A Pedagogical Reinforcement of the Ideal (Hard Sphere) Gas Using a Lattice Model: From Quantized Volume to Mechanical Equilibrium
Abstract
1. Introduction
2. A Lattice Gas at Mechanical Equilibrium with Its Surroundings
2.1. Sampling Local Equilibrium States: A Discrete Thermostatistical Analysis of a Non-Interacting Gas
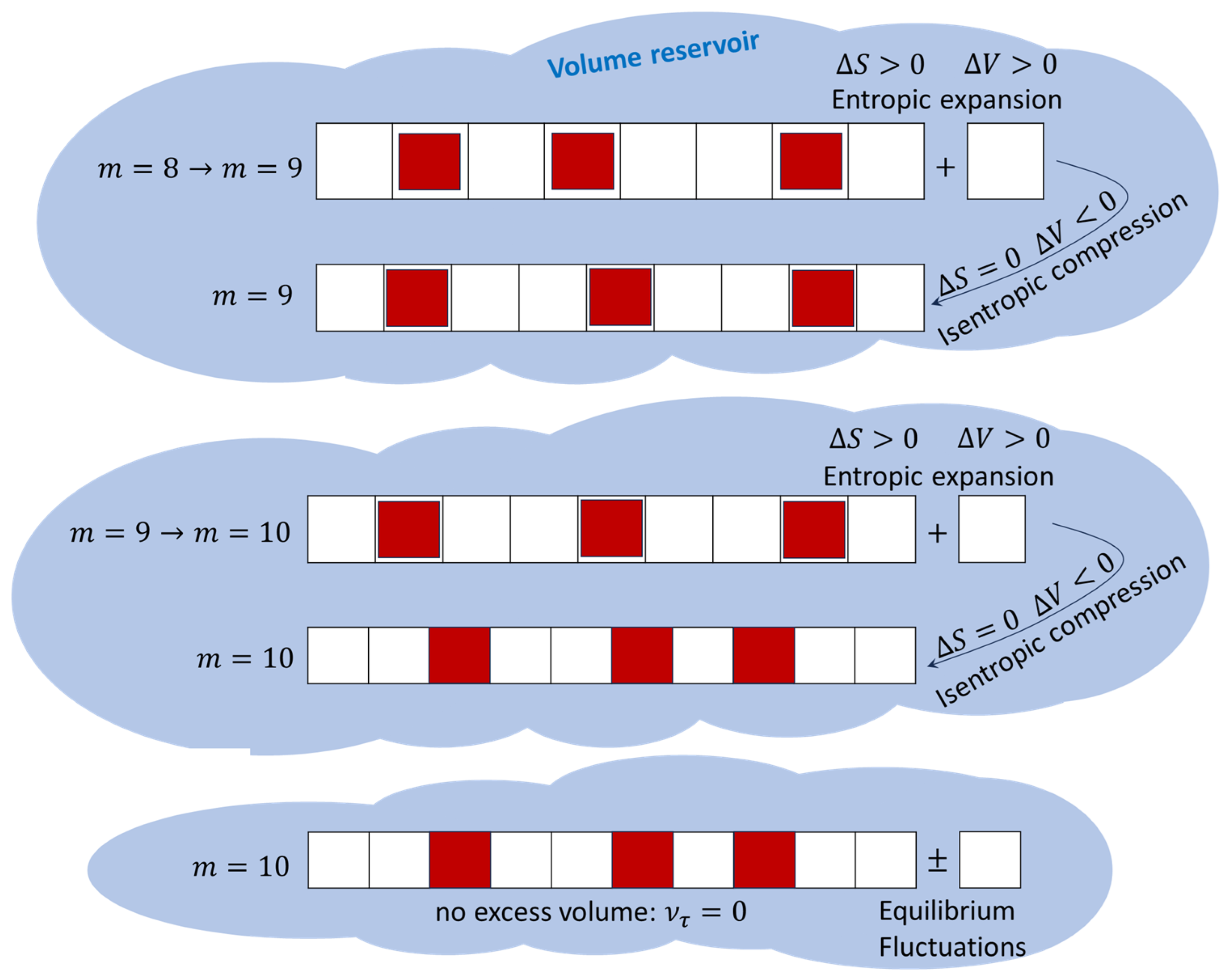
2.2. Discrete Volume, Entropy, and Irreversibility
- An irreversible entropic expansion via the absorption of a quantum of volume (a new lattice site);
- An isentropic compression whereby the volume of each lattice site is reduced.
