1. Introduction
Light plays an important role in non-equilibrium thermodynamics not only in light-driving processes, but also in some basic processes such as atoms interacting with each other by exchanging photons. Therefore, proper and accurate evaluation of the entropy of a single photon in a light beam is crucial for the formulation of the light-related thermodynamics. Although the entropy of a beam of light with a certain frequency
is well derived as
with
the average number of photons [
1], there still exist several different definitions of the entropy of a single photon in a beam of light. The first one is the average entropy,
[
2], which is only a mathematical definition lacking proper physical realization [
3]. The second one is the intrinsic entropy of a single photon [
3,
4,
5], which is based on the observation that photons do not interact with each other and each of them thus forms an isolated thermodynamical system with the intrinsic entropy a constant independent of its frequency and the source temperature. The third definition cares about the effective entropy of a single photon, which is defined as the entropy change due to adding or removing a photon from the light [
6,
7,
8,
9]. Apart from the three definitions above, there still exists the fourth definition as
based on the classical definition of entropy change
[
10]. Coexistence of these definitions of single-photon entropy reflects the complexity involved in the thermodynamics of lights. In this work, we shall limit our attention only to the third definition, and the term “single-photon entropy”, in this work, will specially refer to effective single-photon entropy.
Previously, based on the following two equilibrium assumptions, (i) the incoming light is initially in equilibrium and (ii) photons in the light can quickly equilibrate again after adding or removing a photon from the light; it can be proven that the single-photon entropy of this light with the frequency
at
T is
, with
h the Planck constant, and this result has been applied in addressing various problems [
8,
9,
11,
12,
13,
14,
15,
16,
17]. For example, it has been previously applied to evaluate the entropy of a laser beam [
14,
15,
16] and the entropy production in the photosynthesis [
17].
However, little work has been completed to derive the single-photon entropy from first principles without resorting to the above equilibrium assumptions [
6,
7], which will obviously restrict its wider applications. Accordingly, several problems regarding the single-photon entropy still exist. First, in what circumstances is the formula
accurate or how long will it take for the light to equilibrate? Second, what is the single-photon entropy if the light is initially
not in equilibrium? Third, even for an equilibrium light, can we derive, from first principles, the photon entropy of this light without assuming the light will quickly equilibrate again?
In Dirac’s work [
18], he introduced the idea of second quantization and gracefully derived the Einstein coefficient relation from first principles without resorting to the equilibrium assumptions. Deeply inspired by Dirac’s work, this work closely follows the first principles to re-derive the single-photon entropy and address the above problems from first principles. We also extend this way of derivation to re-derive Jüttner velocity distribution [
19] of two-level atoms in blackbody radiation fields. Apart from the derivation, the result itself is also an obvious example to demonstrate the importance of light in non-equilibrium thermodynamics and a connection between thermal motion and thermal radiation.
2. Single-Photon Entropy Evaluated by Examining the Light–Atom Interaction
Consider a beam of light with the frequency and the number distribution function of photons (or with the state specified by the density operator ). The total entropy of this light is obviously given by or . If the light is in equilibrium, then satisfies the Bose–Einstein distribution, i.e., , and the temperature of this light can be determined by as . Here, the light is in equilibrium means that, if this beam of light interacts with atoms, the number density distribution of photons in the light is, on average, constant, and equating the absorption rate to the emission rate of photons leads to the equilibrium number distribution.
However, in the following derivation, we assume that
can be any number distribution function to make the conclusion more general. Note that, even for a light that is not in equilibrium, it is possible to define its temperature according to recent works [
20,
21]. Furthermore, we assume that this beam of light is shining on some atoms
A and some of the photons will be absorbed by
A. After the time span
, the number distribution of the outgoing light will change a little due to the absorption. We emphasize that the following derivation does not assume that the light beam will equilibrate again after a photon of the beam has been absorbed by some atoms. Comparing the incoming and outgoing number distributions will lead to the entropy change of the beam.
