Global Stability of the Curzon-Ahlborn Engine with a Working Substance That Satisfies the van der Waals Equation of State
Abstract
1. Introduction
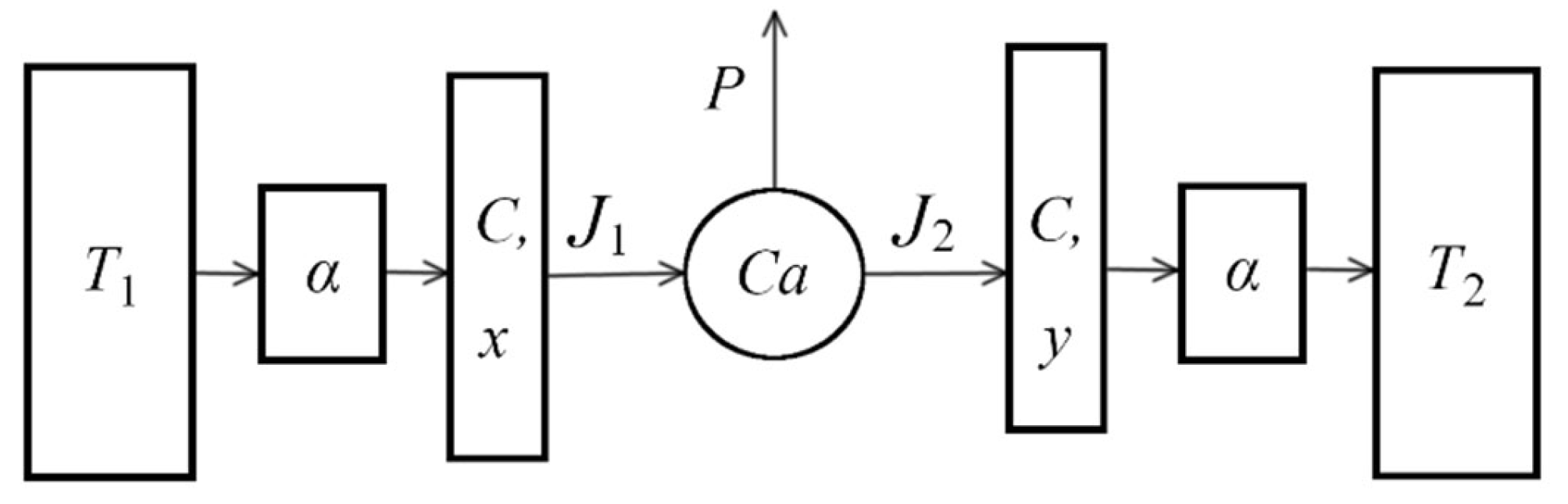
2. Steady-State Characteristics of the CAEVW
3. Stability Analysis and Effects of Time Delays of the CAEVW
3.1. Dynamic Model of the CA Engine
3.2. Local Stability Analysis
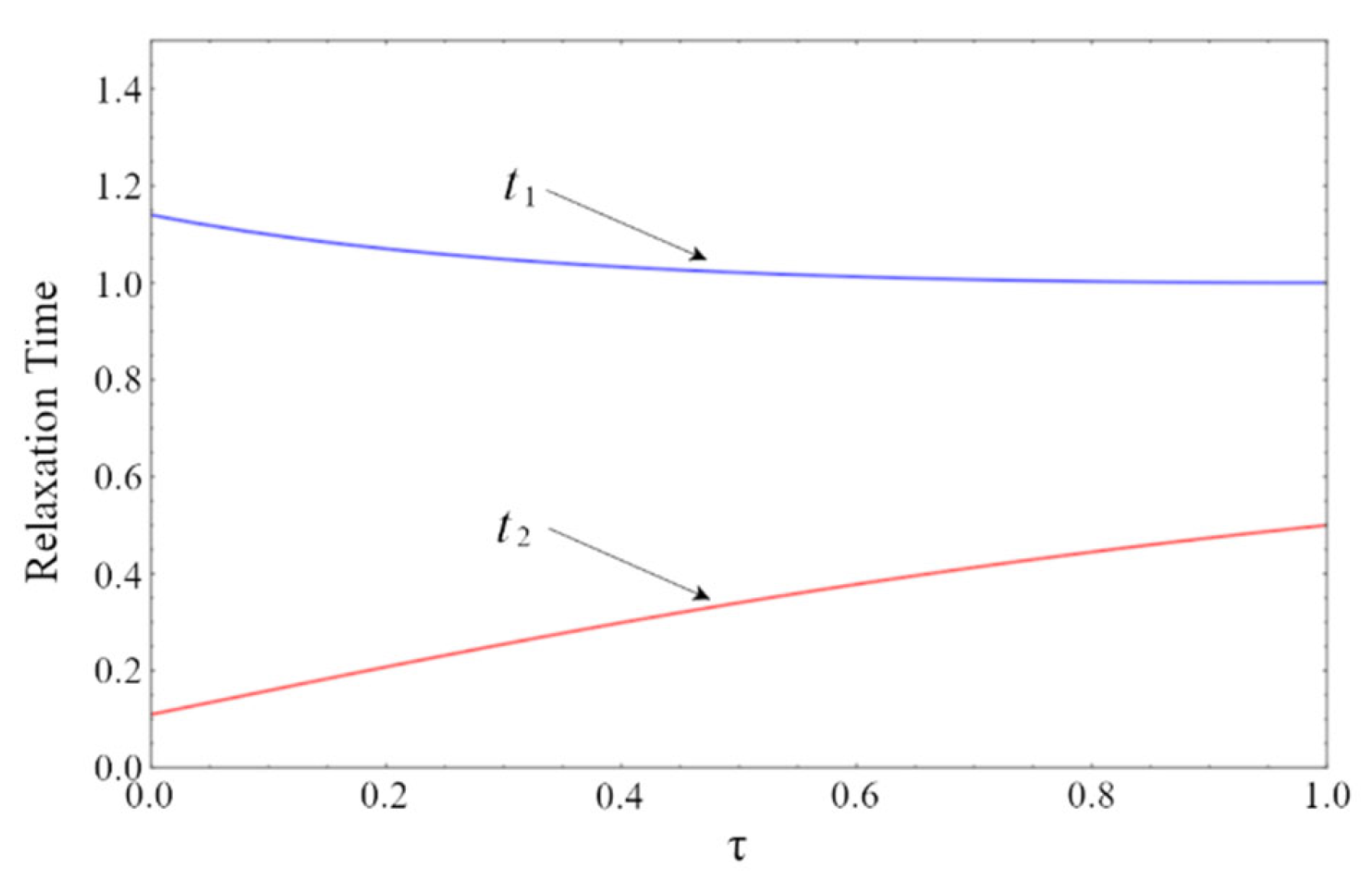
3.3. Dynamic Effects of the Time Delay
4. Global Stability of the CAEVW
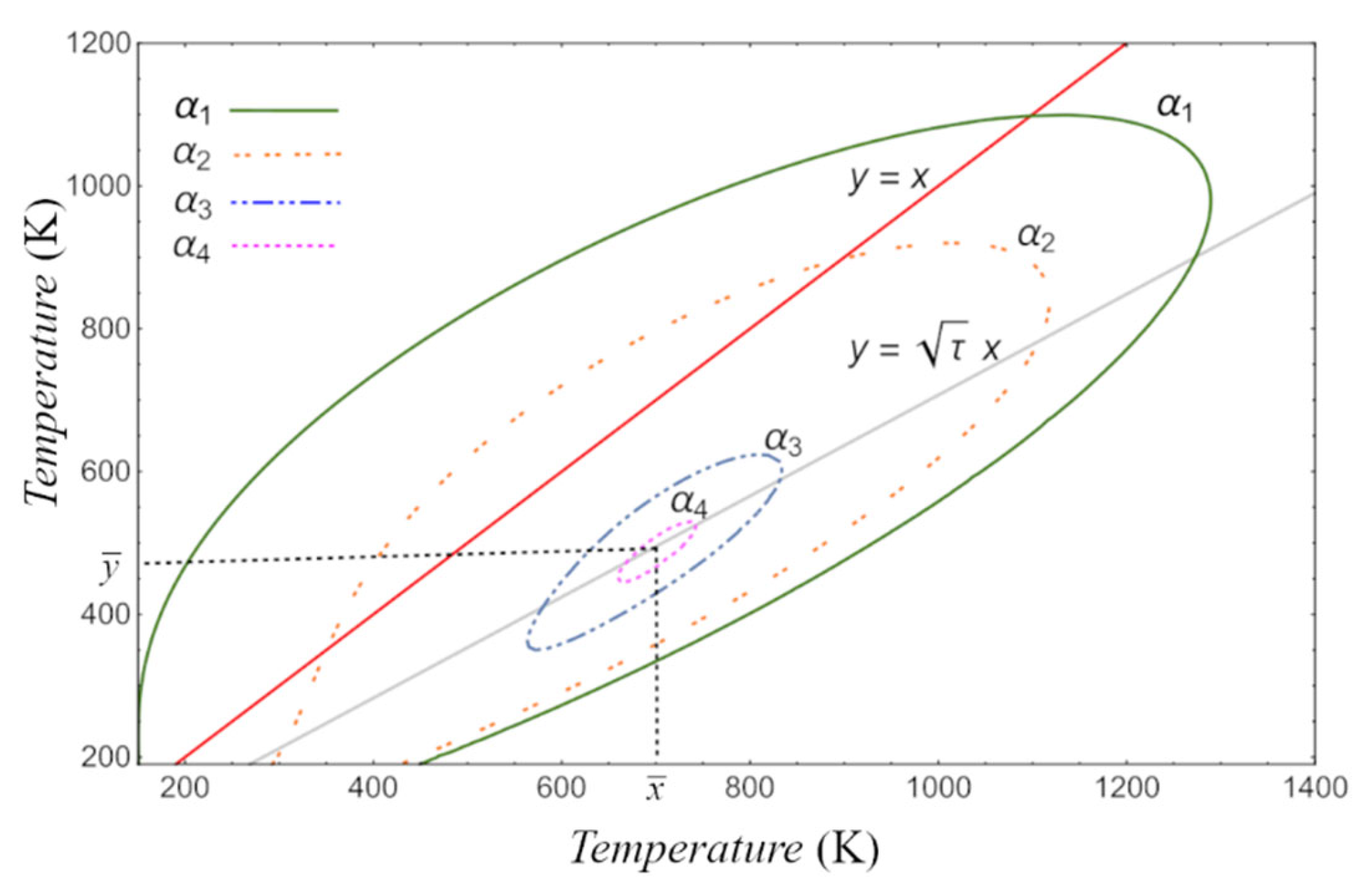
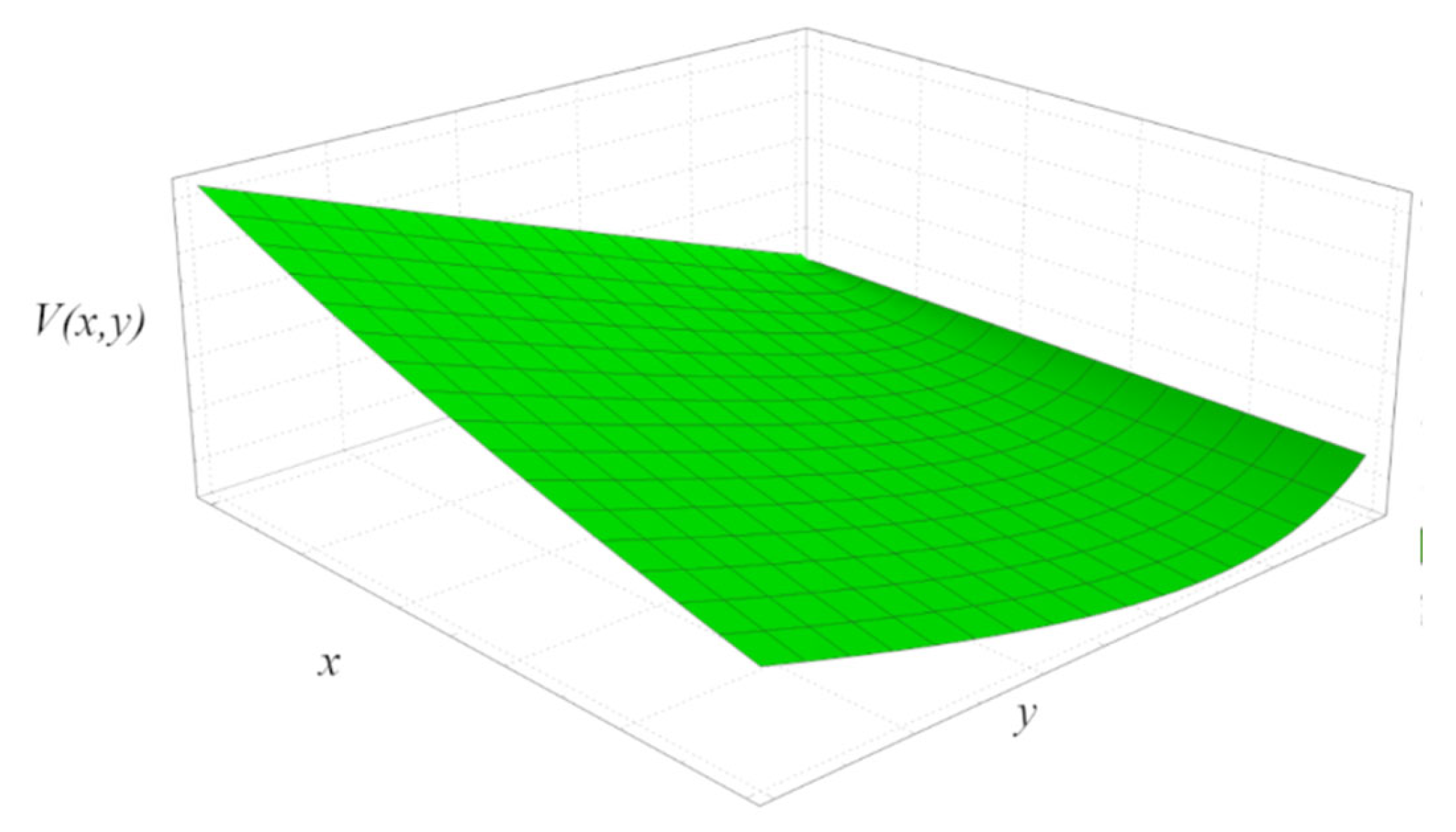
4.1. Lyapunov Function Description
- (1)
- must be positive definite in a region around the steady state;
- (2)
- must be negative definite, that is, .
4.2. Lyapunov Function for CA Engine Model
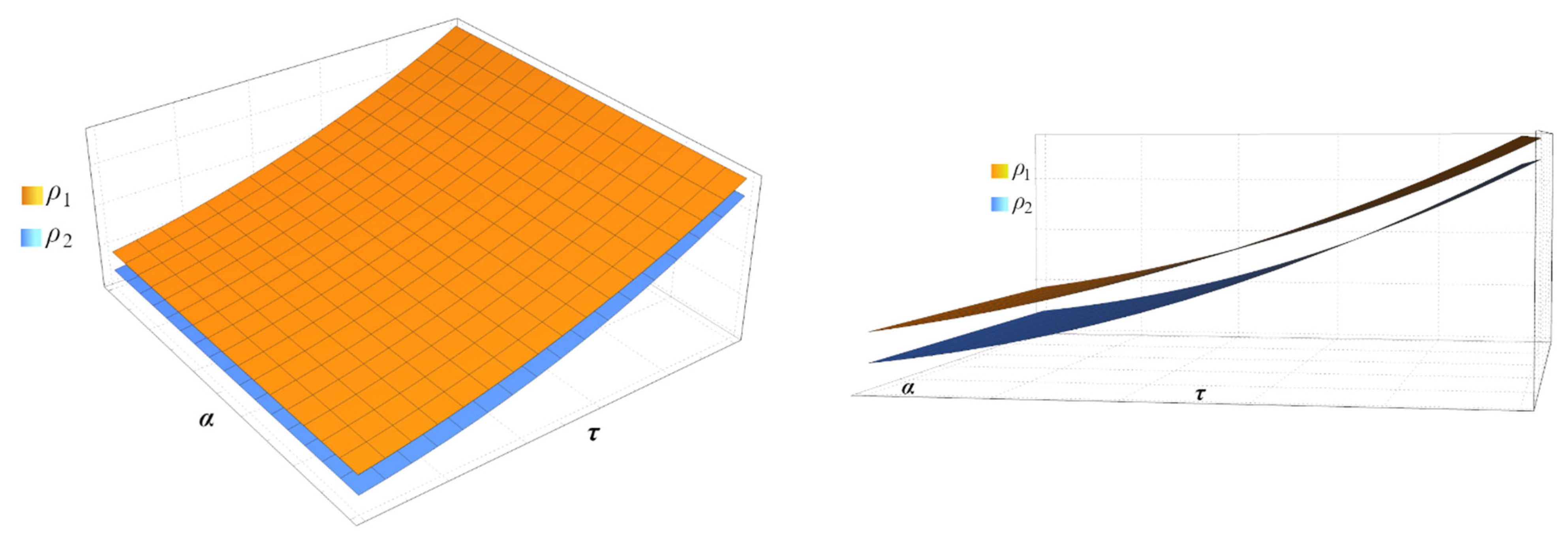
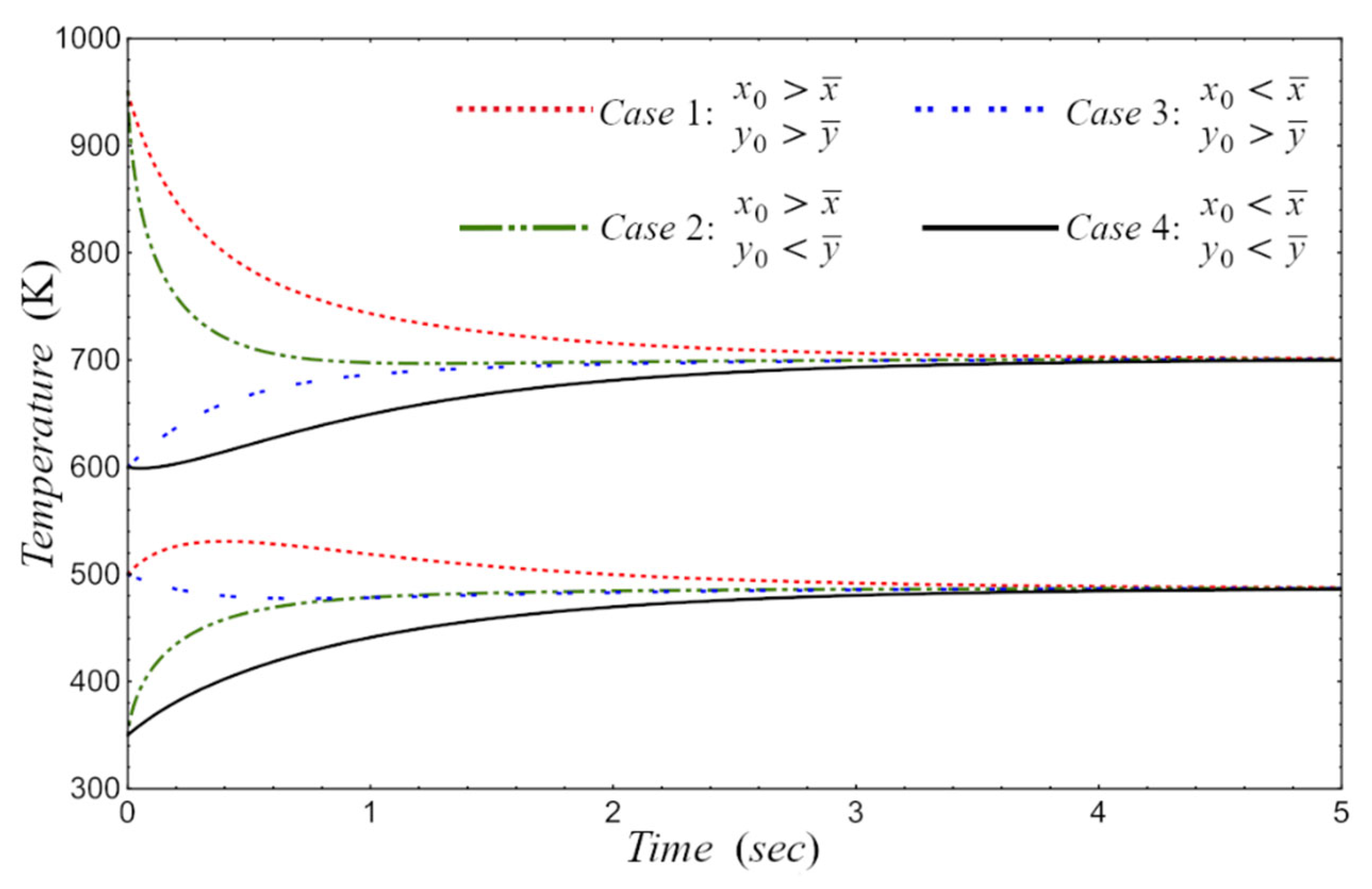
5. Results
6. Conclusion and Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Van der Waals parameter, (kPa·m6)/(kg·K) | |
| Van der Waals parameter, m3/kg | |
| Heat capacity, kJ/(kg·K) | |
| Molar constant pressure specific heat capacity, (W/K) | |
| Molar constant volume specific heat capacity, (W/K) | |
| Heat flux, (J/s) | |
| Steady-state heat flux, (J/s) | |
| Mole number, mol | |
| Power output, (W) | |
| Pressure, (Pa) | |
| Heat, (J) | |
| Universal gas constant, J/(mol•K) | |
