Estradiol Upregulates the Expression of the TGF-β Receptors ALK5 and BMPR2 during the Gonadal Development of Schizothorax prenanti
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tissue Sampling
2.3. Total RNA Isolation and Sequence Cloning
2.4. Real-Time Quantitative PCR Analysis
2.5. Western Blotting Analysis
2.6. Immunohistochemistry Analysis
2.7. Effect of ALK5 and BMPR2 Expression on E2-Fed Gonads
2.8. Data Analysis
3. Results
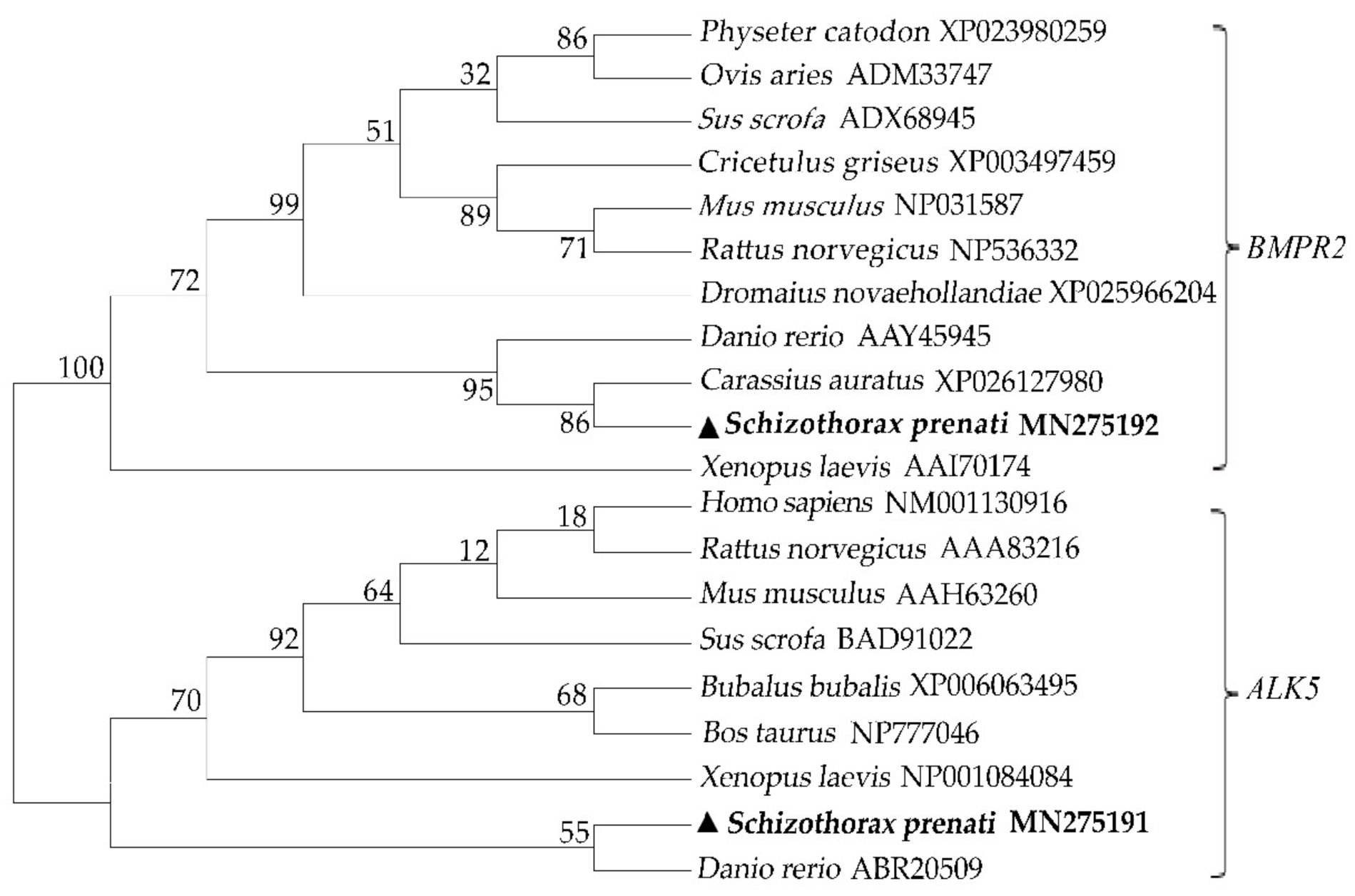
3.1. Nucleotide and Deduced Amino Acid Sequences
3.2. Tissue Distribution of ALK5 and BMPR2
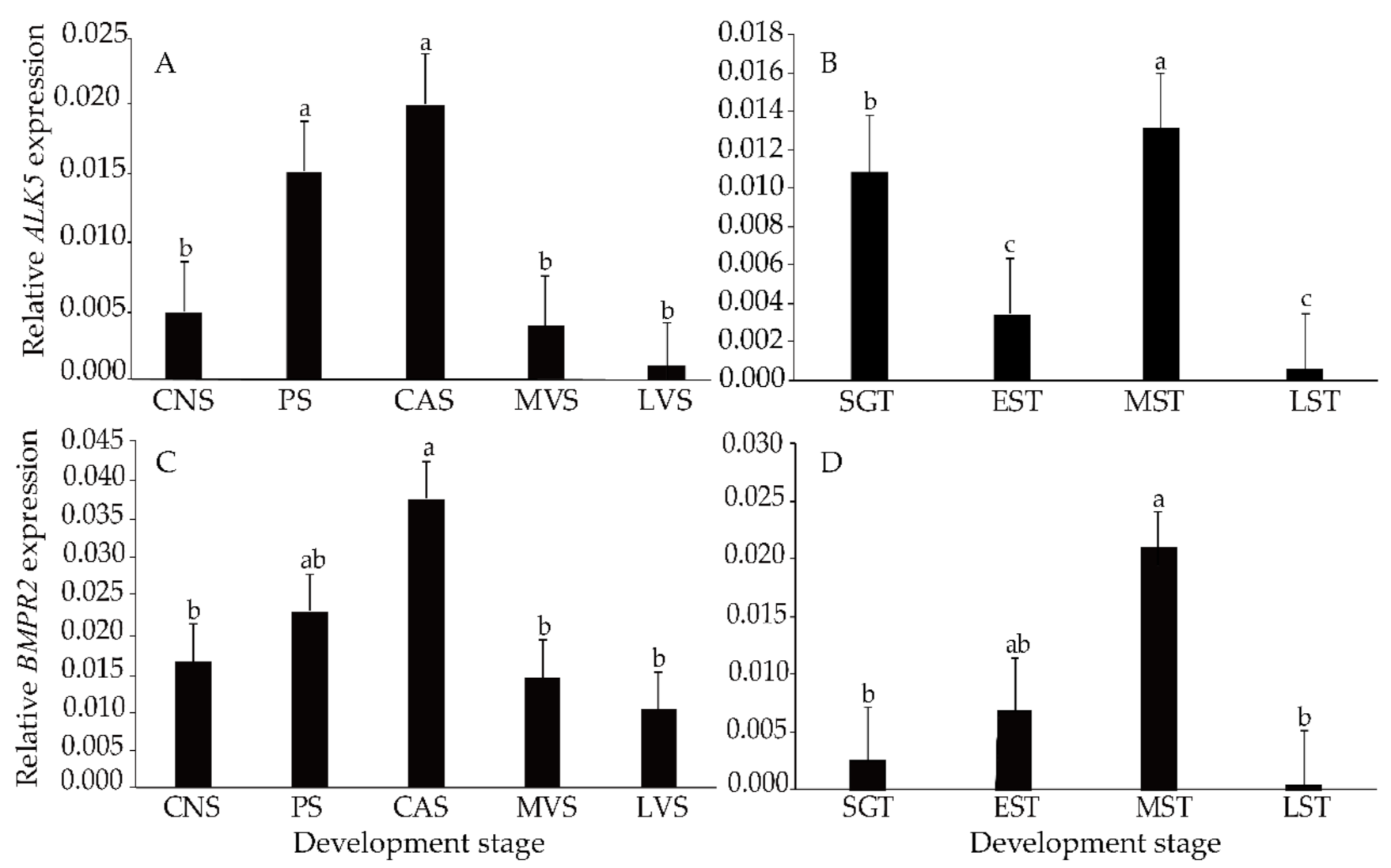
3.3. Expression Levels of ALK5 and BMPR2 mRNAs during Gonad Development
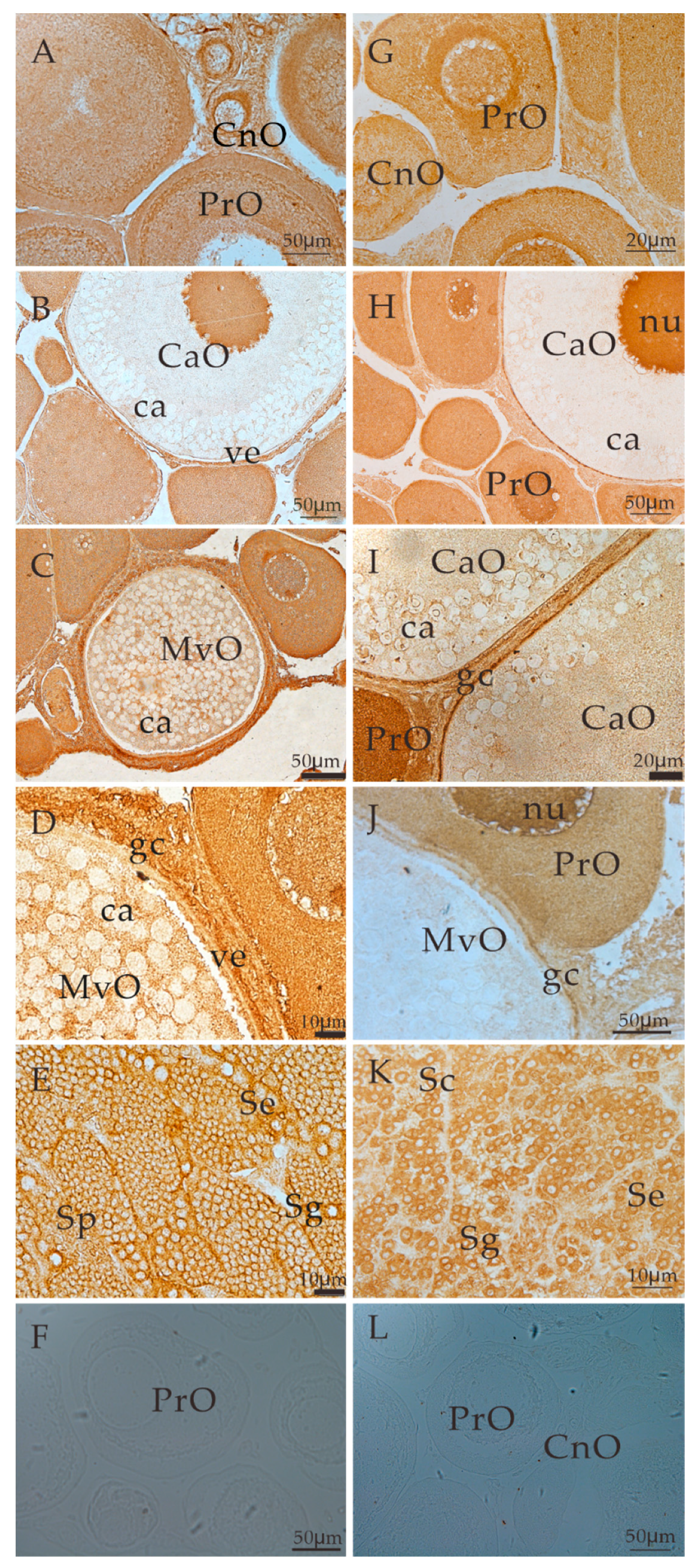
3.4. Localization of ALK5 and BMPR2 during Gonadal Development
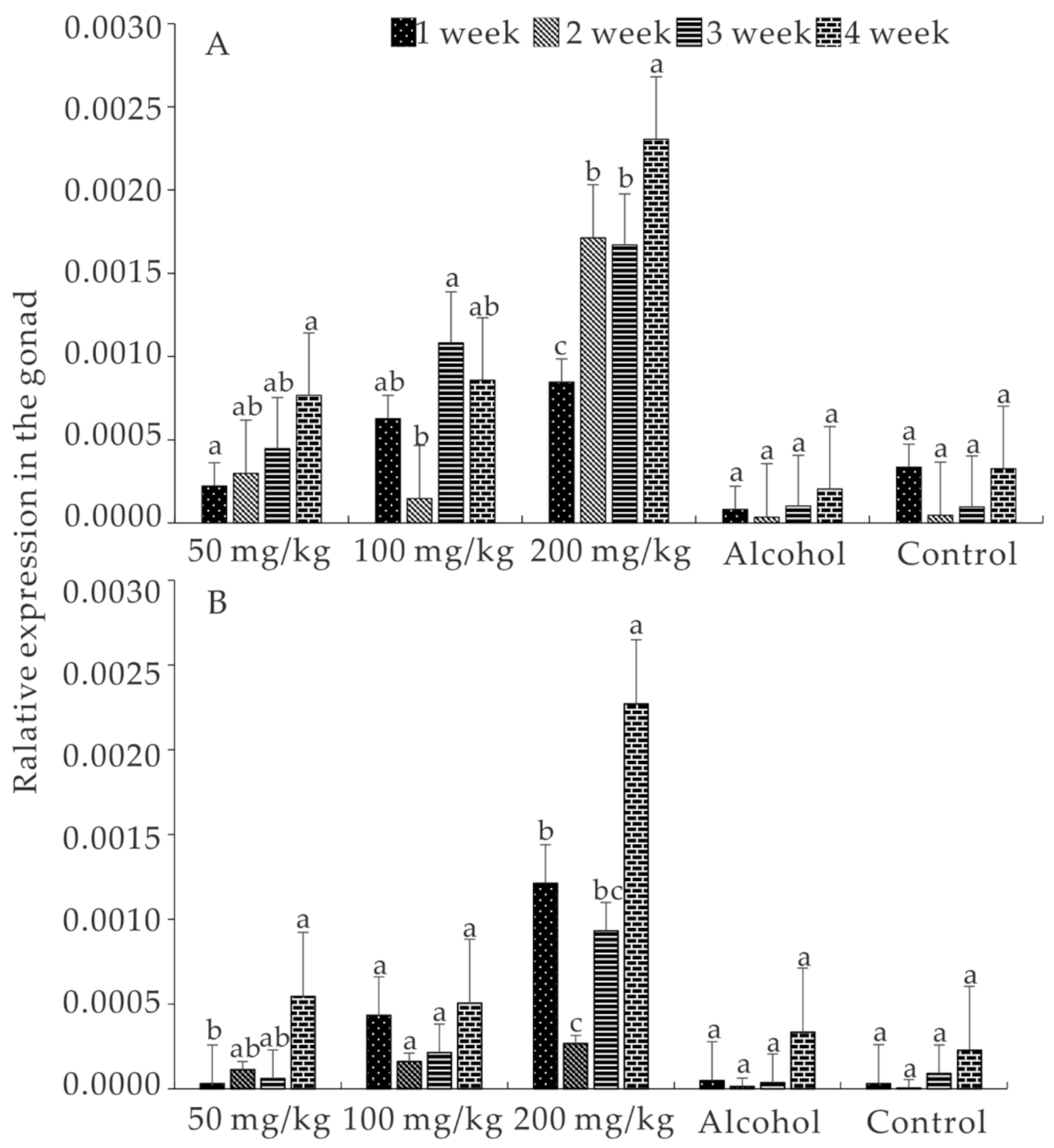
3.5. Expression of ALK5 and BMPR2 Induced by E2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macias, M.J.; Martin, M.P.; Massagué, J. Structural determinants of Smad function in TGF-β signaling. Trends Biochem. Sci. 2015, 40, 296–308. [Google Scholar] [PubMed]
- Li, Q.; Agno, J.E.; Edson, M.A.; Nagaraja, A.K.; Nagashima, T.; Matzuk, M.M. Transforming growth factor β receptor type 1 is essential for female reproductive tract integrity and function. PLoS Genet. 2011, 7, e1002320. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.J.H.; Campbell, B.K.; Mcneilly, A.S.; Baird, D.T. Effect of bone morphogenetic protein 2 (BMP2) on oestradiol and inhibin A production by sheep granulosa cells, and localization of BMP receptors in the ovary by immunohistochemistry. Reproduction 2002, 123, 363–369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [PubMed]
