Antimicrobial Property of Polypropylene Composites and Functionalized Copper Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composite Preparation
2.3. Characterization
3. Results and Discussion
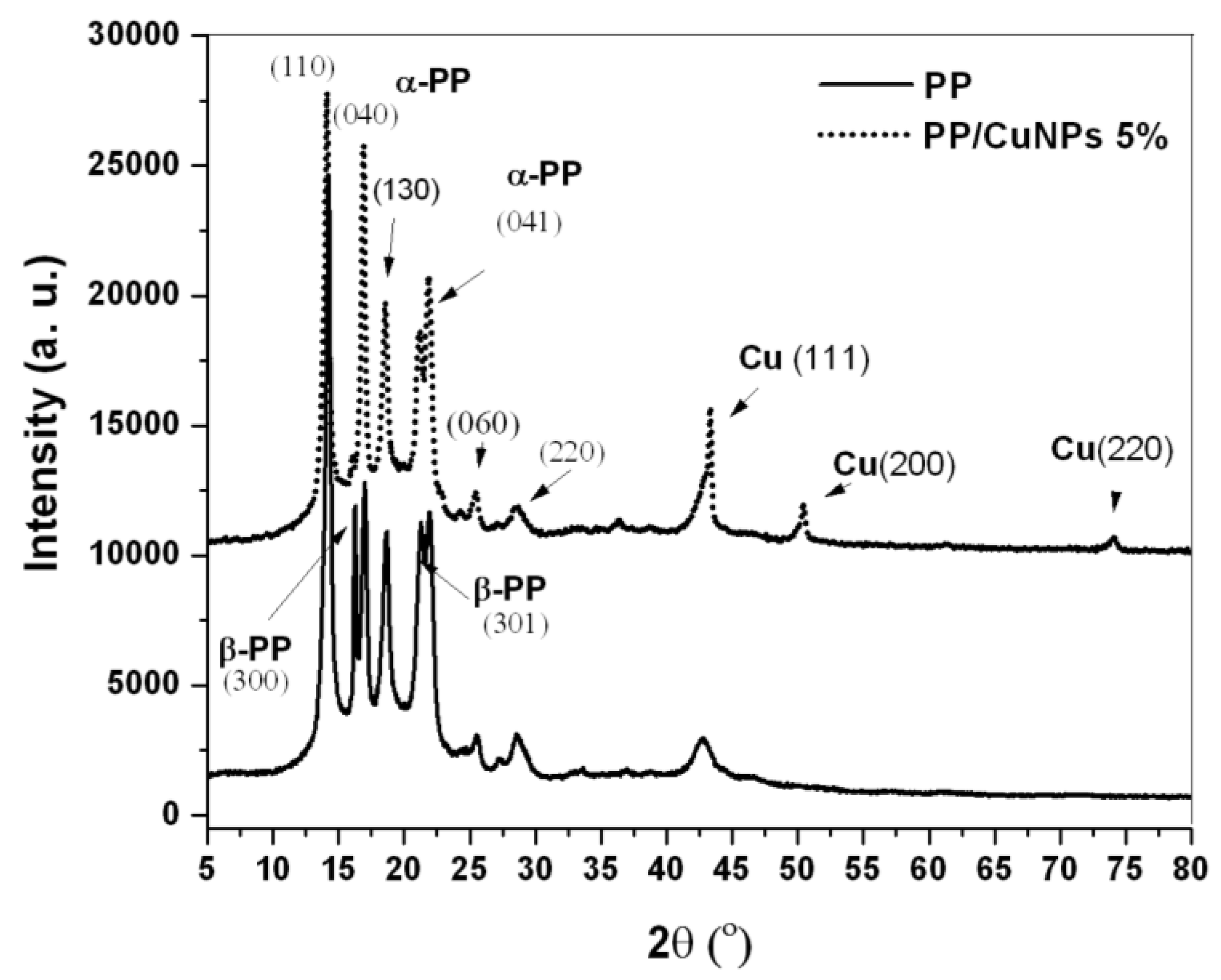
3.1. Wide Angle X-ray Diffraction (WAXD)
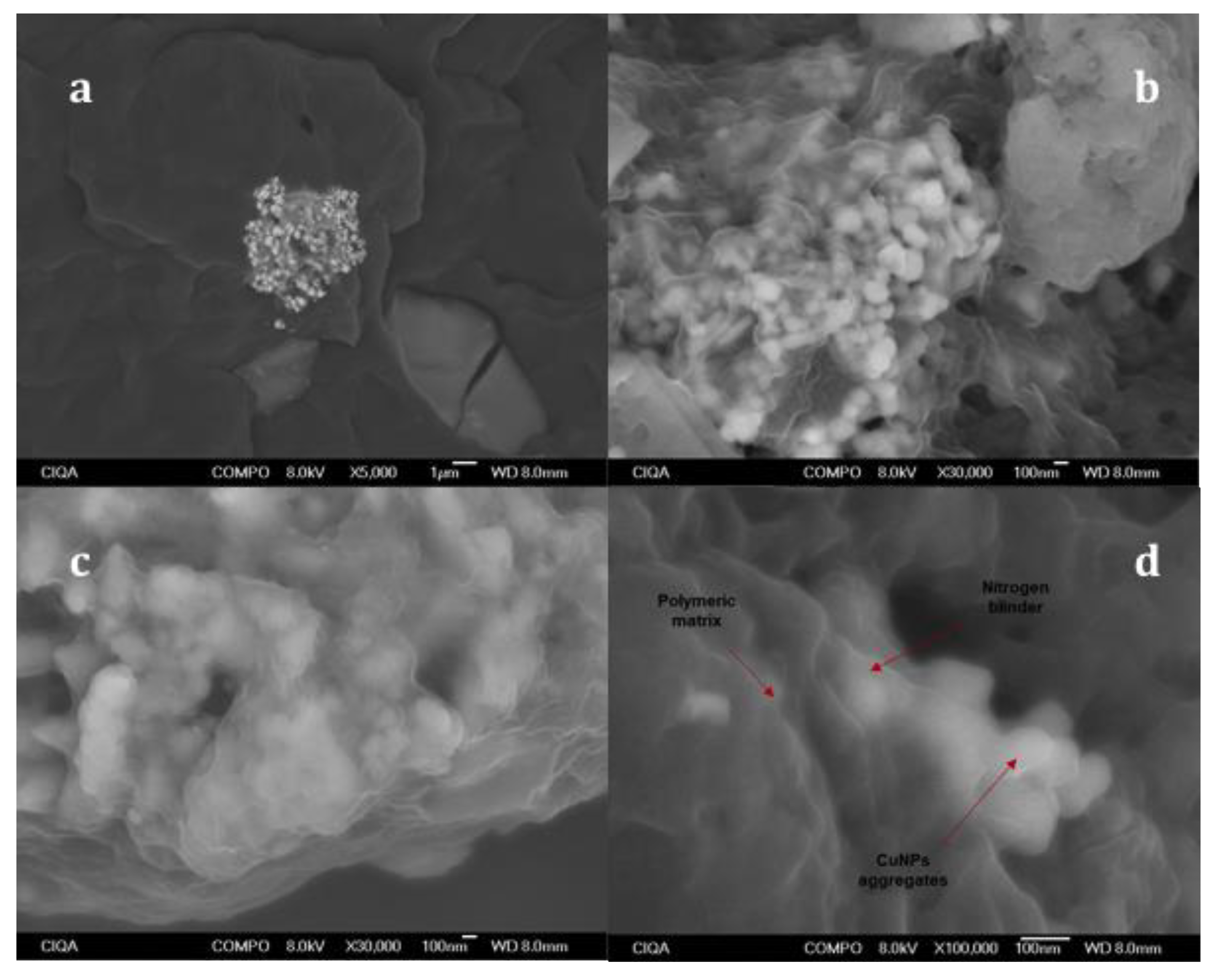
3.2. SEM Analysis
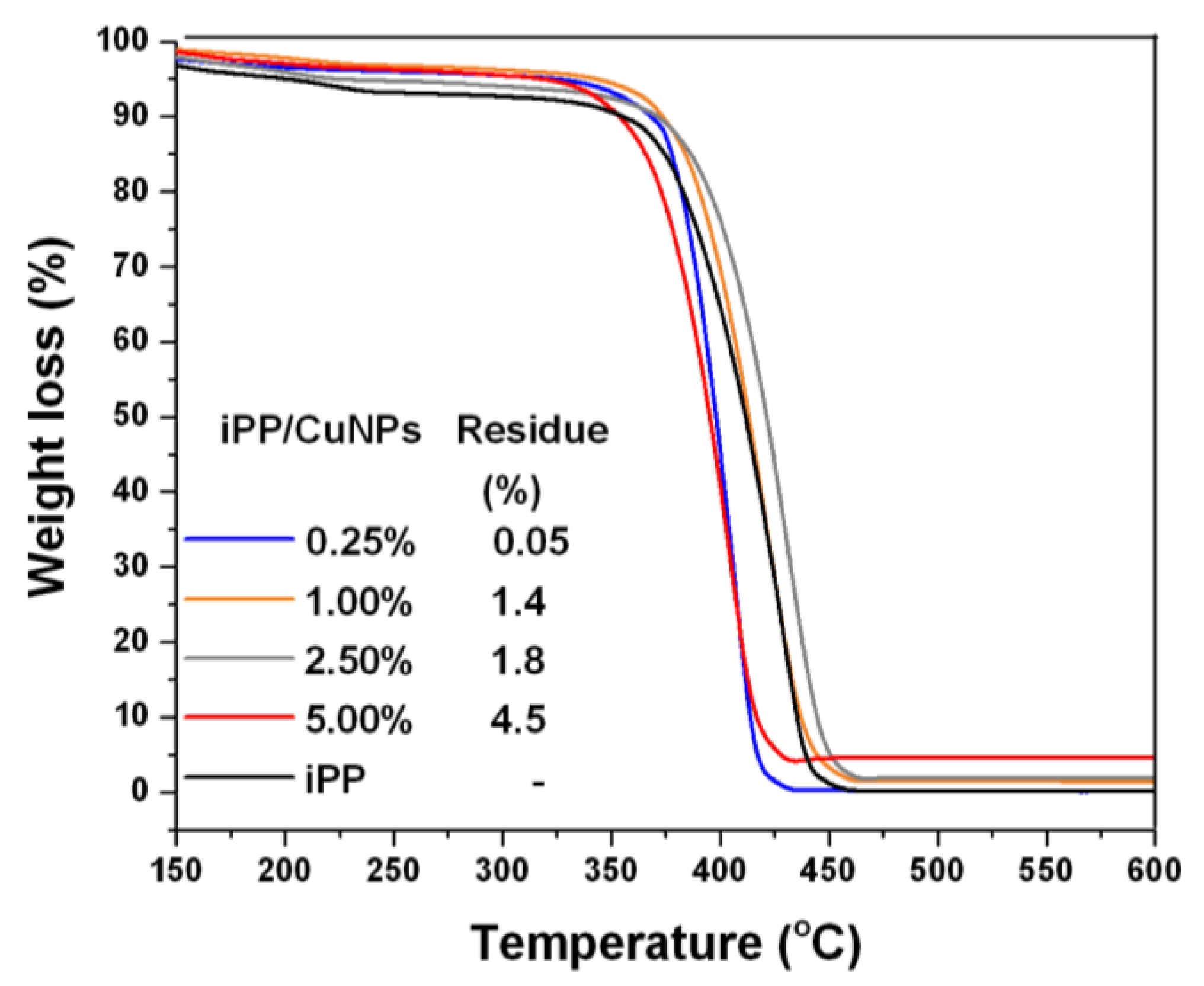
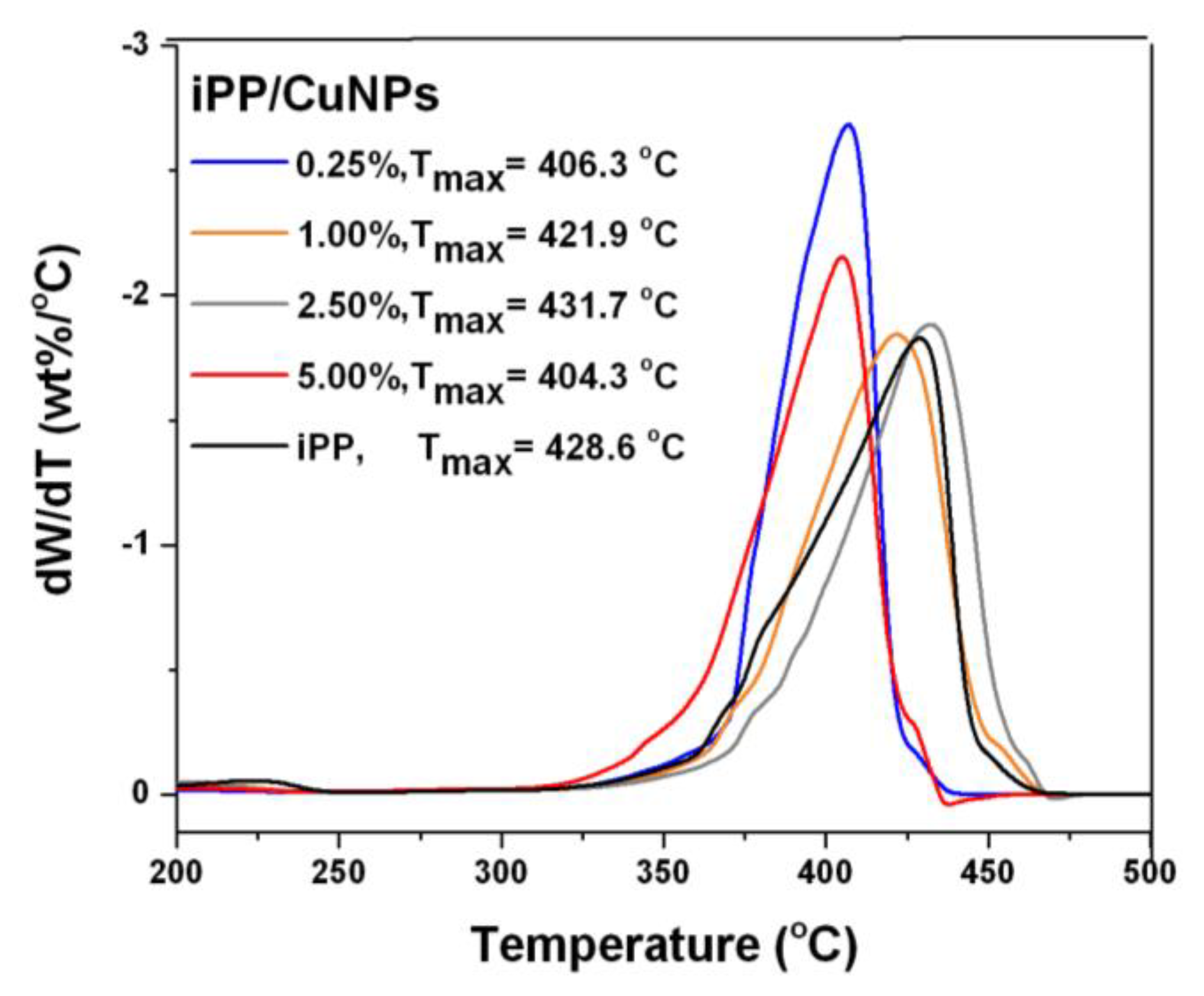
3.3. TGA Analysis
3.4. DSC and DMA Analyses
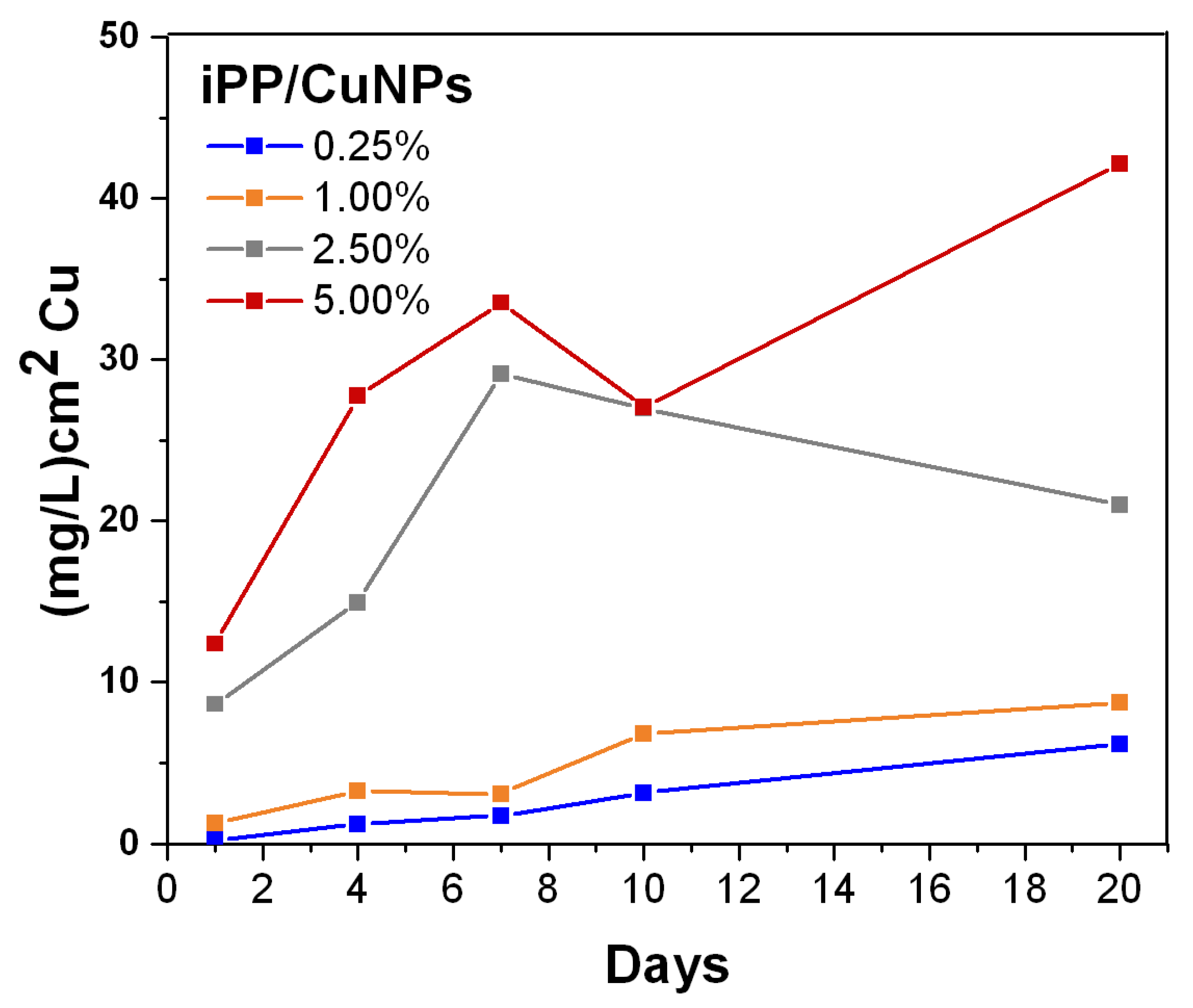
3.5. Cupric Ion Release from PP/CuNPs Composites
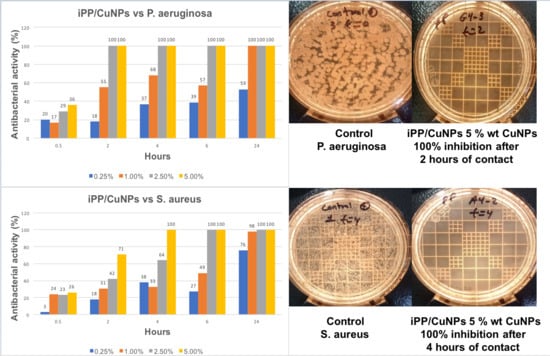
3.6. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nowak, A.; Szade, J.; Talik, E.; Zubko, M.; Wasikowski, D. Physicochemical and antibacterial characterization of ionocity Ag/Cu powder nanoparticles. Mat. Charact. 2016, 117, 9–16. [Google Scholar] [CrossRef]
- Medellín-Banda, D.I.; Navarro-Rodríguez, D.; Fernández-Tavizón, S.; Ávila-Orta, C.A.; Cadenas-Pliego, G.; Comparán-Padilla, V.E. Enhancement of the thermal conductivity of polypropylene with low loadings of CuAg alloy nanoparticles and graphene nanoplatelets. Mat. Tdy. Commun. 2019, 21, 100695. [Google Scholar] [CrossRef]
- Prado, J.V.; Vidal, A.R.; Duran, T.C. Application of copper bactericidal properties in medical practice. Rev. Med. Chil. 2012, 140, 1325–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hans, M.; Erbe, A.; Mathews, S.; Chen, Y.; Solioz, M.; Mücklich, F. Role of copper oxides in contact killing of bacteria. Langmuir 2013, 29, 16160–16166. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.S.; EI Zowalaty, M.E.; Shameli, K.; Zainuddin, N.; Salama, M.; Ibrahim, N.A. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int. J. Nanomed. 2013, 8, 4467–4479. [Google Scholar] [CrossRef]
