Classification of Kinematic and Electromyographic Signals Associated with Pathological Tremor Using Machine and Deep Learning
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Data Acquisition
2.2. Data Pre-Processing
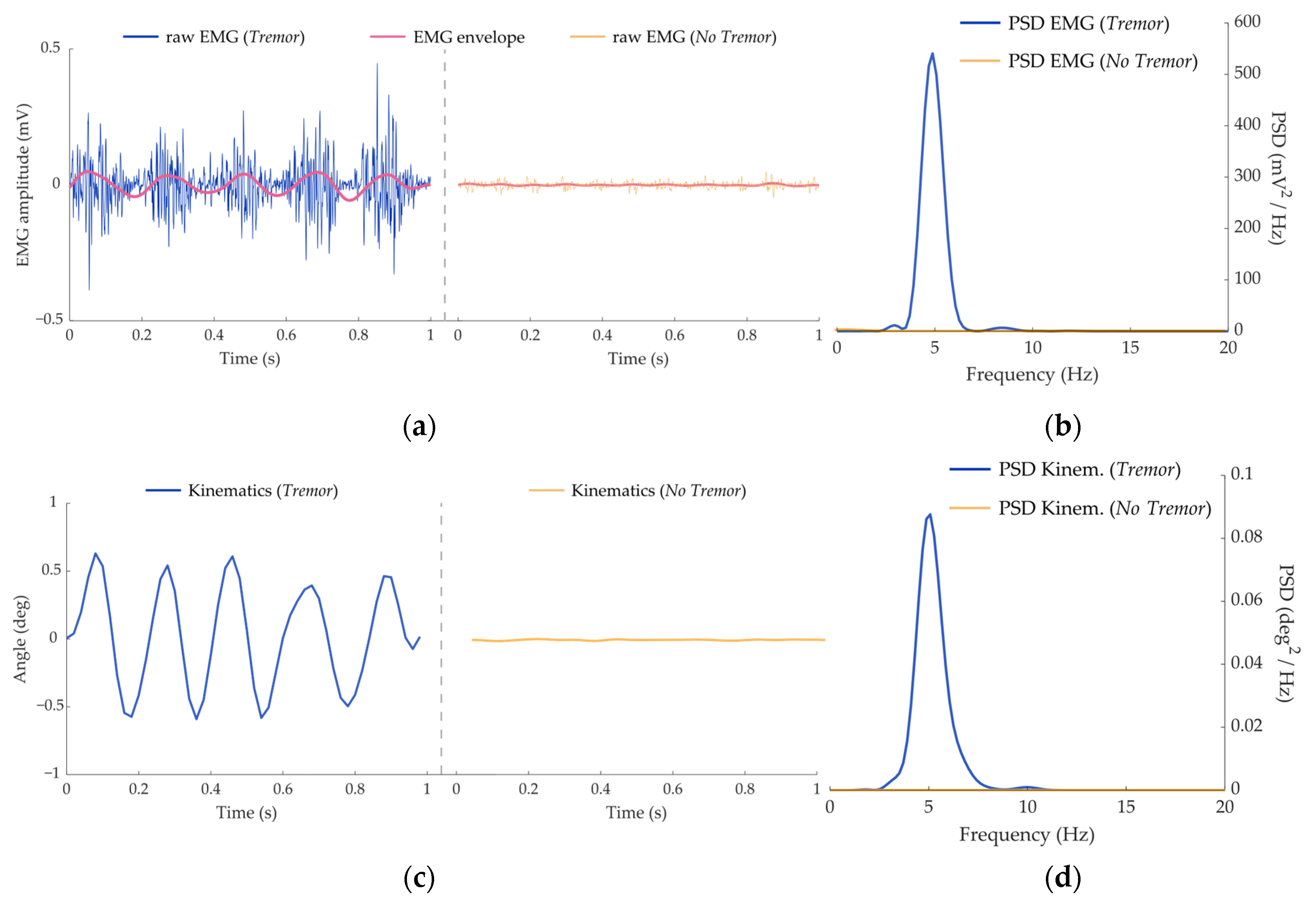
2.2.1. Kinematics
2.2.2. Electromyography
2.3. Classication Algortihms
2.3.1. Machine Learning Classifiers
- K-nearest neighbors (KNN). The fixed parameters selected included the algorithm (Ball tree) and weights (Distance). The variable parameters were the number of neighbors, the leaf size and the distance algorithm [43].