- Moustakas, A.; Heldin, C.H. The regulation of TGFbeta signal transduction. Development 2009, 136, 3699–3714. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zheng, L.; Yuan, Q.; Zhen, G.; Crane, J.L.; Zhou, X.; Cao, X. Transforming growth factor-β in stem cells and tissue homeostasis. Bone Res. 2018, 6, 2. [Google Scholar] [PubMed]
- Yang, Q.; Ma, B.; Qiao, H.; Ma, H.; Dong, Y.; Cao, L.; Ma, J.; Li, Z. TGFB1 represses the expression of SF1 and LRH1 to inhibit E production in rat LCs. Reproduction 2017, 153, 621–629. [Google Scholar]
- Zhu, P.; Zhao, X.; Su, X.; Su, J.; Liu, J.; Liu, Q.; Shi, D. Cloning analysis of buffalo ALK5 gene and the study of its tissue expression pattern. Heilongjiang Anim. Sci. Vet. Med. 2015, 4, 9–13. [Google Scholar]
- Oron, G.; Fisch, B.; Ao, A.; Zhang, X.Y.; Farhi, J.; Ben-Haroush, A.; Haroush, A.B.; Kesseler-Icekson, G.; Abir, R. Expression of growth-differentiating factor 9 and its type 1 receptor in human ovaries. Reprod. Biomed. Online 2010, 21, 109–117. [Google Scholar]
- Sun, R.Z.; Lei, L.; Cheng, L.; Jin, Z.F.; Zu, S.J.; Shan, Z.Y.; Wang, Z.D.; Zhang, J.X.; Liu, Z.H. Expression of GDF-9, BMP-15 and their receptors in mammalian ovary follicles. J. Mol. Histol. 2010, 41, 325–332. [Google Scholar] [CrossRef]
- Galvão, A.; Wolodko, K.; Rebordão, M.R.; Skarzynski, D.; Ferreira-Dias, G. TGFB1 modulates in vitro secretory activity and viability of equine luteal cells. Cytokine 2018, 110, 316–327. [Google Scholar] [CrossRef]
- Maehr, T.; Wang, T.; González-Vecino, J.L.; Wadsworth, S.; Secombes, C.J. Cloning and expression analysis of the transforming growth factor-beta receptors type 1 and 2 in the rainbow trout Oncorhynchus mykiss. Dev. Comp. Immunol. 2012, 37, 115–126. [Google Scholar] [CrossRef]
- Zhan, X.L.; Ma, T.Y.; Wu, J.Y.; Yi, L.Y.; Wang, J.Y.; Gao, X.K.; Li, W.S. Cloning and primary immunological study of TGF-β1 and its receptors TβR I /TβR II in tilapia (Oreochromis niloticus). Dev. Comp. Immunol. 2015, 51, 134–140. [Google Scholar] [CrossRef]
