Heart Rate Complexity and Autonomic Modulation Are Associated with Psychological Response Inhibition in Healthy Subjects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
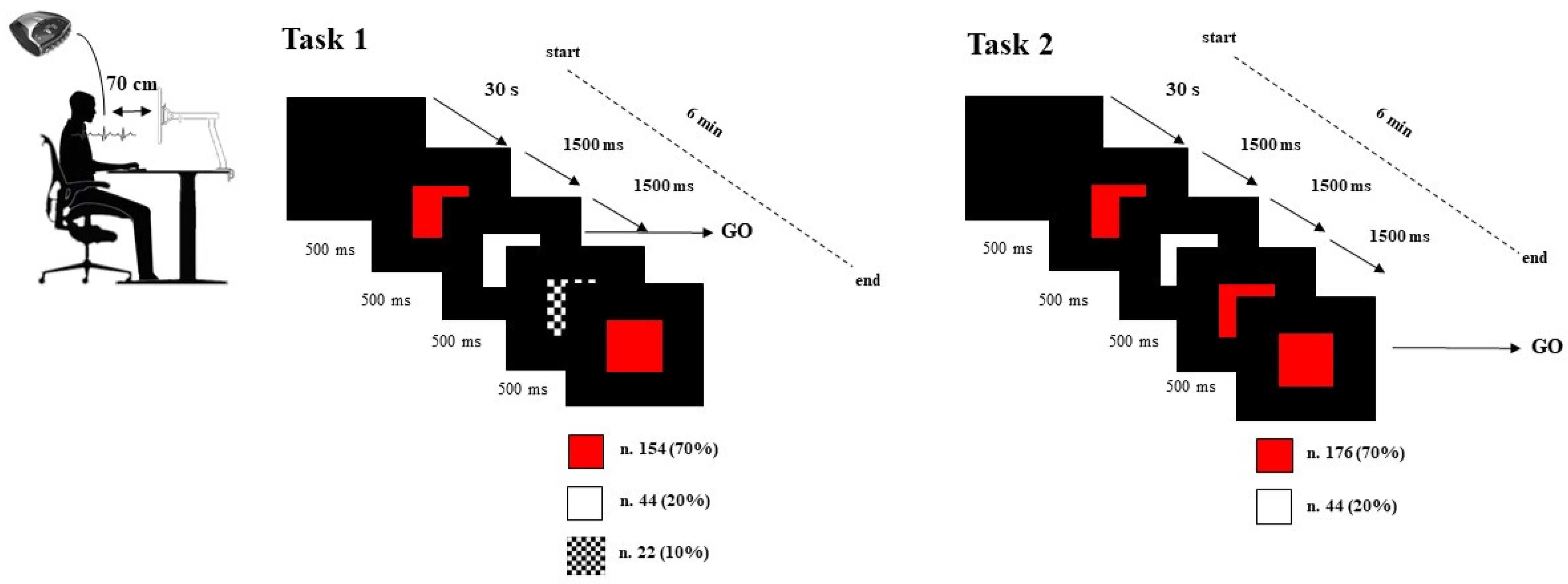
2.2. Procedure
2.3. Data Acquisition
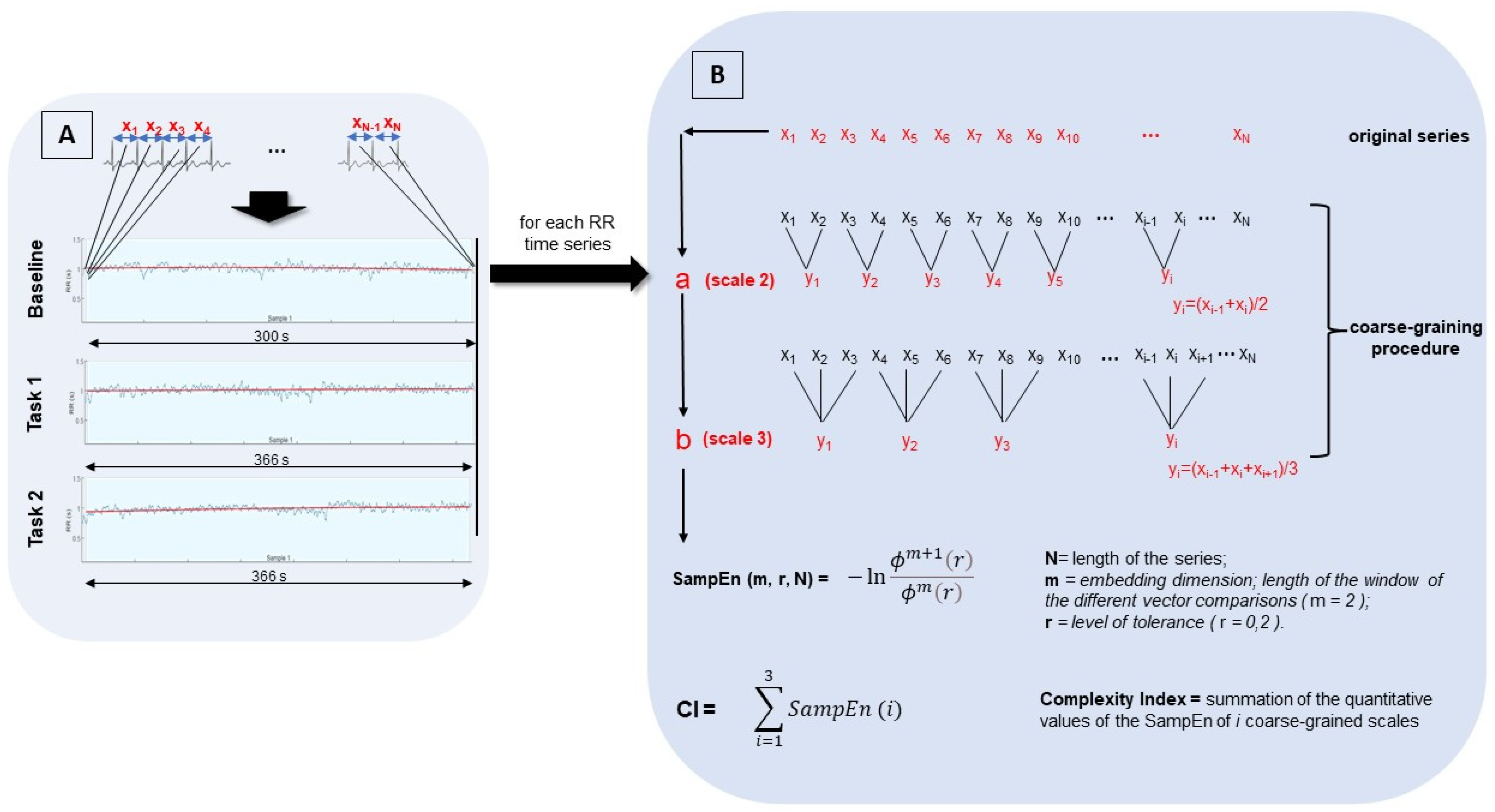
2.4. Data Analysis
2.5. Statistical Analysis
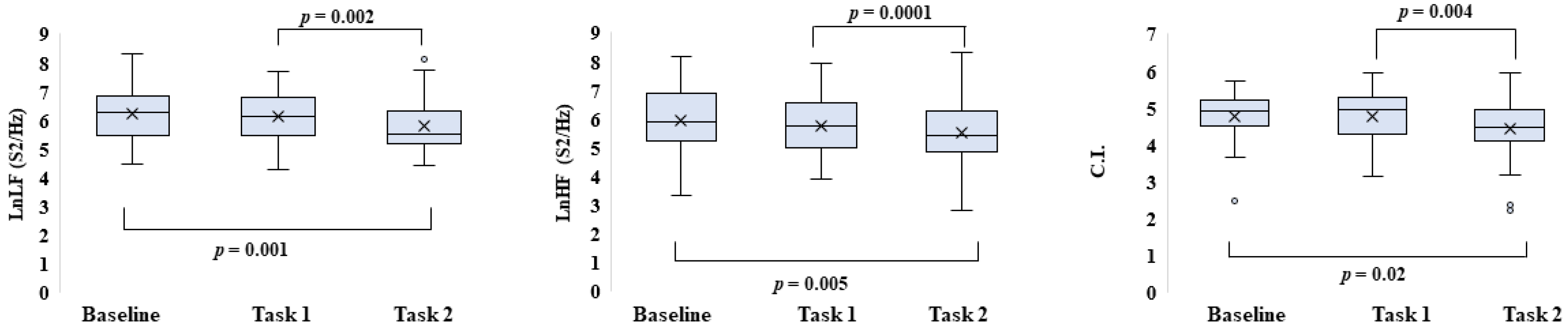
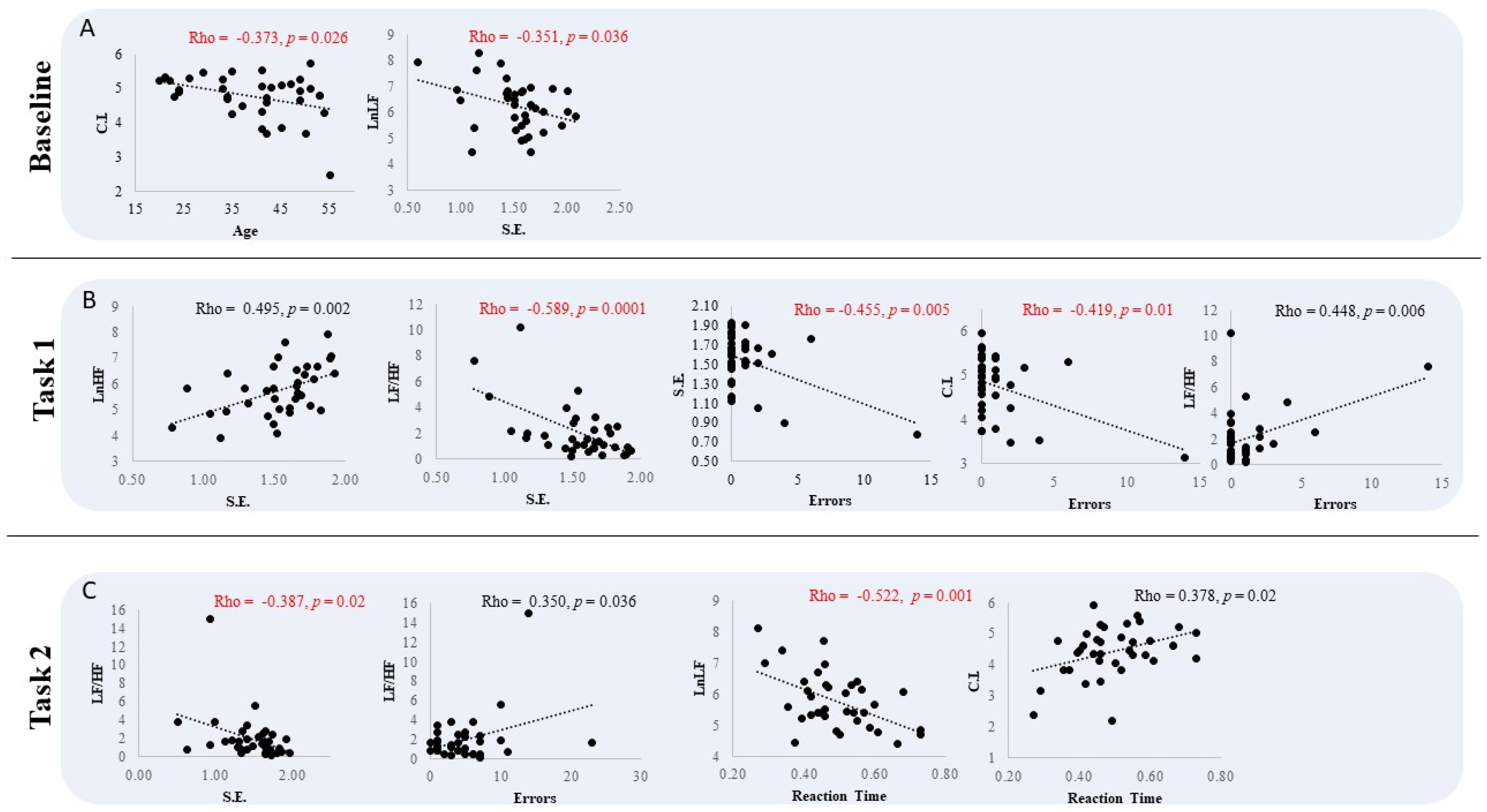
3. Results
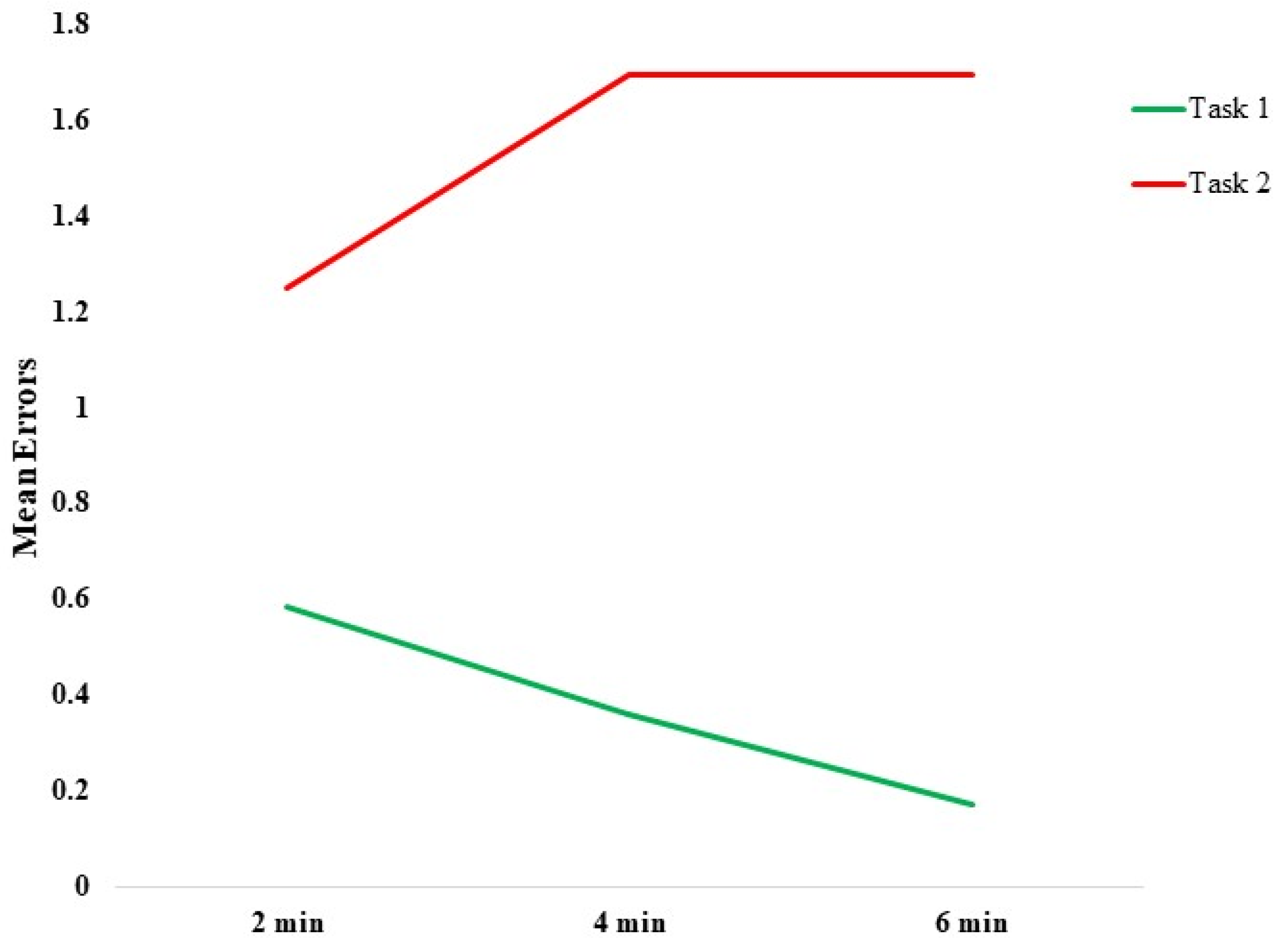
Behavioral Data
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hansen, A.L.; Johnsen, B.H.; Thayer, J.F. Vagal Influence on Working Memory and Attention. Int. J. Psychophysiol. 2003, 48, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Duschek, S.; Muckenthaler, M.; Werner, N.; del Paso, G.A.R. Relationships between Features of Autonomic Cardiovascular Control and Cognitive Performance. Biol. Psychol. 2009, 81, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Favieri, F.; Casagrande, M. Heart Rate Variability and Cognitive Function: A Systematic Review. Front. Neurosci. 2019, 13, 710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napadow, V.; Dhond, R.; Conti, G.; Makris, N.; Brown, E.N.; Barbieri, R. Brain Correlates of Autonomic Modulation: Combining Heart Rate Variability with FMRI. Neuroimage 2008, 42, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the Heart–Brain Connection: Further Elaboration of a Model of Neurovisceral Integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef]
