 2018, 30, 136–146. [Google Scholar] [CrossRef]
- Morillas, H.; Maguregui, M.; Gallego-Cartagena, E.; Marcaida, I.; Carral, N.; Madariaga, J.M. The influence of marine environment on the conservation state of Built Heritage: An overview study. Sci. Total Environ. 2020, 745, 140899. [Google Scholar] [CrossRef]
- Radulescu, C.; Stihi, C.; Ion, R.-M.; Dulama, I.-D.; Stanescu, S.-G.; Stirbescu, R.M.; Teodorescu, S.; Gurgu, I.-V.; Let, D.-D.; Olteanu, L.; et al. Seasonal Variability in the Composition of Particulate Matter and the Microclimate in Cultural Heritage Areas. Atmosphere 2019, 10, 595. [Google Scholar] [CrossRef]
- Park, S.S.; Kim, Y.J. Source contributions to fine particulate matter in an urban atmosphere. Chemosphere 2005, 59, 217–226. [Google Scholar] [CrossRef] [PubMed]
- England, G.C.; Watson, J.G.; Chow, J.C.; Zielinska, B.; Chang, M.C.O.; Loos, K.R.; Hidy, G.M. Dilution-Based Emissions Sampling from Stationary Sources: Part 1—Compact Sampler Methodology and Performance. J. Air Waste Manag. Assoc. 2007, 57, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Planquette, H.; Sherrell, R.M. Sampling for particulate trace element determination using water sampling bottles: Methodology and comparison to in situ pumps. Limnol. Oceanogr. Methods 2012, 10. [Google Scholar] [CrossRef]
- Hassan, S.A.M. Multiscientific approach for the characterization and assessment of the degradation state of the historical Al-Shafi’i mosque walls (Jeddah, Kingdom of Saudi Arabia). Sci. Cult. 2021, 7, 1–19. [Google Scholar]
- Abu-Allaban, M.; El-Khalili, M.M.M. Antiquity impact of air pollution at Gadara, Jordan. Mediterr. Archaeol. Archaeom. 2014, 14, 191–199. [Google Scholar]
- Abdel-Kareem, O. Monitoring, controlling and prevention of the fungal deterioration of textile artifacts in the museum of jordanian heritage. Mediterr. Archaeol. Archaeom. 2010, 10, 85–96. [Google Scholar]
- Naif, A.H.; Fakhoury, L.A.; Sakr, Y.M. A critical anthology of international charters, conventions & principles on documentation of cultural heritage for conservation, monitoring & management. Mediterr. Archaeol. Archaeom. 2021, 21, 291–310. [Google Scholar]
- Lamhasni, T.; El-Marjaoui, H.; El Bakkali, A.; Lyazidi, S.A.; Haddad, M.; Ben-Ncer, A.; Benyaich, F.; Bonazza, A.; Tahri, M. Air pollution impact on architectural heritage of Morocco: Combination of synchronous fluorescence and ATR-FTIR spectroscopies for the analyses of black crusts deposits. Chemosphere 2019, 225, 517–523. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Pinto, D.; Neckel, A.; Dotto, G.L.; Oliveira, M.L.S. The impact of air pollution on the rate of degradation of the fortress of Florianópolis Island, Brazil. Chemosphere 2020, 251, 126838. [Google Scholar] [CrossRef]
- Spezzano, P. Mapping the susceptibility of UNESCO World Cultural Heritage sites in Europe to ambient (outdoor) air pollution. Sci. Total Environ. 2021, 754, 142345. [Google Scholar] [CrossRef] [PubMed]
- La Russa, M.F.; Fermo, P.; Comite, V.; Belfiore, C.M.N.; Barca, D.; Carioni, A.; De Santis, M.; Barbagallo, L.F.; Ricca, M.; Ruffolo, S.A. The Oceanus statue of the Fontana di Trevi (Rome): The analysis of black crust as a tool to investigate the urban air pollution and its impact on the stone degradation. Sci. Total Environ. 2017, 593–594, 297–309. [Google Scholar] [CrossRef]
- Comite, V.; Fermo, P. The effects of air pollution on cultural heritage: The case study of Santa Maria delle Grazie al Naviglio Grande (Milan). Eur. Phys. J. Plus 2018, 133, 556. [Google Scholar] [CrossRef]
- Marrocchino, E.; Telloli, C.; Vaccaro, C. Geochemical and Mineralogical Characterization of Construction Materials from Historical Buildings of Ferrara (Italy). Geosciences 2021, 11, 31. [Google Scholar] [CrossRef]
- Directive 2004/107/EC of the European Parliament and of the Council Relating to Arsenic, Cadmium, Mercury, Nickel and Polycyclic Aromatic Hydrocarbons in Ambient Air (Fourth Daughter Directive). Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2005:023:0003:0016:EN:PDF (accessed on 15 April 2021).
