Abstract
The use of questionnaire survey results as a clinical diagnostic method for schizophrenia lacks a certain degree of objectivity; thus, markers of schizophrenia in different brain signals have been widely investigated. The objective of this investigation was to explore potential markers of schizophrenia by investigating nonequilibrium features in magnetoencephalography (MEG) signals. We propose a new method to quantify the nonequilibrium features of MEG signals: the multiscale permutation time irreversibility (MsPTIRR) index. The results revealed that the MsPTIRR indices of the MEG recordings of patients with schizophrenia were significantly lower than those of the healthy controls (HCs). Moreover, the MsPTIRR indices of the MEG recordings of patients with schizophrenia and HCs differed significantly in the frontal, occipital, and temporal lobe regions. Furthermore, the MsPTIRR indices of the MEG recordings differed significantly between patients with schizophrenia and HCs in the , and bands. Abnormal nonequilibrium features mined in MEG recordings using the MsPTIRR index may be used as potential markers for schizophrenia, assisting in the clinical diagnosis of this disorder.
1. Introduction
Schizophrenia is a severe mental disorder characterized by various abnormalities in thinking, emotion, perception and behavior [1,2,3]. Patients with schizophrenia often experience symptoms such as hallucinations, delusions, speech and behavioral disturbances, and emotional indifference or incoherence [4,5,6]. Approximately 0.3% of the population worldwide suffers from schizophrenia, with different individuals showing varied symptoms [7,8,9]. The mechanisms underlying the pathophysiology of schizophrenia are still unknown [10,11]. The clinical diagnosis of schizophrenia is often based on the patient’s description of their behavior and questionnaire results; thus, diagnoses are subjective and lack objectivity [12,13,14,15]. Therefore, researchers have aimed to identify markers of schizophrenia in a variety of brain signals to improve the objectivity of the diagnosis of this disorder [16,17,18]. Magnetoencephalography (MEG) is highly suitable for studying schizophrenia because of its high temporal and spatial resolution [19,20].
The human brain is a very large and complex system; furthermore, the nervous system consists of several complex subsystems, and the connections between subsystems are nonlinear. Moreover, each subsystem consists of many neurons, and the interrelationships between these neurons are also nonlinear; thus, the output time series of the nervous system should have nonlinear characteristics [21,22]. Therefore, the nonlinear characteristics of the MEG signals of patients with schizophrenia can be analyzed to identify potential markers in MEG recordings, which is of practical significance for the clinical diagnosis of schizophrenia. The complexity and nonequilibrium features of complex systems are important nonlinear features that are usually measured using the information entropy and time irreversibility index of the time series [23,24]. Dynamic complex systems usually exhibit a certain degree of nonequilibrium (directionality) on the time axis, which means that the features have significantly different statistical properties when viewed in the forward and reverse directions on the time axis. This irreversible characteristic of the time series is prevalent in many practical systems, including those in physical, biological, medical, economic and social domains [24,25,26,27]. Therefore, analyzing the irreversibility indices of the output time series of complex systems is crucial to elucidate the dynamic evolution laws of these systems and provides an important basis for forecasting and decision-making.
Time irreversibility indices of electrophysiological time series have been widely used to analyze the dynamics of complex activities in physiological systems. For example, Andrzejewska et al. reported that the time irreversibility indices of electrocardiogram (ECG) signals during the QT interval were lower in patients with long Q-T syndrome than in healthy individuals [28]. Zhang et al. used an irreversibility index quantified by symbolic relative entropy and reported that energy losses in the brains of older people are significantly greater than those in the brains of younger people [29]. Moreover, Yao et al. reported that the time irreversibility index of electroencephalography (EEG) signals was significantly greater in patients with epilepsy than in healthy individuals and during seizure intervals rather than during nonseizure intervals [27]. Zanin et al. also studied the time irreversibility index and reported that the time asymmetry of EEG signals is significantly reduced when the brain is lesioned [30]. Furthermore, Yao et al. used the time irreversibility index and reported a nonlinear decrease in EEG signals during seizure-free intervals in individuals with epilepsy [31]. Gadhoumi et al. successfully differentiated between seizure and nonseizure intervals in patients with epilepsy using the time irreversibility index [32]. Yao et al. successfully classified sleep EEG signals using the time irreversibility index [33]. Bernardi et al. reported that compared with that in healthy controls (HCs), the irreversibility of MEG signals in individuals with obsessive–compulsive disorder (OCD) was concentrated at a fast timescale and evenly distributed within the same hemisphere [34]. Fan et al. achieved good classification results when ECG signals were classified using time-irreversible indices based on the permutation Jensen–Shannon distance [35].
By analyzing the application of the time irreversibility index to study electrophysiological time series, we reached the following two conclusions. First, analyzing the time irreversibility index of brain signals can improve our understanding of how the brain processes information via specific neural pathways and temporal sequences, and this directionality aids in understanding how efficiently the brain filters, integrates and understands external stimuli. Second, analyzing the time irreversibility index of brain signals can elucidate the temporal dynamics of the brain during information integration processes owing to the temporal sequencing and irreversibility of interactions among different brain regions. Studying this irreversibility can reveal how the brain coordinates information from different senses in the time dimension to form a complete perceptual experience. We hypothesized that the statistical properties of nonequilibrium features of MEG signals in schizophrenia (SZ) patients at different time scales differed from those of HC. Therefore, we suggest that the time irreversibility indices of the MEG signals of SZ patients can be distinguished from those of HCs. To test our hypothesis, we designed a multiscale permutation time irreversibility index algorithm to study the nonlinear nonequilibrium characteristics of MEGs at different time scales.
2. Methods
2.1. Participants
In this investigation, 34 participants, including 19 people with schizophrenia (age: , 2 females) and 15 healthy volunteers (age: , 3 females), were included. All patients included in this investigation were sourced from the outpatient clinic of the Nanjing Brain Hospital. All healthy volunteers (the HCs in this study) were recruited by verbal invitation from the researchers. After being made aware of the details of the study, all the participants signed an informed consent form. All the participants were required to meet the following conditions: (a) no other psychiatric illnesses, (b) no history of severe traumatic brain injury, (c) demonstration of normal intellectual development, (d) not pregnant, and (e) no record of alcohol or drug abuse during the two weeks before the MEG data were obtained. This study was approved by the Ethics Committee of the Nanjing Brain Hospital (2017-KY015).
