The Composition of Saturated Vapor over 1-Butyl-3-methylimidazolium Tetrafluoroborate Ionic Liquid: A Multi-Technique Study of the Vaporization Process
Abstract
:1. Introduction
2. Experimental
3. Computational Details
4. Results
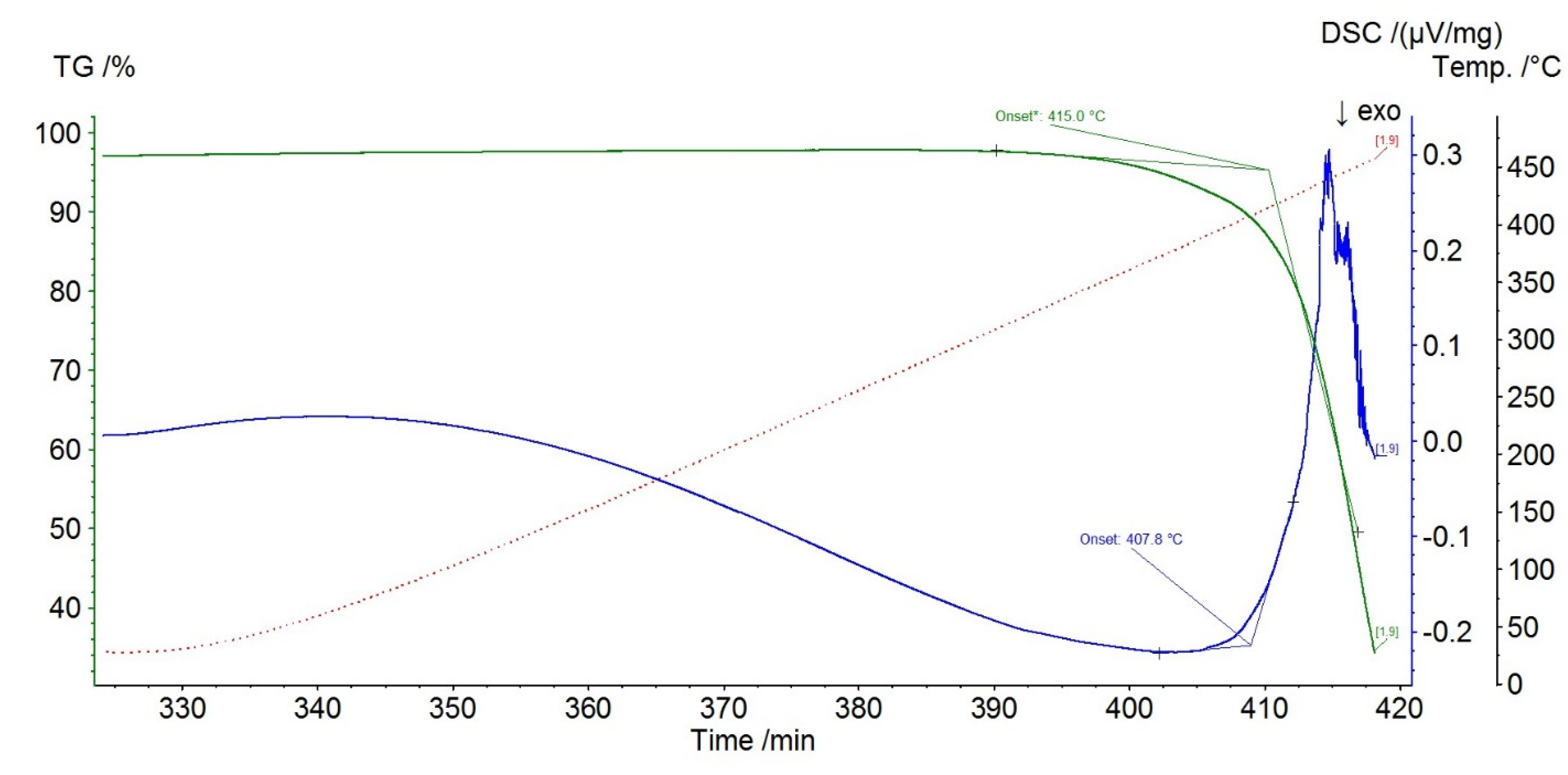
4.1. Thermal Analysis
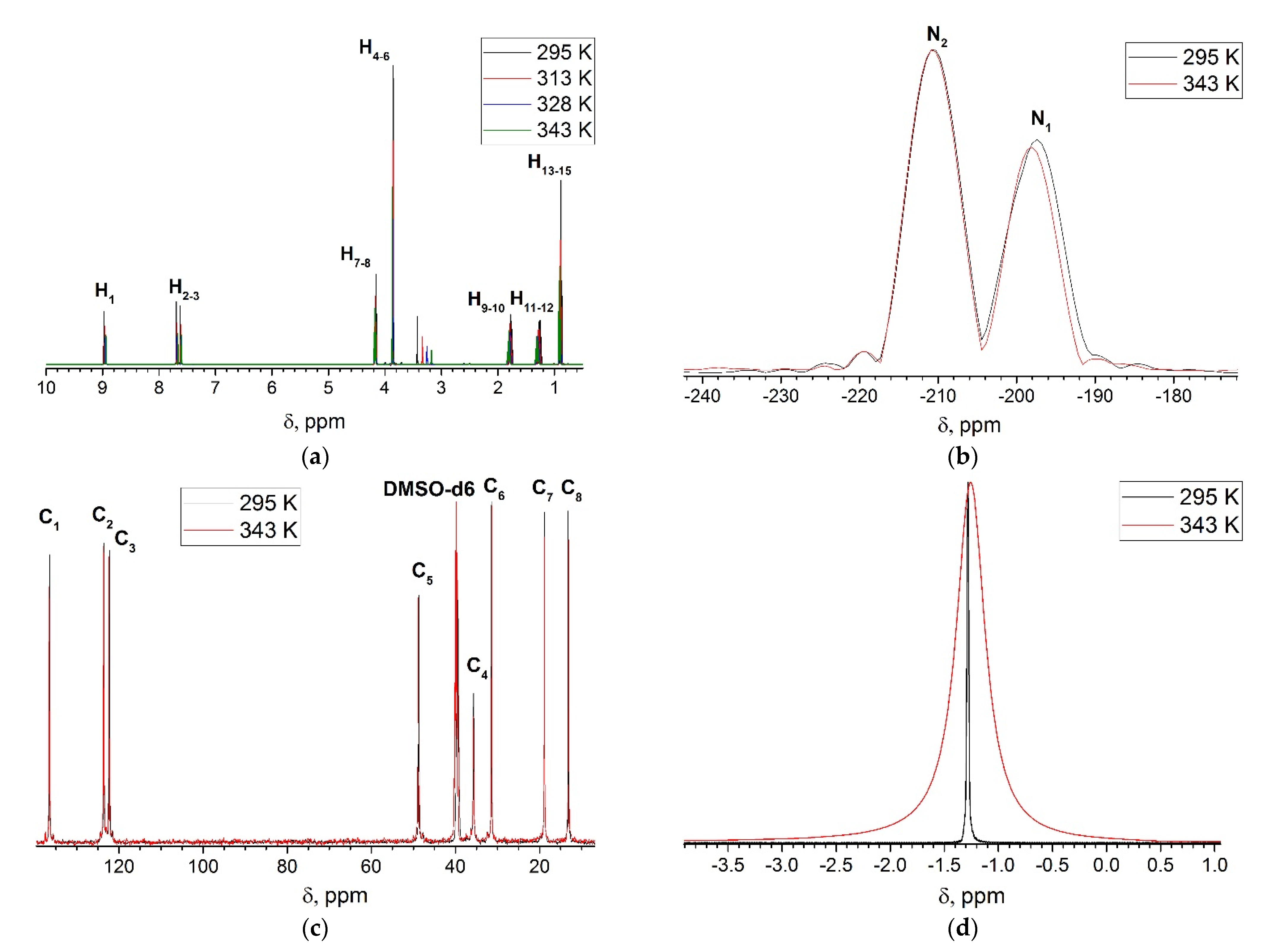
4.2. NMR Analysis
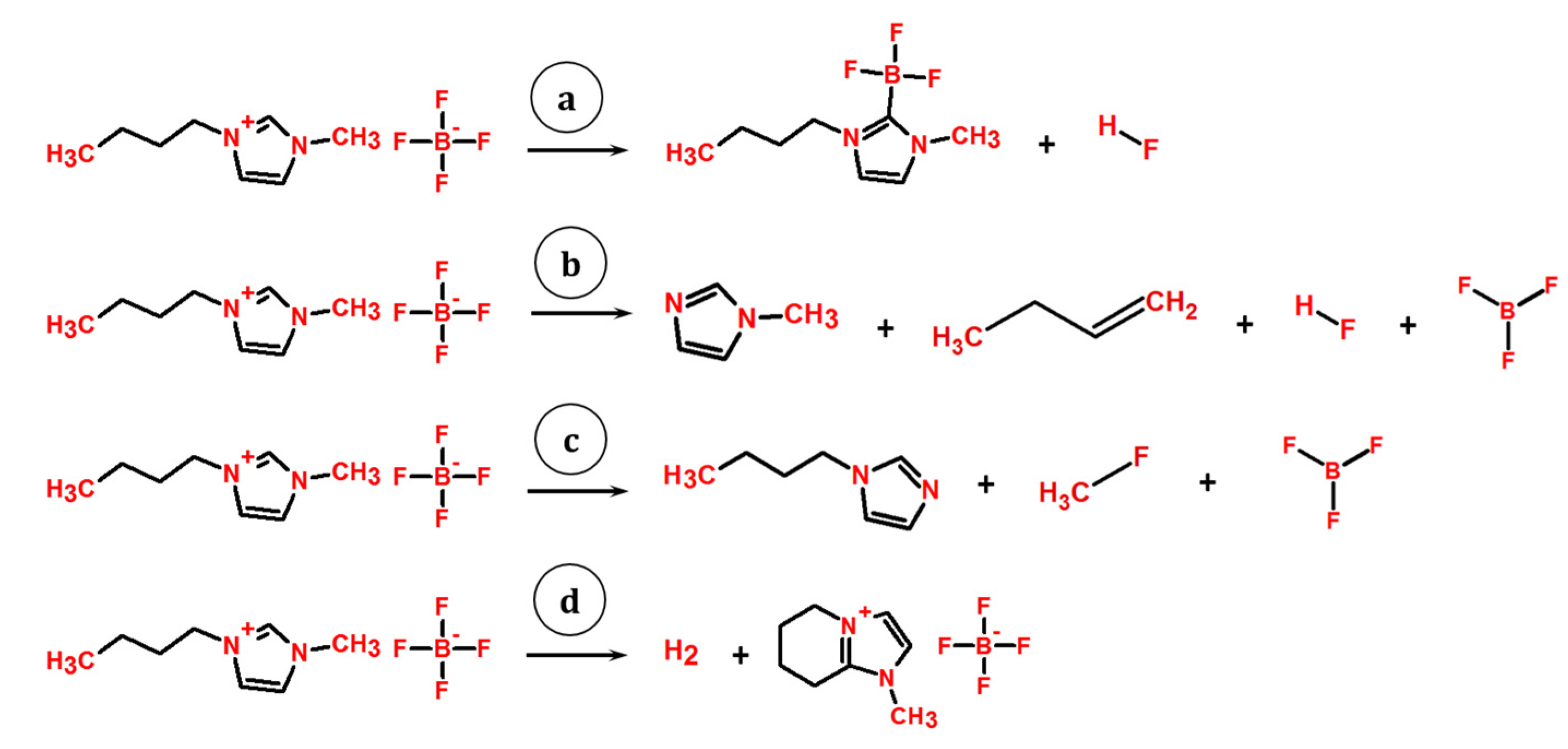
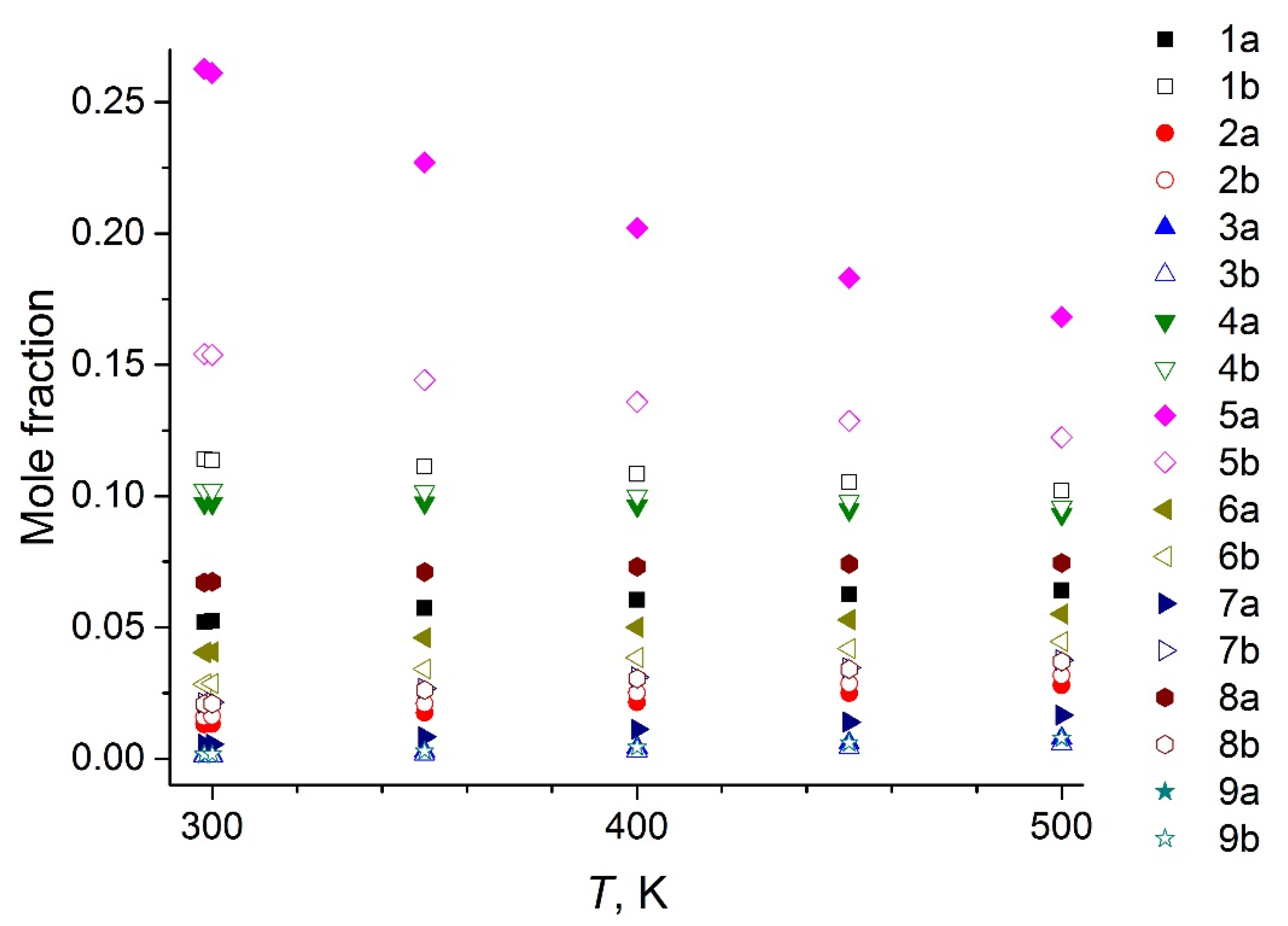
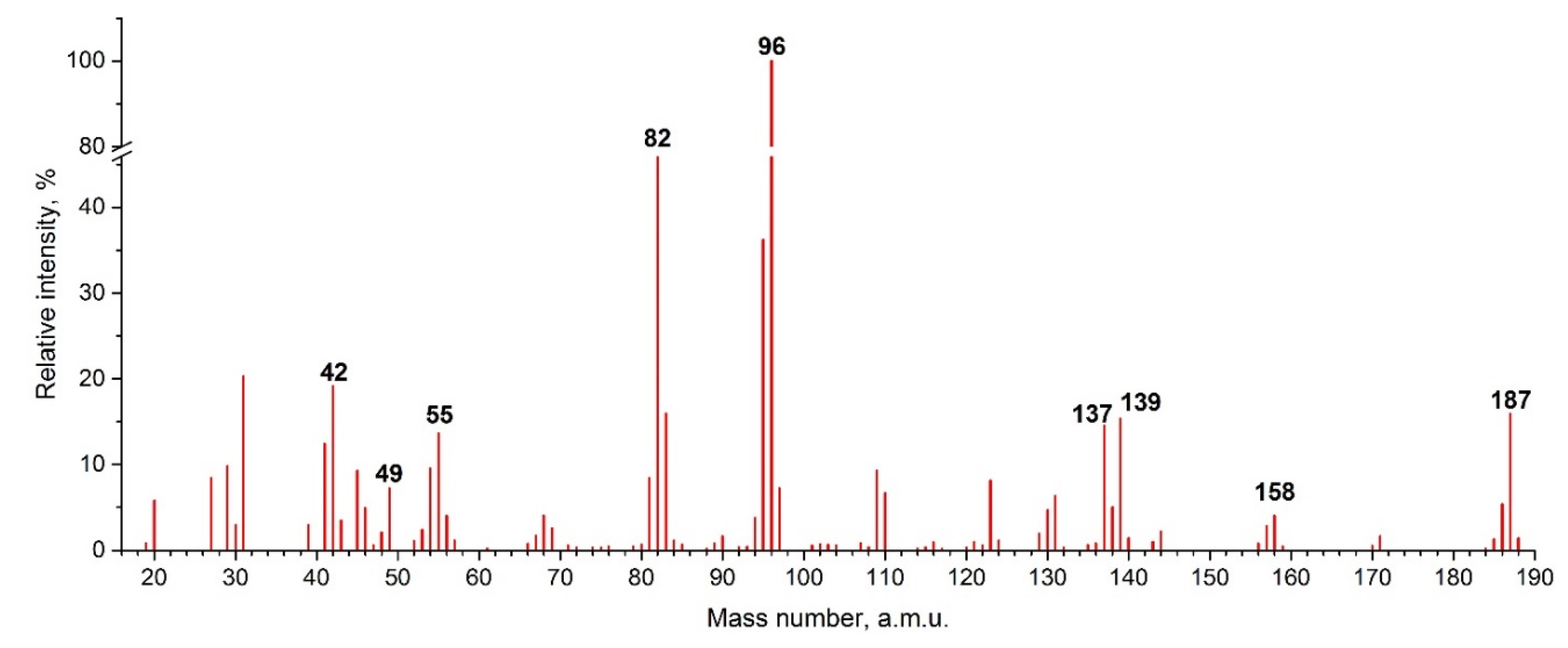
4.3. KEMS
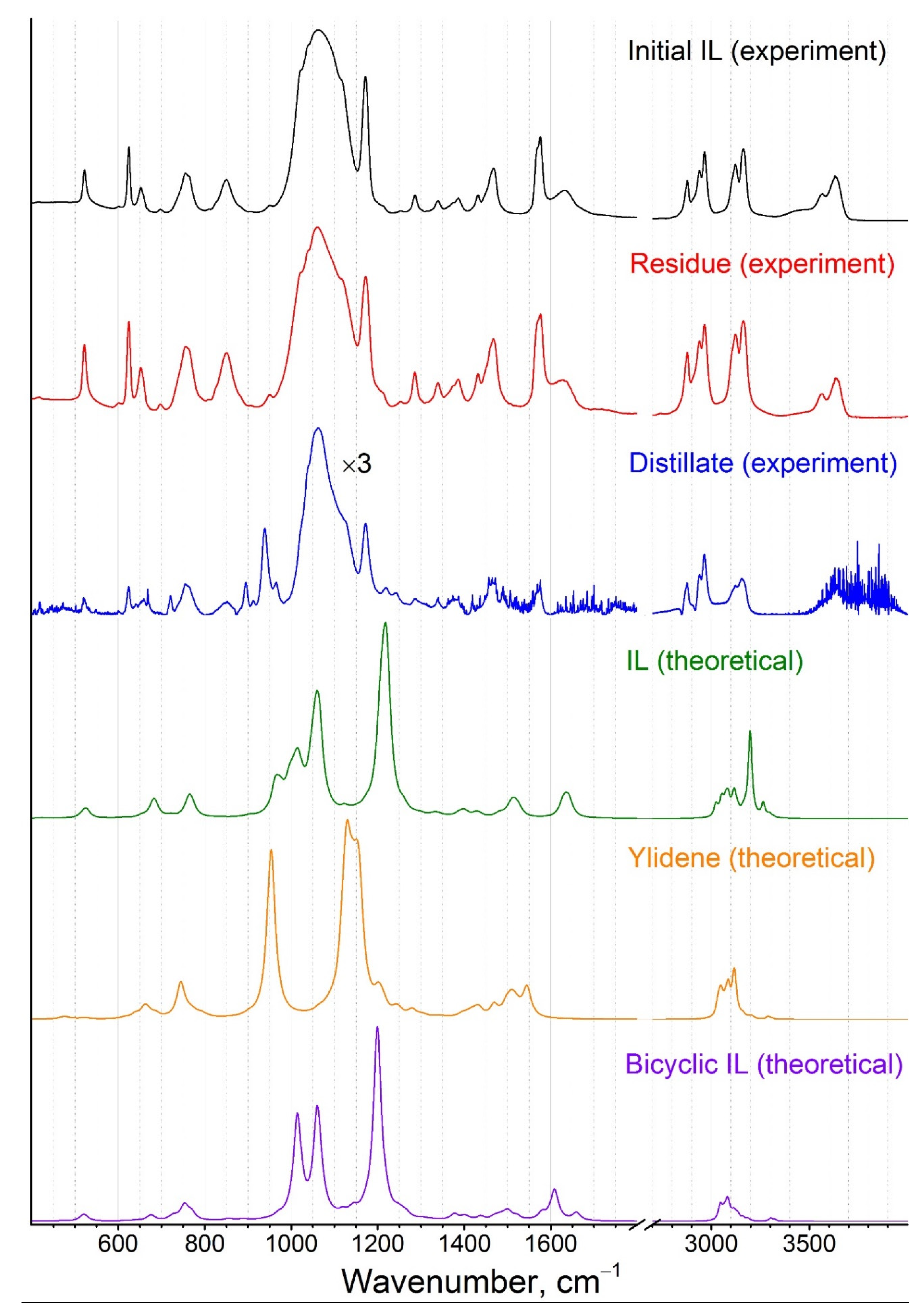
4.4. IR-Spectroscopy
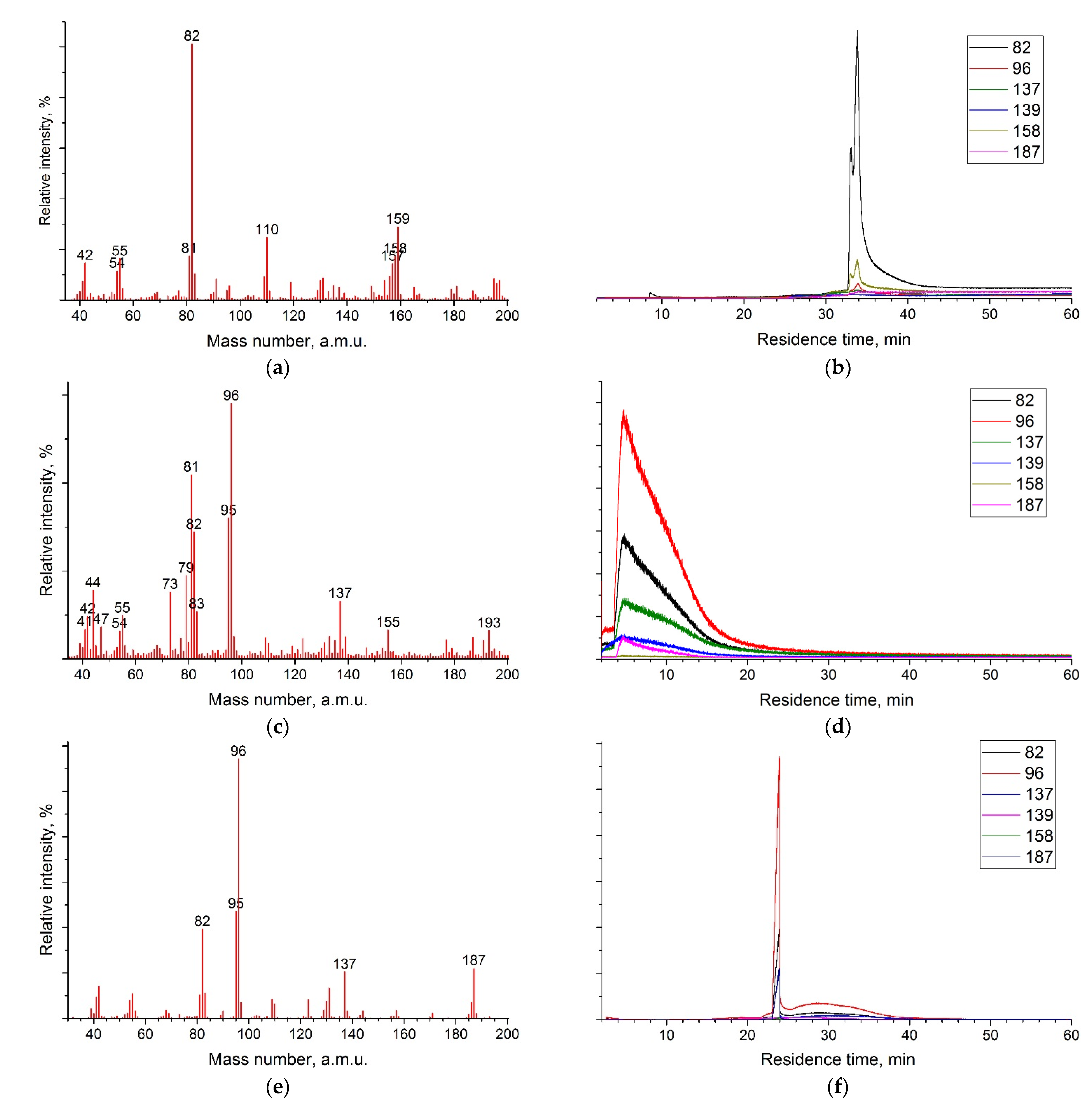
4.5. GCMS
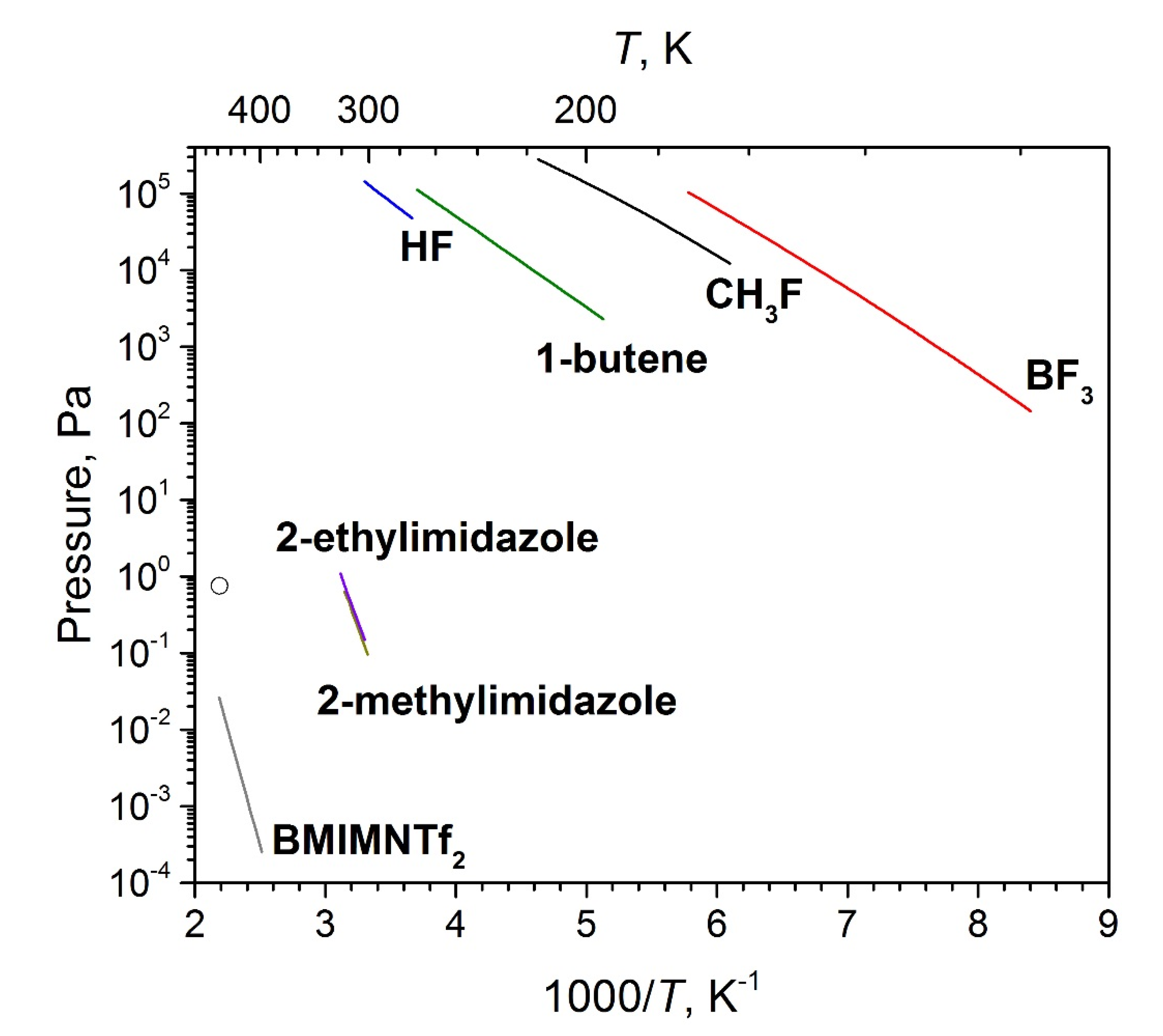
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wasserscheid, P.; Welton, T. (Eds.) Ionic Liquids in Synthesis; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2002; p. 355. [Google Scholar]
- Earle, M.J.; Esperança, J.M.S.S.; Gilea, M.A.; Lopes, J.N.C.; Rebelo, L.P.N.; Magee, J.W.; Seddon, K.R.; Widegren, J.A. The distillation and volatility of ionic liquids. Nature 2006, 439, 831–834. [Google Scholar] [CrossRef]
- Armstrong, J.P.; Hurst, C.; Jones, R.G.; Licence, P.; Lovelock, K.R.J.; Satterley, C.J.; Villar-Garcia, I.J. Vapourisation of ionic liquids. Phys. Chem. Chem. Phys. 2007, 9, 982. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.P.; Esperança, J.M.S.S.; da Piedade, M.E.M.; Lopes, J.N.C.; Rebelo, L.P.N.; Seddon, K.R. The nature of ionic liquids in the gas phase. J. Phys. Chem. A 2007, 111, 6176–6182. [Google Scholar] [CrossRef] [PubMed]
- Zaitsau, D.H.; Kabo, G.J.; Strechan, A.A.; Paulechka, Y.U.; Tschersich, A.; Verevkin, S.P.; Heintz, A. Experimental vapor pressures of 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imides and a correlation scheme for estimation of vaporization enthalpies of ionic liquids. J. Phys. Chem. A 2006, 110, 7303. [Google Scholar] [CrossRef] [PubMed]