Let us first compute the outgoing number distribution. For instance, at the time
t, there are exactly
n photons in the beam that are hitting the atom
A. Then, there will be some probability of the state
turning into
with
the excited state of
A and the energy gap between
and
A being
. According to quantum field theory [
18,
22], the Hamiltonian of this process must look like
, where
is an operator with non-zero matrix elements between different atom states,
is its Hermitian conjugate and their precise expressions are not important here [
18], and
and
a are the creation and annihilation operators of the photon, respectively. Then, during a small time span
, the amount of
that has been excited is proportional to
with
,
and
(see ref. [
22]). Here, we define a new parameter
(
) for convenience, which is small for the small time interval
.
Therefore, the outgoing number distribution function becomes
where
accounts for the probability of the occurrence of
while
accounts for the probability of
not being transformed into
.
Finally, the entropy of a single photon in a beam of light can be obtained by evaluating the entropy decrease per number of photons absorbed by
A as
where
accounts for the number of photons absorbed by
A and the averaged number of photons in the light is
. Thesubscript
is used to emphasize that this single-photon entropy is defined by evaluating the entropy decrease per number of photons absorbed by
A. Detailed derivation of Equation (
2) is referred to
Appendix A. Note that Equation (
2) is valid for all kinds of light sources.
Similarly, the entropy of a single photon in a beam of light can be also obtained by evaluating the entropy increase per number of photons emitted by
through
(both spontaneous and stimulated emissions have been included in this single equation), as follows (see
Appendix A):
Therefore, for an arbitrary number distribution, there will be two different ways, depending on whether the photon is being added (Equation (
3)) or being removed (Equation (
2)) from the light, of defining the photon entropy, and these two definitions are usually not equivalent. When there are
photons being absorbed and
photons being emitted by
A and
, respectively, the entropy change can be evaluated as
. Quite interestingly, the definition of the temperature of non-equilibrium quantum systems [
20,
21] is similar to that of the non-equilibrium light entropy. Just like the light has two possible definitions of entropy (Equations (2) and (3)) when the light is not in equilibrium, there are also two effective temperatures for the non-equilibrium light, depending on whether the heat is flowing towards the environment or is absorbed by the system, i.e., cool-down temperature
and heat-up temperature
. In terms of
,
and
according to Lipka-Bartosik et al.’s work [
21]. However, unfortunately, there are no clear relations between
and
as far as we are concerned.
If
is independent of
n, then we can define the single-photon entropy as
with
; and, similarly,
. When the incoming light is in equilibrium, then
, and we have
which agrees with previous works [
6,
7,
8,
9] and has been previously applied to evaluate the entropy of a monochromatic laser [
16] and the entropy production in photosynthesis [
17].
3. The Velocity Distribution of Two-Level Atoms in Blackbody Radiation Fields
Inspired by the first-principle derivation above, we find that we can derive the equilibrium velocity distribution of two-level atoms placed in blackbody radiation fields without referring to Boltzmann factor.
Assume that there are a number of two-level atoms which will not interact with each other moving in a blackbody radiation field, and the energy difference between
A and the excited state
is
. Since the atoms are moving, the frequency of the photon absorbed/emitted may not be
but will be altered by the Doppler effect. It is expressed as
where
is the angle between the directions of light and particle momentum.
At the same time, when the atoms absorb or emit photons, their momenta or velocities will change. Based on this kinetics, we can derive the equilibrium velocity distribution.