- Zuniga, J.M.; Cortes, A. The role of additive manufacturing and antimicrobial polymers in the COVID-19 pandemic. Exp. Rv. Med. Dev. 2020, 17, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Karmacharya, M.; Joshi, S.R.; Gulenko, O.; Park, J.; Kim, G.H.; Cho, Y.K. Photoactive Antiviral Face Mask with Self-Sterilization and Reusability. Nan. Lett. 2021, 21, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Quiterio-Gutiérrez, T.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Hernández-Fuentes, A.D.; Sandoval-Rangel, A.; Benavides-Mendoza, A.; Cabrera-de la Fuente, M.; Juárez-Maldonado, A. The Application of Selenium and Copper Nanoparticles Modifies the Biochemical Responses of Tomato Plants under Stress by Alternaria solani. Int. J. Mol. Sci. 2019, 20, 1950. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Labrada, F.; López-Vargas, E.R.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Responses of Tomato Plants under Saline Stress to Foliar Application of Copper Nanoparticles. Plants 2019, 8, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Hernández, H.; Quiterio-Gutiérrez, T.; Cadenas-Pliego, G.; Ortega-Ortiz, H.; Hernández-Fuentes, A.D.; Cabrera de la Fuente, M.; Valdés-Reyna, J.; Juárez-Maldonado, A. Impact of Selenium and Copper Nanoparticles on Yield, Antioxidant System, and Fruit Quality of Tomato Plants. Plants 2019, 8, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-García, Y.; Cárdenas-Álvarez, C.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Cabrera-de-la-Fuente, M.; Sandoval-Rangel, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Effect of Three Nanoparticles (Se, Si and Cu) on the Bioactive Compounds of Bell Pepper Fruits under Saline Stress. Plants 2021, 10, 217. [Google Scholar] [CrossRef]
- Bashir, F.; Irfan, M.; Ahmad, T.; Iqbal, J.; Butt, M.T.; Sadef, Y.; Moniruzzaman, M. Efficient utilization of low cost agro materials for incorporation of copper nanoparticles to scrutinize their antibacterial properties in drinking water. Environ. Technol. Innov. 2020, 101228. [Google Scholar] [CrossRef]
- Garcia, K.A.; Peroja, K.A.G.; Tuberon, N.A.L.; Cambiador, C.J.B.; Cid-Andres, A.P. Metal incorporated Philippine Abaca fiber (Manila hemp) as a potential novel filter for water disinfection. J. Phys. Conf. Ser. 2021, 1, 012064. [Google Scholar] [CrossRef]