2.2. Collection of MEGs
MEG recordings were collected by the staff of the Magnetoencephalography Unit of the Nanjing Brain Hospital using a Canadian CTF/VSM 275 full-head superconducting quantum interference device (SQUID)-MEG system (Canadian VSM Company, Coquitlam, BC, Canada). Because metallic zippers, jewelry, and gems can interfere with electromagnetic signals, individuals were required to remove these objects before the MEG recording was performed. When the MEG recordings were being collected, the individuals involved were told to enter an electromagnetically protected room, sit still on a test bed with their eyes open, place their head within a helmet-like sensor array, and remain relaxed. Three small magnetic coils were placed in front of the tip of the subject’s nose, and the openings of both ear canals were used to determine the position of the subject’s head relative to the SQUID sensors. During the MEG data collection process, the data collectors monitored and guided the participants through the cameras and intercoms installed in the electromagnetically shielded room. After confirming that the individuals were in a relaxed state, the MEG recordings were obtained, and the resting-state data were continuously recorded for two minutes. The process of digitizing the recorded MEG data was subsequently carried out at a sampling frequency of 1200 Hz. To facilitate manual checking of the MEG data at a later stage, the ECG and electrooculogram (EOG) signals from each participant were also collected simultaneously. Participants were instructed to remain still throughout the MEG recording session, avoiding any movements, including blinking and muscle contractions, that might have interfered with the accuracy of the data. If the participants made movements that could have affected the accuracy of the research conclusions, the collected signals were abandoned, and new data were collected.
2.3. Preprocessing of MEGs
First, the MEG data were manually inspected using the MATLAB EEGLAB 12.0 toolbox to eliminate data with excessive interference. Independent component analysis (ICA) was used to remove other biological noise and artifacts from the MEG recordings. Different independent components are decomposed via ICA. Interference components (ECG and EOG) are identified by combining multi-dimensional features, which are then removed to finally reconstruct clean signals. The FieldTrip toolbox within MATLAB 2013 was subsequently employed to conduct offline preprocessing of the MEG data. The raw data were filtered with a digital low-pass filter with a frequency of 65 Hz, and the power line component at 50 Hz was notch-filtered with the relevant bandstop filter. Finally, the MEG signals were divided into five frequency bands using appropriate bandpass filters: (1–4 Hz), (5–8 Hz), (9–12 Hz), (13–29 Hz), and (30–65 Hz).
2.4. Multiscale Permutation Time Irreversibility
The core concept of the multiscale permutation time irreversibility algorithm is to first obtain a coarse-grained version of the original time series to obtain a multiscale time series [36,37] and then to calculate the permutation-based time irreversibility parameters at each scale to determine the multiscale permutation time irreversibility parameters of the original sequence. The detailed calculation process is as follows.
First, a coarse-grained version of the original time series with a scale of s is obtained via Formula (1), yielding , where l is the length of the original time series.
With an embedding dimension of m and an embedding delay of , phase space reconstruction is subsequently performed on the basis of the coarse-grained time series according to Formula (2) to obtain . The optimal values of the embedding dimension m and embedding delay can be set according to the false nearest neighbors (FNN) [38] and C-C [39] algorithms.
Afterward, the embedding vector in phase space is arranged in ascending order, and the arranged sequence is . If the vector contains elements with equal values, they are arranged according to the order in which they appear, and their coordinates are modified to the minimum value of the elements in the same group. To directly reflect the spatial structural characteristics of the vector, the positions of the original vector elements in the permuted vector are used to form the amplitude permutation , subsequently obtaining the backward permutation of . The probability of and the probability of its backward permutation are calculated, and the permutation entropy and the permutation time irreversibility with scale s are defined as shown in Formulas (3) and (4), respectively, where U denotes the maximum number of forward permutations and V denotes the sum of the number of forward permutations and backward permutations. Calculating the permutation entropy and permutation time irreversibility at each scale factor yields the multiscale permutation entropy (MsPE) and multiscale permutation time irreversibility (MsPTIRR) of the original time series, respectively.
2.5. Statistical Methods
All the statistical analyses were performed using Statistical Product and Service Solutions (SPSS) software (SPSS25). Kolmogorov-Smirnov tests and F tests were conducted to test whether the data met the assumptions of a normal distribution and homogeneity of variance, respectively. An independent samples t test was adopted to explore significant differences in the MsPTIRR indices between the patients with schizophrenia and HCs. Two-factor analysis of variance was used for results from different frequency bands and different brain regions under a single scale factor.
3. Results
The optimal embedding dimension and embedding delay for each subsequence of the MEG data of all individuals were obtained via the FNN algorithm and C-C algorithm. The final m and were set to 3 and 2, respectively, according to their modes. The permutation time irreversibility and permutation entropy values of the 275 subsequences of the MEG recordings of each participant at each scale condition were computed separately, and their average values were subsequently taken as the permutation time irreversibility index and permutation entropy index for that participant at the given scale condition. The two indices were subsequently averaged for all SZ patients and HCs to obtain the permutation time irreversibility and permutation entropy indices of the patient and HC groups under the given scale conditions. Multiscale analyses were performed for scale factors ranging from 1 to 100, with the scale factor s increasing by 1.
The distributions of the MsPE and MsPTIRR indices for the patients and HCs are shown in Figure 1a and Figure 1b, respectively.
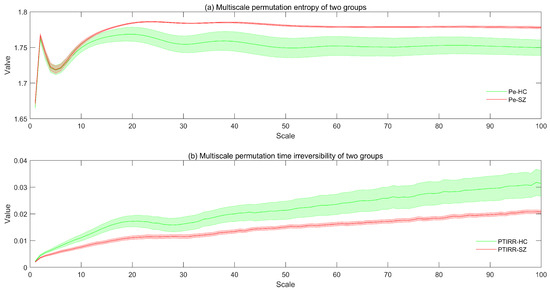
Figure 1.
Distributions of the MsPE and MsPTIRR indices for the patients and HCs. The colored lines in the figure represent the mean values of the MsPE and MsPTIRR indices for the patients and HCs, and the shading of the lines represents the standard errors. In (a), Pe-HC and Pe-SZ denote the MsPE values for the patients and HCs, respectively. In (b), PTIRR-HC and PTIRR-SZ represent the MsPTIRR indices of the patients and HCs, respectively.