- Tonhajzerova, I.; Ondrejka, I.; Turianikova, Z.; Javorka, K.; Calkovska, A.; Javorka, M. Heart Rate Variability: An Index of the Brain–Heart Interaction. In Tachycardia; InTechOpen: London, UK, 2012; pp. 185–202. [Google Scholar]
- Benarroch, E.; Singer, W.; Mauermann, M. Autonomic Neurology; Oxford University Press: New York, NY, USA, 2014; ISBN 978-0-19-992019-8. [Google Scholar]
- Palma, J.-A.; Benarroch, E.E. Neural Control of the Heart: Recent Concepts and Clinical Correlations. Neurology 2014, 83, 261–271. [Google Scholar] [CrossRef]
- Porges, S.W. The Polyvagal Theory: New Insights into Adaptive Reactions of the Autonomic Nervous System. Clevel. Clin. J. Med. 2009, 76, S86–S90. [Google Scholar] [CrossRef]
- Yeh, C.-H.; Juan, C.-H.; Yeh, H.-M.; Wang, C.-Y.; Young, H.-W.V.; Lin, J.-L.; Lin, C.; Lin, L.-Y.; Lo, M.-T. The Critical Role of Respiratory Sinus Arrhythmia on Temporal Cardiac Dynamics. J. Appl. Physiol. 2019, 127, 1733–1741. [Google Scholar] [CrossRef]
- Lewis, G.F.; Furman, S.A.; McCool, M.F.; Porges, S.W. Statistical Strategies to Quantify Respiratory Sinus Arrhythmia: Are Commonly Used Metrics Equivalent? Biol. Psychol. 2012, 89, 349–364. [Google Scholar] [CrossRef] [Green Version]
- Miyake, A.; Friedman, N.P. The Nature and Organization of Individual Differences in Executive Functions: Four General Conclusions. Curr. Dir. Psychol. Sci. 2012, 21, 8–14. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The Unity and Diversity of Executive Functions and Their Contributions to Complex “Frontal Lobe” Tasks: A Latent Variable Analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehto, J.E.; Juujärvi, P.; Kooistra, L.; Pulkkinen, L. Dimensions of Executive Functioning: Evidence from Children. Br. J. Dev. Psychol. 2003, 21, 59–80. [Google Scholar] [CrossRef]
