1. Introduction
Understanding how critical behavior emerges from finite systems remains a foundational challenge in statistical mechanics. The Ising model [
1,
2] is structurally simple, yet captures the essential mechanisms of cooperative behavior in lattice systems and therefore continues to serve as a versatile and influential testing ground for theories of phase transitions and critical phenomena. Despite its long history, determining specific parameters of the Ising model in the thermodynamic limit from finite-sized lattices with high accuracy remains a benchmark challenge [
3]. A wide array of methods has been developed to determine the critical temperature
of the Ising model [
3,
4]. The most common approach is to associate the finite maximum in the heat capacity
of a finite lattice as a proxy for
. From theory, it is known that the heat capacity
for the 2D-Ising model shows a logarithmic singularity
in the vicinity of
with a critical exponent of
[
5]. Monte Carlo (MC) simulations yield accurate numeric estimates but often at high computational cost for large system sizes [
6]. The renormalization group framework provides fundamental insights into universality and scaling laws, yet its practical implementations on complex or finite geometries are technically demanding [
7]. Finite-size scaling [
8,
9], particularly through observables like the Binder cumulant [
10] or heat capacity peaks, has become the standard route to extrapolate
from finite lattices but requires extensive data from multiple sizes to achieve high precision.
Alternatively, exact treatments of small systems offer valuable insight. Kaufman’s solution for periodic boundary conditions (PBCs) [
11] and subsequent modern implementations [
12] provide access to the density of states (DOS) for small lattices, which in principle allows for the exact evaluation of thermodynamic functions. For Ising systems with free (i.e., open) boundaries (FBC), on the other hand, no such solution approaches have been found to date [
13]. Notably, Stošić et al. [
14] employed the transfer-matrix (TM) method to obtain the exact DOS for a set of finite squared FBC-Ising systems, using their results to test finite-size scaling predictions. However, the lack of compact closed-form expressions for the partition function of arbitrary finite lattices [
15] limits the general applicability of such approaches, and the direct extrapolation of
based on these exact calculations remains challenging. Recent advances in quantum computing have revived interest in finite-size Ising models [
16]. While several methods exist for ground-state energies, direct computation of partition functions is far from routine and remains challenging due to unfavorable qubit scaling with interaction complexity [
17]. This shows that even in 2025, there is still a need for the systematic use of exactly solvable small lattices for extrapolation towards the thermodynamic limit.
Building on our previous analytic framework for free energy expressions for finite 2D-Ising systems [
18], the current study systematically compares different extrapolation schemes aimed at estimating the critical temperature of the infinite lattice from a minimal set of exactly solvable finite lattices with both free and periodic boundary conditions. In particular, we analyze scaling behavior of heat capacity peak temperatures (
) across sizes and construct common envelope functions. From theory, we expect
[
9], which is why we primarily focus on estimation approaches for
. For alternative temperature measures, we impose the general requirement that, although they do not have to follow the same scaling behavior as
, they must converge towards the same limiting value
and, ideally, be as simple and robust as possible to calculate. We uncover a surprisingly simple two-parameter scaling law following an
-form, which converges robustly towards Onsager’s exact
, that delivers excellent results even in simplified form with only one adjustable parameter.
This work ultimately aims to demonstrate how minimal yet exact finite-size information can deliver precise critical estimates, offering an efficient alternative to resource-intensive traditional methods and potentially serving as a template for other lattice gas models where analytic solutions are elusive.
2. Materials and Methods
In this work, we consider the classical Ising model, based on isotropic nearest-neighbor interactions with uniform coupling constant
J and two possible values for the spin number of the
i’th spin
, corresponding to the spin-up and spin-down state. In 2D, for finite-sized rectangular lattices of dimensions
(with
and
spins placed in
x- and
y-direction, respectively), the field-free Hamiltonian reads as (see also [
18])
where FBC and PBC denote the case of free and periodic boundary conditions, respectively.
denotes the spin value of the associated spin at lattice position
. Here, we solely focus on square (
) lattices, i.e.,
. Throughout the paper,
J will be set to unity, i.e., only the ferromagnetic case (
) will be considered.
Calculations are based on the exact representation of the canonical partition function as function of spin number and temperature T, using the density of states formalism: . The sum runs over distinct energy levels with associated statistical weights, i.e., degeneracies which obey . For convenience, the inverse temperature will be generally used instead of T, where Boltzmann’s constant will be set to unity to make the heat capacity dimensionless. With this convention, , and we do not need to distinguish between dimensional and dimensionless variants of C, T, and , i.e., , and .
