An Extended Entropic Model for Cohesive Sediment Flocculation in a Piecewise Varied Shear Environment
Abstract
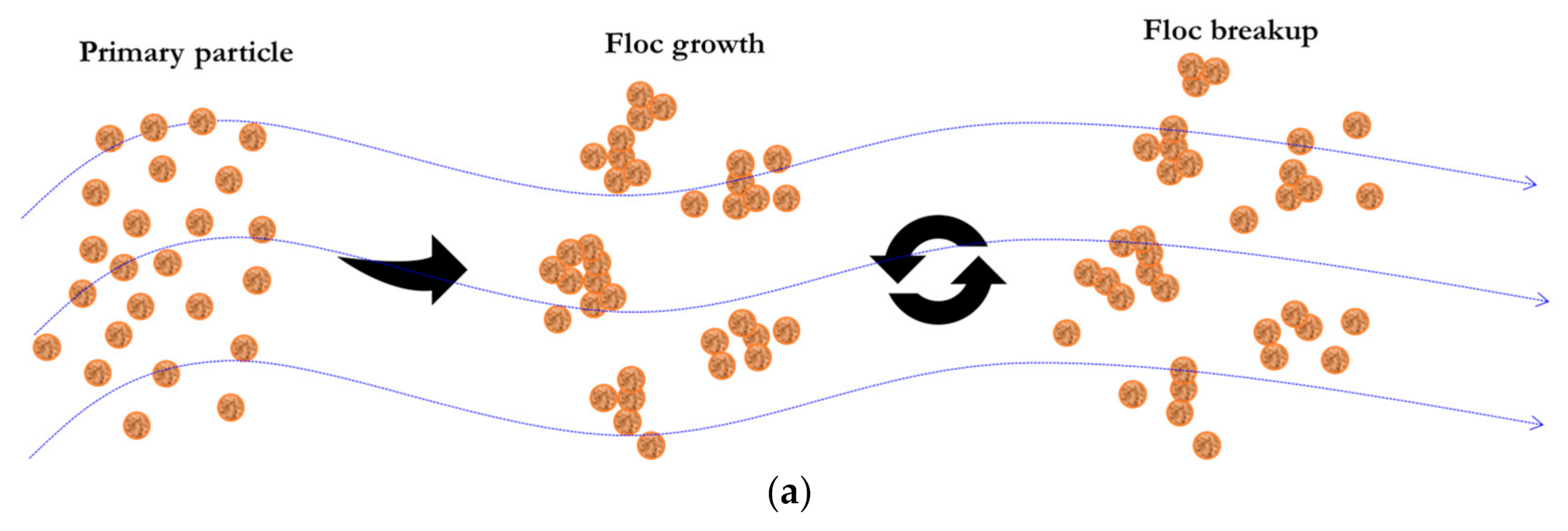
:1. Introduction
2. Multi-Step Entropic Model for Sediment Flocculation
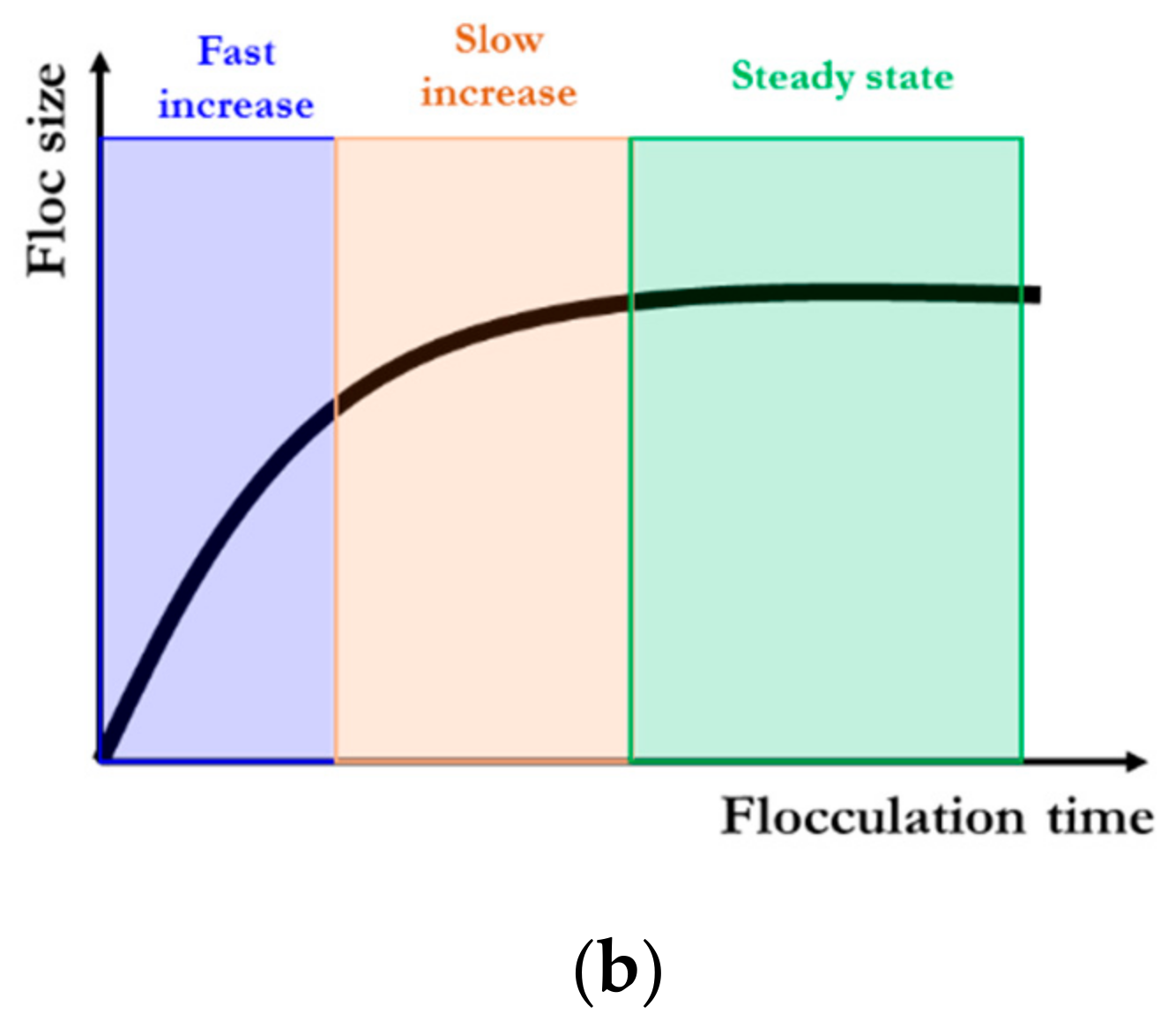
2.1. Flocculation Model for a Constant Flow Shear Environment
2.1.1. Floc Size Growth toward the Steady State at a Constant Shear Rate