- Chen, A.Q.; Liu, Z.; Wei, Q.L.; Yang, Z.; Zhong, Y.J. The expression pattern of GDF9 and MP15 I and II receptor genes during follicle development in gibel carp. J. Shanghai Ocean. Univ. 2015, 24, 656–661. [Google Scholar]
- Zhu, G.; Guo, B.; Pan, D.; Mu, Y.; Feng, S. Expression of bone morphogenetic proteins and receptors in porcine cumulus-oocyte complexes during in vitro maturation. Anim. Reprod. Sci. 2008, 104, 275–283. [Google Scholar] [CrossRef]
- Onagbesan, O.M.; Bruggeman, V.; Van-As, P.; Tona, K.; Williams, J.; Decuypere, E. BMPs and BMPRs in chicken ovary and effects of BMP-4 and -7 on granulosa cell proliferation and progesterone production in vitro. Am. J. Physiol. Endoc. Metab. 2003, 285, 973–983. [Google Scholar] [CrossRef]
- Quinn, R.L.; Shuttleworth, G.; Hunter, M.G. Immunohistochemical localization of the bone morphogenetic protein receptors in the porcine ovary. J. Anat. 2004, 205, 15–23. [Google Scholar] [CrossRef]
- Li, C.W.; Ge, W. Spatiotemporal expression of bone morphogenetic protein family ligands and receptors in the zebrafish ovary: A potential paracrine-signaling mechanism for oocyte-follicle cell communication. Biol. Reprod. 2011, 85, 977–986. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, J.; Pan, Z.; Du, X.; Li, X.; Ma, B.; Yao, W.; Li, Q.; Liu, H. The let-7g microRNA promotes follicular granulosa cell apoptosis by targeting transforming growth factor-β type 1 receptor. Mol. Cell. Endocrinol. 2015, 409, 103–112. [Google Scholar] [CrossRef]
- Larsson, J.; Goumans, M.J.; Sjöstrand, L.J.; Van, R.M.A.; Ward, D.; Levéen, P.; Xu, X.; Ten, D.P.; Mummery, C.L.; Karlsson, S. Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. EMBO J. 2001, 20, 1663–1673. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, B.; Chen, H.; Xu, M.; Sun, X.; Xu, J.; Gao, Y.; Chen, C.; Jiang, H.; Zhang, J. Effects of MiR-375-BMPR2 as a key factor downstream of BMP15/GDF9 on the Smad1/5/8 and Smad2/3 signaling pathways. Cell. Physiol. Biochem. 2018, 46, 213–225. [Google Scholar] [CrossRef]