- Support Vector Machine (SVM). The selected kernel was the Radial Basis Function (RBF), whereas the hyperparameters c and γ were explored in the validation process [44].
- Random Forest (RF). The criterion function (Entropy) and the minimum number of samples per leaf were fixed, whereas the number of trees and the maximum number of features were varied across the cross-validation method [45].
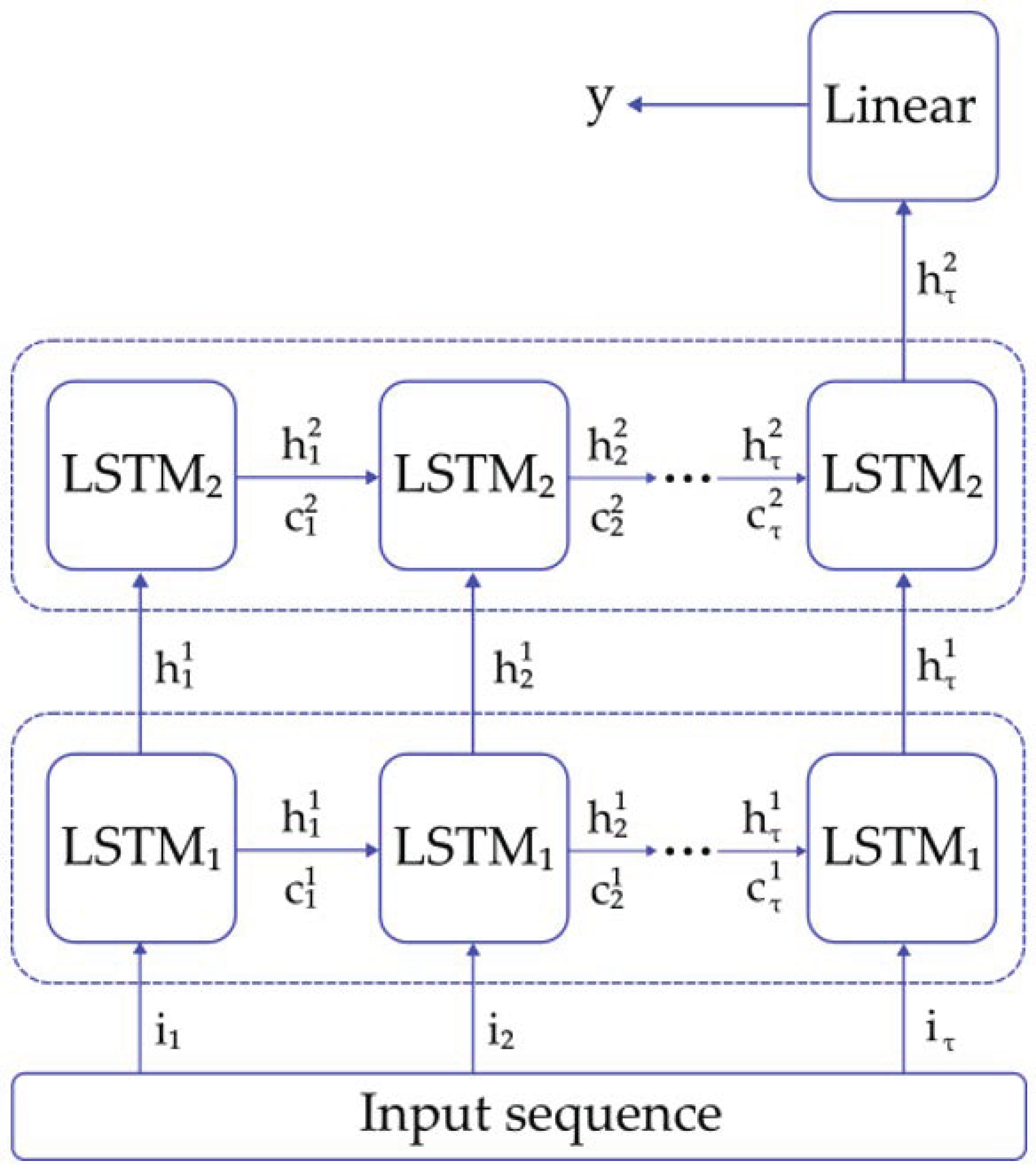
2.3.2. LSTM Classifier
2.4. Performance Metrics
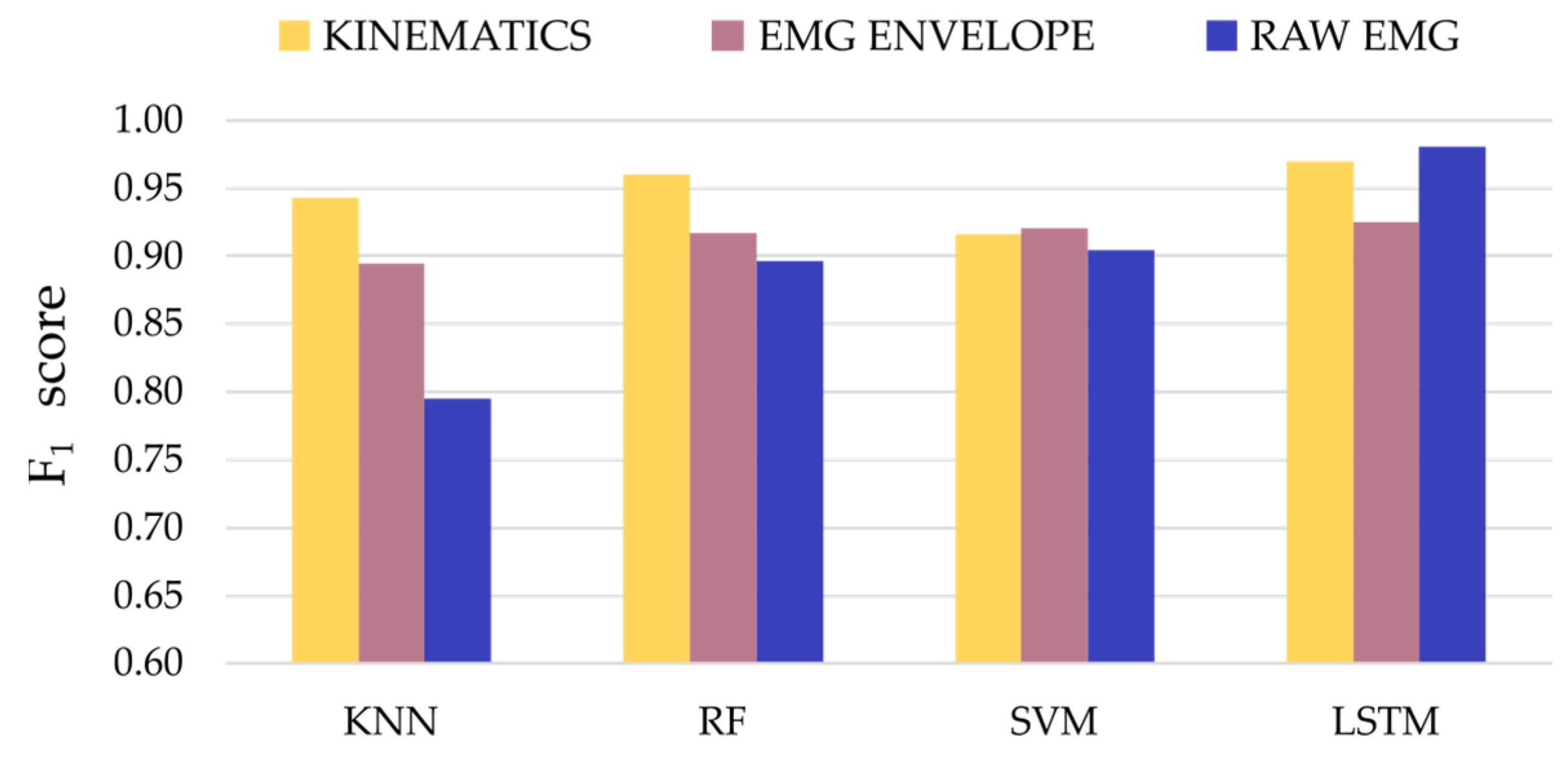
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Thanvi, B.; Lo, N.; Robinson, T. Essential tremor—The most common movement disorder in older people. Age Ageing 2006, 35, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Lenka, A.; Jankovic, J. Tremor Syndromes: An Updated Review. Front. Neurol. 2021, 12, 684835. [Google Scholar] [CrossRef]
- Louis, E.D. Essential Tremor. N. Engl. J. Med. 2001, 345, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Elble, R.J. Physiologic and essential tremor. Neurology 1986, 36, 225–231. [Google Scholar] [CrossRef] [PubMed]
- McAuley, J.H. Physiological and pathological tremors and rhythmic central motor control. Brain 2000, 123, 1545–1567. [Google Scholar] [CrossRef]
- Shanker, V. Essential tremor: Diagnosis and management. BMJ 2019, 366, l4485. [Google Scholar] [CrossRef]
- Latorre, A.; Hallett, M.; Deuschl, G.; Bhatia, K.P. The MDS consensus tremor classification: The best way to classify patients with tremor at present. J. Neurol. Sci. 2022, 435, 120191. [Google Scholar] [CrossRef]
- Louis, E.D.; Faust, P.L. Essential tremor: The most common form of cerebellar degeneration? Cerebellum Ataxias 2020, 7, 12. [Google Scholar] [CrossRef]
- Louis, E.D. Treatment of Essential Tremor: Are there Issues We are Overlooking? Front. Neurol. 2012, 2, 91. [Google Scholar] [CrossRef]
- Ondo, W.G. Current and Emerging Treatments of Essential Tremor. Neurol. Clin. 2020, 38, 309–323. [Google Scholar] [CrossRef]
- Voges, J.; Hilker, R.; Bötzel, K.; Kiening, K.L.; Kloss, M.; Kupsch, A.; Schnitzler, A.; Schneider, G.; Steude, U.; Deuschl, G.; et al. Thirty days complication rate following surgery performed for deep-brain-stimulation. Mov. Disord. 2007, 22, 1486–1489. [Google Scholar] [CrossRef] [PubMed]