In the case of FBC systems, calculation of the DOS (i.e., the collected distribution of discrete energy levels and weights
) was performed using the transfer-matrix (TM) method [
19,
20] for system sizes
. The TM method was implemented in Python 3.11 using standard libraries (numpy, itertools, collections). Energy contributions of individual rows and row pairs were precomputed and combined recursively to obtain the full DOS. The TM-implementation was validated for small system sizes up to
against brute force calculations where all possible
configurations were systematically generated using the Python itertools-module. Up to a size of
, the systems can be calculated in moderate computing time via the TM method on a current laptop without the need for access to specialized high-performance computing infrastructure. For larger system sizes,
, which were not included for model training but for assessing prediction quality, we used the optimized Pfaffian-based algorithm by Karandashev et al. [
21], which evaluates the exact partition function of a given 2D-FBC-Ising lattice at specified
. Since the code (which is freely available [
22] ) does not deliver the DOS directly but returns
,
, and
with
, the formalism for calculating the maximum heat capacity (
,
) presented below is not applicable. In this case, the heat capacity curve
was computed on a
-interval of
with 50 equidistant points around the expected maximum from a finite-difference (second order forward) approach.
was then estimated from the smoothened profile via interpolation of the discretized function values using cubic splines.
For DOS calculations in case of periodic boundaries (PBC), we used the publicly available Mathematica-implementation [
23], developed by Beale [
12], based on Kaufman’s generalization [
11] of Onsager’s solution [
24]. For PBC, system sizes up to
were considered. Note that all the derivations given in the following (such as the calculation of
C and
) are exact and general and hold for all types of boundary conditions in all dimensions; it is only the input in form of the DOS that differs for different systems.
Heat capacity profiles
for the considered
-lattices were calculated based on the DOS from standard thermodynamic relations [
25]:
where
and
denote the first (i.e., the mean value) and second moment of the DOS, respectively. Though it is not explicitly represented for reasons of better readability, it should be noted that all moments
(see also Equation (
5a)) and combinations thereof such as the energy variance
are temperature-dependent (i.e.,
-dependent). The dependency on system size
N (either in the form of an explicit argument or as a subscript to emphasize its meaning as curve parameter) is only explicitly stated when it serves to improve comprehensibility. Throughout the paper, we use short-hand notations for (partial) derivative operators such as