| Temperature, (K) | |
| Time, (s) | |
| Volume, (m3) | |
| Temperature of heat reservoir, (K) | |
| Steady-state temperature of heat reservoir, (K) | |
| Temperature of heat reservoir, (K) | |
| Steady-state temperature of heat reservoir, (K) | |
| Greek symbol | |
| Thermal conductance, W/ (m2·K) | |
| Ratio temperature | |
| Carnot efficiency | |
| Curzon–Ahlborn efficiency | |
| Efficiency of the heat engine with Van der Waals gas substance | |
References
- Curzon, F.L.; Ahlborn, B. Efficiency of Carnot Engine at Maximum Power Out. Am. J. Phy. 1975, 43, 22–25. [Google Scholar] [CrossRef]
- Andresen, B.; Salamon, P.; Berry, R.S. Thermodynamics in finite time: Extremals for imperfect heat engines. J. Chem. Phys. 1977, 66, 1571. [Google Scholar] [CrossRef]
- Sieniutycz, S.; Salamon, P. Finite-Time Thermodynamics and Thermoeconomics; Taylor & Francis: New York, NY, USA, 1990. [Google Scholar]
- De Vos, A. Endoreversible Thermodynamics of Solar Energy Conversion; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Bejan, A. Entropy generation minimization: The new thermodynamics of finite size devices and finite-time processes. J. Appl. Phys. 1996, 79, 1191–1218. [Google Scholar] [CrossRef]
- Hoffmann, K.H.; Burzler, J.M.; Shubert, S. Endoreversible thermodynamics. J. Non-Equilib. Thermodyn. 1997, 22, 311–355. [Google Scholar]