- Geva, R.; Zivan, M.; Warsha, A.; Olchik, D. Alerting, Orienting or Executive Attention Networks: Differential Patters of Pupil Dilations. Front. Behav. Neurosci. 2013, 7, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howells, F.M.; Stein, D.J.; Russell, V.A. Perceived Mental Effort Correlates with Changes in Tonic Arousal during Attentional Tasks. Behav. Brain Funct. 2010, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, E.; Ortega, A.R.; Reyes Del Paso, G.A. Anxiety, Attention, and Decision Making: The Moderating Role of Heart Rate Variability. Int. J. Psychophysiol. 2015, 98, 490–496. [Google Scholar] [CrossRef]
- Sørensen, L.; Wass, S.; Osnes, B.; Schanche, E.; Adolfsdottir, S.; Svendsen, J.L.; Visted, E.; Eilertsen, T.; Jensen, D.A.; Nordby, H.; et al. A Psychophysiological Investigation of the Interplay between Orienting and Executive Control during Stimulus Conflict: A Heart Rate Variability Study. Physiol. Behav. 2019, 211, 112657. [Google Scholar] [CrossRef]
- Aboy, M.; Cuesta-Frau, D.; Austin, D.; Mico-Tormos, P. Characterization of Sample Entropy in the Context of Biomedical Signal Analysis. In Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 5942–5945. [Google Scholar]
- Bouny, P.; Deschodt-Arsac, V.; Touré, E.; Arsac, L. Entropy and Multifractality in Heart Rate Dynamics as Markers of Specific Brain-Heart Coordinations When Adapting to Cognitive Tasks. In Proceedings of the ACAPS 2021 Neurosciences—Contrôle moteur—Methodology and Technology, Montpellier, France, 27–29 October 2021. [Google Scholar]
