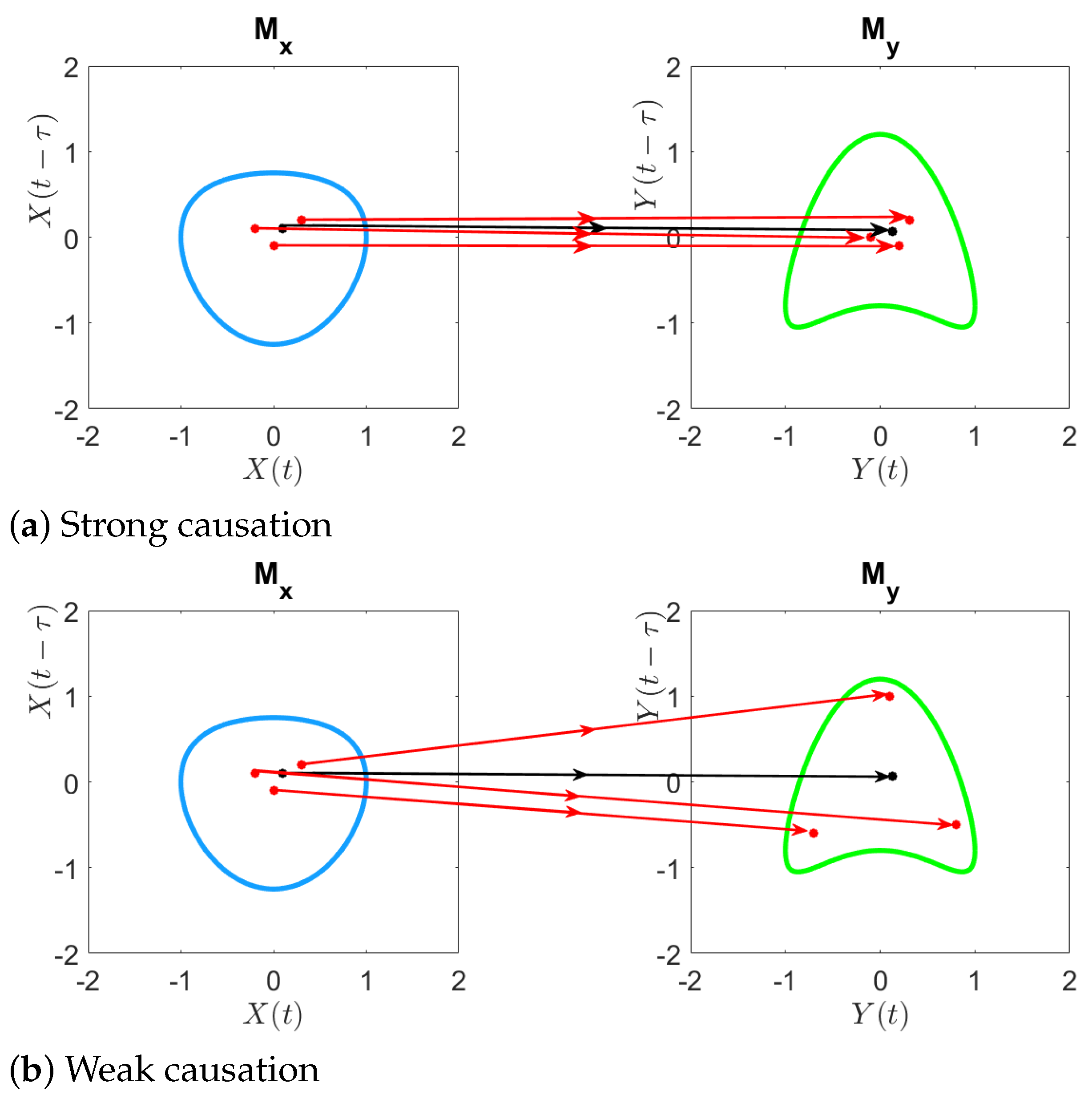
In this section, we illustrate the performance of cCCM (together with CCM) in causality detection through simulation examples, including autoregressive models, stochastic processes with a dominant spectral component embedded in noise, deterministic chaotic maps, and systems with memory. As will be seen, these examples show that CCM and cCCM are sensitive to changes in coupling strength. It can be observed that CCM tends to result in larger causation values than cCCM; this is expected since CCM uses both the past and future values of X to predict the current value of Y (and vice versa), while cCCM only uses both the past values of X to predict the current value of Y (and vice versa).
It should be noted that for an accurate assessment of the causation, the sampling rate should always be chosen to be larger than the Nyquist rate. Otherwise, the causal relationship identified by cCCM may be invalid since the under-sampled sequences cannot capture the total information in the original signals.
3.2.4. Examples on Systems with Memory
In this subsection, we examined the causal relationship in systems with memory (Examples 10–14) using CCM and cCCM under different choices of E and .