- Wu, C.; Chen, L.; Chen, J. (Eds.) Recent Advances in Finite Thermodynamics; Nova Science: New York, NY, USA, 1999. [Google Scholar]
- Santillán, M.; Maya, G.; Angulo-Brown, F. Local stability analysis of an endoreversible Curzon–Ahlborn–Novikov engine working in a maximum-power-like regime. J. Phys. D Appl. Phys. 2001, 34, 2068–2072. [Google Scholar] [CrossRef]
- Páez-Hernández, R.T.; Angulo-Brown, F.; Santillán, M. Dynamic Robustness and Thermodynamic Optimization in a Non-Endoreversible Curzon-Ahlborn engine. J. Non-Equilib. Thermodyn. 2006, 31, 173–188. [Google Scholar] [CrossRef]
- Páez-Hernández, R.T. Thermodynamic Optimization and robustness Dynamic of Thermal Engines and Some Biological Systems. Ph.D. Thesis, Escuela Superior de Física y Matemáticas, IPN, Mexico City, Mexico, 2008. (In Spanish). [Google Scholar]
- Páez-Hernández, R.; Ladino-Luna, D.; Portillo-Díaz, P. Dynamic properties in an endoreversible Curzon-Ahlborn engine using a Van der Waals gas as working substance. Phys. A 2011, 390, 3275–3282. [Google Scholar] [CrossRef]
- Páez-Hernández, R.T.; Ladino-Luna, D.; Portillo-Díaz, P. Effects of the time delays in endoreversible and non-endoreversible thermal engines working at different regimes. In Proceedings of the 12th Joint European Thermodynamics Conference, Brescia, Italy, 1–5 July 2013. [Google Scholar]