- Bornas, X.; Llabrés, J.; Noguera, M.; López, A.M.; Gelabert, J.M.; Vila, I. Fear Induced Complexity Loss in the Electrocardiogram of Flight Phobics: A Multiscale Entropy Analysis. Biol. Psychol. 2006, 73, 272–279. [Google Scholar] [CrossRef]
- Voss, A.; Heitmann, A.; Schroeder, R.; Peters, A.; Perz, S. Short-Term Heart Rate Variability--Age Dependence in Healthy Subjects. Physiol. Meas. 2012, 33, 1289–1311. [Google Scholar] [CrossRef]
- Riganello, F.; Larroque, S.K.; Bahri, M.A.; Heine, L.; Martial, C.; Carrière, M.; Charland-Verville, V.; Aubinet, C.; Vanhaudenhuyse, A.; Chatelle, C.; et al. A Heartbeat Away from Consciousness: Heart Rate Variability Entropy Can Discriminate Disorders of Consciousness and Is Correlated with Resting-State FMRI Brain Connectivity of the Central Autonomic Network. Front. Neurol. 2018, 9, 769. [Google Scholar] [CrossRef] [Green Version]
- Deschodt-Arsac, V.; Blons, E.; Gilfriche, P.; Spiluttini, B.; Arsac, L.M. Entropy in Heart Rate Dynamics Reflects How HRV-Biofeedback Training Improves Neurovisceral Complexity during Stress-Cognition Interactions. Entropy 2020, 22, 317. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.-K. Multiscale Entropy Analysis of Biological Signals. Phys. Rev. E 2005, 71, 021906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado-Bonal, A.; Marshak, A. Approximate Entropy and Sample Entropy: A Comprehensive Tutorial. Entropy 2019, 21, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, H.; Benton, D. We Should Be Using Non-linear Indices When Relating Heart-Rate Dynamics to Cognition and Mood. Sci. Rep. 2015, 5, 16619. [Google Scholar] [CrossRef] [Green Version]