Example 10:
Consider a system with memory
Here, (i) the MATLAB command “randn(1024,1)” returns an 1024-by-1 matrix of normally distributed random numbers; (ii)
and
while
. The results corresponding to different
E or
values are displayed in
Table 2.
We can see that the causation from cannot be fully captured when and
Example 11:
Consider
where
and
while
. Then, for different
E or
values, the results are displayed in
Table 3.
We can see that the causation from cannot be fully captured when and
Example 12:
Consider a system with different dominant delays from
Example 11:
where
and
while
. Then, for different
E or
values, the results are displayed in
Table 4.
We can see that the causation from cannot be fully captured when and
Example 13:
Consider
where
and
while
. Then, for different
E or
values, the results are displayed in
Table 5.
From this example, we can see the following: (i) when , we have and the causation corresponding to item cannot be captured; (ii) when and , we have and the causation corresponding to items and cannot be captured; and (iii) when and , we have , and the causation corresponding to all the items can be captured.
Now, if we consider the time-delayed causality, in which
remains the same and
, then this is equivalent to considering the causality from
to
. In this case, as shown in
Table 6, even when
and
, we have
and the causation corresponding to all the items can be captured.
Example 14:
Consider
where
and
while
. Then, for different
E or
values, the results are displayed in
Table 7.
In this example, both and can only capture the causation corresponding to , and and can capture the overall causation accurately.
Now, if we consider the time-delayed causality, in which
remains the same and
, then this is equivalent to considering the causality from
to
. In this case, as shown in
Table 8,
and
work even better than
and
since
leads to a manifold with a lower dimension and, hence, a higher nearest neighbor density.
From Examples 10–14, it can be seen that in systems with memory, the selection of the shadow manifold dimension E and the signal lag largely rely on the positions of the dominant delays in the channel impulse response.
It can be seen that in systems with memory, for the accurate evaluation of CCM and cCCM causality, the following conditions need to be satisfied:
- (a)
, where denotes the largest dominant delay.
- (b)
For each t, the shadow manifold constructing vector should contain all the samples corresponding to the dominant delays.
It is also observed that if the conditions above are not satisfied, time-delayed cCCM from to might still capture the causation accurately if the instantaneous information exchange between and is not significant. More specifically, if we consider a linear time-invariant (LTI) system , where denotes the channel impulse response, when is negligibly small, we say that there is no significant instantaneous information exchange between and .
In the following two examples, we compare the performance between cCCM and Granger causality (GC) for systems with memory.
Example 15:
Consider a system with memory:
where
, and
while
. Here, we assume that
is independent of
X. We then compare the performances of GC and CCM under different noise powers, and the results are shown in
Table 9.
As can be seen, as long as the signal-to-noise ratio (SNR) is not too small, cCCM can capture the strong bidirectional causality between and , but GC cannot. This is mainly because cCCM takes the instantaneous information exchange between and into consideration, but GC does not. That is, when there exists instantaneous information exchange between and , GC may fail to capture the causal coupling between and .
It is also observed that the
value decreases as the noise power increases, which is consistent with our analysis in
Section 3.1. When
and SNR
dB, both cCCM and GC can no longer deliver valid results due to the strong noise effect.
Recall that the most commonly used method in Granger causality [
10,
11,
12] analysis is to compare the following two prediction errors
and
:
And the Granger causality is defined to be the log-likelihood ratio
where
,
, and
stands for the determinant of the covariance matrix.
Our results in
Table 9 and the definition of GC suggest that the small fluctuations in the GC values as the noise variance increases from
to 4 are more likely to reflect the impact of the noise rather than the detection of the causality.
Example 16:
Consider
where
while
, and
is an independently generated Gaussian noise. Then, the Granger causality between
X and
Y is
and the causality detected by cCCM is
In this example, there is no instantaneous information exchange between and , and both GC and cCCM detect the strong unidirectional causality from X to Y and deliver consistent results.
3.2.5. Additional Examples on the Selection of the Dimension of the Shadow Manifold E and Time Lag
In this subsection, we illustrate the impact of E and on the performance of cCCM through some additional simulation examples, including single-tone time series embedded in noise (Example 13) and Gaussian stochastic process (Example 14). As in Example 3, it was found that a large value may help enhance the performance of cCCM under noise. However, it is also noticed that if E is too large, cCCM may no longer deliver valid results, as the excessively high dimension of the shadow manifold significantly reduces the density of the nearest neighbors, leading to inaccurate state-space reconstruction and causality evaluation.
Example 17:
This is a revisit of Example 3, with additional discussions on the selection of
E and
and different sampling instants. Consider the following noisy single-tone time series:
where
, and
and
are independent AWGN noises with SNRs varying in 0, 5, 10, 15, and 20 dB, or equal to 0 for all
t in the noise-free case. By changing the values for
E and
, we are able to observe different noise effects. The simulation results for
and
are shown in
Table 10 below.