- Chimal-Eguía, J.C.; Barranco-Jiménez, M.A.; Angulo-Brown, F. Stability analysis of an endoreversible heat engine with Stefan-Boltzmann heat transfer law working in a maximum power-like regime. Open Syst. Inf. Dyn. 2006, 13, 43–54. [Google Scholar] [CrossRef]
- Guzmán-Vargas, L.; Reyes-Ramírez, I.; Sánchez-Salas, N. The effect of heat transfer laws and thermal conductance on the local stability of an endoreversible heat engine. J. Phys. D Appl. Phys. 2005, 38, 1282–1291. [Google Scholar] [CrossRef]
- Barranco-Jiménez, M.A.; Páez-Hernández, R.T.; Reyes-Ramírez, I.; Guzmán-Vargas, L. Local stability analysis of a thermo-economic model of a Novikov-Curzon-Ahlborn heat engine. Entropy 2011, 13, 1584–1594. [Google Scholar] [CrossRef]
- Chimal-Eguía, J.C.; Reyes-Ramírez, I.; Guzmán-Vargas, L. Local stability of an endoreversible heat engine working an ecological regime. Open Syst. Inf. Dyn. 2007, 14, 411–424. [Google Scholar] [CrossRef]
- Sánchez-Salas, N.; Chimal-Eguía, J.C.; Guzmán-Aguilar, F. On the Dynamic robustness of a non-endoreversible engine working in different operating regimes. Entropy 2011, 13, 422–436. [Google Scholar] [CrossRef]
- Huang, Y.W.; Sun, D.X.; Kang, Y.M. Local stability analysis of a class of endoreversible heat pumps. J. Appl. Phys. 2007, 102, 034905. [Google Scholar] [CrossRef]