- Lora-Millan, J.S.; Delgado-Oleas, G.; Benito-León, J.; Rocon, E. A Review on Wearable Technologies for Tremor Suppression. Front. Neurol. 2021, 12, 700600. [Google Scholar] [CrossRef] [PubMed]
- Castrillo-Fraile, V.; Peña, E.C.; Galán, J.M.T.G.Y.; Delgado-López, P.D.; Collazo, C.; Cubo, E. Tremor Control Devices for Essential Tremor: A Systematic Literature Review. Tremor Other Hyperkinetic Mov. 2019, 9. [Google Scholar] [CrossRef]
- Pascual-Valdunciel, A.; Rajagopal, A.; Pons, J.L.; Delp, S. Non-invasive electrical stimulation of peripheral nerves for the management of tremor. J. Neurol. Sci. 2022, 435, 120195. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Valdunciel, A.; Gonzalez-Sanchez, M.; Muceli, S.; Adan-Barrientos, B.; Escobar-Segura, V.; Perez-Sanchez, J.R.; Jung, M.K.; Schneider, A.; Hoffmann, K.-P.; Moreno, J.C.; et al. Intramuscular Stimulation of Muscle Afferents Attains Prolonged Tremor Reduction in Essential Tremor Patients. IEEE Trans. Biomed. Eng. 2020, 68, 1768–1776. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Valdunciel, A.; Hoo, G.W.; Avrillon, S.; Barroso, F.O.; Goldman, J.G.; Hernandez-Pavon, J.C.; Pons, J.L. Peripheral electrical stimulation to reduce pathological tremor: A review. J. Neuroeng. Rehabil. 2021, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Alder, G.; Signal, N.; Olsen, S.; Taylor, D. A Systematic Review of Paired Associative Stimulation (PAS) to Modulate Lower Limb Corticomotor Excitability: Implications for Stimulation Parameter Selection and Experimental Design. Front. Neurosci. 2019, 13, 895. [Google Scholar] [CrossRef]
- Puttaraksa, G.; Muceli, S.; Gallego, J.; Holobar, A.; Charles, S.K.; Pons, J.L.; Farina, D. Voluntary and tremorogenic inputs to motor neuron pools of agonist/antagonist muscles in essential tremor patients. J. Neurophysiol. 2019, 122, 2043–2053. [Google Scholar] [CrossRef]
- Dosen, S.; Muceli, S.; Dideriksen, J.L.; Romero, J.P.; Rocon, E.; Pons, J.; Farina, D. Online Tremor Suppression Using Electromyography and Low-Level Electrical Stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 23, 385–395. [Google Scholar] [CrossRef]
