1. Introduction
1.1. Motivation
The COVID-19 pandemic has sparked renewed interest in understanding how behavioral and structural heterogeneity affects the spread of infectious diseases in social systems. Although classical compartmental models in epidemiology treat the population as homogeneous, real-world societies are composed of individuals with diverse behavioral responses, including varying levels of adherence to public health interventions such as mask wearing, vaccination, and social distancing.
Recent empirical studies have shown that people tend to cluster in social and spatial proximity based on their health behaviors and beliefs [
1,
2,
3], a phenomenon known as homophily. Such clustering can have profound implications for epidemic dynamics, particularly when individuals who neglect anti-epidemic measures form tightly connected subgroups. These clusters can serve as reservoirs for sustained transmission, reducing the overall effectiveness of interventions aimed at the population level.
Our study focuses on understanding how homophily in adherence to anti-epidemic measures affects the final size of an epidemic in a population composed of two behavioral groups. We distinguish between compliant individuals who follow preventive measures and non-compliant individuals who do not. By embedding these groups into synthetic networks with tunable levels of inter-group connectivity, we explore whether increasing separation between behavioral groups protects compliant individuals or, paradoxically, makes them more vulnerable.
We show that under certain conditions, separating groups may inadvertently amplify transmission within the non-compliant cluster, increasing secondary exposure for the compliant group. This finding underscores the importance of considering network structure and asymmetric behavioral effects in the design and evaluation of epidemic control strategies.
1.2. Related Work
The role of behavioral clustering in epidemic dynamics has been studied in multiple disciplines, including epidemiology, network science, and computational social science. Early work by May and Silverman [
1] highlighted the potential threat posed by spatial clusters of individuals who refuse vaccination, showing that such clustering can lower herd immunity thresholds. Omer et al. [
2] extended this observation by identifying geographic clustering of non-medical exemptions and its association with pertussis outbreaks.
From a network-theoretic perspective, Salathé and Bonhoeffer [
4] showed that opinion clustering can dramatically affect outbreak size, even when the overall fraction of protected individuals remains constant. More recent studies have investigated the dual role of social influence and selection in producing clusters of vaccine hesitancy [
5] and how assortative mixing based on behaviors such as mask-wearing [
6] or vaccination [
7,
8,
9] alters epidemic thresholds and dynamics. In particular, Are et al. [
9] show that vaccine status homophily can amplify infection risk among the unvaccinated, even when overall coverage is high.
The interaction between homophily and the effectiveness of non-pharmaceutical interventions (NPIs) has recently been linked to paradoxical phenomena. Klimek et al. [
10] demonstrate that, under specific behavioral correlations, low mask coverage can be more protective for mask wearers than medium coverage—a result attributed to network-level feedbacks and assortativity. These findings resonate with our observation that increased separation between compliant and non-compliant individuals can elevate risk for the compliant group, depending on which aspect of transmission is modulated by interventions.
From a modeling perspective, our use of networks with controlled clustering and modularity builds on recent advances in network generation. In particular, the STC model proposed in [
11] enables explicit control of triadic closure while preserving degree distributions. The importance of higher-order motifs has been emphasized in studies of complex contagion [
12,
13], showing that clustering and triangle overlap influence both outbreak thresholds and final epidemic size. Bizzarri et al. [
14] theoretically predict that homophily may first amplify and then suppress infections as it increases, depending on the epidemic regime.
These studies collectively suggest that homophily in protective behavior is not merely a passive reflection of social preferences but an active driver of epidemic risk. Our work contributes to this literature by demonstrating that, under realistic constraints on transmission asymmetry, increased homophily can produce counterintuitive outcomes. Unlike most prior models, we examine this effect in networks with controlled modularity and clustering, and we isolate the conditions under which increased separation between compliant and non-compliant individuals harms rather than protects the compliant population.
1.3. Research Questions and Contribution
This work addresses the following research question: under what conditions does increasing behavioral homophily—interpreted as the separation between compliant and non-compliant individuals—lead to reduced epidemic impact in the compliant group? More specifically, we aim to understand how network structure and asymmetric transmission dynamics influence the final size of the epidemic within each behavioral group.
To answer this question, we develop a modified SIR model in which the probability of transmission depends asymmetrically on the behaviors of both the infected and susceptible individuals. We embed the population into synthetic networks generated by two models that allow control over inter-group connection probabilities: the stochastic block model (SBM), and a triadic closure (TC) model, which captures clustering typical of real-world social networks.