- Tamayo, L.; Azócar, M.; Kogan, M.; Riveros, A.; Páez, M. Copper-polymer nanocomposites: An excellent and cost-effective biocide for use on antibacterial surfaces. Mat. Sci. Eng. C 2016, 69, 1391–1409. [Google Scholar] [CrossRef]
- Gurianov, Y.; Nakonechny, F.; Albo, Y.; Nisnevitch, M. Antibacterial Composites of Cuprous Oxide Nanoparticles and Polyethylene. Int. J. Mol. Sci. 2019, 20, 439. [Google Scholar] [CrossRef] [Green Version]
- Gurianov, Y.; Nakonechny, F.; Albo, Y.; Nisnevitch, M. LLDPE Composites with Nanosized Copper and Copper Oxides for Water Disinfection. Polymers 2020, 12, 1713. [Google Scholar] [CrossRef] [PubMed]
- Jung-Huh, A.; Jik-Kwon, Y. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Contr. Release 2011, 156, 128–145. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; Jimenez de Aberasturi, D.; Ruis de Larramendi, I.; Rojo, T.; SErpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Tren. Biotech. 2012, 30, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Raffi, M.; Mehrwan, S.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Yawar, W.; Hasan, M.M. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Act. Biomat. 2008, 4, 707–716. [Google Scholar] [CrossRef]
- Chen, K.L.; Bothun, G.D. Nanoparticles Meet Cell Membranes: Probing Nonspecific Interactions using Model Membranes. Environ. Sci. Technol. 2014, 48, 873–880. [Google Scholar] [CrossRef]
- Palza, H. Antimicrobial Polymers with Metal Nanoparticles. Int. J. Mole. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horst, A.M.; Vukanti, R.; Priester, J.H.; Holden, P.A. An Assessment of Fluorescence and Absorbance-Based Assays to Study Metal-Oxide Nanoparticle ROS Production and Effects on Bacterial Membranes. Small 2013, 9, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Radheshkumar, C.; Munstedt, H. Antimicrobial polymers from polypropylene/silver composites-Ag+ release measured by anode stripping voltammetry. React. Funct. Polym. 2006, 66, 780–788. [Google Scholar] [CrossRef]