- Dideriksen, J.L.; Gianfelici, F.; Popovic-Maneski, L.Z.; Farina, D. EMG-based demodulation of pathological tremor using the Iterated Hilbert Transform. In Proceedings of the 2011 5th International IEEE/EMBS Conference on Neural Engineering, Cancun, Mexico, 27 April–1 May 2011; pp. 116–119. [Google Scholar] [CrossRef]
- Xu, F.L.; Hao, M.Z.; Xu, S.Q.; Hu, Z.X.; Xiao, Q.; Lan, N. Development of a closed-loop system for tremor suppression in patients with Parkinson’s disease. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 1782–1785. [Google Scholar] [CrossRef]
- Hao, M.-Z.; Xu, S.-Q.; Hu, Z.-X.; Xu, F.-L.; Niu, C.-X.M.; Xiao, Q.; Lan, N. Inhibition of Parkinsonian tremor with cutaneous afferent evoked by transcutaneous electrical nerve stimulation. J. Neuroeng. Rehabil. 2017, 14, 75. [Google Scholar] [CrossRef]
- Alam, N.; Johnson, B.; Gendreau, J.; Tavakolian, K.; Combs, C.; Fazel-Rezai, R. Tremor quantification of Parkinson’s disease—A pilot study. In Proceedings of the 2016 IEEE International Conference on Electro Information Technology (EIT), Grand Forks, ND, USA, 19–21 May 2016; pp. 0755–0759. [Google Scholar] [CrossRef]
- Stamatakis, J.; Ambroise, J.; Crémers, J.; Sharei, H.; Delvaux, V.; Macq, B.; Garraux, G. Finger Tapping Clinimetric Score Prediction in Parkinson’s Disease Using Low-Cost Accelerometers. Comput. Intell. Neurosci. 2013, 2013, 717853. [Google Scholar] [CrossRef]
- De Araújo, A.C.A.; Santos, E.G.D.R.; De Sá, K.S.G.; Furtado, V.K.T.; Santos, F.A.; De Lima, R.C.; Krejcová, L.V.; Santos-Lobato, B.L.; Pinto, G.H.L.; Cabral, A.D.S.; et al. Hand Resting Tremor Assessment of Healthy and Patients with Parkinson’s Disease: An Exploratory Machine Learning Study. Front. Bioeng. Biotechnol. 2020, 8, 778. [Google Scholar] [CrossRef]
- Jeon, H.; Lee, W.-W.; Park, H.; Lee, H.J.; Kim, S.K.; Kim, H.B.; Jeon, B.; Park, K.S. Automatic Classification of Tremor Severity in Parkinson’s Disease Using a Wearable Device. Sensors 2017, 17, 2067. [Google Scholar] [CrossRef]