As shown in Figure 1a, as the scale factor increases, the MsPE values of the SZ and HC groups first increase, then decrease, then increase again and finally stabilize. When the scaling factor was greater than 10, the MsPE values of the two groups differed, with the values of the SZ group being greater than those of the HC group. This figure also shows that the standard error of the MsPE value of the SZ group was greater than that of the HC group. Moreover, as shown in Figure 1b, the MsPTIRR indices of both groups increase slowly with increases in the scale factor. In addition, the standard error of this indicator was greater in the HC group than in the SZ group. However, the MsPTIRR index of the HC group decreased when the scale factor was in the range of 20–30. Additionally, when the scale factor was increased to 2, the MsPTIRR index of the HC group was greater than that of the SZ group. These results suggest that the MEG data of the SZ group are highly complex with low nonequilibrium. This occurs because the brain activities of SZ patients are disorganized; therefore, the various oscillations are in a somewhat chaotic state and entangled, thus increasing the complexity and decreasing the nonequilibrium of the MEG signals in patients.
Furthermore, the MsPE and MsPTIRR indices differed at various scale factors, suggesting that the dynamics of MEG data have obvious multiscale characteristics; therefore, the dynamics are different at various time scales. A comparison of the trends of the MsPE and MsPTIRR indices at different scale factors in the SZ and HC groups revealed that the MsPTIRR index is more suitable for mining abnormal nonlinear features in the MEG recordings of the SZ group. Therefore, we used a two-tailed independent-samples t test to statistically analyze the MsPTIRR indices of the two groups, and the distributions of the p values of the t tests for different scale factors are shown in Figure 2. As shown in Figure 2, the differences in the MsPTIRR indices of the SZ and HC groups differed at different scale factors. The difference in the MsPTIRR index between the two groups was greatest at a scale factor of 16 .

Figure 2.
Distribution of the p values after logarithmic transformations at different scale factors. The y-axis displays the logarithm of p with a base of 0.05, and the horizontal black line indicates a p value of 0.05.
In this section, we explore the differences in the MsPTIRR indices of the MEG signals of SZ patients and HCs in different brain regions. First, the brain was divided into two hemispheres, left and right; subsequently, each hemisphere was divided into five brain regions: the central, frontal, parietal, occipital, and temporal regions. The distributions of the MsPTIRR indicators within the different brain regions for SZ patients and HCs are shown in Figure 3. As shown in Figure 3, the overall trends of the MsPTIRR indices in different brain regions in SZ patients and HCs were approximately the same. In addition, as shown in Figure 3, the MsPTIRR indices of the brain regions in the left hemisphere of the SZ patients and HCs were slightly greater than those of the brain regions in the right hemisphere. This is because there is some degree of asymmetry in the structures of the left and right hemispheres of the human brain. Careful observation of the curves in Figure 3 reveals some differences in the trends of the MsPTIRR indices in different brain regions in SZ patients and HCs. In particular, the differences in the MsPTIRR indices between the two groups were greater in the frontal, occipital, and temporal regions than in the central and parietal regions.
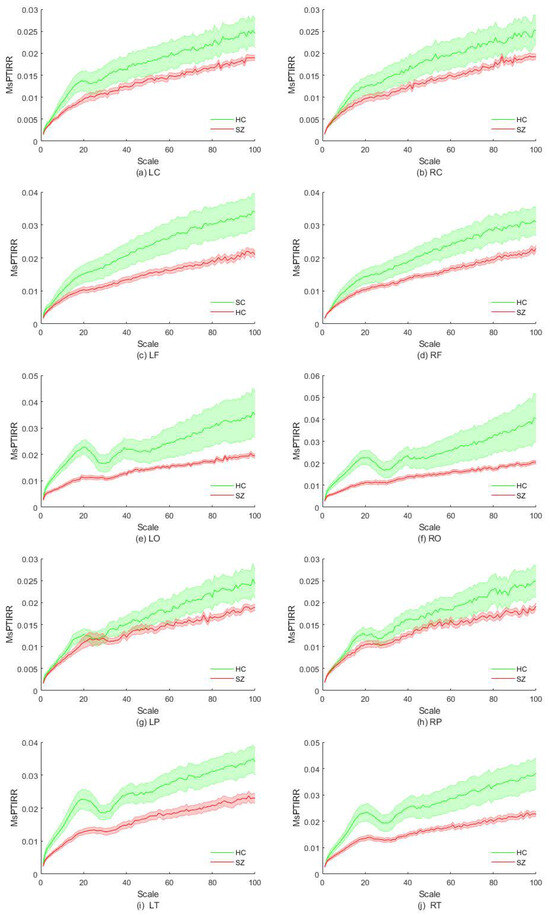
Figure 3.
Distribution of the MsPTIRR indices in different brain regions. HC indicates healthy controls, and SZ indicates schizophrenia patients. L represents the left hemisphere of the brain, R represents the right hemisphere of the brain, C denotes the central region, F represents the frontal region, O represents the occipital region, P represents the parietal region, and T represents the temporal region.
The statistical results revealed a statistically significant difference in the MsPTIRR indices of the two groups within the central region of the left hemisphere at scale factors of 15–20, whereas no significant differences in the MsPTIRR indices of the two groups were found within the central region of the right hemisphere at any scale. These results are consistent with the results in Figure 3a,b. In the frontal regions of the left hemisphere, the MsPTIRR indices of the two groups significantly differed as the scale factor increased to 43, whereas in the frontal regions of the right hemisphere, the MsPTIRR indices of the two groups significantly differed only when the scale factor exceeded 75. The significant differences in the MsPTIRR indices of the two groups within these two brain regions increased with increasing scale factors. Furthermore, in the occipital region of the left hemisphere, there was a significant difference in the MsPTIRR indices of the two groups when the scale factor ranged from 2 to 27 and from 34 to 45. The greatest difference in the MsPTIRR indices of the two groups was observed at a scale factor of 16 . The greatest differences in the MsPTIRR indices of the two groups in the occipital region of the right hemisphere were obtained for the scale factor ranges of 2–26 and 37–41. Furthermore, no statistically significant differences in the MsPTIRR indices of the two groups were found in the parietal regions of the left or right hemisphere at any scale factor. Moreover, in the temporal region of the left hemisphere, significant differences in the MsPTIRR indices of the two groups were found for scale factors greater than 2. However, in the temporal region of the right hemisphere, no significant difference in the MsPTIRR indices of the two groups was found at a scale factor of 28–32. The detailed statistics are shown in Table 1. In addition, the results of the ANOVA showed that the differences between brain regions in the frontal, occipital and temporal regions in the patient group and other brain regions were significantly stronger.