- Palza, H.; Quijada, R.; Delgado, K. Antimicrobial polymer composite with copper micro and nanoparticles: Effect of particle size and polymer matrix. J. Bioact. Compat. 2015, 30, 366–380. [Google Scholar] [CrossRef]
- Damm, C.; Münstedt, H.; Rösch, A. The antimicrobial efficacy of polyamide6/silver-nano- and microcomposites. Mat. Chem. Phys. 2008, 108, 61–66. [Google Scholar] [CrossRef]
- Sierra-Ávila, R.; Pérez-Alvarez, M.; Valdez-Garza, J.; Avila-Orta, C.A.; Jiménez-Regalado, E.J.; Mata-Padilla, J.M.; Soto-Castruita, E.; Cadenas-Pliego, G. Synthesis and Thermomechanical Characterization of Nylon 6/Cu Nanocomposites Produced by an Ultrasound-Assisted Extrusion Method. Adv. Mat. Sci. Eng. 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Xu, L.; Jiang, Y. Preparation and Thermal Properties of Modified Cu2O/Polypropylene (PP) Composite. Materials 2020, 13, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramazanov, M.A.; Hajiyeva, F.V. Copper and copper oxide nanoparticles in polypropylene matrix: Synthesis, characterization, and dielectric properties. Comp. Interf. 2020, 1–14. [Google Scholar] [CrossRef]
- Palza, H.; Delgado, K.; Pinochet, I. Improving the Metal Ion Release from Nanoparticles Embedded in a Polypropylene Matrix for Antimicrobial Applications. J. App. Polym. Sci. 2015, 41232, 1–8. [Google Scholar] [CrossRef]
- Molaba, M.P.; Dudic, D.; Luyt, A.S. Influence of the presence of medium-soft paraffin wax on the morphology and properties of iPP/silver nanocomposites. eXPRESS Polym. Lett. 2015, 9, 901–915. [Google Scholar] [CrossRef]
- Jardón-Maximino, N.; Pérez-Alvarez, M.; Sierra-Ávila, R.; Ávila-Orta, C.A.; Jiménez-Regalado, E.; Bello, A.M.; González-Morones, P.; Cadenas-Pliego, P. Oxidation of Copper Nanoparticles Protected with Different Coatings and Stored under Ambient Conditions. J. Nanomat. 