- Moon, S.; Song, H.-J.; Sharma, V.D.; Lyons, K.E.; Pahwa, R.; Akinwuntan, A.E.; Devos, H. Classification of Parkinson’s disease and essential tremor based on balance and gait characteristics from wearable motion sensors via machine learning techniques: A data-driven approach. J. Neuroeng. Rehabil. 2020, 17, 125. [Google Scholar] [CrossRef]
- Zanini, R.A.; Colombini, E.L.; de Castro, M.C.F. Parkinson’s Disease EMG Signal Prediction Using Neural Networks. In Proceedings of the 2019 IEEE International Conference on Systems, Man and Cybernetics (SMC), Bari, Italy, 6–9 October 2019. [Google Scholar] [CrossRef]
- Pascual-Valdunciel, A.; Lopo-Martinez, V.; Sendra-Arranz, R.; Gonzalez-Sanchez, M.; Perez-Sanchez, J.R.; Grandas, F.; Torricelli, D.; Moreno, J.C.; Barroso, F.O.; Pons, J.L.; et al. Prediction of Pathological Tremor Signals Using Long Short-Term Memory Neural Networks. IEEE J. Biomed. Health Inform. 2022, 26, 5930–5941. [Google Scholar] [CrossRef]
- Shahtalebi, S.; Atashzar, S.F.; Patel, R.V.; Mohammadi, A. WAKE: Wavelet decomposition coupled with adaptive Kalman filtering for pathological tremor extraction. Biomed. Signal Process. Control 2018, 48, 179–188. [Google Scholar] [CrossRef]
- López-De-Ipiña, K.; Solé-Casals, J.; Faundez-Zanuy, M.; Calvo, P.M.; Sesa, E.; De Lizarduy, U.M.; De La Riva, P.; Marti-Masso, J.F.; Beitia, B.; Bergareche, A. Selection of Entropy Based Features for Automatic Analysis of Essential Tremor. Entropy 2016, 18, 184. [Google Scholar] [CrossRef]
- Lopez-De-Ipina, K.; Solé-Casals, J.; Faúndez-Zanuy, M.; Calvo, P.M.; Sesa, E.; Roure, J.; Martinez-De-Lizarduy, U.; Beitia, B.; Fernández, E.; Iradi, J.; et al. Automatic Analysis of Archimedes’ Spiral for Characterization of Genetic Essential Tremor Based on Shannon’s Entropy and Fractal Dimension. Entropy 2018, 20, 531. [Google Scholar] [CrossRef]
- Dideriksen, J.L.; Laine, C.M.; Dosen, S.; Muceli, S.; Rocon, E.; Pons, J.L.; Benito-Leon, J.; Farina, D. Electrical Stimulation of Afferent Pathways for the Suppression of Pathological Tremor. Front. Neurosci. 2017, 11, 178. [Google Scholar] [CrossRef]
- Caramia, C.; Torricelli, D.; Schmid, M.; Munoz-Gonzalez, A.; Gonzalez-Vargas, J.; Grandas, F.; Pons, J.L. IMU-Based Classification of Parkinson’s Disease from Gait: A Sensitivity Analysis on Sensor Location and Feature Selection. IEEE J. Biomed. Health Inform. 2018, 22, 1765–1774. [Google Scholar] [CrossRef]