- Chilingarov, N.S.; Medvedev, A.A.; Deyko, G.S.; Kustov, L.M.; Chernikova, E.A.; Glukhov, L.M.; Markov, V.Y.; Ioffe, I.N.; Senyavin, V.M.; Polyakova, M.V.; et al. Mass spectrometric studies of 1-ethyl-3-methylimidazolium and 1-propyl-2,3-dimethylimidazolium bis(trifluoromethyl)-sulfonylimides. Rapid Commun. Mass Spectrom. 2015, 29, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Luo, H.; Li, H.; Dai, S. Direct UV-spectroscopic measurement of selected ionic-liquid vapors. Phys. Chem. Chem. Phys. 2010, 12, 7246–7250. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, B.; Ciccioli, A.; Gigli, G.; Lapi, A.; Misceo, N.; Tanzi, L.; Ciprioti, S.V. Vaporization of the prototypical ionic liquid BMImNTf2 under equilibrium conditions: A multitechnique study. Phys. Chem. Chem. Phys. 2014, 16, 15653–15661. [Google Scholar] [CrossRef] [PubMed]
- Dunaev, A.M.; Motalov, V.B.; Kudin, L.S.; Sergeev, D.N.; Akopyan, A.V. Decomposition temperatures, IR and mass spectra of some chiral ionic liquids based on alkylimidazolium. Russ. Chem. J. 2017, 61, 35–41. [Google Scholar]
- Clarke, C.J.; Puttick, S.; Sanderson, T.J.; Taylor, A.W.; Bourne, R.A.; Lovelock, K.R.J.; Licence, P. Thermal stability of dialkylimidazolium tetrafluoroborate and hexafluorophosphate ionic liquids: Ex situ bulk heating to complement in situ mass spectrometry. Phys. Chem. Chem. Phys. 2018, 20, 16786–16800. [Google Scholar] [CrossRef]
- Chambreau, S.D.; Schenk, A.C.; Sheppard, A.J.; Yandek, G.R.; Vaghjiani, G.L.; Maciejewski, J.; Koh, C.J.; Golan, A.; Leone, S.R. Thermal Decomposition Mechanisms of Alkylimidazolium Ionic Liquids with Cyano-Functionalized Anions. J. Phys. Chem. A 2014, 118, 11119–11132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volpe, V.; Brunetti, B.; Gigli, G.; Lapi, A.; Ciprioti, S.V.; Ciccioli, A. Toward the Elucidation of the Competing Role of Evaporation and Thermal Decomposition in Ionic Liquids: A Multitechnique Study of the Vaporization Behavior of 1-Butyl-3-methylimidazolium Hexafluorophosphate under Effusion Conditions. J. Phys. Chem. B 2017, 121, 10382–10393. [Google Scholar] [CrossRef] [PubMed]