2018, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sierra-Ávila, R.; Pérez-Álvarez, M.; Cadenas-Pliego, C.; Ávila-Orta, C.A.; Betancourt-Galindo, R.; Jiménez-Regalado, E.; Jiménez-Barrera, R.M.; Martínez-Colunga, J.G. Synthesis of Copper Nanoparticles Coated with Nitrogen Ligands. J. Nanomat. 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sierra-Ávila, R.; Pérez-Álvarez, M.; Cadenas-Pliego, G.; Comparán-Padilla, V.E.; Ávila-Orta, C.A.; Pérez-Camacho, O.; Jiménez-Regalado, E.; Hernández-Hernández, E.; Jiménez-Barrera, R.M. Synthesis of Copper Nanoparticles Using Mixture of Allylamine and Polyallylamine. J. Nanomat. 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Cadenas-Pliego, G.; Pérez Alvarez, M.; Avila-Orta, C.A.; Ortega-Ortiz, H.; Betancourt-Galindo, R.; Comparán-Padilla, V.E.; Neira-Velázquez, M.G.; Sierra-Avila, R.; Jiménez-Regalado, E.; Rivera-Orta, V.H.; et al. Síntesis de Nanopartículas Metálicas Modificadas con Polímeros Mediante Ligantes Nitrogenados. Mx. Patent MX/E/2012/081565, 13 2012. [Google Scholar]
- España-Sánchez, B.L.; Ávila-Orta, C.A.; Padilla-Vaca, F.; Neira-Velázquez, M.G.; González-Morones, P.; Rodriguez-Gonzalez, J.A.; Hernández-Hernández, E.; Rangel-Serrano, A. Enhanced antibacterial activity of melt processed polypropylene Ag and Cu nanocomposites by argon plasma treatment. Plasm. Pross. Polym. 2014, 11, 353–365. [Google Scholar] [CrossRef]
- Camacho, P.H.; Morales-Cepeda, A.B.; Salas-Papayanopolos, H.; Bautista, J.E.; Castro, C.; Lozano, T. Crystallization behavior of polypropylene/silver nanocomposites using polyethylene glycol as reducing agent and interface modifier. J. Thermopl. Comp. Mat. 2015, 1–16. [Google Scholar] [CrossRef]
- Van Erp, T.N.; Balzano, L.; Peters, G.W.M. Oriented Gamma Phase in Isotactic Polypropylene Homopolymer. ACS Macr. Lett. 2012, 1, 618–622. [Google Scholar] [CrossRef]
- Abad, M.J.; Arribas, J.M.; Gómez, M.A.; Marco, C. Análisis de la cristalización dinámica de polipropileno isotáctico en presencia de ácido pimélico. Rev. Iber. Polim. 2005, 6, 93–110. [Google Scholar]