- Huang, Y.W. Local asymptotic stability of an irreversible heat pump subject to total thermal conductance constraint. Energy Convers. Manag. 2009, 50, 1444–1449. [Google Scholar] [CrossRef]
- Wu, X.H.; Chen, L.G.; Sun, F.R. Local stability of an endoreversible heat pump working at the maximum ecological function. Chin. J. Eng. Thermophys. 2011, 32, 1811–1815. [Google Scholar]
- Wu, X.H.; Chen, L.G.; Sun, F.R. Local stability of an endoreversible heat pump with linear phenomenological heat transfer law working in an ecological regime. Sci. Iran. B 2012, 19, 1519–1525. [Google Scholar] [CrossRef][Green Version]
- Chimal-Eguía, J.C. Dynamic Stability in an endoreversible Chemical reactor. Rev. Mex. Quim. 2015, 14, 703–710. [Google Scholar]
- Gonzalez-Ayala, J.; Mateos-Roco, J.M.; Medina, A.; Calvo-Hernández, A. Optimization, Stability, and Entropy in Endoreversible Heat Engines. Entropy 2020, 22, 1323. [Google Scholar] [CrossRef]
- Páez-Hernández, R.T.; Santillán, M. Comparison of the energetic properties and the dynamical stability in a mathematical model of the stretch reflex. Phys. A 2008, 387, 3574–3582. [Google Scholar] [CrossRef]
- Guo, H.; Xu, Y.; Zhang, X.; Zhu, Y.; Chen, H. Finite-time thermodynamics modeling and analysis on compressed air energy storage systems with thermal storage. Renew. Sustain. Energy Rev. 2021, 138, 110656. [Google Scholar] [CrossRef]