- Dimitriev, D.A.; Saperova, E.V.; Dimitriev, A.D. State Anxiety and Nonlinear Dynamics of Heart Rate Variability in Students. PLoS ONE 2016, 11, e0146131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byun, S.; Kim, A.Y.; Jang, E.H.; Kim, S.; Choi, K.W.; Yu, H.Y.; Jeon, H.J. Entropy Analysis of Heart Rate Variability and Its Application to Recognize Major Depressive Disorder: A Pilot Study. Technol. Health Care 2019, 27, 407–424. [Google Scholar] [CrossRef] [Green Version]
- Georgiou, G.; Essau, C.A. Go/No-Go Task. In Encyclopedia of Child Behavior and Development; Goldstein, S., Naglieri, J.A., Eds.; Springer: Boston, MA, USA, 2011; pp. 705–706. ISBN 978-0-387-79061-9. [Google Scholar]
- Espinosa, A.; Alegret, M.; Boada, M.; Vinyes, G.; Valero, S.; Martínez-Lage, P.; Peña-Casanova, J.; Becker, J.T.; Wilson, B.A.; Tárraga, L. Ecological Assessment of Executive Functions in Mild Cognitive Impairment and Mild Alzheimer’s Disease. J. Int. Neuropsychol. Soc. 2009, 15, 751–757. [Google Scholar] [CrossRef] [Green Version]
- Ottaviani, C.; Zingaretti, P.; Petta, A.M.; Antonucci, G.; Thayer, J.F.; Spitoni, G.F. Resting Heart Rate Variability Predicts Inhibitory Control above and beyond Impulsivity. J. Psychophysiol. 2019, 33, 198–206. [Google Scholar] [CrossRef]
- Antonucci, G.; Spitoni, G.F.; Orsini, A.; D’Olimpio, F.; Cantagallo, A. Taratura Italiana Della Batteria per La Valutazione Della Sindrome Disesecutiva: BADS. Available online: https://iris.unicampania.it/handle/11591/159383?mode=full.274 (accessed on 22 April 2022).