- Iwata, N.; Hasegawa, T.; Fujita, S.; Nagao, S.; Nakano, Y.; Nada, T.; Nishiyama, Y.; Hosoya, T.; Otsuka, F. Effect of the interaction of metformin and bone morphogenetic proteins on ovarian steroidogenesis by human granulosa cells. Biochem. Biophys. Res. Commun. 2018, 503, 1422–1427. [Google Scholar] [CrossRef]
- Fujita, S.; Hasegawa, T.; Nishiyama, Y.; Fujisawa, S.; Nakano, Y.; Nada, T.; Iwata, N.; Kamada, Y.; Masuyama, H.; Otsuka, F. Interaction between orexin A and bone morphogenetic protein system on progesterone biosynthesis by rat granulosa cells. J. Steroid. Biochem. Mol. Biol. 2018, 181, 73–79. [Google Scholar] [CrossRef]
- Patiño, L.C.; Silgadom, D.; Laissue, P. A potential functional association between mutant BMPR2 and primary ovarian insufficiency. Syst. Biol. Reprod. Med. 2017, 63, 145–149. [Google Scholar] [CrossRef]
- Yan, T.; Cai, Y.; He, J.; Zhang, Q.; Wang, X.; Zhang, S.; He, L.; He, Z. Characterization and expression profiles of cyp19a1a in the schizothoracine fish Schizothorax prenanti. Tissue Cell 2019, 58, 70–75. [Google Scholar] [CrossRef]
- Yan, T.; Zhang, S.; Zhang, Q.; Deng, F.; Cai, Y.; He, J.; Ma, Z.; He, L.; Luo, J.; Yang, D.; et al. Expression patterns and oestradiol regulation of growth differentiation factor 9 in Schizothorax prenanti. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 248–249, 110470. [Google Scholar] [CrossRef]
- Blazer, V.S. Histopathological assessment of gonadal tissue in wild fishes. Fish Physiol. Biochem. 2002, 26, 85–101. [Google Scholar] [CrossRef]