- Dunaev, A.M.; Motalov, V.B.; Kudin, L.S.; Butman, M.F. Molecular and Ionic Composition of Saturated Vapor over EMImNTf2 Ionic Liquid. J. Mol. Liq. 2016, 219, 599–601. [Google Scholar] [CrossRef]
- Deyko, A.; Lovelock, K.R.J.; Licence, P.; Jones, R.G. The vapour of imidazolium-based ionic liquids: A mass spectrometry study. Phys. Chem. Chem. Phys. 2011, 13, 16841–16850. [Google Scholar] [CrossRef] [PubMed]
- Meine, N.; Benedito, F.; Rinaldi, R. Thermal stability of ionic liquids assessed by potentiometric titration. Green Chem. 2010, 12, 1711–1714. [Google Scholar] [CrossRef]
- Swiderski, K.; McLean, A.; Gordon, C.M.; Vaughana, D.H. Estimates of internal energies of vaporisation of some room temperature ionic liquids. Chem. Commun. 2004, 19, 2178–2179. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Yermalayeu, A.V.; Schubert, T.J.S.; Verevkin, S.P. Alkyl-imidazolium tetrafluoroborates: Vapor pressure, thermodynamics of vaporization, and enthalpies of formation. Mol. Liq. 2017, 242, 951–957. [Google Scholar] [CrossRef]
- Liang, R.; Yang, M.; Zhou, Q. Thermal Stability, Equilibrium Vapor Pressure and Standard Enthalpy of Vaporization of 1-Butyl,3-methylimidazoliumTetrafluoroborate. Acta Phys.-Chim. Sin. 2010, 26, 1468–1472. [Google Scholar] [CrossRef]
- Krannich, M.; Heym, F.; Jess, A. Characterization of Six Hygroscopic Ionic Liquids with Regard to Their Suitability for Gas Dehydration: Density, Viscosity, Thermal and Oxidative Stability, Vapor Pressure, Diffusion Coefficient, and Activity Coefficient of Water. J. Chem. Eng. Data 2016, 61, 1162–1176. [Google Scholar] [CrossRef]
- Deyko, A.; Lovelock, K.R.J.; Corfield, J.-A.; Taylor, A.W.; Gooden, P.N.; Villar-Garcia, I.J.; Licence, P.; Jones, R.G.; Krasovskiy, V.G.; Chernikova, E.A.; et al. Measuring and predicting ΔvapH298 values of ionic liquids. Phys. Chem. Chem. Phys. 2009, 11, 8544–8555. [Google Scholar] [CrossRef]