Table 1.
Summary of t test results in different brain regions.
We next explored the MsPTIRR indices of the MEG data of the two groups in different frequency bands. The distributions of the MsPTIRR indices of the MEG data of both groups in the , , , , and bands are shown in Figure 4, and the statistical results for each frequency band are shown in Figure 5. As shown in Figure 4, the MsPTIRR indices of the MEG signals of the two groups increased with increasing scale factor in the , and bands. However, in the and bands, as the scale factor increased, the MsPTIRR indices of the two groups showed complex change trends. Instead of only increasing, the values changed considerably, particularly in the band. Moreover, the MsPTIRR indices of the SZ group were greater than those of the HC group for several scale factors in the and bands. This result occurred owing to the highly abnormal and oscillations that occurred in the resting state in the SZ patients. As shown in Figure 4, the change trends of the MsPTIRR indices in SZ patients and HCs were similar in each frequency band. However, as shown in Figure 5, as the scale factor increased, significant differences in the MsPTIRR values emerged between the two groups in the , , and bands. The difference is particularly noticeable in the frequency band when the scale factor is between 2 and 17. In addition, the results of the ANOVA show that the differences between the and bands and other frequency bands in patients are greater than those between other frequency bands.

Figure 4.
Distribution of the MsPTIRR indices in different frequency bands. HC indicates healthy controls, and SZ indicates schizophrenia patients.
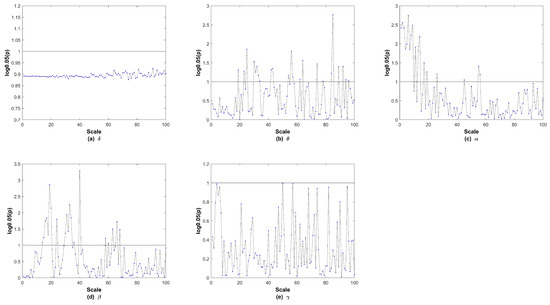
Figure 5.
The p values in each frequency band after logarithmic transformations. In the subgraph, the y-axis displays the logarithm of p with a base of 0.05, and the horizontal black line indicates a p value of 0.05.
4. Discussion
In this investigation, we explored the nonlinear nonequilibrium characteristics of MEG data in SZ patients using the proposed multiscale permutation time irreversibility algorithm. Overall, the findings revealed that the MsPTIRR indices of the MEG data of SZ patients were lower than those of HCs. The brain region study results revealed significant differences in the MsPTIRR indices of SZ patients and HCs, mainly in the frontal, occipital and temporal regions. The cross-frequency study results revealed significant differences in the MsPTIRR indices between SZ patients and HCs, mainly in the , and bands. An in-depth discussion of these results is presented below.
First, our results show that the MsPTIRR indices of the MEG data of SZ patients are significantly lower than those of HCs. It demonstrates that alterations have occurred in the dynamic characteristics of oscillatory signals in the cerebral nervous system of SZ patients [40,41]. These findings suggest that, normally, MEG signals in the human brain exhibit different characteristics for forward and reverse processes on the time axis. The transmission of information between neurons in the brain is purposeful and sequential. The individual neuronal components involved in this information transfer process are highly sequential and cannot be easily altered. This is because the human brain must process information via specific neural pathways and time sequences to integrate and categorize relevant information effectively, thus preventing confusion during information processing. Even in the resting state, brain activity is more dynamic in healthy individuals, with frequent neurotransmitter release and neuronal excitation–inhibition regulation; thus, the MsPTIRR index tends to be greater. However, the brains of patients with schizophrenia are characterized by highly disordered states, with reduced brain activity and enhanced synchronization between neurons, resulting in lower MsPTIRR indices. Related studies have shown abnormalities in the synchronization of neurons in the brains of SZ patients [42,43,44].
There are several possible causes of this abnormal neuronal synchronization. (1) SZ patients have imbalances in neurotransmitter levels in the brain, mainly involving dysregulation of the glutamatergic system and abnormalities in the dopaminergic system. There is much evidence that N-methyl-D-aspartate (NMDA) receptors function abnormally in SZ patients, resulting in dysfunction of the glutamatergic system and imbalanced excitation–inhibition in the cortex, leading to abnormal neuronal synchronization [45,46,47,48,49]. The results of this study indicate that dopaminergic neuronal activity is abnormal in SZ patients, affecting the normal discharge of neurons, thereby interfering with normal signaling between neurons and ultimately leading to abnormal neuronal synchronization across the brain [50,51,52,53]. (2) Abnormalities in brain structure and function can also lead to abnormalities in neuronal synchronization. Cerebral white matter fiber tracts are involved in neural pathways that connect different brain regions, and their integrity is crucial for achieving neuronal synchronization. Previous findings suggest that the myelination of white matter fibers is impaired in the brains of SZ patients [54,55,56]. Myelin acts as an insulating layer that wraps around nerve fibers and accelerates nerve signal transmission. Myelination damage slows nerve signaling, attenuates signals, and affects information exchange between different brain regions, leading to abnormal neuronal synchronization. Altered functional connectivity of brain networks can also lead to abnormal neuronal synchronization. Previous studies suggest that people with SZ have abnormal functional connectivity in the brain, leading to altered connections between brain regions, which can impair signal transmission between these regions, in turn leading to abnormal neuronal synchronization [57,58,59,60]. (3) Neurodevelopmental abnormalities can also disrupt neuronal synchronization. Problems with neuronal migration and differentiation during the early stages of brain development can interfere with the normal formation of neural circuits in the brain. Disordered neuronal migration and differentiation in the fetus or during early childhood can result in neurons failing to migrate to their intended locations in the brain or connections between neurons not being established properly, leading to abnormal neuronal synchronization in these brain regions in adults with schizophrenia [61,62]. Furthermore, abnormal synaptic pruning in SZ patients, especially during critical periods such as puberty, disrupts neuronal connectivity patterns in the frontal, occipital, and temporal lobe regions, ultimately leading to abnormal neuronal synchronization [63,64].