- Lin, H.Y.; Wang, X.F.; Ng-Eaton, E.; Weinberg, R.A.; Lodish, H.F. Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase. Cell 1992, 68, 775–785. [Google Scholar] [CrossRef]
- Zhu, H.J.; Sizeland, A.M. A pivotal role for the transmembrane domain in transforming growth factor-beta receptor activation. J. Biol. Chem. 1999, 274, 11773–11781. [Google Scholar] [CrossRef]
- Wehrenberg, U.; Giebel, J.; Rune, G.M. Possible involvement of transforming growth factor-β1 and transforming growth factor-β receptor type II during luteinization in the marmoset ovary. Tissue Cell 1998, 30, 360–367. [Google Scholar] [CrossRef]
- Haas, C.S.; Rovani, M.T.; Oliveira, F.C. Expression of growth and differentiation factor 9 and cognate receptors during final follicular growth in cattle. Anim. Reprod. 2016, 13, 756–761. [Google Scholar] [CrossRef]
- Wu, F.; Lin, T.; Sung, L.; Chang, W.; Wu, P.; Luo, C. BMP8A sustains spermatogenesis by activating both SMAD1/5/8 and SMAD2/3 in spermatogonia. Sci. Signal. 2017, 10, eaal1910. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Q.; Hogan, B.L. Evidence that mouse Bmp8a (Op2) and Bmp8b are duplicated genes that play a role in spermatogenesis and placental development. Mech. Dev. 1996, 57, 159–168. [Google Scholar] [CrossRef]
- Shimizu, T.; Yokoo, M.; Miyake, Y.; Sasada, H.; Sato, E. Differential expression of bone morphogenetic protein 4–6 (BMP-4, -5, and -6) and growth differentiation factor-9 (GDF-9) during ovarian development in neonatal pigs. Domest. Anim. Endocrinol. 2004, 27, 397–405. [Google Scholar] [CrossRef]
- Brankin, V.; Quinn, R.L.; Webb, R.; Hunter, M.G. Evidence for a functional bone morphogenetic protein (BMP) system in the porcine ovary. Domest. Anim. Endocrinol. 2005, 28, 367–379. [Google Scholar] [CrossRef]
- Prochazka, R.; Nemcova, L.; Nagyova, E.; Kanka, J. Expression of growth differentiation factor 9 messenger RNA in porcine growing and preovulatory ovarian follicles. Biol. Reprod. 2004, 71, 1290–1295. [Google Scholar] [CrossRef]