- Chen, L.; Meng, Z.; Ge, Y.; Wu, F. Performance Analysis and Optimization for Irreversible Combined Carnot Heat Engine Working with Ideal Quantum Gases. Entropy 2021, 23, 536. [Google Scholar] [CrossRef] [PubMed]
- Susu, Q.; Zemin, D.; LinGen, C.; YanLin, G.E. Performance optimization of thermionic refrigerators based on vander Waals heterostructures. Sci. China Technol. Sci. 2021, 64, 1007–1016. [Google Scholar]
- Hamedani Raja, S.; Maniscalco, S.; Paraoanu, G.S.; Pekola, J.P.; Lo Gullo, N. Finite-Time quantum Stirling heat engine. New. J. Phys. 2021, 23, 033034. [Google Scholar] [CrossRef]
- Andresen, B.; Salamon, P. Future Perspectives of Finite-Time Thermodynamics. Entropy 2022, 24, 690. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, G.; Minami, Y. Finite-Time Thermodynamics in microscopic heat engines. Phys. Rev. Res. 2022, 4, L01008. [Google Scholar] [CrossRef]
- Rojas-Pacheco, A.; Obregón-Quintana, L.S.; Guzmán-Vargas, L. Time-delay effects on dynamics of a two-actor conflict model. Phys. A 2013, 392, 458–467. [Google Scholar] [CrossRef]
- Reyes-Ramírez, I.; Barranco-Jiménez, M.A.; Rojas-Pacheco, A.; Guzmán-Vargas, L. Global stability analysis of an endoreversible Curzon-Ahlborn Heat engine under different regimes. Entropy 2014, 16, 5796–5809. [Google Scholar] [CrossRef]