In addition, significant differences in the MsPTIRR indices of the two groups were found only in the frontal, occipital and temporal regions. These findings suggest that the abnormal features discussed earlier are more prominent in these three brain regions. The findings of the brain region analysis do not suggest that the abnormal features discussed above are not present in the central or parietal regions of the brains of patients with schizophrenia; rather, they are present less prominently. Our previous study revealed that the difference in MEG complexity between the two groups was particularly prominent in the frontal, occipital and temporal lobe regions, which is consistent with the findings of the present study [65]. Additionally, previous researchers have reported a variety of abnormal activities in the frontal lobe [66,67], occipital lobe [68,69], and temporal lobe [70,71], supporting our findings.
The frequency band analysis revealed differences in the nonequilibrium features of the MEG signals between the patients and HCs, mainly in the , and bands. This is because waves are dominant in the resting state, and the band in the MEG recordings is closer to the and bands than to the and bands. Our findings are consistent with the findings of several previous studies. Koshiyama et al. reported abnormal phase discontinuities in and waves in schizophrenia patients [72]. Arora et al. identified potential markers of schizophrenia in EEG signals in the , , and bands [73]. Furthermore, Ramsay et al. revealed that the strength and consistency of neural oscillations in schizophrenia patients were significantly reduced in the , , and bands [74]. Lin et al. reported that and band power was significantly increased in schizophrenia patients, whereas band power was significantly increased and band power was decreased in HCs [75]. These results support the reliability of our findings.
We successfully identified abnormal nonlinear nonequilibrium (directionality on the time axis) features in the MEG data of SZ patients, and these features could serve as potential markers of schizophrenia. Our findings provide a quantifiable and objective foundation for the clinical diagnosis of schizophrenia, which could increase the accuracy of the diagnosis of this disorder. However, this study has several limitations that should be considered. First, the number of schizophrenia patients included in the study was small. The findings should be validated, and the impact of confounding factors such as age and gender on the results should be considered with a larger sample size. In addition, the nonequilibrium characteristics of MEG signals in SZ patients have only been studied at the sensor level, with no research conducted in the MEG source space. Third, only abnormal nonequilibrium features in the MEG recordings of SZ patients and HCs were investigated, and owing to the small sample size, these features were not utilized in combination with relevant deep learning methods for classification analyses. Therefore, it is necessary to conduct relevant research in the MEG source space in future studies. The findings could be further validated in future studies by collecting more data with a larger sample to enhance their reliability. When substantial data are collected, the identified features can be combined with deep learning methods to conduct recognition studies of schizophrenia. Finally, the most suitable parameters of the MEG recordings could be used as markers of schizophrenia.
5. Conclusions
In conclusion, we combined the time irreversibility algorithm with a multiscale time series algorithm and proposed the MsPTIRR algorithm. We used the proposed algorithm to study the nonequilibrium features of the MEG recordings of SZ patients. Comparative analyses revealed that the MsPTIRR index is more suitable than the MsPE for mining nonlinear features in the MEG data of SZ patients. The results revealed that the MsPTIRR indices of the MEG signals of SZ patients were significantly lower than those of HCs for various time scale factors. Thus, schizophrenia appears to disturb the nonequilibrium characteristics of the nervous system. The brain region analysis results revealed significant differences in the nonequilibrium characteristics of MEG signals between SZ patients and HCs at various time scales in the frontal, occipital and temporal lobe regions. Moreover, the analysis of different frequency bands revealed significant differences in the nonequilibrium characteristics between the two groups, which were present mainly in the , and bands. Abnormal nonequilibrium features in MEG signals found at various time scales using the MsPTIRR method may be potential markers of schizophrenia in MEG recordings and may provide an objective basis for the clinical diagnosis of this disorder.
Author Contributions
D.B.: writing—original draft, funding acquisition, conceptualization, validation; M.X., Y.W., Z.Z. and X.C.: software, visualization; W.Y.: methodology; J.W.: supervision, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
The project was supported by the National Natural Science Foundation of China (Grant No. 62461021), the Support Program for Young PhDs in Gansu Universities (Project Number 2024QB-109), the Doctoral Research Initiation Fund Project of Hexi University (Project Number KYQD2024011), and the College Students’ Innovation Training Program (S202510740053).
Institutional Review Board Statement
This study was approved by the Ethics Committee of the Nanjing Brain Hospital (2017-KY015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data in this paper may be requested from the corresponding author upon reasonable request.