3. A More Advanced Thermostatistical Analysis Allowing for Discrete Volume
4. Instructional Context
5. Discussion and Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Atkins, P. The Evolution of Physical Chemistry Courses, The evolution of physical chemistry courses. In Advances in Teaching Physical Chemistry; Ellison, M.D., Schoolcraft, T.A., Eds.; American Chemical Society/Oxford University Press: Washington, DC, USA, 2008; pp. 44–55. [Google Scholar] [CrossRef]
- Tsaparlis, G. The logical and psychological structure of physical chemistry and its relevance to graduate students’ opinions about the difficulties of the major areas of the subject. Chem. Educ. Res. Pract. 2016, 17, 320. [Google Scholar] [CrossRef]
- Tsaparlis, G. The logical and psychological structure of physical chemistry and its relevance to the organization/sequencing of the major areas covered in physical chemistry textbooks. Chem. Educ. Res. Pract. 2014, 15, 391. [Google Scholar] [CrossRef]
- Baierlein, R. Entropy and the second law: A pedagogical alternative. Am. J. Phys. 1994, 62, 15–26. [Google Scholar] [CrossRef]
- Reif, F. Thermal physics in the introductory physics course: Why and how to teach it from a unified atomic perspective. Am. J. Phys. 1999, 67, 1051–1062. [Google Scholar] [CrossRef]
- Schoepf, D.C. A statistical development of entropy for the introductory physics course. Am. J. Phys. 2002, 70, 128–136. [Google Scholar] [CrossRef]
- Chabay, R.W.; Sherwood, A. Bringing atoms into first-year physics. Am. J. Phys. 1999, 67, 1045–1050. [Google Scholar] [CrossRef]
- Moore, T.A.; Schroeder, D.V. A different approach to introducing statistical mechanics. Am. J. Phys. 1997, 65, 26–36. [Google Scholar] [CrossRef]
- Yu, T.H. Teaching thermodynamics with the quantum volume. J. Chem. Educ. 2020, 97, 736–740. [Google Scholar] [CrossRef]
- Kraska, T. Establishing a Connection for Students between the Reacting System and the Particle Model with Games and Stochastic Simulations of the Arrhenius Equation. J. Chem. Educ. 2020, 97, 1951–1959. [Google Scholar] [CrossRef]
- Malgieri, M.; Onorato, P.; Valentini, A.; de Ambrosis, A. Improving the connection between the microscopic and macroscopic approaches to thermodynamics in high school. Phys. Educ. 2016, 51, 65010. [Google Scholar] [CrossRef]
- McGlashan, M.L. The use and misuse of the laws of thermodynamics. J. Chem. Educ. 1966, 43, 226–232. [Google Scholar] [CrossRef]
- Denbigh, K.G. Note on entropy, disorder and disorganization. Br. J. Philos. Sci. 1989, 40, 323–332. [Google Scholar] [CrossRef]
- Leff, H.S. Thermodynamic entropy: The spreading and sharing of energy. Am. J. Phys. 1996, 64, 1261–1271. [Google Scholar] [CrossRef]
- Lambert, F.L. Shuffled cards, messy desks, and disorderly dorm rooms—Examples of entropy increase? Nonsense! J. Chem. Educ. 1999, 76, 1385–1387. [Google Scholar] [CrossRef]
- Styer, D.F. Insights into entropy. Am. J. Phys. 2000, 68, 1090–1096. [Google Scholar] [CrossRef]
- Lambert, F.L. Disorder—A cracked crutch for supporting entropy discussions. J. Chem. Educ. 2002, 79, 187–192. [Google Scholar] [CrossRef]
- Carson, E.M.; Watson, J.R. Undergraduate students’ understanding of entropy and Gibbs free energy. Univ. Chem. Educ. 2002, 6, 4–12. [Google Scholar]