- Erickson, G.F.; Shimasaki, S. The spatiotemporal expression pattern of the bone morphogenetic protein family in rat ovary cell types during the estrous cycle. Reprod. Biol. Endocrinol. 2003, 1, 9. [Google Scholar] [CrossRef]
- Young, J.C.; Wakitani, S.; Loveland, K.L. TGF-β superfamily signaling in testis formation and early male germline development. Semin. Cell Dev. Biol. 2015, 45, 94–103. [Google Scholar] [CrossRef]
- Ciller, I.M.; Palanisamy, S.K.; Ciller, U.A.; Mcfarlane, J.R. Postnatal expression of bone morphogenetic proteins and their receptors in the mouse testis. Physiol. Res. 2016, 65, 673–682. [Google Scholar] [CrossRef]
- Yi, S.E.; Lapolt, P.S.; Yoon, B.S.; Chen, J.Y.; Lu, J.K.; Lyons, K.M. The type I BMP receptor BmprIB is essential for female reproductive function. Proc. Natl. Acad. Sci. USA 2001, 98, 7994–7999. [Google Scholar] [CrossRef]
- Paradis, F.; Novak, S.; Murdoch, G.K.; Dyck, M.K.; Dixon, W.T.; Foxcroft, G.R. Temporal regulation of BMP2, BMP6, BMP15, GDF9, BMPR1A, BMPR1B, BMPR2 and TGFBR1 mRNA expression in the oocyte, granulosa and theca cells of developing preovulatory follicles in the pig. Reproduction 2009, 138, 115–129. [Google Scholar] [CrossRef]
- Jayawardana, B.C.; Shimizu, T.; Nishimoto, H.; Kaneko, E.; Tetsuka, M.; Miyamoto, A. Hormonal regulation of expression of growth differentiation factor-9 receptor type I and II genes in the bovine ovarian follicle. Reproduction 2006, 131, 545–553. [Google Scholar] [CrossRef]
- Knapczyk, S.K.; Grzesiak, M.; Witek, P.; Duda, M.; Koziorowski, M.; Slomczynska, M. Neonatal exposure to agonists and antagonists of sex steroid receptors induces changes in the expression of oocyte-derived growth factors and their receptors in ovarian follicles in gilts. Theriogenology 2019, 134, 42–52. [Google Scholar] [CrossRef]