- Lovelock, K.R.J.; Deyko, A.; Licence, P.; Jones, R.G. Vaporisation of an ionic liquid near room temperature. Phys. Chem. Chem. Phys. 2010, 12, 8893–8901. [Google Scholar] [CrossRef] [PubMed]
- Deyko, A.; Hessey, S.G.; Licence, P.; Chernikova, E.A.; Krasovskiy, V.G.; Kustov, L.M.; Jones, R.G. The enthalpies of vaporisation of ionic liquids: New measurements and predictions. Phys. Chem. Chem. Phys. 2012, 14, 3181–3193. [Google Scholar] [CrossRef]
- Fredlake, C.P.; Crosthwaite, J.M.; Hert, D.G.; Aki, S.N.V.K.; Brennecke, J.F. Thermophysical Properties of Imidazolium-Based Ionic Liquids. J. Chem. Eng. Data 2004, 49, 954–964. [Google Scholar] [CrossRef]
- Ngo, H.L.; LeCompte, K.; Hargens, L.; McEwen, A.B. Thermal properties of imidazolium ionic liquids. Thermochim. Acta 2000, 357, 97–102. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Eapen, T.; Deepthi, T.; Kunduchi, P.V.; Benny, K.G. Mechanistic outlook on thermal degradation of 1,3-dialkyl imidazolium ionic liquids and organoclays. RSC Adv. 2016, 6, 9421–9428. [Google Scholar] [CrossRef]
- Ohtani, H.; Ishimura, S.; Kumai, M. Thermal decomposition behaviors of imidazolium-type ionic liquids studied by pyrolysis-gas chromatography. Anal. Sci. 2008, 24, 1335–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arduengo, A.J.; Harlow, R.L.; Kline, M. A stable crystalline carbine. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
- Taylor, A.W.; Lovelock, K.R.J.; Jones, R.G.; Licence, P. Borane-substituted imidazol-2-ylidenes: Syntheses in vacuo. Dalton Trans. 2011, 40, 1463–1470. [Google Scholar] [CrossRef]
- Kan, H.; Tsengy, M.; Chu, Y. Bicyclic imidazolium-based ionic liquids: Synthesis and characterization. Tetrahedron 2007, 63, 1644–1653. [Google Scholar] [CrossRef]
- Knorr, M.; Icker, M.; Efimova, A.; Schmidt, P. Reactivity of Ionic Liquids: Studies on Thermal Decomposition Behavior of 1-Butyl-3-methylimidazolium Tetrafluoroborate. Thermochim. Acta 2020, 694, 178786. [Google Scholar] [CrossRef]