- Kozliak, E.I. Introduction of entropy via the Boltzmann distribution in undergraduate physical chemistry: A molecular approach. J. Chem. Educ. 2004, 81, 1595–1598. [Google Scholar] [CrossRef]
- Ben-Naim, A. An information-theoretical formulation of the second law of thermodynamics. J. Chem. Educ. 2009, 86, 99–105. [Google Scholar] [CrossRef]
- Ben-Naim, A. Entropy: Order or Information. J. Chem. Educ. 2011, 88, 594. [Google Scholar] [CrossRef]
- Barzi, F.; Fethi, K. Reformulation of classical thermodynamics from information theory. Phys. Educ. 2025, 60, 025501. [Google Scholar] [CrossRef]
- Langbeheim, E.; Livne, S.; Safran, S.A.; Yerushalmi, E. Introductory physics going soft. Am. J. Phys. 2012, 80, 51–60. [Google Scholar] [CrossRef]
- Langbeheim, E.; Safran, S.A.; Yerushalmi, E. Visualizing the Entropy Change of a Thermal Reservoir. J. Chem. Educ. 2014, 91, 380–385. [Google Scholar] [CrossRef]
- Zhang, K. Illustrating the Concepts of Entropy, Free Energy, and Thermodynamic Equilibrium with a Lattice Model. J. Chem. Educ. 2020, 97, 1903–1907. [Google Scholar] [CrossRef]
- Dill, K.A.; Bromberg, S. Molecular Driving Forces: Statistical Thermodynamics in Biology, Chemistry, Physics and Nanoscience, 2nd ed.; Garland Science: New York, NY, USA, 2011. [Google Scholar]
- Åstrand, P.O.; de Miguel, R. Lattice models in teaching thermodynamics: Merging the configurational and translational entropies. ChemRxiv 2024, preprint. [Google Scholar] [CrossRef]
- Åstrand, P.O.; de Miguel, R. Lattice models in molecular thermodynamics: Merging the configurational and translational entropies. J. Phys. Chem. B 2024, 128, 12752–12759. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, D.V. An Introduction to Thermal Physics; Addison Wesley Longman: San Francisco, CA, USA, 2000. [Google Scholar]
- Onsager, L. Reciprocal relations in irreversible processes. I. Phys. Rev. 1931, 37, 405–426. [Google Scholar] [CrossRef]
- Onsager, L. Reciprocal relations in irreversible processes. II. Phys. Rev. 1931, 38, 2265–2279. [Google Scholar] [CrossRef]
- Onsager, L.; Malchup, S. Fluctuations in irreversible processes. Phys. Rev. 1953, 91, 1505–1512. [Google Scholar] [CrossRef]
- de Groot, S.R.; Mazur, P. Non-Equilibrium Thermodynamics; Dover: New York, NY, USA, 1984. [Google Scholar]
- Chandler, D. Introduction to Modern Statistical Mechanics; Oxford University Press: New York, NY, USA, 1987. [Google Scholar]
- Elcock, E.W.; Landsberg, P.T. Temperature Dependent Energy Levels in Statistical Mechanics. Proc. Phys. Soc. Lond. Sect. B 1957, 70, 161. [Google Scholar] [CrossRef]
- Pathria, R.K.; Beale, P.D. Statistical Mechanics, 3rd ed.; Elsevier: Oxford, UK, 2011. [Google Scholar]
- Cuden, C.B. The Temperature Dependence of the Energy Gaps in Semiconductors. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 1969. [Google Scholar] [CrossRef]
- Bendt, P.J.; Cowan, R.D.; Yarnell, J.L. Excitations in Liquid Helium: Thermodynamic Calculations. Phys. Rev. 1959, 113, 1386. [Google Scholar] [CrossRef]
- Donnelly, R.J.; Roberts, P.H. A theory of temperature-dependent energy levels: Thermodynamic properties og He II. Low Temp. Phys. 1977, 27, 687. [Google Scholar] [CrossRef]
- Allen, P.V.; Heine, V. Theory of the temperature dependence of electronic band structures. J. Phys. C Solid State Phys. 1976, 9, 2305. [Google Scholar] [CrossRef]