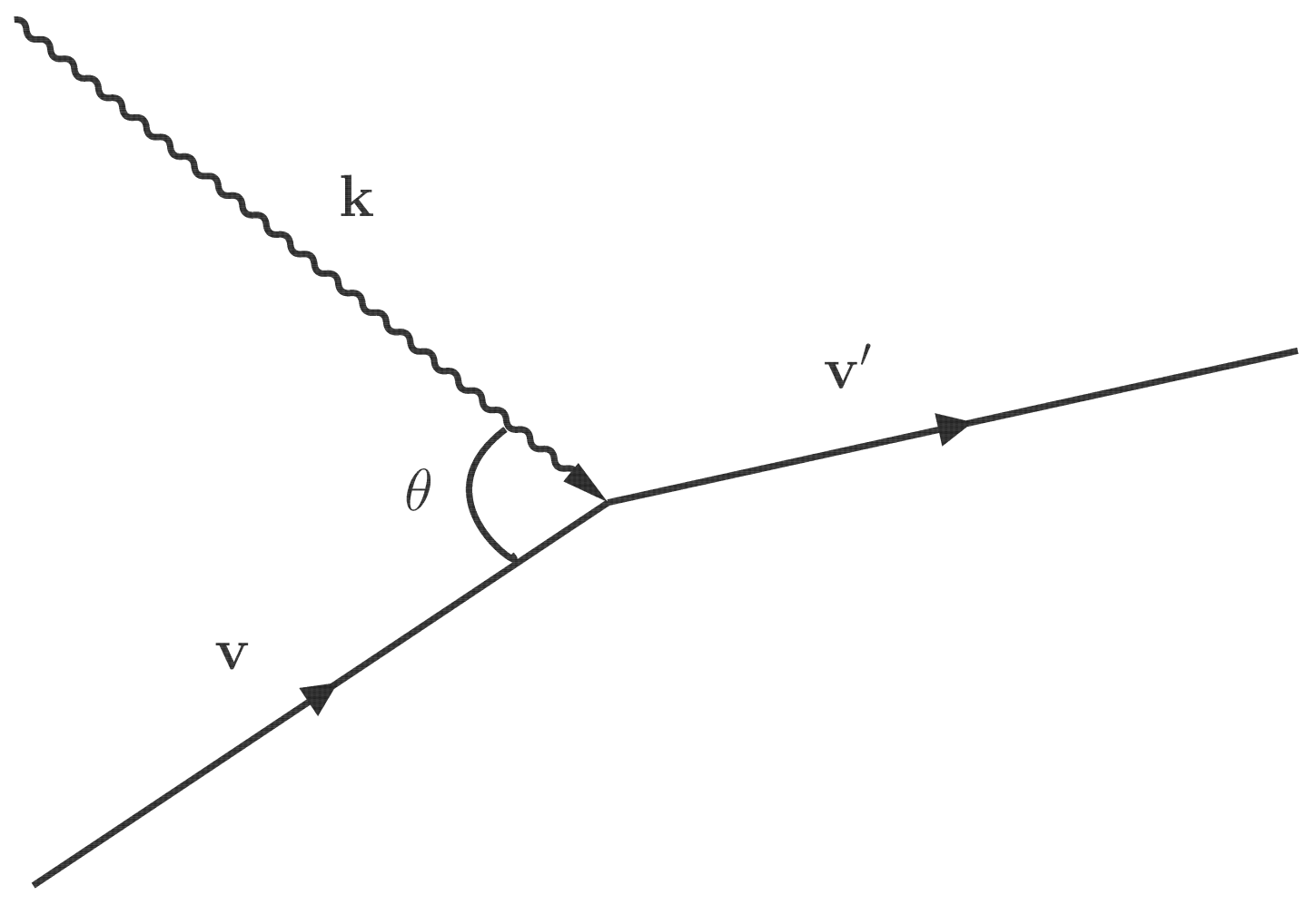
For instance, consider an atom
A with velocity
absorbing a photon with the wave vector
and changing into the excited state
with velocity
(see
Figure 1), and assume that the Hamiltonian of this process can be written as
. Note that the velocity
, the wave vector
or the photon frequency
, and the frequency
satisfy Equation (
5). The probability for this absorption process is
. The excited state
with velocity
can also emit a photon with wave vector
(both spontaneous and stimulated emissions) and change back to atom
A with velocity
. The probability for this emission process is
.