- European Parliament. Consolidated Text: Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on Ambient Air Quality and Cleaner Air for Europe; European Parliament: Brussels, Belgium, 2008. [Google Scholar]
- Grau-Bové, J.; Strlič, M. Fine particulate matter in indoor cultural heritage: A literature review. Herit. Sci. 2013, 1, 8. [Google Scholar] [CrossRef]
- Kuzmichev, A.A.; Azarov, V.N.; Stefanenko, I.V. The Impact of Dust Particles on Cultural Heritage Objects in the Field of Environmental Mechanics. Appl. Mech. Mater. 2018, 878, 259–262. [Google Scholar] [CrossRef]
- Fettweis, M.; Francken, F.; Pison, V.; Van den Eynde, D. Suspended particulate matter dynamics and aggregate sizes in a high turbidity area. Mar. Geol. 2006, 235, 63–74. [Google Scholar] [CrossRef]
- Cates, A.M.; Ruark, M.D.; Hedtcke, J.L.; Posner, J.L. Long-term tillage, rotation and perennialization effects on particulate and aggregate soil organic matter. Soil Tillage Res. 2016, 155, 371–380. [Google Scholar] [CrossRef]
- Willis, R.D.; Blanchard, F.T.; Conner, T.L. Guidelines for the Application of SEM/EDX Analytical Techniques to Particulate Matter Samples; EPA-600/R-02-070; U.S. Environmental Protection Agency: Research Triangle Park, NC, USA, 2002. [Google Scholar]
- Shi, Z.; Shao, L.; Jones, T.P.; Whittaker, A.G.; Lu, S.; Bérubé, K.A.; He, T.; Richards, R.J. Characterization of airborne individual particles collected in an urban area, a satellite city and a clean air area in Beijing, 2001. Atmos. Environ. 2003, 37, 4097–4108. [Google Scholar] [CrossRef]
- Ebert, M.; Weinbruch, S.; Hoffmann, P.; Ortner, H.M. The chemical composition and complex refractive index of rural and urban influenced aerosols determined by individual particle analysis. Atmos. Environ. 2004, 38, 6531–6545. [Google Scholar] [CrossRef]
- Ghirardo, D.Y. Lucrezia Borgia’s Palace in Renaissance Ferrara. J. Soc. Archit. Hist. 2005, 64, 474–497. [Google Scholar] [CrossRef]
- Caglioti, B. Costabili Palace and the Architecture “All’Antica” in Ferrara at the End of the XV Century. Athens J. Archit. 2021, 7, 173–198. [Google Scholar]
- Arpae Emilia Romagna. Rapporto Sulla Qualità dell’Aria del Comune di Ferrara—Dati 2006; Arpae Emilia Romagna: Bologna, Italy, 2006. [Google Scholar]
- Telloli, C. Metal concentrations in snow samples in an urban area in the Po Valley. Int. J. Geosci. 2014, 5, 116–1136. [Google Scholar] [CrossRef]
- Telloli, C.; Chicca, M.; Leis, M.; Vaccaro, C. Fungal spores and pollen in particulate matter collected during agricultural activities in the Po Valley (Italy). J. Environ. Sci. 2016, 46, 229–240. [Google Scholar] [CrossRef]
- Wilkinson, K.; Lundkvist, J.; Seisenbaeva, G.; Kessler, V. New tabletop SEM-EDS-based approach for cost-efficient monitoring of airborne particulate matter. Environ. Pollut. 2011, 159, 311–318. [Google Scholar] [CrossRef]
- Moreno, T.; Gibbons, W.; Jones, T.; Richards, R. The geology of ambient aerosols: Characterising urban and rural/coastal silicate PM10–2.5 and PM2.5 using high-volume cascade collection and scanning electron microscopy. Atmos. Environ. 2003, 37, 4265–4276. [Google Scholar] [CrossRef]
- Telloli, C.; Malaguti, A.; Mircea, M.; Tassinari, R.; Vaccaro, C.; Berico, M. Properties of agricultural aerosol released during wheat harvest threshing, plowing and sowing. J. Environ. Sci. 2014, 26, 1903–1912. [Google Scholar] [CrossRef]
- Telloli, C.; Chicca, M.; Pepi, S.; Vaccaro, C. Saharan dust particles in snow samples of Alps and Apennines during an exceptional event of transboundary air pollution. Environ. Monit. Assess. 2018, 190, 37. [Google Scholar] [CrossRef]