and
for the first and second partial derivatives, respectively (in this case with respect to
), while all other independent variables (such as
) are held constant.
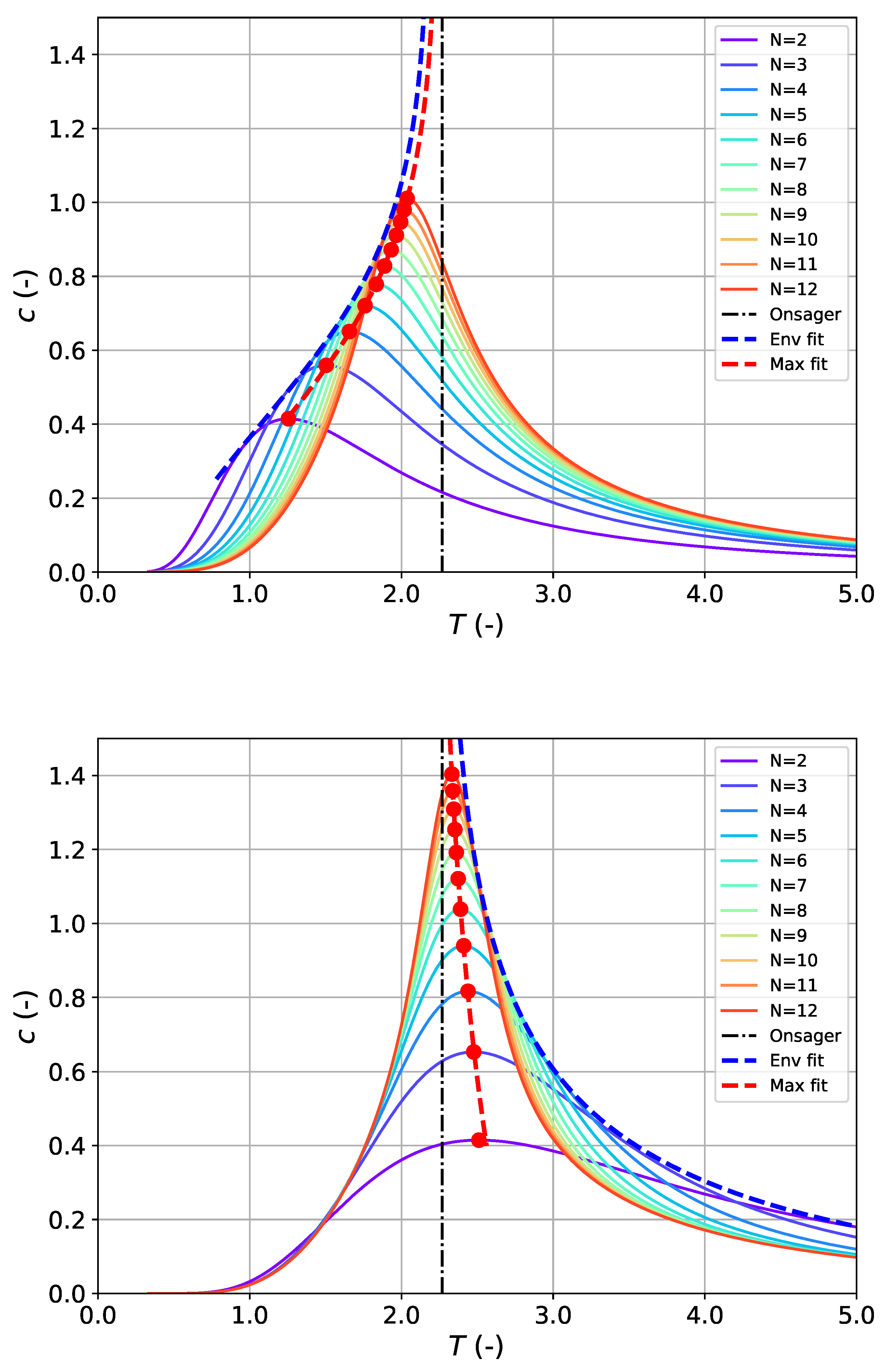
Finding the (inverse) temperature (
) at the peak maximum of the heat capacity profile follows from derivation of Equation (
2):
By setting the right-hand side to zero (
), the problem of calculating
is reduced to finding the zeroes of the following function:
with the supplementary relations
Obviously, for the Ising model featuring single-peaked heat capacity profiles, finding the maximum of
is equivalent to finding the maximum of
, i.e.,
with
. Since Equation (
4) cannot be solved analytically and therefore no closed expression can be derived for
, it was solved numerically via Newton’s method according to
, using Equation (
5e) and Equation (
5f).
denotes the numeric value for
at the
k-th iteration. Iterations were stopped once consecutive updates for
differed by less than
on an absolute scale and written to file with a precision of 14 decimal places. Numerical results for heat capacity peak data for FBC and PBC systems calculated by the approach described above are listed in the
Appendix A in
Table A1 and
Table A2, respectively. It should be stressed that the only approximation involved in the procedure arises from the numerical determination of
from Equation (
4).
While the maximum temperature is obtained from differentiation of
, another possible temperature measure that can be used as a proxy for the true critical temperature
can be constructed from integration of the heat capacity curve. Changes in the mean energy
(for the Ising model, the thermodynamic concepts of (total) mean energy, internal energy, and potential energy coincide) and the entropy
S between the two limiting temperature values of
and
are related to integrals of
and can be calculated analytically due to their state function property [
18]. For FBC one obtains
where the Jacobian factor of
for the transformation from
T- to
-space has to be considered in the second step. Note that the integral of
without the Jacobian factor would lack a direct thermodynamic interpretation. The subscript “cal” recalls the analogy to calorimetric experiments. The ratio of the two integral quantities introduces the corresponding calorimetric temperature which, in case of FBC, shows a
-scaling law with system size
where the Binomial formula was applied in the first step and
. It is evident that the limiting value of this temperature measure
is not identical to the true 2D-critical temperature
[
19,
24]. However, as it turns out,
seems to be almost perfectly correlated with
(for FBC and PBC), implying that it obeys the identical
-scaling law (compare with the
Section 3). Different interpretation approaches for
are summarized in
Appendix B.