- Reyes-Ramírez, I.; Barranco-Jiménez, M.A.; Rojas-Pacheco, A.; Guzmán-Vargas, L. Global stability of the Curzon-Ahlborn heat engine using the Lyapunov method. Phys. A 2014, 399, 98–105. [Google Scholar] [CrossRef]
- Valencia-Ortega, G.; Levario-Medina, S.; Barranco-Jiménez, M.A. Local and Global Stability of a Curzon-Ahlborn model applied to power plants working at maximum power κ-efficient power. arXiv 2020, arXiv:2005.10397. [Google Scholar] [CrossRef]
- Gutkowicz-Krusin, D.; Procacia, I.; Ross, J. On the efficiency of rate processes, Power and Efficiency of heat engines. J. Chem. Phys. 1978, 69, 3898. [Google Scholar] [CrossRef]
- Ladino-Luna, D. Ciclo de Cuzon y Ahlbon para un gas de van de Waals. Rev. Mex. Fís. 2002, 48, 575–578. [Google Scholar]
- Heywood, J.B. Internal Combustion Engine Fundamentals; Mc Graw Hill Publishing Company: New York, NY, USA, 1988. [Google Scholar]
- Bejan, A. Advanced Engineering Thermodynamics; John Wiley & Sons: Hoboken, NJ, USA, 1988. [Google Scholar]
- Velasco, S.; Roco, J.M.M.; Medina, A.; White, J.A.; Calvo-Hernández, A. Optimization of heat engines including the saving of natural resources and the reduction of thermal pollution. J. Phys. D Appl. Phys. 2000, 33, 355. [Google Scholar] [CrossRef]