As can be seen, as we increase the length of the data span , the noise effect is reduced. In particular, compared with and when we choose and , a much better noise immunity is achieved, since is sufficiently long.
Note that increasing leads to the downsampling of the time series, and increasing E expands the dimension of the shadow manifolds. An over-increase in E or might downgrade the performance of cCCM. From Example 14, it can be seen that if E is much larger than , cCCM may deliver inaccurate results.
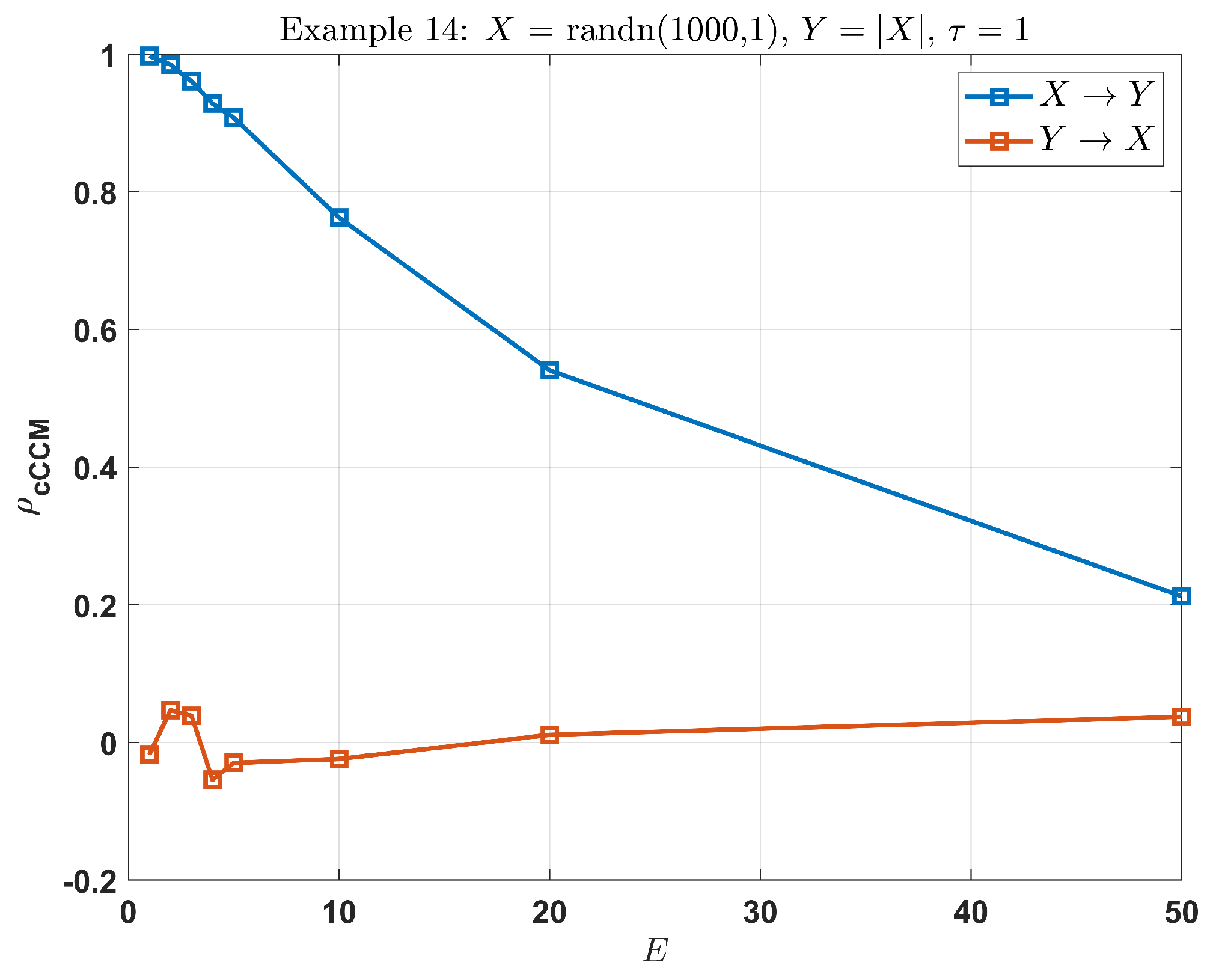
Example 18:
In this example, there is a strong unidirectional causality from
X to
Y, but very weak causation in the inverse direction. Choose
. From
Figure 5, we can see that as
E increases, the cCCM value keeps on decreasing and reduces to
when
, which no longer reflects the strong unidirectional causality from
X to
Y.