Acknowledgments
We would like to express our sincere thanks to all those who participated in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jauhar, S.; Johnstone, M.; McKenna, P.J. Schizophrenia. Lancet 2022, 399, 473–486. [Google Scholar] [CrossRef]
- Makhlouf, A.T.; Drew, W.; Stubbs, J.L.; Taylor, J.J.; Liloia, D.; Grafman, J.; Silbersweig, D.; Fox, M.D.; Siddiqi, S.H. Heterogeneous patterns of brain atrophy in schizophrenia localize to a common brain network. Nature Mental Health 2025, 3, 19–30. [Google Scholar] [CrossRef]
- Huang, J.; Wang, M.; Ju, H.; Ding, W.; Zhang, D. AGBN-Transformer: Anatomy-guided brain network transformer for schizophrenia diagnosis. Biomed. Signal Process. Control 2025, 102, 107226. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Marques, T.R.; Howes, O.D. Schizophrenia—An overview. JAMA Psychiatry 2020, 77, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Wen, P.; Song, B.; Li, Y. 3D convolutional neural network for schizophrenia detection using as EEG-based functional brain network. Biomed. Signal Process. Control 2024, 89, 105815. [Google Scholar] [CrossRef]
- Pain, S.; Sarma, M.; Samanta, D. Graph signal processing and graph learning approaches to Schizophrenia pattern identification in brain Electroencephalogram. Biomed. Signal Process. Control 2025, 100, 106954. [Google Scholar] [CrossRef]
- Cai, M.; Ji, Y.; Zhao, Q.; Xue, H.; Sun, Z.; Wang, H.; Zhang, Y.; Chen, Y.; Zhao, Y.; Zhang, Y.; et al. Homotopic functional connectivity disruptions in schizophrenia and their associated gene expression. Neuroimage 2024, 289, 120551. [Google Scholar] [CrossRef]
- Gosala, B.; Kapgate, P.D.; Jain, P.; Chaurasia, R.N.; Gupta, M. Wavelet transforms for feature engineering in EEG data processing: An application on Schizophrenia. Biomed. Signal Process. Control 2023, 85, 104811. [Google Scholar] [CrossRef]
- Wang, C.; Ren, Y.; Zhang, R.; Wang, C.; Ran, X.; Shen, J.; Zhao, Z.; Tao, W.; Yang, Y.; Ren, W.; et al. Schizophrenia classification and abnormalities reveal of brain region functional connection by deep-learning multiple sparsely connected network. Biomed. Signal Process. Control 2024, 96, 106580. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Keefe, R.S.; McGuire, P.K. Cognitive impairment in schizophrenia: Aetiology, pathophysiology, and treatment. Mol. Psychiatry 2023, 28, 1902–1918. [Google Scholar] [CrossRef]
- Javitt, D.C. Cognitive impairment associated with schizophrenia: From pathophysiology to treatment. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Barlati, S.; Nibbio, G.; Vita, A. Evidence-based psychosocial interventions in schizophrenia: A critical review. Curr. Opin. Psychiatry 2024, 37, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Nasrallah, H.; Akbarian, S.; Carpenter, W.T., Jr.; DeLisi, L.E.; Gaebel, W.; Green, M.F.; Gur, R.E.; Heckers, S.; Kane, J.M.; et al. The schizophrenia syndrome, circa 2024: What we know and how that informs its nature. Schizophr. Res. 2024, 264, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Haider, U.; Hanif, M.; Rashid, A.; Qaisar, S.M.; Subasi, A. EEG-based schizophrenia classification using penalized sequential dictionary learning in the context of mobile healthcare. Biomed. Signal Process. Control 2024, 90, 105856. [Google Scholar] [CrossRef]
- Sahu, P.K. Artificial intelligence system for verification of schizophrenia via theta-EEG rhythm. Biomed. Signal Process. Control 2023, 81, 104485. [Google Scholar] [CrossRef]
- Warren, N.; O’Gorman, C.; Horgan, I.; Weeratunga, M.; Halstead, S.; Moussiopoulou, J.; Campana, M.; Yakimov, V.; Wagner, E.; Siskind, D. Inflammatory cerebrospinal fluid markers in schizophrenia spectrum disorders: A systematic review and meta-analysis of 69 studies with 5710 participants. Schizophr. Res. 2024, 266, 24–31. [Google Scholar] [CrossRef]
- Cao, T.; Zhang, S.; Chen, Q.; Zeng, C.; Wang, L.; Jiao, S.; Chen, H.; Zhang, B.; Cai, H. Long non-coding RNAs in schizophrenia: Genetic variations, treatment markers and potential targeted signaling pathways. Schizophr. Res. 2023, 260, 12–22. [Google Scholar] [CrossRef]
- Swathi, N.; Sankaran, K.S. Advances and clinical Implications of neuroanatomical biomarkers in schizophrenia by optimizing brain tissue segmentation. Biomed. Signal Process. Control 2025, 102, 107215. [Google Scholar] [CrossRef]
- Fred, A.L.; Kumar, S.N.; Kumar Haridhas, A.; Ghosh, S.; Purushothaman Bhuvana, H.; Sim, W.K.J.; Vimalan, V.; Givo, F.A.S.; Jousmäki, V.; Padmanabhan, P.; et al. A brief introduction to magnetoencephalography (MEG) and its clinical applications. Brain Sci. 2022, 12, 788. [Google Scholar] [CrossRef]
- Brookes, M.J.; Leggett, J.; Rea, M.; Hill, R.M.; Holmes, N.; Boto, E.; Bowtell, R. Magnetoencephalography with optically pumped magnetometers (OPM-MEG): The next generation of functional neuroimaging. Trends Neurosci. 2022, 45, 621–634. [Google Scholar] [CrossRef]
- Lau, Z.J.; Pham, T.; Chen, S.A.; Makowski, D. Brain entropy, fractal dimensions and predictability: A review of complexity measures for EEG in healthy and neuropsychiatric populations. Eur. J. Neurosci. 2022, 56, 5047–5069. [Google Scholar] [CrossRef]
- Ahamed, S.I.; Rabbani, M.; Povinelli, R.J. A comprehensive survey on detection of non-linear analysis techniques for EEG signal. In Proceedings of the 2023 IEEE International Conference on Digital Health (ICDH), Chicago, IL, USA, 2–8 July 2023; IEEE: New York, NY, USA, 2023; pp. 184–194. [Google Scholar]
- Bandt, C.; Pompe, B. Permutation entropy: A natural complexity measure for time series. Phys. Rev. Lett. 2002, 88, 174102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shang, P.; Shang, B. Time irreversibility analysis and abnormality detection based on Riemannian geometry for complex time series. Commun. Nonlinear Sci. Numer. Simul. 2023, 117, 106985. [Google Scholar] [CrossRef]