The key contributions of this study are:
We introduce a variant of the SIR model with behavior-dependent transmission asymmetries, distinguishing between the infectiousness of non-compliant individuals and the susceptibility of compliant individuals.
We systematically explore epidemic dynamics across a range of homophily levels and model parameters on both SBM and TC networks, ensuring constant network density for fair comparison (mean degree ).
We identify a counterintuitive effect: in clustered networks (TC), increasing the separation between groups can increase the fraction of recovered nodes in the compliant group, due to intensified intra-group transmission among non-compliant individuals.
We show that this effect does not appear in SBM networks, underscoring the role of local clustering in mediating epidemic outcomes.
By isolating the conditions under which homophily exacerbates risk rather than mitigates it, our findings contribute to the theoretical understanding of behavioral–epidemic interactions and offer cautionary insight into the design of intervention strategies.
2. Model and Methods
2.1. Epidemic Dynamics with Behavior-Based Transmission
We use a discrete-time SIR (Susceptible–Infected–Recovered) model implemented on a static undirected network. Each node represents an individual and can be in one of three epidemiological states: susceptible (S), infected (I), or recovered (R). At each time step, infected individuals attempt to transmit the disease to their susceptible neighbors, and then recover with fixed probability.
To model behavioral heterogeneity, we divide the population into two equally sized groups:
Compliant nodes: those who adhere to anti-epidemic measures (e.g., mask-wearing, isolation, vaccination);
Non-compliant nodes: those who ignore such measures.
We introduce two parameters that control the effect of behavior on transmission probability:
: scales the infectiousness of compliant infected nodes (e.g., impact of mask wearing);
: scales the susceptibility of compliant susceptible nodes (e.g., impact of vaccination).
In what follows, smaller values of or denote more effective interventions (e.g., implies strong source-control effectiveness), whereas represents no effect. Given a baseline transmission probability p, the probability of disease transmission along an edge depends on the behavioral types of both individuals involved:
Non-compliant (I) → Non-compliant (S): transmission with probability p;
Compliant (I) → Non-compliant (S): probability ;
Non-compliant (I) → Compliant (S): probability ;
Compliant (I) → Compliant (S): probability .
All infected nodes recover independently with fixed probability r. This recovery probability is the same across both groups.
Each simulation begins with a fixed number of initially infected nodes, randomly selected from both groups. The epidemic evolves until no infected nodes remain. We record the final fraction of nodes in the recovered (R) state within each behavioral group.
2.2. Network Models
To study the interplay between network structure and behavioral homophily in epidemic spreading, we generate synthetic social networks using two complementary approaches: the Stochastic Block Model (SBM) and a network model based on Triadic Closure (TC). Both approaches allow us to manipulate the level of group separation (homophily) while preserving the average degree of the network, ensuring that any differences in epidemic outcomes are attributable to structural and behavioral factors, not changes in connectivity.
2.2.1. Stochastic Block Model (SBM)
In the SBM, nodes are divided into two groups—compliant and non-compliant—each containing
nodes. Edges are placed probabilistically between node pairs according to their group membership. We define the connection probability matrix:
where
is the desired global network density,
controls the level of between-group connectivity (asymmetry), and
are the sizes of the two groups. This formulation ensures that the expected total number of edges remains constant regardless of
a, allowing us to isolate the effect of homophily. For the equal-size case
, Equation (
1) simplifies to
which we use in all simulations in this study. When
, connections are distributed randomly across the network (well-mixed); as
a increases toward 1, the network becomes fully separated with no inter-group edges.
2.2.2. Triadic Closure (TC)
The TC model [
15] is designed to capture an essential feature of real social networks: local clustering. We begin by generating an Erd
s–Rényi (ER) random graph with
N nodes and average degree
and then iteratively rewire edges to increase clustering without changing the number of edges.
The TC construction proceeds in two phases:
- 1.
Thermalization. Randomly select an edge and replace it with an edge that closes a triangle by connecting a random endpoint to a second-order neighbor. Repeat this process until the global clustering coefficient reaches a plateau.
- 2.