- Boyanova, M.; Balta-Calleja, F.J.; Fakirov, S. New aspects of the β→α polymorphic transition in plastically deformed isotactic polypropylene studied by microindentation hardness. J. Mat. Sci. 2006, 41, 5504–5509. [Google Scholar] [CrossRef]
- Slouf, M.; Pavlova, E.; Krejcikova, S.; Ostafinska, A.; Zhigunov, A.; Krzyzanek, V. Relations between morphology and micromechanical properties of alpha, beta and gamma phases of iPP. Polygr. Test. 2018, 1–18. [Google Scholar] [CrossRef]
- Garbarczyk, J. A study on the mechanism of polymorphic transition β→α in isotactic polypropylene. Makr. Chem. 1985, 186, 2145–2151. [Google Scholar] [CrossRef]
- Machado, G.; Denardin, E.L.G.; Kinast, E.J.; Goncalves, M.C.; de Luca, M.A.; Teixeira, S.R. Crystalline properties and morphological changes in plastically deformed isotatic polypropylene evaluated by X-ray diffraction and transmission electron microscopy. Europ. Polym. J. 2005, 41, 129–138. [Google Scholar] [CrossRef]
- Lloyd, G.E. Atomic number and crystallographic contrast images with the SEM: A review of backscattered electron techniques. Mineral. Mag. 1987, 51, 3–19. [Google Scholar] [CrossRef] [Green Version]
- Bai, F.; Li, F.; Clahoun, B.H.; Quirk, R.P.; Cheng, S.Z.D. Polymer Handbook, 4th ed.; Brandrup, J., Immergut, E.H., Grulke, E.A., Eds.; Wiley-Interscience: New York, NY, USA, 1999; Volume 1, Chapter V. [Google Scholar]
- Sahoo, R.K.; Mohanty, S.; Nayak, S.K. Effect of silver nanoparticles on the morphology, crystallization, and melting behavior of polypropylene: A study on non-isothermal crystallization kinetics. Polym. Sci. Ser. A 2016, 58, 443–453. [Google Scholar] [CrossRef]
- <named-content content-type="background:white">Luyt, A.S.; Molefi, J.A.; Krump, H. Thermal, mechanical and electrical properties of copper powder filled low-density and linear low-density polyethylene composites. Polym. Deg. Stab. 2006, 91, 1629–1636. [Google Scholar] [CrossRef]