- O’Byrne, J.; Kafri, Y.; Tailleur, J.; van Wijland, F. Time irreversibility in active matter, from micro to macro. Nat. Rev. Phys. 2022, 4, 167–183. [Google Scholar] [CrossRef]
- Yao, W.; Wang, J.; Perc, M.; Yao, W.; Dai, J.; Guo, D.; Yao, D. Time irreversibility and amplitude irreversibility measures for nonequilibrium processes. Commun. Nonlinear Sci. Numer. Simul. 2021, 96, 105688. [Google Scholar] [CrossRef]
- Petelczyc, M.; Czechowski, Z. Effect of nonlinearity and persistence on multiscale irreversibility, non-stationarity, and complexity of time series—Case of data generated by the modified Langevin model. Chaos Interdiscip. J. Nonlinear Sci. 2023, 33, 053107. [Google Scholar] [CrossRef]
- Andrzejewska, M.; Zebrowski, J.J.; Rams, K.; Ozimek, M.; Baranowski, R. Assessment of time irreversibility in a time series using visibility graphs. Front. Netw. Physiol. 2022, 2, 877474. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J. Modified symbolic relative entropy based electroencephalogram time irreversibility analysis. Acta Phys. Sin. 2013, 62, 038701. [Google Scholar] [CrossRef]
- Zanin, M.; Güntekin, B.; Aktürk, T.; Hanoğlu, L.; Papo, D. Time irreversibility of resting-state activity in the healthy brain and pathology. Front. Physiol. 2020, 10, 1619. [Google Scholar] [CrossRef]
- Yao, W.; Yao, W.; Wang, J.; Dai, J. Quantifying time irreversibility using probabilistic differences between symmetric permutations. Phys. Lett. A 2019, 383, 738–743. [Google Scholar] [CrossRef]
- Gadhoumi, K.; Lina, J.; Gotman, J. Discriminating Preictal and Interictal States Using Time Irreversibility of the Intracerebral Eeg in Patients with Mesial Temporal Lobe Epilepsy. In Epilepsia; Wiley-Blackwell: Hoboken, NJ, USA, 2013; Volume 54, pp. 259–260. [Google Scholar]
- Yao, W. Permutation time irreversibility in sleep electroencephalograms: Dependence on sleep stage and the effect of equal values. Phys. Rev. E 2024, 109, 054104. [Google Scholar] [CrossRef]
- Bernardi, D.; Shannahoff-Khalsa, D.; Sale, J.; Wright, J.A.; Fadiga, L.; Papo, D. The time scales of irreversibility in spontaneous brain activity are altered in obsessive compulsive disorder. Front. Psychiatry 2023, 14, 1158404. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Song, J.; Li, D. Multiscale Irreversibility Analysis of Time Series Based on Permutation Jensen-Shannon Distance. Fluct. Noise Lett. 2025, 24, 2550013. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of complex physiologic time series. Phys. Rev. Lett. 2002, 89, 068102. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of biological signals. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2005, 71, 021906. [Google Scholar] [CrossRef]
- Kennel, M.B.; Brown, R.; Abarbanel, H.D. Determining embedding dimension for phase-space reconstruction using a geometrical construction. Phys. Rev. A 1992, 45, 3403. [Google Scholar] [CrossRef]
- Kim, H.; Eykholt, R.; Salas, J. Nonlinear dynamics, delay times, and embedding windows. Phys. D Nonlinear Phenom. 1999, 127, 48–60. [Google Scholar] [CrossRef]
- Seifert, U. Stochastic thermodynamics, fluctuation theorems and molecular machines. Rep. Prog. Phys. 2012, 75, 126001. [Google Scholar] [CrossRef]
- Roldán, É.; Parrondo, J.M. Entropy production and Kullback-Leibler divergence between stationary trajectories of discrete systems. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2012, 85, 031129. [Google Scholar] [CrossRef]
- Yao, R.; Xue, J.; Li, H.; Wang, Q.; Deng, H.; Tan, S. Dynamics and synchronization control in schizophrenia for EEG signals. Biomed. Signal Process. Control 2022, 71, 103118. [Google Scholar] [CrossRef]
- Lee, H.; Dvorak, D.; Fenton, A.A. Targeting neural synchrony deficits is sufficient to improve cognition in a schizophrenia-related neurodevelopmental model. Front. Psychiatry 2014, 5, 15. [Google Scholar] [CrossRef]
- Braus, D. Temporal perception and organisation, neuronal synchronisation and schizophrenia. Fortschritte Neurol. Psychiatr. 2002, 70, 591–600. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, R.A.; Krystal, J.H.; Howes, O.D. Dopamine and glutamate in schizophrenia: Biology, symptoms and treatment. World Psychiatry 2020, 19, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.C.; McCollum, L.A.; Schoonover, K.E.; Mabry, S.J.; Roche, J.K.; Lahti, A.C. Ultrastructural evidence for glutamatergic dysregulation in schizophrenia. Schizophr. Res. 2022, 249, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Li, N.; Cai, H. Candidate metabolic biomarkers for schizophrenia in CNS and periphery: Do any possible associations exist? Schizophr. Res. 2020, 226, 95–110. [Google Scholar] [CrossRef]
- Tong, J.; Zhou, Y.; Huang, J.; Zhang, P.; Fan, F.; Chen, S.; Tian, B.; Cui, Y.; Tian, L.; Tan, S.; et al. N-methyl-D-aspartate receptor antibody and white matter deficits in schizophrenia treatment-resistance. Schizophr. Bull. 2021, 47, 1463–1472. [Google Scholar] [CrossRef]
- Howes, O.D.; Shatalina, E. Integrating the neurodevelopmental and dopamine hypotheses of schizophrenia and the role of cortical excitation-inhibition balance. Biol. Psychiatry 2022, 92, 501–513. [Google Scholar] [CrossRef]
- McCutcheon, R.; Beck, K.; Jauhar, S.; Howes, O.D. Defining the locus of dopaminergic dysfunction in schizophrenia: A meta-analysis and test of the mesolimbic hypothesis. Schizophr. Bull. 2018, 44, 1301–1311. [Google Scholar] [CrossRef]
- Tan, T.; Wang, W.; Williams, J.; Ma, K.; Cao, Q.; Yan, Z. Stress exposure in dopamine D4 receptor knockout mice induces schizophrenia-like behaviors via disruption of GABAergic transmission. Schizophr. Bull. 2019, 45, 1012–1023. [Google Scholar] [CrossRef]
- Brugger, S.P.; Angelescu, I.; Abi-Dargham, A.; Mizrahi, R.; Shahrezaei, V.; Howes, O.D. Heterogeneity of striatal dopamine function in schizophrenia: Meta-analysis of variance. Biol. Psychiatry 2020, 87, 215–224. [Google Scholar] [CrossRef]