- Corvini, G.; Holobar, A.; Moreno, J.C. On Repeatability of MU Fatiguing in Low-Level Sustained Isometric Contractions of Tibialis Anterior Muscle. In Converging Clinical and Engineering Research on Neurorehabilitation IV; Torricelli, D., Akay, M., Pons, J.L., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 909–1113. [Google Scholar]
- Elble, R.J.; McNames, J. Using Portable Transducers to Measure Tremor Severity. Tremor Other Hyperkinetic Mov. 2016, 6, 375. [Google Scholar] [CrossRef]
- Farina, D.; Negro, F.; Muceli, S.; Enoka, R.M. Principles of Motor Unit Physiology Evolve with Advances in Technology. Physiology 2016, 31, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Merletti, R.; Muceli, S. Surface EMG detection in space and time: Best practices. J. Electromyogr. Kinesiol. 2019, 49, 102363. [Google Scholar] [CrossRef] [PubMed]
- Sendra-Arranz, R.; Gutiérrez, A. A long short-term memory artificial neural network to predict daily HVAC consumption in buildings. Energy Build. 2020, 216, 109952. [Google Scholar] [CrossRef]
- Guido, S.; Mueller, A.C. Introduction to Machine Learning with Python: A Guide for Data Scientists; O’Reilly Media: Sebastopol, CA, USA, 2016. [Google Scholar]
- Geron, A. Hands-On Machine Learning with Scikit-Learn, Keras, and TensorFlow: Concepts, Tools, and Techniques to Build Intelligent Systems, 2nd ed.; O’Reilly Media, Inc.: Sebastopol, CA, USA, 2019. [Google Scholar]
- Paszke, A.; Gross, S.; Chintala, S.; Chanan, G.; Yang, E.; DeVito, Z.; Lin, Z.; Desmaison, A.; Antiga, L.; Lerer, A. Automatic Differentiation in PyTorch. In Proceedings of the NIPS 2017 Workshop on Autodiff, Long Beach, CA, USA, 9 December 2017. [Google Scholar]
- Sajal, S.R.; Ehsan, T.; Vaidyanathan, R.; Wang, S.; Aziz, T.; Al Mamun, K.A. Telemonitoring Parkinson’s disease using machine learning by combining tremor and voice analysis. Brain Inform. 2020, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Cai, G.; Lin, Z.; Wang, Z.; Ye, Q. Validation of Inertial Sensing-Based Wearable Device for Tremor and Bradykinesia Quantification. IEEE J. Biomed. Health Inform. 2020, 25, 997–1005. [Google Scholar] [CrossRef]