Behavioral modularity. Assign nodes to two groups of equal size (compliant and non-compliant), then further rewire edges to increase the proportion of intra-group connections. This is carried out by selecting edges and reconnecting them to second-order neighbors of a random endpoint but only if it belongs to the same group, gradually increasing modularity while maintaining the total number of edges. For selected asymmetry level
, the process ends when
We use the standard Newman–Girvan modularity with the two behavioral groups as communities. The stopping rule (
3) provides a simple monotone link between
a and the achieved inter-group sparsity; higher
a corresponds to higher modularity (stronger separation). This two-step rewiring algorithm results in networks with nontrivial clustering and tunable behavioral homophily. Importantly, the procedure preserves the average degree and network size, enabling meaningful comparisons with SBM.
In both models, the parameter a serves as a homophily control: denotes full mixing, while represents strong separation between groups. Although a does not yield the same graph modularity in TC and SBM, it monotonically tunes the group separation in both models. Comparing epidemic outcomes across SBM and TC networks for the same a and transmission parameters allows us to isolate the role of local clustering in shaping infection dynamics.
2.3. Simulation Procedure
We simulate epidemic outbreaks on undirected networks of nodes, evenly split into two behavioral groups: compliant and non-compliant, each with 2500 nodes. The average degree is fixed at , ensuring consistent network density across all scenarios. We focus on a baseline transmission probability and a recovery probability for all infected individuals.
Each simulation begins by randomly infecting 50 compliant and 50 non-compliant nodes. All remaining nodes are initially in the susceptible state S. The simulation evolves in discrete synchronous time steps. The disease spreads solely through direct contact with infected neighbors; there is no environmental transmission or memory of prior states. Each realization proceeds until the absorbing state is reached (i.e., the set of infected nodes is empty).
To account for randomness in both the network generation and the initial conditions, we conduct 1000 independent simulations for each parameter configuration. In every run a new network is generated according to the selected model (SBM or TC) and asymmetry level a.
We explore the full grid of parameter combinations: . The results are aggregated by averaging across realizations and by analyzing the full distribution of outcomes within each condition, enabling robust conclusions about trends and variability in epidemic size and the direction of transmission.
3. Results
We begin our analysis by investigating how the final epidemic size varies between the compliant and non-compliant groups, as a function of behavioral homophily. Specifically, we focus on the fraction of nodes in the recovered (R) state at the end of the simulation, separately for each group. This provides a measure of the total epidemic burden experienced by compliant and non-compliant individuals under different network and behavioral configurations.