- Patrick, C.E.; Giustino, F. Unified theory of electron-phonon renormalization and phono-assisted optical absorption. J. Phys. Condens. Matter 2014, 26, 365503. [Google Scholar] [CrossRef]
- Dykman, M.I.; Kono, K.; Kostantinov, D.; Lea, M.J. Ripplonic Lamb Shift for Electrons on Liquid Helium. Phys. Rev. Lett. 2017, 119, 256802. [Google Scholar] [CrossRef]
- Erez, A.; Meir, Y. Effect of amplitude fluctuations on the Berezinskii-Kosterlitz-Thouless transition. Phys. Rev. B 2013, 88, 184510. [Google Scholar] [CrossRef]
- Kolář, M.; Ryabov, A.; Filip, R. Optomechanical oscillator controlled by variatioons in its heat bath temperature. Phys. Rev. A 2017, 95, 042105. [Google Scholar] [CrossRef]
- Yamano, T. Efficiencies of thermodynamics when temperature-dependent energy levels exist. Phys. Chem. Chem. Phys. 2016, 18, 7011. [Google Scholar] [CrossRef] [PubMed]
- Yamano, T. Effect of temperature-dependent energy levels on exergy. J. Phys. Commun. 2017, 1, 055007. [Google Scholar] [CrossRef]
- de Miguel, R.; Rubí, J.M. Thermodynamics far from the thermodynamic limit. J. Phys. Chem. B 2017, 121, 10429. [Google Scholar] [CrossRef] [PubMed]
- Yunt, E.; Fadaie, M.; Müstecaplıoğlu, Ö.E.; Smith, C.M. Internal geometric friction in a Kitaev-chain heat engine. Phys. Rev. B 2020, 102, 155423. [Google Scholar] [CrossRef]
- Shental, O.; Kanter, I. Shannon meets Carnot: Generalized second thermodynamic law. Europhys. Lett. 2009, 85, 10006. [Google Scholar] [CrossRef]
- de Miguel, R.; Rubí, J.M. Statistical Mechanics at Strong Coupling: A Bridge Between Landsberg’s Energy Levels and Hill’s Nanothermodynamics. Nanomaterials 2020, 10, 2471. [Google Scholar] [CrossRef] [PubMed]
- Talkner, P.; Hanggi, P. Colloquium: Statistical mechanics and thermodynamics at strong coupling: Quantum and classical. Rev. Mod. Phys. 2020, 92, 041002. [Google Scholar] [CrossRef]
- Kolář, M.; Ryabov, A.; Filip, R. Heat capacities of thermally manipulated mechanical oscillator at strong coupling. Sci. Rep. 2019, 9, 10855. [Google Scholar] [CrossRef] [PubMed]
- de Miguel, R.; Rubí, J.M. Strong Coupling and Nonextensive Thermodynamics. Entropy 2020, 22, 975. [Google Scholar] [CrossRef] [PubMed]
- de Miguel, R.; Rubí, J.M. Negative thermophoretic force in the strong coupling regime. Phys. Rev. Lett. 2019, 123, 200602. [Google Scholar] [CrossRef]
- de Miguel, R. Rayleigh-Schrödinger Perturbation Theory and Nonadditive Thermodynamics. J. Phys. Chem. B. 2023, 127, 5089. [Google Scholar] [CrossRef]
- Young, H.D.; Friedman, R.A. Sears & Zemansky’s University Physics with Modern Physcs, 14th ed.; Pearson: Essex, UK, 2015; Volume 1. [Google Scholar]
- Castellan, G.W. Physical Chemistry, 3rd ed.; The Banjamin/Cummings Publishing Company: Menlo Park, CA, USA, 1983. [Google Scholar]
- McQuarrie, D.A. Statistical Mechanics; HarperCollins: New York, NY, USA, 1976. [Google Scholar]
- Onsager, L. Crystal Statistics. I. A Two-Dimensional Model with an Order-Disorder Transition. Phys. Rev. 1944, 65, 117–149. [Google Scholar] [CrossRef]
- Ningrum, V.P.; Liu, B.; Wang, W.; Yin, Y.; Cao, Y.; Zha, C.; Xie, H.; Jiang, X.; Sun, Y.; Qin, S.; et al. Recent Advances in Two-Dimensional Magnets: Physics and Devices towards Spintronic Applications. Research 2020, 2020, 1768918. [Google Scholar] [CrossRef]