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart Rate Variability: Standards of Measurement, Physiological Interpretation and Clinical Use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Singh, M.; Banga, V.K. Sample Entropy Based HRV: Effect of ECG Sampling Frequency. Biomed. Sci. Eng. 2014, 2, 68–72. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological Time-Series Analysis Using Approximate Entropy and Sample Entropy. Am. J. Physiol. -Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef]
- Bursac, Z.; Gauss, C.H.; Williams, D.K.; Hosmer, D.W. Purposeful Selection of Variables in Logistic Regression. Source Code Biol. Med. 2008, 3, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Midi, H.; Sarkar, S.K.; Rana, S. Collinearity Diagnostics of Binary Logistic Regression Model. J. Interdiscip. Math. 2010, 13, 253–267. [Google Scholar] [CrossRef]
- Appelhans, B.M.; Luecken, L.J. Heart Rate Variability as an Index of Regulated Emotional Responding. Rev. Gen. Psychol. 2006, 10, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.P.; Thayer, J.F.; Koenig, J. Resting Cardiac Vagal Tone Predicts Intraindividual Reaction Time Variability during an Attention Task in a Sample of Young and Healthy Adults. Psychophysiology 2016, 53, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.L.; Johnsen, B.H.; Sollers, J.J.; Stenvik, K.; Thayer, J.F. Heart Rate Variability and Its Relation to Prefrontal Cognitive Function: The Effects of Training and Detraining. Eur. J. Appl. Physiol. 2004, 93, 263–272. [Google Scholar] [CrossRef]
- Colzato, L.S.; Steenbergen, L. High Vagally Mediated Resting-State Heart Rate Variability Is Associated with Superior Action Cascading. Neuropsychologia 2017, 106, 1–6. [Google Scholar] [CrossRef]
- Beevers, C.G.; Ellis, A.J.; Reid, R.M. Heart Rate Variability Predicts Cognitive Reactivity to a Sad Mood Provocation. Cogn. Ther. Res. 2011, 35, 395–403. [Google Scholar] [CrossRef]
- Hansen, A.L.; Johnsen, B.H.; Thayer, J.F. Relationship between Heart Rate Variability and Cognitive Function during Threat of Shock. Anxiety Stress Coping 2009, 22, 77–89. [Google Scholar] [CrossRef]
- Hovland, A.; Pallesen, S.; Hammar, Å.; Hansen, A.L.; Thayer, J.F.; Tarvainen, M.P.; Nordhus, I.H. The Relationships among Heart Rate Variability, Executive Functions, and Clinical Variables in Patients with Panic Disorder. Int. J. Psychophysiol. 2012, 86, 269–275. [Google Scholar] [CrossRef]
- Moretta, T.; Sarlo, M.; Buodo, G. Problematic Internet Use: The Relationship Between Resting Heart Rate Variability and Emotional Modulation of Inhibitory Control. Cyberpsychol. Behav. Soc. Netw. 2019, 22, 500–507. [Google Scholar] [CrossRef]
- Thayer, J.F.; Friedman, B.H.; Borkovec, T.D. Autonomic Characteristics of Generalized Anxiety Disorder and Worry. Biol. Psychiatry 1996, 39, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Verkuil, B.; Brosschot, J.F.; Borkovec, T.D.; Thayer, J.F. Acute Autonomic Effects of Experimental Worry and Cognitive Problem Solving: Why Worry about Worry? Int. J. Clin. Health Psychol. 2009, 9, 439–453. [Google Scholar]
- Melo, H.M.; Nascimento, L.M.; Takase, E. Mental Fatigue and Heart Rate Variability (HRV): The Time-on-Task Effect. Psychol. Neurosci. 2017, 10, 428–436. [Google Scholar] [CrossRef]
- Barber, A.D.; John, M.; DeRosse, P.; Birnbaum, M.L.; Lencz, T.; Malhotra, A.K. Parasympathetic Arousal-Related Cortical Activity Is Associated with Attention during Cognitive Task Performance. Neuroimage 2020, 208, 116469. [Google Scholar] [CrossRef]
- Barber, A.D.; Gallego, J.A.; DeRosse, P.; Birnbaum, M.L.; Lencz, T.; Ali, S.A.; Moyett, A.; Malhotra, A.K. Contributions of Parasympathetic Arousal-Related Activity to Cognitive Performance in Patients with First-Episode Psychosis and Control Subjects. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021. ahead of print. [Google Scholar] [CrossRef]
- Schaich, C.L.; Malaver, D.; Chen, H.; Shaltout, H.A.; Zeki Al Hazzouri, A.; Herrington, D.M.; Hughes, T.M. Association of Heart Rate Variability with Cognitive Performance: The Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2020, 9, e013827. [Google Scholar] [CrossRef]