- Chen, A.; Xu, Z.; Yu, S.; Yang, Z. Effects of daidzein on mRNA expression of bone morphogenetic protein receptor type I and II genes in the ovine granulosa cells. Asian-Australas. J. Anim. Sci. 2010, 23, 326–332. [Google Scholar] [CrossRef]
- Chen, A.Q.; Yu, S.D.; Wang, Z.G.; Xu, Z.R.; Yang, Z.G. Stage-specific expression of bone morphogenetic protein type I and type II receptor genes: Effects of follicle-stimulating hormone on ovine antral follicles. Anim. Reprod. Sci. 2009, 111, 391–399. [Google Scholar] [CrossRef]
- Reader, K.L.; Heath, D.A.; Lun, S.; Mcintosh, C.J.; Western, A.H.; Littlejohn, R.P.; Mcnatty, K.P.; Juengel, J.L. Signalling pathways involved in the cooperative effects of ovine and murine GDF9+BMP15-stimulated thymidine uptake by rat granulosa cells. Reproduction 2011, 142, 123–131. [Google Scholar] [CrossRef]
- Rol, N.; Kurakula, K.B.; Happé, C.; Bogaard, H.J.; Goumans, M.J. TGF-β and BMPR2 signaling in PAH: Two black sheep in one family. Int. J. Mol. Sci. 2018, 19, 2585. [Google Scholar] [CrossRef]
- Vitt, U.A.; Mazerbourg, S.; Klein, C.; Hsueh, A.J.W. Bone morphogenetic protein receptor type II is a receptor for growth differentiation factor-9. Biol. Reprod. 2002, 67, 473–480. [Google Scholar] [CrossRef][Green Version]

| Primers | Sequence (5′-3′) | Use |
|---|---|---|
| ALK5-F1 | GGACCATCGCCAGGACCATC | Sequence amplification |
| ALK5-F2 | CTTCTACATCTGCCACAGC | |
| ALK5-R1 | GCCTCRCAGCTCTGCC | |
| ALK5-R2 | GTTRGCGTACCAGCACTC | |
| BMPR2-F1 | AACGAACGCTCCATATAC | Sequence amplification |
| BMPR2-R1 | TGGGAGTGCTCCATCAGG | |
| BMPR2-F2 | CHSHRGGRGGYGGAGC | |
| BMPR2-F3 | GTCCGCCACAACTACAACG | |
| RACE ALK5-F1 | TCCGTGCCAACATCCCA | Sequence amplification |
| RACE ALK5-F2 | GAGGGAGTGCTGGTACGCTA | |