- Chaudhary, G.R.; Bansal, S.; Mehta, S.K.; Ahluwalia, A.S. Thermophysical and Spectroscopic Studies of Pure 1-Butyl-3-methylimidazolium Tetrafluoroborate and Its Aqueous Mixtures. J. Solut. Chem. 2014, 43, 340–359. [Google Scholar] [CrossRef]
- Cha, S.; Ao, M.; Sung, W.; Moon, B.; Ahlström, B.; Johansson, P.; Ouchi, Y.; Kim, D. Structures of ionic liquid-water mixtures investigated by IR and NMR spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 9591–9601. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Wang, N.-N.; Luo, J.-J.; Zhou, Y.; Yu, Z.-W. Hydrogen-bonding interactions between [BMIM][BF4] and acetonitrile. Phys. Chem. Chem. Phys. 2013, 15, 18055–18064. [Google Scholar] [CrossRef] [PubMed]
- Dharaskar, S.A.; Wasewar, K.L.; Varma, M.N.; Shende, D.Z.; Yoo, C. Synthesis, characterization and application of 1-butyl-3-methylimidazolium tetrafluoroborate for extractive desulfurization of liquid fuel. Arab. J. Chem. 2016, 9, 578–587. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Hu, Y.; Chen, G.; Wang, Z.; Jin, X. Rapid proton diffusion in hydroxyl functionalized imidazolium ionic liquids. Sci. China Ser. B Chem. 2017, 60, 734–739. [Google Scholar] [CrossRef]
- Dunaev, A.M.; Motalov, V.B.; Kudin, L.S. A High-Temperature Mass-Spectrometric Method for Determination of the Electron Work Function of Ionic Crystals: Lanthanum, Cerium, and Praseodymium Triiodides. Russ. J. Gen. Chem. 2017, 87, 632–638. [Google Scholar] [CrossRef]
- Dunaev, A.M.; Kryuchkov, A.S.; Kudin, L.S.; Butman, M.F. Automatic complex for high temperature investigation on basis of mass spectrometer MI1201. Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. 2011, 54, 73–77. (In Russian) [Google Scholar]