When the system reaches equilibrium, the distribution will not change, which leads to
. Since the radiation field is blackbody-like,
with
and
T is the blackbody temperature. Therefore, we have
Now, suppose that another atom with velocity
can absorb a photon with the wave vector
and change into the same excited state
with the velocity
. Similarly, we have
in equilibrium and, comparing it with Equation (
6), we obtain
According to the energy conservation, the following relation must hold:
where the left-hand side accounts for the total energy of the state
and a photon with
while the right-hand side is the total energy of the state
and a photon with
. Substituting Equation (
8) into Equation (
7) and letting
, we can obtain the equation that the velocity distribution satisfies,
More details about this equation: the left side of Equation (
7) gives
and the right-hand side of Equation (
7) gives
after Equation (
8) has been plugged into this equation; equating these two expressions leads to the above equation. Noting that, in this section, we assume that all functions of
are isotropic in
and can be expressed in terms of
, then the operator
in the above equation can be defined as
for some function
f.
Finally, the velocity distribution can be obtained, by integrating the above equation, as
This velocity distribution is identical to the Jüttner distribution [
19,
23], which is reduced to the Maxwell velocity distribution for the small
. Note that the derivation above is unique and from first principles since we do not need to introduce the Boltzmann factor [
24].
Finally, several comments are made.
(i) In the original derivation [
19] of Jüttner distribution, quantum effects were not considered. However, this work shows that, even when quantum effects are explicitly considered, through the second-quantization formulation, the atoms will still satisfy the distribution in the blackbody radiation field.
(ii) Apart from this unique way of derivation, the result itself is also interesting. Since these two-level atoms have no other internal energy level and no collisions (interactions) with each other, it is hard to believe that the velocity distribution can still relax to Maxwell–Jüttner form only by a blackbody radiation field because of the Doppler effect. Thus, we think this may be a new perspective to understand the relation between thermal motion and thermal radiation.
(iii) Even though we have proved, in theory, that atoms in a blackbody radiation field will relax to the Jüttner distribution even without interatomic collisions, it is still hard to imagine how this occurs. Therefore, we have performed a Monte Carlo (MC) simulation showing that the velocity distribution function of 50,000 atoms with the same initial absolute velocity (
) will gradually evolve to the Jüttner distribution (see
Figure 2) without considering interatomic interactions. During each MC simulation step, we randomly select an atom and select all the model variables, such as
and
, according to the probability or distributions given by the theory. According to Equation (
5), an atom with the speed
has the possibility to absorb photons with the frequency ranging from
to
thanks to the Doppler effect, which might be one of the key reasons that the distribution widening without interatomic collisions is possible.
(iv) Note that Equation (
4) in the last section can be also applied to estimate the entropy change of photons due to the absorption and emission processes discussed here, and it is
with
T the temperature of the blackbody.
Figure 2.
Radial distribution functions (dash-dotted lines) of 50,000 atoms’ velocities at different Monte Carlo (MC) simulation steps. The pale red solid line shows the Jüttner distribution at the same temperature. In this MC simulation, all atoms with the same velocity but with different speed directions are placed in a blackbody radiation field, and interatomic collisions have not been considered. Parameter setting of the simulation is as follows: , , , and a MC step . For simplicity, it is assumed that does not depend on atom’s velocities. c is the speed of light.
Figure 2.
Radial distribution functions (dash-dotted lines) of 50,000 atoms’ velocities at different Monte Carlo (MC) simulation steps. The pale red solid line shows the Jüttner distribution at the same temperature. In this MC simulation, all atoms with the same velocity but with different speed directions are placed in a blackbody radiation field, and interatomic collisions have not been considered. Parameter setting of the simulation is as follows: , , , and a MC step . For simplicity, it is assumed that does not depend on atom’s velocities. c is the speed of light.