| RACE ALK5-R1 | CCTTCCCTATGCTCTCCTG | |
| RACE ALK5-R2 | GATGATGGTCCTGGCGA | |
| β-Actin Q-F1 | GATTCGCTGGAGATGATGCT | Real-time quantitative PCR analysis |
| β-Actin Q-R1 | CGTTCTAGAAGGTGTGATGCC | |
| 18S rRNA Q-F1 | ACCACCCACAGAATCGAGAAA | Real-time quantitative PCR analysis |
| 18S rRNA Q-R1 | GCCTGCGGCTTAATTTGACT | |
| ALK5 Q-F1 | ACCCGAGGTGCTGGATGACT | Real-time quantitative PCR analysis |
| ALK5 Q-R1 | TTGGGATGTTGGCACGGAG | |
| BMPR2 Q-F1 | TGACGGGAAATCGCCTC | Real-time quantitative PCR analysis |
| BMPR2 Q-R1 | CTCAAAGGAGGGGTGGTTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, T.; Zhang, S.; Cai, Y.; Ma, Z.; He, J.; Zhang, Q.; Deng, F.; Ye, L.; Chen, H.; He, L.; et al. Estradiol Upregulates the Expression of the TGF-β Receptors ALK5 and BMPR2 during the Gonadal Development of Schizothorax prenanti. Animals 2021, 11, 1365. https://doi.org/10.3390/ani11051365
Yan T, Zhang S, Cai Y, Ma Z, He J, Zhang Q, Deng F, Ye L, Chen H, He L, et al. Estradiol Upregulates the Expression of the TGF-β Receptors ALK5 and BMPR2 during the Gonadal Development of Schizothorax prenanti. Animals. 2021; 11(5):1365. https://doi.org/10.3390/ani11051365
Chicago/Turabian StyleYan, Taiming, Songpei Zhang, Yueping Cai, Zhijun Ma, Jiayang He, Qian Zhang, Faqiang Deng, Lijuan Ye, Hongjun Chen, Liang He, and et al. 2021. "Estradiol Upregulates the Expression of the TGF-β Receptors ALK5 and BMPR2 during the Gonadal Development of Schizothorax prenanti" Animals 11, no. 5: 1365. https://doi.org/10.3390/ani11051365
APA StyleYan, T., Zhang, S., Cai, Y., Ma, Z., He, J., Zhang, Q., Deng, F., Ye, L., Chen, H., He, L., Luo, J., Yang, D., & He, Z. (2021). Estradiol Upregulates the Expression of the TGF-β Receptors ALK5 and BMPR2 during the Gonadal Development of Schizothorax prenanti. Animals, 11(5), 1365. https://doi.org/10.3390/ani11051365