Applying the same procedure for PBC, it is
instead of Equation (
6a) due to the different value for the ground-state energy, yielding
instead of Equation (
7). However, since it was found that this approach does not deliver a satisfactory description of the data, it was not explored further. For the sake of completeness, it should be noted that if
would be formally treated as a (non-normalized) probability density function,
would correspond to the mode of this distribution, and other characteristic distribution parameters, such as the mean and median temperatures, could be derived. However, since these parameters neither exhibit the same scaling behavior as
(in contrast to
) nor tend towards the true
, they are not further considered as relevant temperature measures.
In order to assess the quality of the presented approaches, we compared them in the case of FBC systems with the established cumulant intersection method proposed by Binder [
10]. Therein, the fourth-order (“Binder”) cumulant
is calculated according to
with the moments of the (instantaneous) magnetization
according to
where
denotes an instantaneous spin configuration,
is the energy level associated with the magnetization value
and
corresponds to the joint DOS, i.e., the multiplicity of microstates with energy level
E and magnetization
M. In contrast to the energy-DOS introduced beforehand, this represents a 2D or joint DOS since it involves both energy and magnetization levels. The joint DOS was obtained exactly by two complementary numerical methods implemented in Python. For small lattices, we performed a full enumeration of all
configurations and accumulated counts into an (
E,
M)-histogram. For larger lattices, we used a TM/dynamic-programming extension that carries the row pattern and the running magnetization as state variables: row energies and row–row interaction energies were precomputed, and counts were propagated row-by-row while tracking both energy and magnetization. Results were stored as integer counts and validated by checking the closure relation
.
All implementations were performed in Python 3.11 using standard libraries (numpy, itertools, collections).
4. Discussion
In this work, we focused on extrapolation strategies based on the heat capacity profile of finite Ising lattices that are used as a proxy for the critical temperature of the infinite lattice
and which we expect to converge towards
for
. While this is a common approach it is by no means unique. Another possible choice is to focus on the maximum of the zero-field susceptibility [
26]. However, it appears that these different approaches do not necessarily lead to the same extrapolation result in practice due to the different underlying methods used for their determination or their limited accuracy [
10]. For this reason, we have deliberately focused on the investigation of small systems for which the exact calculation of the partition function (or equivalently the DOS) is in principal possible, using suitable methods, even if no direct analytical expression can be given.
We have identified two approaches: “approach 1” is focused on modeling the maximum (or peak) temperature
(c.f. Equation (10)); “approach 2” is focused on modeling the sequence of heat capacity maxima
together with (one-sided) tangent envelopes to the family of heat capacity profiles (c.f. Equation (12)). The appeal of approach 1 is that it involves a minimal number of adjustable parameters (in the simplified form of Equation (
11), only 1), requires a minimum of exact data points from DOS calculations for adjustment, and can be converted into a linear representation, which allows straightforward and robust solution to the optimization problem. Further, it turns out that approach 1 can be applied to both types of boundary conditions and allows very accurate estimation of
with a maximum relative deviation significantly below 1%. The results of approach 2 show a greater deviation from the true
than approach 1, although the predictive power is similar. However, (slightly) different approaches must be chosen for FBC and PBC, each with three parameters, and a linear representation as for approach 1 is not possible, which makes optimization more challenging. An advantage is that, for a given boundary condition type, the same approach can be used to describe both the maxima and the common envelope. However, no method could be found to combine these partial results in a way that would yield a better estimate than the individual estimates. Overall, it can be said that approach 1 enables a more robust and accurate estimate for
.
In the case of PBC maxima, it was found that, for both tested extrapolation approaches to be necessary, the data from the smallest lattice with needed to be excluded from the modeling in order to obtain satisfactory results, which was not the case for FBC. This observation seems counterintuitive, as finite-size effects would be expected to be particularly pronounced in small FBC systems rather than PBC, and we are currently unable to provide an explanation for this.