- Molefi, J.A.; Luyt, A.S.; Krupa, I. Comparison of the influence of copper micro- and nano-particles on the mechanical properties of polyethylene/copper composites. J. Mater. Sci. 2009, 45, 82–88. [Google Scholar] [CrossRef]
- Delgado, K.; Quijada, R.; Palma, R.; Palza, H. Polypropylene with embedded copper metal or copper oxide nanoparticles as a novel plastic antimicrobial agent. Lett. App. Microb. 2011, 53, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, L.A.; Zapata, P.A.; Vejar, N.D.; Azócar, M.I.; Gulppi, M.A.; Zhou, X. Release of silver and copper nanoparticles from polyethylene nanocomposites and their penetration into Listeria monocytogenes. Mat. Sci. Eng. C 2014, 40, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotech. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Quiñones-Jurado, Z.V.; Waldo-Mendoza, M.A.; Aguilera-Bandin, H.M.; Villabona-Leal, E.G.; Cervantes-González, E.; Pérez, E. Silver nanoparticles supported on TiO2 and their antibacterial properties: Effect of Surface confinement and nonexistence of plasmon resonance. Mat. Sci. App. 2014, 5, 895–903. [Google Scholar] [CrossRef] [Green Version]
- Kori, M.A.; Kulthe, M.G.; Goyal, R.K. Influence of Cu Micro Particles on Mechanical Properties of Injection Molded Polypropylene/Cu Composites. Int. J. Innov. Res. Sci. Eng. Techn. 2014, 3, 14034–14043. [Google Scholar]
- Boudenne, A.; Ibos, L.; Fois, M.; Majesté, J.C.; Géhin, E. Electrical and thermal behavior of polypropylene filled with copper particles. Comp. Part A 2005, 36, 1545–1554. [Google Scholar] [CrossRef]
- Ghassan, A.N. Effect of Temperature and Nickel Concentration on the Electrical and Dielectric Properties of Polyethylene-Nickel Composites. Int. J. Eng. Res. Dev. 2015, 11, 29–38. [Google Scholar]
- Shu, X.; Feng, J.; Liao, J.; Zhang, D.; Peng, R.; Shi, Q.; Xie, X. Amorphous carbon-coated nano-copper particles: Novel synthesis by Sol–Gel and carbothermal reduction method and extensive characterization. J. Alloy. Compd. 2020, 156556. [Google Scholar] [CrossRef]