- Sergeev, D.N.; Dunaev, A.M.; Ivanov, D.A.; Golovkina, Y.A.; Gusev, G.I. Automatization of mass spectrometer for the obtaining of ionization efficiency functions. Prib. Tekhnika Eksperimenta 2014, 1, 139–140. (In Russian) [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parameterization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Dunning, J. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- van Valkenburg, M.E.; Vaughn, R.L.; Williams, M.; Wilkes, J.S. Thermochemistry of ionic liquid heat-transfer fluids. Thermochim. Acta 2005, 425, 181–188. [Google Scholar] [CrossRef]
- Schreiner, C. Synthese und Charakterisierung neuer Ionischer Flüssigkeiten auf der Basis gemischter Fluoroborat-Anionen. Ph.D. Thesis, University of Regensburg, Regensburg, Germany, 2009. [Google Scholar]
- Holbrey, J.D.; Seddon, K.R. The phase behaviour of 1-alkyl-3-methylimidazolium tetrafluoroborates; ionic liquids and ionic liquid crystals. Chem. Soc. Dalton Trans. 1999, 2133–2140. [Google Scholar] [CrossRef]
- Erdmenger, T.; Vitz, J.; Wiesbrock, F.; Schubert, U.S. Influence of different branched alkyl side chains on the properties of imidazolium-based ionic liquids. J. Mater. Chem. 2008, 18, 5267–5273. [Google Scholar] [CrossRef]
- Hog, M.; Schneider, M.; Studer, G.; Bäuerle, M.; Föhrenbacher, S.A.; Scherer, H.; Krossing, I. An Investigation of the Symmetric and Asymmetric Cleavage Products in the System Aluminum Trihalide/1-Butylimidazole. Chem. A Eur. J. 2017, 23, 11054–11066. [Google Scholar] [CrossRef] [PubMed]
- Pachler, K.G.R.; Pachter, R.; Wessels, P.L. Carbon-13proton coupling constants in N-substituted imidazoles. A 13C NMR study and MO calculations. Magn. Reson. Chem. 1981, 17, 278–284. [Google Scholar] [CrossRef]
- Kimura, K.; Katsumata, S.; Achiba, Y.; Yamazaki, T.; Iwata, S. Ionization energies, Ab initio assignments, and valence electronic structure for 200 molecules. In Handbook of HeI Photoelectron Spectra of Fundamental Organic Compounds; Japan Scientific Societies Press: Tokyo, Japan, 1981; p. 268. [Google Scholar]
- Strasser, D.; Goulay, F.; Kelkar, M.S.; Maginn, E.J.; Leone, S.R. Photoelectron Spectrum of Isolated Ion-Pairs in Ionic Liquid Vapor. J. Phys. Chem. A 2007, 111, 3191–3195. [Google Scholar] [CrossRef]