- Rodrigues, N.T.; Oliveira, T.J. Thermodynamic behavior of binary mixtures of hard spheres: Semianalytical solutions on a Husimi lattice built with cubes. Phys. Rev. E 2019, 100, 032112. [Google Scholar] [CrossRef]
- Riccardo, J.J.; Pasinetti, P.M.; Riccardo, J.L.; Ramirez-Pastor, A.J. Statistical Mechanics of Linear k-mer Lattice Gases: From Theory to Applications. Entropy 2025, 27, 750. [Google Scholar] [CrossRef]
- Schmitz, F.; Virnau, P.; Binder, K. Monte Carlo tests of nucleation concepts in the lattice gas model. Phys. Rev. E 2013, 87, 053302. [Google Scholar] [CrossRef]
- Binder, K.; Virnau, P. Overview: Understanding nucleation phenomena from simulations of lattice gas models. J. Chem. Phys. 2016, 145, 211701. [Google Scholar] [CrossRef]
- Schmitz, F.; Virnau, P.; Binder, K. Determination of the Origin and Magnitude of Logarithmic Finite-Size Effects on Interfacial Tension: Role of Interfacial Fluctuations and Domain Breathing. Phys. Rev. Lett. 2014, 112, 125701. [Google Scholar] [CrossRef]
- Grassberger, P. Pruned-enriched Rosenbluth method: Simulations of θ polymers of chain length up to 1,000,000. Phys. Rev. E 1997, 56, 3682–3693. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Bouzid, M.; Rocklin, D.Z.; Del Gado, E.; Mao, X. Correlated Rigidity Percolation and Colloidal Gels. Phys. Rev. Lett. 2019, 123, 058001. [Google Scholar] [CrossRef]
- Dill, K.A.; Ozkan, S.B.; Shell, M.S.; Weikl, T.R. The Protein Folding Problem. Annu. Rev. Biophys. 2008, 37, 289–316. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Dar, F.; Pappu, R.V. LASSI: A lattice model for simulating phase transitions of multivalent proteins. PLoS Comput. Biol. 2019, 15, e1007028. [Google Scholar] [CrossRef]
- Takada, S. Gō model revisited. Biophys. Physicobiol. 2019, 16, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Seveno, D.; D. Blake, T.; De Coninck, J. Young’s Equation at the Nanoscale. Phys. Rev. Lett. 2013, 111, 096101. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cao, G. Effectiveness of the Young-Laplace equation at nanoscale. Sci. Rep. 2016, 6, 23936. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Riordon, J.; Hadi Zandavi, S.; Xu, Y.; H. Persad, A.; Mostowfi, F.; Sinton, D. Capillary Condensation in 8 nm Deep Channels. J. Phys. Chem. Lett. 2018, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Latella, I.; Peréz-Madrid, A.; Campa, A.; Casetti, L.; Ruffo, S. Thermodynamics of Nonadditive Systems. Phys. Rev. Lett. 2015, 114, 230601. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
de Miguel, R. A Pedagogical Reinforcement of the Ideal (Hard Sphere) Gas Using a Lattice Model: From Quantized Volume to Mechanical Equilibrium. Entropy 2026, 28, 45. https://doi.org/10.3390/e28010045
de Miguel R. A Pedagogical Reinforcement of the Ideal (Hard Sphere) Gas Using a Lattice Model: From Quantized Volume to Mechanical Equilibrium. Entropy. 2026; 28(1):45. https://doi.org/10.3390/e28010045
Chicago/Turabian Stylede Miguel, Rodrigo. 2026. "A Pedagogical Reinforcement of the Ideal (Hard Sphere) Gas Using a Lattice Model: From Quantized Volume to Mechanical Equilibrium" Entropy 28, no. 1: 45. https://doi.org/10.3390/e28010045
APA Stylede Miguel, R. (2026). A Pedagogical Reinforcement of the Ideal (Hard Sphere) Gas Using a Lattice Model: From Quantized Volume to Mechanical Equilibrium. Entropy, 28(1), 45. https://doi.org/10.3390/e28010045