2.1.2. Floc Size Decay toward Another Steady State at a Higher Constant Shear Rate
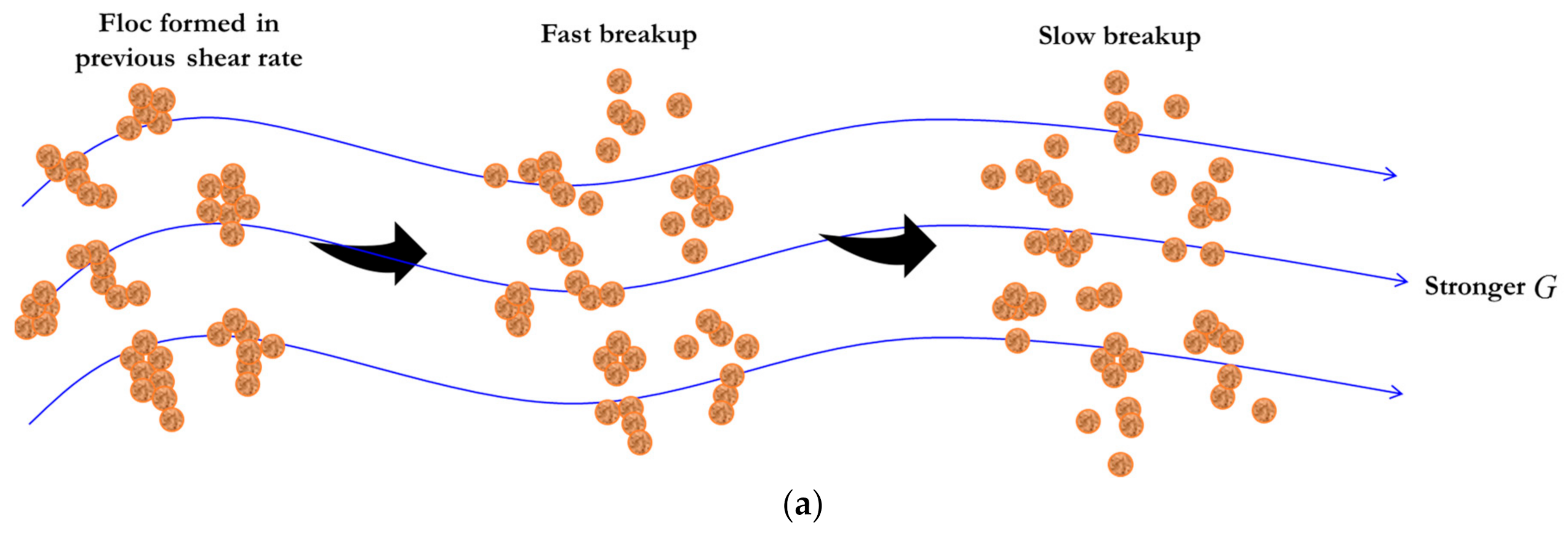
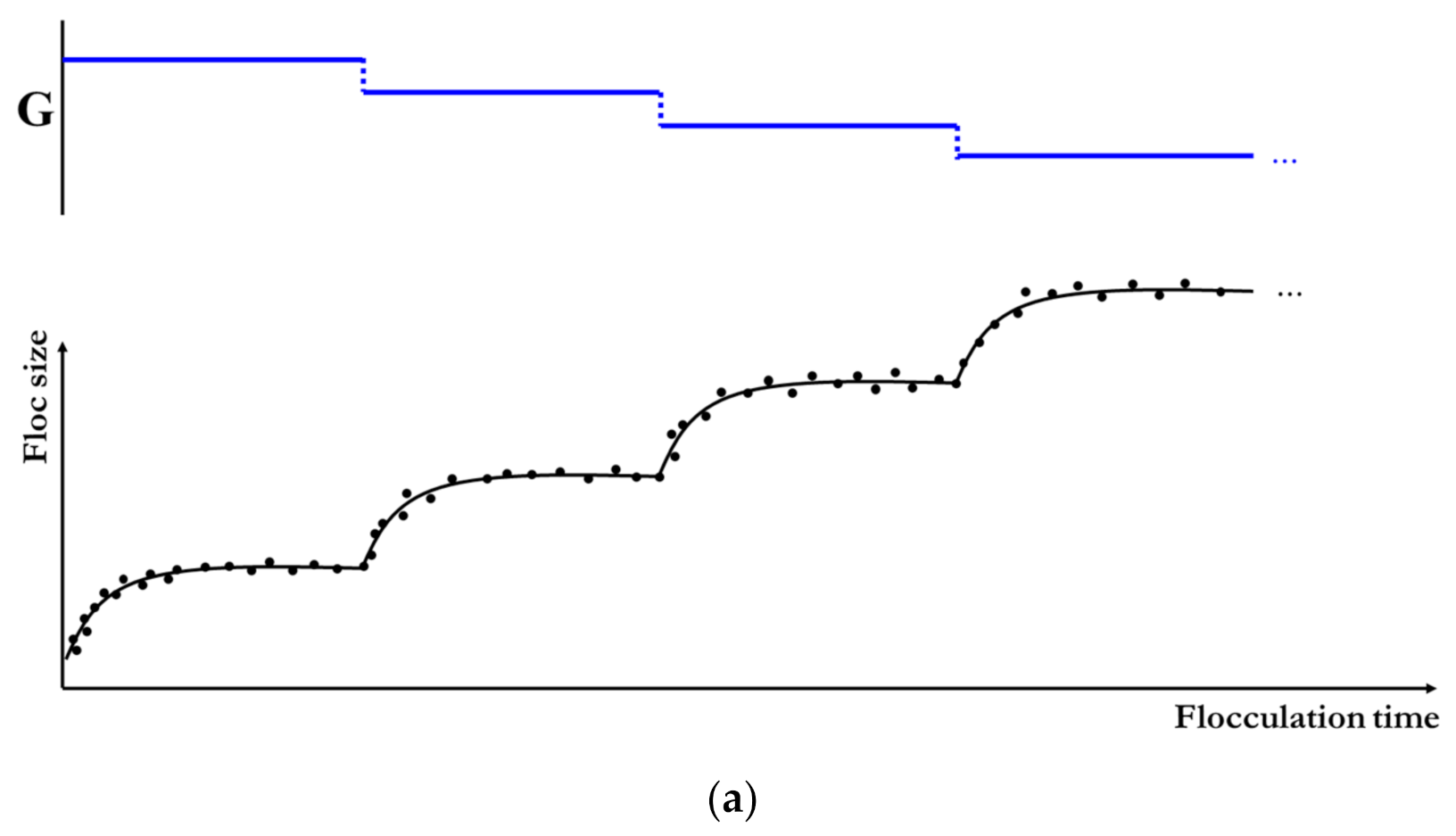
2.2. Flocculation Model for a Piecewise Varied Shear
3. Test with Experimental Data
3.1. Performance Evaluation of the Model
- (1)
- Correlation coefficient R2 between the observed data points and the modeled data points;
- (2)
- The average relative error (RE) between the observed data points and the modeled data, which is calculated as follows: , where and are the observed data and the modeled data, and is the total number of data points;
- (3)
- The root mean square error (RMSE) between the observed data points and the modeled data points: RMSE=.