Figure 1 presents the logarithm (base 10) of the expected fraction of recovered nodes in the compliant group, shown for various combinations of
,
, and asymmetry levels, separately for TC and SBM networks.
A key observation is that for small values of (e.g., ), the fraction of recovered nodes in the compliant group initially increases with group separation, reaching a maximum around before decreasing again. Analogous results for the non-compliant group are consistently monotonic across all parameters and network types; for brevity, we do not include them here.
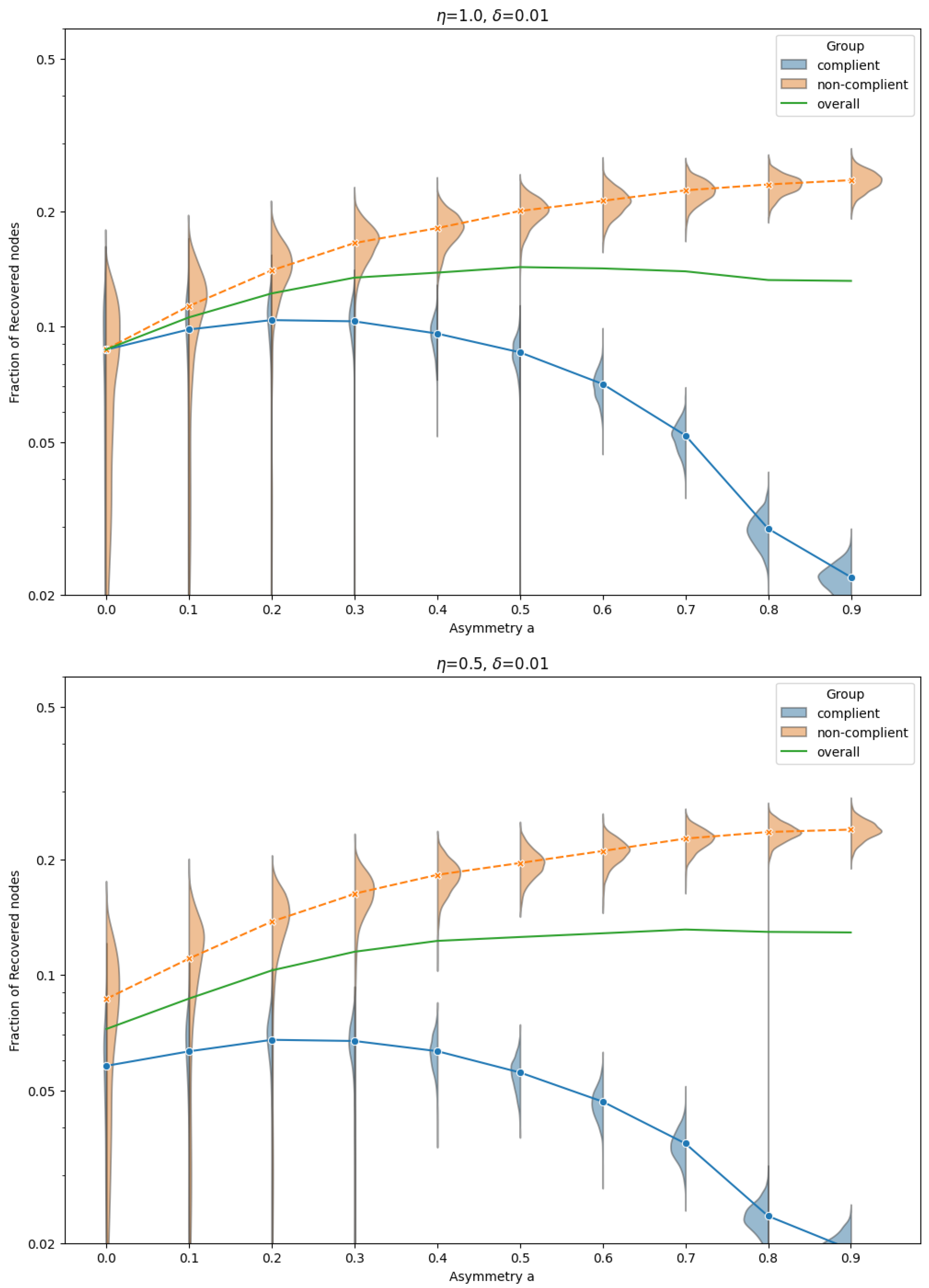
Figure 2 shows the distribution and mean fraction of recovered nodes in each group for the TC model, across a range of asymmetry levels
a, and for transmission asymmetry parameters
,
fixed. This non-monotonic behavior is unexpected: stronger separation from the high-risk non-compliant group intuitively suggests better protection. However, the increased clustering among non-compliant individuals accelerates within-group transmission and leads to more secondary infections that eventually reach compliant individuals via residual inter-group links.
This paradoxical effect is most pronounced when , but it persists for smaller values of as well. Importantly, the effect emerges consistently when , regardless of , suggesting that it is the asymmetry in infectiousness—not susceptibility—that drives the non-monotonic outcomes.
By contrast, in the non-compliant group, the fraction of recovered nodes increases monotonically with homophily: as separation strengthens, their denser internal connectivity facilitates more efficient spread.
For larger values of (e.g., ), the asymmetry in infectiousness disappears, and both groups exhibit nearly identical epidemic sizes that vary little with a.
In the case of SBM, the non-monotonic effect observed in TC is absent. In the compliant group, the epidemic size either decreases monotonically or remains flat as a increases. This confirms that the paradoxical effect seen in TC arises from the interaction between clustering and behavioral heterogeneity.
These findings highlight that network structure plays a crucial role in shaping the impact of behavioral homophily on epidemic dynamics. In particular, the clustering-induced amplification of internal transmission within the non-compliant group can counteract the protective effect of separation, resulting in increased risk for the compliant group.
4. Discussion and Conclusions
4.1. Implications of Homophily for Epidemic Control
Our findings reveal the nuanced and at times counterintuitive role of behavioral homophily in shaping epidemic outcomes. While separating compliant individuals from non-compliant ones may intuitively appear protective, our results show that this strategy can fail—or even backfire—under specific conditions.