For further assessment of the accuracy of the presented approaches, we additionally estimated
from the established cumulant intersection method [
10] in case of FBC systems (calculation details can be found in the
Section 2). The procedure is based on plotting the fourth-order (“Binder”) cumulant
according to Equation (
8) as a function of temperature for different system sizes and estimate
from the common intersection point (denoted as crossing temperature
) between pairs of
-curves (
i,
j) [
6]. In this way, a total of
values for
is obtained with
and
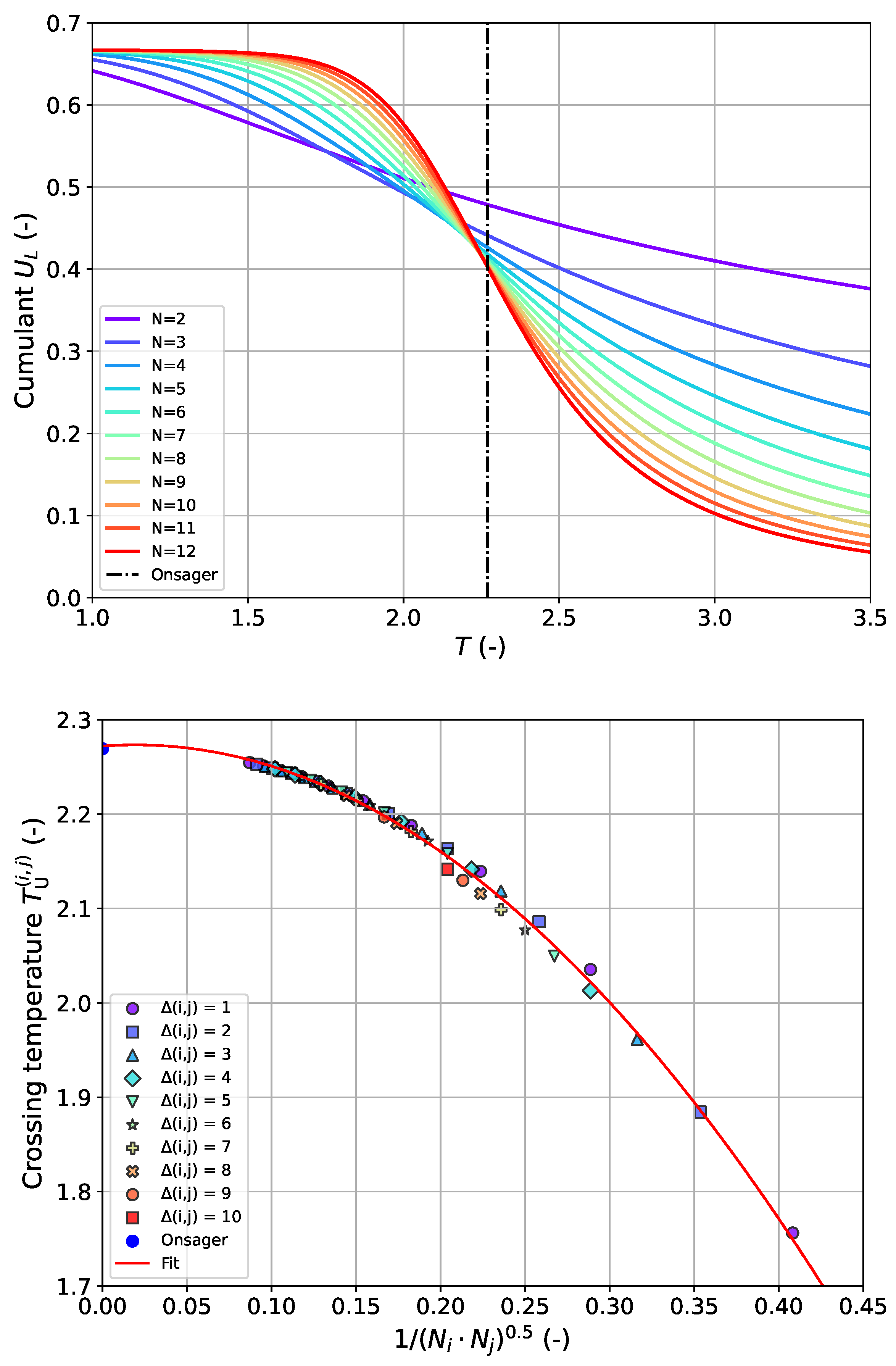
. It is known that the intersection points are scattered in the form of a pronounced size-dependence of the
-values especially in the case of very small systems, preventing an unambiguous determination of
. This effect can be seen in the top panel of
Figure 3. In order to still be able to use the data from all pairs without excluding particular systems, we propose an evaluation by plotting over the inverse effective system size as given by the geometric mean of the sizes for each pair (
). We have found that all points fall onto a single curve to a very good approximation in that way (see lower panel of
Figure 3). The simplest approach to modeling this relationship is a quadratic approach involving three parameters in the following form:
The estimate for the critical temperature
for the infinite lattice is obtained by extrapolation
which corresponds to the offset on the vertical axis in the lower graph of
Figure 3. The success of this approach is shown in
Figure 3 with the metrics listed in
Table 4. As can be seen, the “Binder” cumulant method yields an
with similar accuracy as the corresponding estimate from approach 1; however, more adjustable model parameters are required.