3.2. Comparison with Experimental Data Sets in the Literature
3.2.1. Cohesive Sediment Field
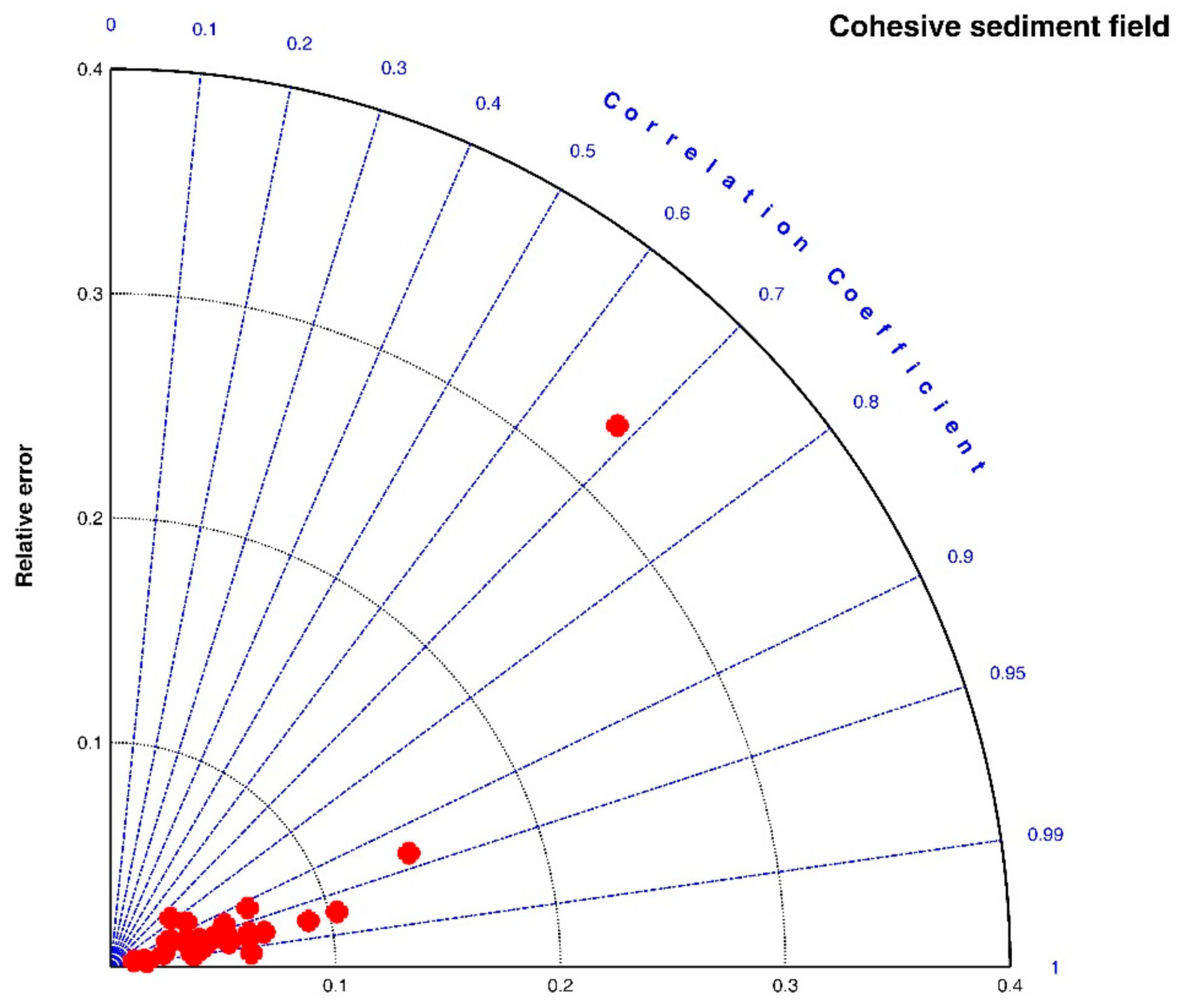
| References | Flocculation Experiment Condition | Fitting Effect | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Material | Flocculation Environment | Flocculation Condition | Flocculation Period | L0 | Ls | M | R2 | RE | RMSE | |
| Tsai et al. (1987) [77] | Sediment from the Detroit River | Couette Viscometer | 100 mg/L Sediment concentration | G = 100/s | 8.89 | 118.75 | 3000 | 0.9754 | 0.0928 | 5.8169 |
| G = 200/s | 102.75 | 75.62; | 40 | 0.9177 | 0.0295 | 3.0310 | ||||
| G = 200/s | 7.06 | 88.43 | 2000 | 0.9341 | 0.1419 | 8.6894 | ||||
| G = 400/s | 78.69 | 53.40 | 120 | 0.8586 | 0.0390 | 3.0530 | ||||
| G = 400/s | 7.84 | 49.48 | 780 | 0.9796 | 0.0537 | 2.2237 | ||||
| G = 100/s | 46.27 | 112.11 | 650 | 0.9544 | 0.0413 | 4.5201 | ||||
| 400 mg/L Sediment concentration | G = 100/s | 7.11 | 60.54 | 560 | 0.9953 | 0.0629 | 2.1620 | |||
| G = 200/s | 60.01 | 41.67 | 130 | 0.9799 | 0.0152 | 0.8524 | ||||
| G = 200/s | 6.83 | 41.15 | 520 | 0.9890 | 0.0380 | 1.2036 | ||||
| G = 400/s | 38.45 | 18.47 | 200 | 0.9606 | 0.0476 | 1.7866 | ||||