In clustered networks such as those generated by the Triadic Closure (TC) model, increasing homophily amplifies internal connectivity within each group. When the asymmetry in transmission is large (i.e., ), non-compliant individuals become the dominant vectors of infection. As internal transmission accelerates within the non-compliant group, the residual inter-group links can serve as bridges, enabling infection to spill over into the compliant group. Paradoxically, this means that partial separation (e.g., ) can result in a higher epidemic burden for compliant individuals than no separation ().
Interestingly, our results show that this paradoxical effect occurs across all examined values of , although it is most pronounced when . This indicates that the effect is driven by source-control effectiveness , not by susceptibility . Since represents the effectiveness of interventions that reduce outward transmission (e.g., mask-wearing), our findings suggest that clustering and partial separation can undermine such measures even when they are highly effective at source control. In contrast, interventions that affect (e.g., vaccines reducing susceptibility) do not produce this paradoxical behavior.
This insight aligns with recent studies such as [
10], which show that the effectiveness of masks may paradoxically increase when fewer people wear them, due to interactions with the network structure, and behavior clustering. Similarly, ref. [
9] found that homophily in vaccination status raises the infection risk among unvaccinated individuals, even when overall coverage is high.
Crucially, the paradoxical pattern emerges only under three conditions: strong behavioral asymmetry (), high clustering (as in TC networks), and partial—incomplete—group separation. It does not occur in random-like networks generated by the Stochastic Block Model (SBM), nor when transmission asymmetry is weak ().
These results suggest that segregation-based mitigation strategies, whether spontaneous or policy-driven, should be evaluated not only based on their local behavioral logic but also in terms of their systemic effects in clustered social structures. Failure to account for network topology may lead to unintended consequences.
4.2. Limitations and Future Work
While our model provides valuable qualitative insights, several limitations must be acknowledged.
Theory-first scope and parameter interpretation. This study is purely theoretical and aims to identify conditions under which the reported counterintuitive effect can emerge. The numerical choices for the transmission–scaling parameters (source control) and (recipient protection) are therefore illustrative and should not be mapped one-to-one onto the real-world effectiveness of specific interventions (e.g., vaccines or masks). Any empirical application would require a dedicated calibration of and to the data and the study context. In this sense, the parameter settings should be read as a modeling device to expose structural mechanisms rather than as direct policy prescriptions.
Network models and realism. We rely on synthetic networks generated by two stylized models: Stochastic Block Model (SBM) and Triadic Closure (TC). These capture complementary structural features—SBM offers tunable inter-group separation with relatively low clustering, while TC enhances local clustering via triadic closure—yet real contact networks also exhibit degree heterogeneity, overlapping communities, multilayer organization, and temporal evolution. Recent advances in higher-order network modeling [
12,
13,
16] provide a path to extend our framework to more realistic settings.
Behavioral dynamics and timescale separation. Behavior is modeled in a binary and static manner: individuals are either compliant or non-compliant, with fixed
and
. This choice is appropriate under a
timescale separation assumption, where epidemic unfolding is much faster than behavioral change. In practice, adherence may be probabilistic, context-dependent, or change over time due to feedback from epidemic progression [
17]. Relaxing the static approximation—by allowing time-varying or feedback-driven adherence—is a promising avenue that could yield additional, potentially nontrivial phenomena.
Outcome metrics and data products. Our analysis focuses on the final epidemic size (group-wise and overall) and directional transmission counts, rather than time-resolved dynamics. While this suffices to uncover structural effects, it limits direct guidance for real-time intervention planning. Extending the analysis to temporal trajectories (e.g., peak timing, incidence profiles) in future work would be natural once more realistic behavioral and network dynamics are included.
Sensitivity and robustness. We present results for ; additional simulations for show qualitatively similar behavior, which we omit for brevity. All conclusions are drawn from many independent simulations and reported as full distributions (violin plots) across network realizations. Occasionally, randomized graphs may exhibit structural failures that appear as outliers in these distributions; such realizations do not materially affect the mean trends aggregated over many runs.
Finally, although our results are not predictive, they clarify
when and why behavioral clustering embedded in the network structure may yield unintended epidemiological consequences, and they delineate concrete directions for extending the framework toward more realistic settings, including multilayer networks [
18], vaccination feedback, or hybrid NPI scenarios. This perspective aligns with broader insights emerging across epidemiology, sociology, and network science [
11,
14].