- Peres, L.B.; Calil, B.C.; da Silva, A.P.S.P.B.; Dionísio, V.C.; Vieira, M.F.; Andrade, A.D.O.; Pereira, A.A. Discrimination between healthy and patients with Parkinson’s disease from hand resting activity using inertial measurement unit. Biomed. Eng. Online 2021, 20, 50. [Google Scholar] [CrossRef]
- Carrillo-Moreno, J.; Pérez-Gandía, C.; Sendra-Arranz, R.; García-Sáez, G.; Hernando, M.E.; Gutiérrez, Á. Long short-term memory neural network for glucose prediction. Neural Comput. Appl. 2020, 33, 4191–4203. [Google Scholar] [CrossRef]
- Kingma, D.P.; Ba, J. Adam: A Method for Stochastic Optimization 2017. arXiv 2017, arXiv:1412.6980. [Google Scholar]
- Prechelt, L. Early Stopping—But When? In Neural Networks: Tricks of the Trade; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar] [CrossRef]
- Martinez Manzanera, O.; Elting, J.W.; van der Hoeven, J.H.; Maurits, N.M. Tremor Detection Using Parametric and Non-Parametric Spectral Estimation Methods: A Comparison with Clinical Assessment. PLoS ONE 2016, 11, e0156822. [Google Scholar] [CrossRef]
- Luft, F.; Sharifi, S.; Mugge, W.; Schouten, A.C.; Bour, L.J.; van Rootselaar, A.-F.; Veltink, P.H.; Heida, T. A Power Spectral Density-Based Method to Detect Tremor and Tremor Intermittency in Movement Disorders. Sensors 2019, 19, 4301. [Google Scholar] [CrossRef]
- Surangsrirat, D.; Thanawattano, C.; Pongthornseri, R.; Dumnin, S.; Anan, C.; Bhidayasiri, R. Support vector machine classification of Parkinson’s disease and essential tremor subjects based on temporal fluctuation. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 6389–6392. [Google Scholar] [CrossRef]
- Bougea, A.M.; Zikos, P.; Spanou, I.; Efthymiopoulou, E. Clock-Drawing Tasks as Predictive Measurements for Disease Classification Among Patients with Parkinson’s Disease and Essential Tremor. Cureus 2021, 13, e13239. [Google Scholar] [CrossRef]


| KNN | Algorithm | Leaf Size | Metric | Neighbors | Weights |
|---|---|---|---|---|---|
| Kinematics | Ball-Tree | Euclidean | 6 | Distance | |
| EMG envelope | Ball-Tree | 30 | Euclidean | 3 | Distance |