- Strogatz, S.H. Nonlinear Dynamics and Chaos; Addison Wesley: Reading, MA, USA, 1994. [Google Scholar]
- McDonald, N. Biological Delay Systems: Linear Stability Theory; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco-Paez, J.C.; Chimal-Eguía, J.C.; Páez-Hernández, R.; Ladino-Luna, D. Global Stability of the Curzon-Ahlborn Engine with a Working Substance That Satisfies the van der Waals Equation of State. Entropy 2022, 24, 1655. https://doi.org/10.3390/e24111655
Pacheco-Paez JC, Chimal-Eguía JC, Páez-Hernández R, Ladino-Luna D. Global Stability of the Curzon-Ahlborn Engine with a Working Substance That Satisfies the van der Waals Equation of State. Entropy. 2022; 24(11):1655. https://doi.org/10.3390/e24111655
Chicago/Turabian StylePacheco-Paez, Juan Carlos, Juan Carlos Chimal-Eguía, Ricardo Páez-Hernández, and Delfino Ladino-Luna. 2022. "Global Stability of the Curzon-Ahlborn Engine with a Working Substance That Satisfies the van der Waals Equation of State" Entropy 24, no. 11: 1655. https://doi.org/10.3390/e24111655
APA StylePacheco-Paez, J. C., Chimal-Eguía, J. C., Páez-Hernández, R., & Ladino-Luna, D. (2022). Global Stability of the Curzon-Ahlborn Engine with a Working Substance That Satisfies the van der Waals Equation of State. Entropy, 24(11), 1655. https://doi.org/10.3390/e24111655