- Farber, M.; Srivastava, R.D.; Moyer, J.W. Mass spectrometric determination of the thermodynamics of potassium hydroxide and minor potassium-containing species required in magnetohydrodynamic power systems. J. Chem. Thermodyn. 1982, 14, 1103–1113. [Google Scholar] [CrossRef]
- Dibeler, V.H.; Liston, S.K. Mass-spectrometric study of photoionization. XII. Boron trifluoride and diboron tetrafluoride. Inorg. Chem. 1968, 7, 1742–1746. [Google Scholar] [CrossRef]
- Pogrebnoi, A.M.; Kudin, L.S.; Motalov, V.B.; Goryushkin, V.F. Vapor species over cerium and samarium trichlorides, enthalpies of formation of (LnCl3)n molecules and Cl−(LnCl3)n ions. Rapid Commun. Mass Spectrom. 2001, 15, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- Acree, W.E., Jr.; Chickos, J.S. NIST Chemistry WebBook, NIST Standard Reference Database Number 69; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1998; p. 20899. [Google Scholar] [CrossRef]
- Stull, D.R. Vapor Pressure of Pure Substances. Organic and Inorganic Compounds. Ind. Eng. Chem. 1947, 39, 517–540. [Google Scholar] [CrossRef]
- Sheft, I.; Perkins, A.J.; Hyman, H.H. Anhydrous Hydrogen Fluoride: Vapor Pressure and Liquid Density. J. Inorg. Nucl. Chem. 1973, 35, 3677–3680. [Google Scholar] [CrossRef]
- Michels, A.; Wassenaar, T. Vapour pressure of methylfluoride. Physica 1948, 14, 104–110. [Google Scholar] [CrossRef]
- Coffin, C.C.; Maass, O. The Preparation and Physical Properties of α-,β- and γ-Butylene and Normal and Isobutane. J. Am. Chem. Soc. 1928, 50, 1427–1437. [Google Scholar] [CrossRef]
- Jiménez, P.; Roux, M.V.; Turrión, C. Thermochemical properties of N-heterocyclic compounds IV. Enthalpies of combustion, vapour pressures and enthalpies of sublimation, and enthalpies of formation of 2-methylimidazole and 2-ethylimidazole. J. Chem. Thermodyn. 1992, 24, 1145–1149. [Google Scholar] [CrossRef]




| m/z | AE, eV | a | Assigned Ion |
|---|---|---|---|
| 49 | 16.9 ± 0.5 | 12.970 ± 0.274 | BF2+ |