- Calparsoro, E.; Maguregui, M.; Giakoumaki, A.; Morillas, H.; Madariaga, J.M. Evaluation of black crust formation and soiling process on historical buildings from the Bilbao metropolitan area (north of Spain) using SEM-EDS and Raman microscopy. Environ. Sci. Pollut. Res. 2017, 24, 9468–9480. [Google Scholar] [CrossRef]
- Silva, L.T.; Mendes, B.; Oliveira, C.; Reis, C.; Silva, P.L.; Silva, J.F. Monitoring the potential effect of particulate matter on cultural heritage. Contribution of an environmental monitoring system. In Proceedings of the IRAS 2019—First International Symposium on Risk Analysis and Safety of Complex Structures and Components, Porto, Portugal, 1–2 July 2019; pp. 312–314. [Google Scholar]
- Chen, X.; Zhang, Y.; Huo, H.; Wu, Z. Study of high tensile strength of natural continuous basalt fibers. J. Nat. Fibers 2020, 17, 214–222. [Google Scholar] [CrossRef]
- D.M. 20 Maggio 1991. Criteri per la Raccolta Dei Dati Inerenti la Qualità dell’Aria. G.U. 1991 n.126. Available online: https://www.isprambiente.gov.it/files/aria/d.m.20maggio-1991.pdf (accessed on 15 April 2021).
- Ozvan, A.; Dincer, I.; Akin, M.; Oyan, V.; Tapan, M. Experimental studies on ignimbrite and the effect of lichens and capillarity on the deterioration of Seljuk Gravestones. Eng. Geol. 2015, 185, 81–95. [Google Scholar] [CrossRef]
- Sohrabi, M.; Favero-Longo, S.F.; Pérez-Ortega, S.; Ascaso, C.; Haghighat, Z.; Talebian, M.H.; Fadaei, H.; de los Rios, A. Lichen colonization and associated deterioration processes in Pasargadae, UNESCO world heritage site, Iran. Int. Biodeter. Biodeg. 2017, 117, 171–182. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals; Longman Scientific & Technical: London, UK, 1992. [Google Scholar]
- Tomohiro, K.; Satoshi, K. Identification of Individual Si-Rich Particles Derived from Kosa Aerosol by the Alkali Elemental Composition. Bull. Chem Soc. Jpn. 2001, 74, 723–729. [Google Scholar]
- Leong, H.Y.; Leong Ong, D.E.; Sanjayan, J.G.; Nazari, A. The effect of different Na2O and K2O ratios of alkali activator on compressive strength of fly ash based-geopolymer. Constr. Build. Mater. 2016, 106, 500–511. [Google Scholar] [CrossRef]
- Li, X.; Zhi, L.; Shi, W.; Kong, L.; Bai, J.; Yu, J.; Reinmoller, M.; Guhl, S.; Meyer, B.; Li, W. Effect of K2O/Na2O on fusion behavior of coal ash with high silicon and aluminum level. Fuel 2020, 265, 116964. [Google Scholar] [CrossRef]
- Henderson, C.M.B. Composition, Thermal Expansion and Phase Transitions in Framework Silicates: Revisitation and Review of Natural and Synthetic Analogues of Nepheline-, Feldspar- and Leucite-Mineral Groups. Solids 2021, 2, 1–49. [Google Scholar] [CrossRef]
- Galindo, N.; Yubero, E.; Clemente, A.; Nicolas, J.F.; Varea, M.; Crespo, J. PM events and changes in the chemical composition of urban aerosols: A case study in the western Mediterranean. Chemosphere 2020, 244, 125520. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, G.; Zhao, W.; Yang, Y.; Wang, L.; Liu, Z.; Wen, T.; Cheng, M.; Wang, Y.; Wang, Y. Different roles of nitrate and sulfate in air pollution episodes in the North China Plain. Atmos. Environ. 2020, 224, 117325. [Google Scholar] [CrossRef]
- Kakavas, S.; Pandis, S.N. Effects of urban dust emissions on fine and coarse PM levels and composition. Atmos. Environ. 2021, 246, 118006. [Google Scholar] [CrossRef]