- Knight, E.L.; Giuliano, R.J.; Shank, S.W.; Clarke, M.M.; Almeida, D.M. Parasympathetic and Sympathetic Nervous Systems Interactively Predict Change in Cognitive Functioning in Midlife Adults. Psychophysiology 2020, 57, e13622. [Google Scholar] [CrossRef]
- Dalise, A.M.; Prestano, R.; Fasano, R.; Gambardella, A.; Barbieri, M.; Rizzo, M.R. Autonomic Nervous System and Cognitive Impairment in Older Patients: Evidence from Long-Term Heart Rate Variability in Real-Life Setting. Front. Aging Neurosci. 2020, 12, 40. [Google Scholar] [CrossRef]
- Blons, E.; Arsac, L.M.; Gilfriche, P.; McLeod, H.; Lespinet-Najib, V.; Grivel, E.; Deschodt-Arsac, V. Alterations in Heart-Brain Interactions under Mild Stress during a Cognitive Task Are Reflected in Entropy of Heart Rate Dynamics. Sci. Rep. 2019, 9, 18190. [Google Scholar] [CrossRef] [Green Version]
- Berry, K.J.; Mielke, P.W. Exact and Monte Carlo Resampling Procedures for the Wilcoxon-Mann-Whitney and Kruskal-Wallis Tests. Percept. Mot. Ski. 2000, 91, 749–754. [Google Scholar] [CrossRef]
- Lipsitz, L.A. Aging as a Process of Complexity Loss. In Complex Systems Science in Biomedicine; Deisboeck, T.S., Kresh, J.Y., Eds.; Topics in Biomedical Engineering International Book Series; Springer: Boston, MA, USA, 2006; pp. 641–654. ISBN 978-0-387-33532-2. [Google Scholar]
- Goldberger, A.L.; Peng, C.-K.; Lipsitz, L.A. What Is Physiologic Complexity and How Does It Change with Aging and Disease? Neurobiol. Aging 2002, 23, 23–26. [Google Scholar] [CrossRef]
- Beckers, F. Aging and Nonlinear Heart Rate Control in a Healthy Population. AJP Heart Circ. Physiol. 2006, 290, H2560–H2570. [Google Scholar] [CrossRef] [PubMed]
- Pikkujamsa, S.M.; Makikallio, T.H.; Sourander, L.B.; Raiha, I.J.; Puukka, P.; Skytta, J.; Peng, C.-K.; Goldberger, A.L.; Huikuri, H.V. Cardiac Interbeat Interval Dynamics from Childhood to Senescence: Comparison of Conventional and New Measures Based on Fractals and Chaos Theory. Circulation 1999, 100, 393–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakhchina, A.V.; Arutyunova, K.R.; Sozinov, A.A.; Demidovsky, A.V.; Alexandrov, Y.I. Sample Entropy of the Heart Rate Reflects Properties of the System Organization of Behaviour. Entropy 2018, 20, 449. [Google Scholar] [CrossRef] [Green Version]
- Manor, B.; Lipsitz, L.A. Physiologic Complexity and Aging: Implications for Physical Function and Rehabilitation. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 45, 287–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, A.C.M.; Porta, A.; Melo, R.C.; Quitério, R.J.; da Silva, E.; Borghi-Silva, A.; Tobaldini, E.; Montano, N.; Catai, A.M. Aging Reduces Complexity of Heart Rate Variability Assessed by Conditional Entropy and Symbolic Analysis. Intern. Emerg. Med. 2012, 7, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Geovanini, G.R.; Vasques, E.R.; de Oliveira Alvim, R.; Mill, J.G.; Andreão, R.V.; Vasques, B.K.; Pereira, A.C.; Krieger, J.E. Age and Sex Differences in Heart Rate Variability and Vagal Specific Patterns—Baependi Heart Study. Glob. Heart 2020, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Porta, A.; Gnecchi-Ruscone, T.; Tobaldini, E.; Guzzetti, S.; Furlan, R.; Montano, N. Progressive Decrease of Heart Period Variability Entropy-Based Complexity during Graded Head-up Tilt. J. Appl. Physiol. 2007, 103, 1143–1149. [Google Scholar] [CrossRef]
- Castaldo, R.; Melillo, P.; Pecchia, L. Acute Mental Stress Detection via Ultra-Short Term HRV Analysis. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, IFMBE, Toronto, Canada, 7–12 June 2015; Jaffray, D.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 51, pp. 1068–1071, ISBN 978-3-319-19386-1. [Google Scholar]
- Kim, H.-G.; Cheon, E.-J.; Bai, D.-S.; Lee, Y.H.; Koo, B.-H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Weippert, M.; Behrens, M.; Rieger, A.; Behrens, K. Sample Entropy and Traditional Measures of Heart Rate Dynamics Reveal Different Modes of Cardiovascular Control During Low Intensity Exercise. Entropy 2014, 16, 5698–5711. [Google Scholar] [CrossRef] [Green Version]