| Samples | Tc a (°C) | ΔHc a (J/g) | Tm a (°C) | ΔHm a (J/g) | Xc b | E’ c (MPa) |
|---|---|---|---|---|---|---|
| PP | 117.1 | 93.7 | 164.2 | 92.4 | 0.44 | 1764 |
| PP/CuNPs 0.25% | 118.9 | 99.3 | 163.7 | 97.8 | 0.47 | 3983 |
| PP/CuNPs 1.00% | 118.6 | 90.4 | 162.5 | 87.9 | 0.42 | 1695 |
| PP/CuNPs 2.50% | 121.1 | 93.4 | 163.6 | 93.5 | 0.45 | 2839 |
| PP/CuNPs 5.00% | 122.1 | 93.9 | 163.5 | 96.7 | 0.46 | 2699 |
| Composite PP/CuNPs | P. aeruginosa Gram (-) | S. aureus Gram (+) |
|---|---|---|
| AA(%)/h | AA(%)/h | |
| 0.25% | 53/24 | 76/24 |
| 1.00% | 100/24 | 98/24 |
| 2.50% | 100/2 | 100/6 |
| 5.00% | 100/2 | 100/4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jardón-Maximino, N.; Cadenas-Pliego, G.; Ávila-Orta, C.A.; Comparán-Padilla, V.E.; Lugo-Uribe, L.E.; Pérez-Alvarez, M.; Tavizón, S.F.; Santillán, G.d.J.S. Antimicrobial Property of Polypropylene Composites and Functionalized Copper Nanoparticles. Polymers 2021, 13, 1694. https://doi.org/10.3390/polym13111694
Jardón-Maximino N, Cadenas-Pliego G, Ávila-Orta CA, Comparán-Padilla VE, Lugo-Uribe LE, Pérez-Alvarez M, Tavizón SF, Santillán GdJS. Antimicrobial Property of Polypropylene Composites and Functionalized Copper Nanoparticles. Polymers. 2021; 13(11):1694. https://doi.org/10.3390/polym13111694
Chicago/Turabian StyleJardón-Maximino, Noemi, Gregorio Cadenas-Pliego, Carlos A. Ávila-Orta, Víctor Eduardo Comparán-Padilla, Luis E. Lugo-Uribe, Marissa Pérez-Alvarez, Salvador Fernández Tavizón, and Gerardo de Jesús Sosa Santillán. 2021. "Antimicrobial Property of Polypropylene Composites and Functionalized Copper Nanoparticles" Polymers 13, no. 11: 1694. https://doi.org/10.3390/polym13111694
APA StyleJardón-Maximino, N., Cadenas-Pliego, G., Ávila-Orta, C. A., Comparán-Padilla, V. E., Lugo-Uribe, L. E., Pérez-Alvarez, M., Tavizón, S. F., & Santillán, G. d. J. S. (2021). Antimicrobial Property of Polypropylene Composites and Functionalized Copper Nanoparticles. Polymers, 13(11), 1694. https://doi.org/10.3390/polym13111694