It is important to clarify that the two presented approaches are not completely new (cf., e.g., Equation (4.1) in [
27]), but only certain aspects. A novel aspect of our analysis that has not yet been discussed to the best of our knowledge is the observed strong correlation of
with the auxiliary quantity of the calorimetric temperature
. The temperature measure
can be computed exactly from the thermodynamic properties of the Ising model in terms of energetic and entropic changes and, importantly, exhibits the correct scaling behavior with system size
N according to
. What both quantities have in common is that they can be calculated from the shape of the heat capacity profile
, while
is obtained from differentiation, i.e., the slope of
,
is obtained from an integration procedure, i.e., the area under
(although it can be calculated without the need to actually perform the numerical integration). In the broader thermodynamic context of heat transfer within thermodynamic cycles,
is also known as the thermodynamic mean temperature
[
28] and can be interpreted as a characteristic average temperature of the system. Different interpretation approaches for
(or
) are presented in
Appendix B. Although the stringent physical/mathematical origin of the observed strong correlation between
and the peak temperature
currently remains unclear, some analogy can be found in the field of protein thermodynamics. Here, one is interested in the energetic and entropic changes that occur during heat-induced (i.e., calorimetric) protein unfolding. The derivation is based on the Gibbs equation (which corresponds to the second approach presented in
Appendix B) and considers protein unfolding as a kind of pseudo-phase transition from an ordered (folded) to a disordered (unfolded) state of the protein. Ref. [
29] uses the example of lysozyme as the model protein to demonstrate that the midpoint temperature of the experimentally recorded heat capacity profile (i.e.,
) agrees very well with the enthalpy/entropy ratio (i.e.,
). Since the experiments are conducted under constant-pressure rather than constant-volume conditions, calculation of
involves the calorimetric enthalpy change rather than
, but otherwise, the definition is identical to Equation (
7). As in the case of the finite Ising lattices, the transition between the two states (from “folded” to “unfolded”) takes place over a wide temperature range and
(including
) remains finite. The main reason behind the applicability of the analogy could be that in both cases, i.e., protein unfolding and finite Ising lattices, the cooperative unit is not made out of bulk material but is of finite size; in case of protein unfolding, the cooperative unit is determined by the size of the macromolecule [
30] and in vitro protein experiments are typically conducted in highly diluted aqueous solutions, which is why the model of a single protein molecule dissolved in a background of low-molecular solvent often represents a relatively good approximation. This state of infinite dilution further resembles the situation of a single Ising lattice of finite size. Another similarity between the two problems is that the thermodynamic behavior of “typical” water-soluble proteins (such as lysozyme) can often be described to good approximation by rather simple statistical–mechanical models such as lattice models [
31]. Within the assumptions of these models, each amino acid residue can, like a spin, only assume two discrete states (hydrophobic or polar).
It is also remarkable but not obvious why the identical approach works not only for FBC but also for PBC, while in the latter case, the corresponding scaling behavior that would follow in analogy according to
(see
Section 2) does not appear to be adequate. Nonetheless, as our results demonstrate, the scaling with system size as obtained from
in the presented way can be employed as a robust estimator of the critical temperature. In the next step, the transferability to 3D lattices will be studied. Since practical calculation of the exact DOS is limited to
in 3D, a sophisticated DOS estimator such as the Wang–Landau method [
32,
33] has to be applied. Another new aspect of the present work is the further simplified variant given by Equation (
11) with only one model parameter. In approach 2, the description of the envelope and the fact that it can be described to a good approximation using the same function as for the maxima curve are, to the best of our knowledge, also new.
In our previous work [
18], we posed the central question whether it is possible to approximate a finite-sized 2D-Ising lattice via 1D Ising chains on the basis of free energy expressions. The approach involved a combination of an exact analytical intra-chain and an approximative inter-chain part through which interactions among adjacent chains are established. While the free energy contribution of these inter-chain bonds could be well approximated as a function of temperature and system size, unfortunately, it has been found that the extrapolation behavior
is not possible without imposing the target solution for
in the form of an additional constraint into the model. With the relatively simple scaling law for
presented in this work, we hope to be able to improve the approach of our previous work in future studies by incorporating it into the free energy construction scheme.