- Porta, A.; Bari, V.; Ranuzzi, G.; De Maria, B.; Baselli, G. Assessing Multiscale Complexity of Short Heart Rate Variability Series through a Model-Based Linear Approach. Chaos Interdiscip. J. Nonlinear Sci. 2017, 27, 093901. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.-K. Multiscale Entropy Analysis of Complex Physiologic Time Series. Phys. Rev. Lett. 2002, 89, 068102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voss, A.; Schulz, S.; Koschke, M.; Bär, K.J. Linear and Nonlinear Analysis of Autonomic Regulation in Depressed Patients. In Proceedings of the 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 2653–2656. [Google Scholar] [CrossRef]
- Voss, A.; Schulz, S.; Schroeder, R.; Baumert, M.; Caminal, P. Methods Derived from Nonlinear Dynamics for Analysing Heart Rate Variability. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Cysarz, D.; Porta, A.; Montano, N.; Van Leeuwen, P.; Kurths, J.; Wessel, N. Quantifying Heart Rate Dynamics Using Different Approaches of Symbolic Dynamics. Eur. Phys. J. Spec. Top. 2013, 222, 487–500. [Google Scholar] [CrossRef]
- Riganello, F.; Chatelle, C.; Schnakers, C.; Laureys, S. Heart Rate Variability as an Indicator of Nociceptive Pain in Disorders of Consciousness? J. Pain Symptom Manag. 2018, 57, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortese, D.; Riganello, F.; Arcuri, F.; Lucca, L.; Tonin, P.; Schnakers, C.; Laureys, S. The Trace Conditional Learning of the Noxious Stimulus in UWS Patients and Its Prognostic Value in a GSR and HRV Entropy Study. Front. Hum. Neurosci. 2020, 14, 97. [Google Scholar] [CrossRef] [PubMed]
- Spangler, D.P.; McGinley, J.J. Vagal Flexibility Mediates the Association Between Resting Vagal Activity and Cognitive Performance Stability Across Varying Socioemotional Demands. Front. Psychol. 2020, 11, 2093. [Google Scholar] [CrossRef]
- Garavaglia, L.; Gulich, D.; Defeo, M.M.; Mailland, J.T.; Irurzun, I.M. The Effect of Age on the Heart Rate Variability of Healthy Subjects. PLoS ONE 2021, 16, e0255894. [Google Scholar] [CrossRef]
- Kumral, D.; Schaare, H.L.; Beyer, F.; Reinelt, J.; Uhlig, M.; Liem, F.; Lampe, L.; Babayan, A.; Reiter, A.; Erbey, M.; et al. The Age-Dependent Relationship between Resting Heart Rate Variability and Functional Brain Connectivity. NeuroImage 2019, 185, 521–533. [Google Scholar] [CrossRef]
- Bai, X.; Li, J.; Zhou, L.; Li, X. Influence of the Menstrual Cycle on Nonlinear Properties of Heart Rate Variability in Young Women. Am. J. Physiol.-Heart Circ. Physiol. 2009, 297, H765–H774. [Google Scholar] [CrossRef]
- Valencia, J.F.; Porta, A.; Vallverdu, M.; Claria, F.; Baranowski, R.; Orlowska-Baranowska, E.; Caminal, P. Refined Multiscale Entropy: Application to 24-h Holter Recordings of Heart Period Variability in Healthy and Aortic Stenosis Subjects. IEEE Trans. Biomed. Eng. 2009, 56, 2202–2213. [Google Scholar] [CrossRef]
- Humeau-Heurtier, A. The Multiscale Entropy Algorithm and Its Variants: A Review. Entropy 2015, 17, 3110–3123. [Google Scholar] [CrossRef]
| Task 1 | Task 2 | |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| RT during GO trials (s) | 0.37 ± 0.08 | 0.49 ± 0.11 |
| % errors during GO trials | 0.04% | 1.38% |
| % errors during NoGo trials | 1.81% | 2.93% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riganello, F.; Vatrano, M.; Tonin, P.; Cerasa, A.; Cortese, M.D. Heart Rate Complexity and Autonomic Modulation Are Associated with Psychological Response Inhibition in Healthy Subjects. Entropy 2023, 25, 152. https://doi.org/10.3390/e25010152
Riganello F, Vatrano M, Tonin P, Cerasa A, Cortese MD. Heart Rate Complexity and Autonomic Modulation Are Associated with Psychological Response Inhibition in Healthy Subjects. Entropy. 2023; 25(1):152. https://doi.org/10.3390/e25010152
Chicago/Turabian StyleRiganello, Francesco, Martina Vatrano, Paolo Tonin, Antonio Cerasa, and Maria Daniela Cortese. 2023. "Heart Rate Complexity and Autonomic Modulation Are Associated with Psychological Response Inhibition in Healthy Subjects" Entropy 25, no. 1: 152. https://doi.org/10.3390/e25010152