- Avram, M.; Brandl, F.; Knolle, F.; Cabello, J.; Leucht, C.; Scherr, M.; Mustafa, M.; Koutsouleris, N.; Leucht, S.; Ziegler, S.; et al. Aberrant striatal dopamine links topographically with cortico-thalamic dysconnectivity in schizophrenia. Brain 2020, 143, 3495–3505. [Google Scholar] [CrossRef]
- Stedehouder, J.; Kushner, S. Myelination of parvalbumin interneurons: A parsimonious locus of pathophysiological convergence in schizophrenia. Mol. Psychiatry 2017, 22, 4–12. [Google Scholar] [CrossRef]
- Saito, Y.; Kubicki, M.; Koerte, I.; Otsuka, T.; Rathi, Y.; Pasternak, O.; Bouix, S.; Eckbo, R.; Kikinis, Z.; von Hohenberg, C.C.; et al. Impaired white matter connectivity between regions containing mirror neurons, and relationship to negative symptoms and social cognition, in patients with first-episode schizophrenia. Brain Imaging Behav. 2018, 12, 229–237. [Google Scholar] [CrossRef]
- Komatsu, H.; Takeuchi, H.; Kikuchi, Y.; Ono, C.; Yu, Z.; Iizuka, K.; Takano, Y.; Kakuto, Y.; Funakoshi, S.; Ono, T.; et al. Ethnicity-dependent effects of schizophrenia risk variants of the OLIG2 gene on OLIG2 transcription and white matter integrity. Schizophr. Bull. 2020, 46, 1619–1628. [Google Scholar] [CrossRef]
- Bai, D.; Yao, W.; Yan, W.; Wang, J. Network analysis of magnetoencephalogram signals in schizophrenia patients when viewing emotional facial stimuli. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Candelaria-Cook, F.T.; Stephen, J.M. Test–retest reliability of magnetoencephalography resting-state functional connectivity in schizophrenia. Front. Psychiatry 2020, 11, 551952. [Google Scholar] [CrossRef] [PubMed]
- Lottman, K.K.; Gawne, T.J.; Kraguljac, N.V.; Killen, J.F.; Reid, M.A.; Lahti, A.C. Examining resting-state functional connectivity in first-episode schizophrenia with 7T fMRI and MEG. NeuroImage Clin. 2019, 24, 101959. [Google Scholar] [CrossRef] [PubMed]
- Sanfratello, L.; Houck, J.M.; Calhoun, V.D. Relationship between MEG global dynamic functional network connectivity measures and symptoms in schizophrenia. Schizophr. Res. 2019, 209, 129–134. [Google Scholar] [CrossRef]
- Sanders, B.; D’Andrea, D.; Collins, M.O.; Rees, E.; Steward, T.G.; Zhu, Y.; Chapman, G.; Legge, S.E.; Pardiñas, A.F.; Harwood, A.J.; et al. Transcriptional programs regulating neuronal differentiation are disrupted in DLG2 knockout human embryonic stem cells and enriched for schizophrenia and related disorders risk variants. Nat. Commun. 2022, 13, 27. [Google Scholar] [CrossRef]
- Kalkman, H.O. Altered growth factor signaling pathways as the basis of aberrant stem cell maturation in schizophrenia. Pharmacol. Ther. 2009, 121, 115–122. [Google Scholar] [CrossRef]
- Yilmaz, M.; Yalcin, E.; Presumey, J.; Aw, E.; Ma, M.; Whelan, C.W.; Stevens, B.; McCarroll, S.A.; Carroll, M.C. Overexpression of schizophrenia susceptibility factor human complement C4A promotes excessive synaptic loss and behavioral changes in mice. Nat. Neurosci. 2021, 24, 214–224. [Google Scholar] [CrossRef]
- Faludi, G.; Mirnics, K. Synaptic changes in the brain of subjects with schizophrenia. Int. J. Dev. Neurosci. 2011, 29, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Yao, W.; Wang, S.; Wang, J. Multiscale weighted permutation entropy analysis of schizophrenia magnetoencephalograms. Entropy 2022, 24, 314. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Zhao, H.; Li, Y.; Cai, H.; Song, Y.; Wang, R.; Yu, Y.; Zhu, J. Network localization of state and trait of auditory verbal hallucinations in schizophrenia. Schizophr. Bull. 2024, 50, 1326–1336. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Q.; Shah, C.; Li, Q.; Sweeney, J.A.; Li, F.; Gong, Q. Cortical thickness abnormalities at different stages of the illness course in schizophrenia: A systematic review and meta-analysis. JAMA Psychiatry 2022, 79, 560–570. [Google Scholar] [CrossRef]
- Uscătescu, L.C.; Said-Yürekli, S.; Kronbichler, L.; Stelzig-Schöler, R.; Pearce, B.G.; Reich, L.A.; Weber, S.; Aichhorn, W.; Kronbichler, M. Reduced intrinsic neural timescales in schizophrenia along posterior parietal and occipital areas. NPJ Schizophr. 2021, 7, 55. [Google Scholar] [CrossRef]
- Cheng, J.; Ren, Y.; Gu, Q.; He, Y.; Wang, Z. QEEG Biomarkers for ECT Treatment Response in Schizophrenia. Clin. EEG Neurosci. 2022, 53, 499–505. [Google Scholar] [CrossRef]
- Brugger, S.P.; Howes, O.D. Heterogeneity and homogeneity of regional brain structure in schizophrenia: A meta-analysis. JAMA Psychiatry 2017, 74, 1104–1111. [Google Scholar] [CrossRef]
- Williams, J.A.; Burgess, S.; Suckling, J.; Lalousis, P.A.; Batool, F.; Griffiths, S.L.; Palmer, E.; Karwath, A.; Barsky, A.; Gkoutos, G.V.; et al. Inflammation and brain structure in schizophrenia and other neuropsychiatric disorders: A Mendelian randomization study. JAMA Psychiatry 2022, 79, 498–507. [Google Scholar] [CrossRef]
- Koshiyama, D.; Miyakoshi, M.; Tanaka-Koshiyama, K.; Joshi, Y.B.; Sprock, J.; Braff, D.L.; Light, G.A. Abnormal phase discontinuity of alpha-and theta-frequency oscillations in schizophrenia. Schizophr. Res. 2021, 231, 73–81. [Google Scholar] [CrossRef]
- Arora, M.; Knott, V.J.; Labelle, A.; Fisher, D.J. Alterations of resting EEG in hallucinating and nonhallucinating schizophrenia patients. Clin. EEG Neurosci. 2021, 52, 159–167. [Google Scholar] [CrossRef]
- Ramsay, I.S.; Pokorny, V.J.; Lynn, P.A.; Klein, S.D.; Sponheim, S.R. Limited consistency and strength of neural oscillations during sustained visual attention in schizophrenia. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2024, 9, 337–345. [Google Scholar] [CrossRef]
- Lin, P.; Zhu, G.; Xu, X.; Wang, Z.; Li, X.; Li, B. Brain network analysis of working memory in schizophrenia based on multi graph attention network. Biomed. Signal Process. Control 2023, 80, 104310. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).