| EMG Raw | Ball-Tree | 30 | Chebyshev | 2 | Distance |
| RF | Criterion | Max. Features | Min. Samples | Trees | |
| Kinematics | Entropy | sqrt | 2 | 100 | |
| EMG envelope | Entropy | sqrt | 2 | 100 | |
| EMG Raw | Entropy | sqrt | 2 | 110 | |
| SVM | C | γ | Kernel | ||
| Kinematics | 10 | 1 | RBF | ||
| EMG envelope | 1 | 0.1 | RBF | ||
| EMG Raw | 10 | 0.01 | RBF | ||
| LSTM | Learning Rate | Hidden Size | |||
| Kinematics | 0.005 | 50 | |||
| EMG envelope | 0.005 | 35 | |||
| EMG Raw | 0.005 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascual-Valdunciel, A.; Lopo-Martínez, V.; Beltrán-Carrero, A.J.; Sendra-Arranz, R.; González-Sánchez, M.; Pérez-Sánchez, J.R.; Grandas, F.; Farina, D.; Pons, J.L.; Oliveira Barroso, F.; et al. Classification of Kinematic and Electromyographic Signals Associated with Pathological Tremor Using Machine and Deep Learning. Entropy 2023, 25, 114. https://doi.org/10.3390/e25010114
Pascual-Valdunciel A, Lopo-Martínez V, Beltrán-Carrero AJ, Sendra-Arranz R, González-Sánchez M, Pérez-Sánchez JR, Grandas F, Farina D, Pons JL, Oliveira Barroso F, et al. Classification of Kinematic and Electromyographic Signals Associated with Pathological Tremor Using Machine and Deep Learning. Entropy. 2023; 25(1):114. https://doi.org/10.3390/e25010114
Chicago/Turabian StylePascual-Valdunciel, Alejandro, Víctor Lopo-Martínez, Alberto J. Beltrán-Carrero, Rafael Sendra-Arranz, Miguel González-Sánchez, Javier Ricardo Pérez-Sánchez, Francisco Grandas, Dario Farina, José L. Pons, Filipe Oliveira Barroso, and et al. 2023. "Classification of Kinematic and Electromyographic Signals Associated with Pathological Tremor Using Machine and Deep Learning" Entropy 25, no. 1: 114. https://doi.org/10.3390/e25010114
APA StylePascual-Valdunciel, A., Lopo-Martínez, V., Beltrán-Carrero, A. J., Sendra-Arranz, R., González-Sánchez, M., Pérez-Sánchez, J. R., Grandas, F., Farina, D., Pons, J. L., Oliveira Barroso, F., & Gutiérrez, Á. (2023). Classification of Kinematic and Electromyographic Signals Associated with Pathological Tremor Using Machine and Deep Learning. Entropy, 25(1), 114. https://doi.org/10.3390/e25010114