| 82 | 13.0 ± 0.5 | 17.486 ± 0.364 | MIm+ |
| 96 | 11.3 ± 0.5 | 17.365 ± 0.442 | MMIm+ |
| 137 | 11.0 ± 0.5 | 17.327 ± 0.455 | C8H13N2+ |
| 139 | 12.4 ± 0.5 | 14.249 ± 0.390 | BMIm+ |
| 158 | 11.7 ± 0.5 | 13.445 ± 0.363 | BMImF+ |
| 187 | 13.8 ± 0.5 | 17.968 ± 0.510 | BMImBF2+ |
| T, K | m/z | ||||||
|---|---|---|---|---|---|---|---|
| 82 | 96 | 137 | 139 | 158 | 187 | ||
| Effusion cell | 487 | 250 | 455 | 95 | 100 | 18 | 68 |
| Open cell | 470 | 157 | 284 | 63 | 100 | ** | - |
| Open surface | 471 | 52 | 52 | 21 | 100 | ** | - |
| Open surface * | 501 | 44 | 23 | 15 | 100 | 13 | - |
| Compound | T, K | m/z | ||||
|---|---|---|---|---|---|---|
| 82 | 96 | 137 | 187 | |||
| GCMS (nonpolar column) | BMImBF4 | 523 | 34 | 100 | 18 | 19 |
| KEMS (effusion cell) | BMImBF4 | 487 | 55 | 100 | 21 | 15 |
| DIMS [10] | Imidazole-2-ylidene | 323 | 28 | 100 | 27 | 64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunaev, A.M.; Motalov, V.B.; Kudin, L.S. The Composition of Saturated Vapor over 1-Butyl-3-methylimidazolium Tetrafluoroborate Ionic Liquid: A Multi-Technique Study of the Vaporization Process. Entropy 2021, 23, 1478. https://doi.org/10.3390/e23111478
Dunaev AM, Motalov VB, Kudin LS. The Composition of Saturated Vapor over 1-Butyl-3-methylimidazolium Tetrafluoroborate Ionic Liquid: A Multi-Technique Study of the Vaporization Process. Entropy. 2021; 23(11):1478. https://doi.org/10.3390/e23111478
Chicago/Turabian StyleDunaev, Anatoliy M., Vladimir B. Motalov, and Lev S. Kudin. 2021. "The Composition of Saturated Vapor over 1-Butyl-3-methylimidazolium Tetrafluoroborate Ionic Liquid: A Multi-Technique Study of the Vaporization Process" Entropy 23, no. 11: 1478. https://doi.org/10.3390/e23111478
APA StyleDunaev, A. M., Motalov, V. B., & Kudin, L. S. (2021). The Composition of Saturated Vapor over 1-Butyl-3-methylimidazolium Tetrafluoroborate Ionic Liquid: A Multi-Technique Study of the Vaporization Process. Entropy, 23(11), 1478. https://doi.org/10.3390/e23111478