- Dimitriou, K.; Paschalidou, A.K.; Kassomenos, P.A. Distinct synoptic patterns and air masses responsible for long-range desert dust transport and sea spray in Palermo, Italy. Theor. Appl. Climatol. 2017, 130, 1123–1132. [Google Scholar] [CrossRef]
- Bertram, T.H.; Cochran, R.E.; Grassian, V.H.; Stone, E.A. Sea spray aerosol chemical composition: Elemental and molecular mimics for laboratory studies of heterogeneous and multiphase reactions. Chem. Soc. Rev. 2018, 47, 2374–2400. [Google Scholar] [CrossRef] [PubMed]
- Gregoris, E.; Morabito, E.; Barbaro, E.; Feltracco, M.; Toscano, G.; Merico, E.; Grasso, F.M.; Cesari, D.; Conte, M.; Contini, D.; et al. Chemical characterization and source apportionment of size-segregated aerosol in the port-city of Venice (Italy). Atmos. Pollut. Res. 2021, 12, 261–271. [Google Scholar] [CrossRef]
- Agbede, T.M.; Adekiya, A.O.; Eifediyi, E.K. Impact of poultry manure and NPK fertilizer on soil physical properties and growth and yield of carrot. J. Hortic. Res. 2017, 25, 81–88. [Google Scholar] [CrossRef]
- Merico, E.; Grasso, F.M.; Cesari, D.; Decesari, S.; Belosi, F.; Manarini, F.; De Nuntiis, P.; Rinaldi, M.; Gambaro, A.; Morabito, E.; et al. Characterisation of atmospheric pollution near an industrial site with a biogas production and combustion plant in southern Italy. Sci. Total Environ. 2020, 717, 137220. [Google Scholar] [CrossRef] [PubMed]
- Bove, M.C.; Brotto, P.; Calzolai, G.; Cassola, F.; Cavalli, F.; Fermo, P.; Hjorth, J.; Massabò, D.; Nava, S.; Piazzalunga, A.; et al. Prati PM10 source apportionment applying PMF and chemical tracer analysis to ship-borne measurements in the Western Mediterranean. Atmos. Environ. 2016, 125, 140–151. [Google Scholar] [CrossRef]
- Daellenbach, K.R.; Stefenelli, G.; Bozzetti, C.; Vlachou, A.; Fermo, P.; Gonzalez, R.; Piazzalunga, A.; Colombi, C.; Canonaco, F.; Hueglin, C.; et al. Prévôt Long-term chemical analysis and organic aerosol source apportionment at nine sites in central Europe: Source identification and uncertainty assessment. Atmos. Chem. Phys. 2017, 17, 13265–13282. [Google Scholar] [CrossRef]
- Malaguti, A.; Mircea, M.; La Torretta, T.M.G.; Telloli, C.; Petralia, E.; Stracquadanio, M.; Berico, M. Comparison of online and offline methods for measuring fine secondary inorganic ions and carbonaceous aerosols in the Central Mediterranean area. Aerosol Air Qual. Res. 2015, 15, 2641–2653. [Google Scholar] [CrossRef]
- Patino, D.; Pérez-Orozco, R.; Porteiro, J.; Lapuerta, M. Characterization of biomass PM emissions using thermophoretic sampling: Composition and morphological description of the carbonaceous residues. J. Aerosol Sci. 2019, 127, 49–62. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrocchino, E.; Telloli, C.; Rizzo, A. Chemical Characterization of Particulate Matter in the Renaissance City of Ferrara. Geosciences 2021, 11, 227. https://doi.org/10.3390/geosciences11060227
Marrocchino E, Telloli C, Rizzo A. Chemical Characterization of Particulate Matter in the Renaissance City of Ferrara. Geosciences. 2021; 11(6):227. https://doi.org/10.3390/geosciences11060227
Chicago/Turabian StyleMarrocchino, Elena, Chiara Telloli, and Antonietta Rizzo. 2021. "Chemical Characterization of Particulate Matter in the Renaissance City of Ferrara" Geosciences 11, no. 6: 227. https://doi.org/10.3390/geosciences11060227
APA StyleMarrocchino, E., Telloli, C., & Rizzo, A. (2021). Chemical Characterization of Particulate Matter in the Renaissance City of Ferrara. Geosciences, 11(6), 227. https://doi.org/10.3390/geosciences11060227