| G = 400/s | 7.69 | 22.19 | 210 | 0.9182 | 0.0663 | 1.2766 | ||||
| G = 100/s | 20.85 | 58.14 | 240 | 0.9607 | 0.0473 | 2.6358 | ||||
| Burban et al. (1989) [50] | Natural bottom sediments from the Detroit River | Horizontal Couette type flocculator | Sediments in fresh water at a concentration of 400 mg/L. | G = 100/s | 5 | 53.37 | 1350 | 0.9715 | 0.1036 | 3.2498 |
| G = 200/s | 51.52 | 40.85 | 75 | 0.9665 | 0.0106 | 0.6365 | ||||
| G = 200/s | 5.46 | 38.95 | 250 | 0.6827 | 0.3300 | 8.2543 | ||||
| G = 400/s | 38.68 | 18.56 | 200 | 0.9815 | 0.0394 | 1.0488 | ||||
| G = 400/s | 5 | 22.60 | 300 | 0.9735 | 0.0903 | 1.3645 | ||||
| G = 100/s | 22.44 | 56 | 500 | 0.9894 | 0.0163 | 1.0980 | ||||
| Sediments in seawater at a concentration of 400 mg/L. | G = 100/s | 5 | 40.25 | 300 | 0.9768 | 0.0428 | 1.7719 | |||
| G = 200/s | 40.00 | 31.86 | 75 | 0.7741 | 0.0343 | 1.4053 | ||||
| G = 200/s | 5 | 31.39 | 250 | 0.9880 | 0.0367 | 1.0293 | ||||
| G = 400/s | 30.83 | 20.19 | 50 | 0.9111 | 0.0276 | 0.8020 | ||||
| G = 400/s | 5 | 18.09 | 100 | 0.9781 | 0.0380 | 0.6666 | ||||
| G = 100/s | 18.89 | 40.12 | 280 | 0.9698 | 0.0242 | 1.1663 | ||||
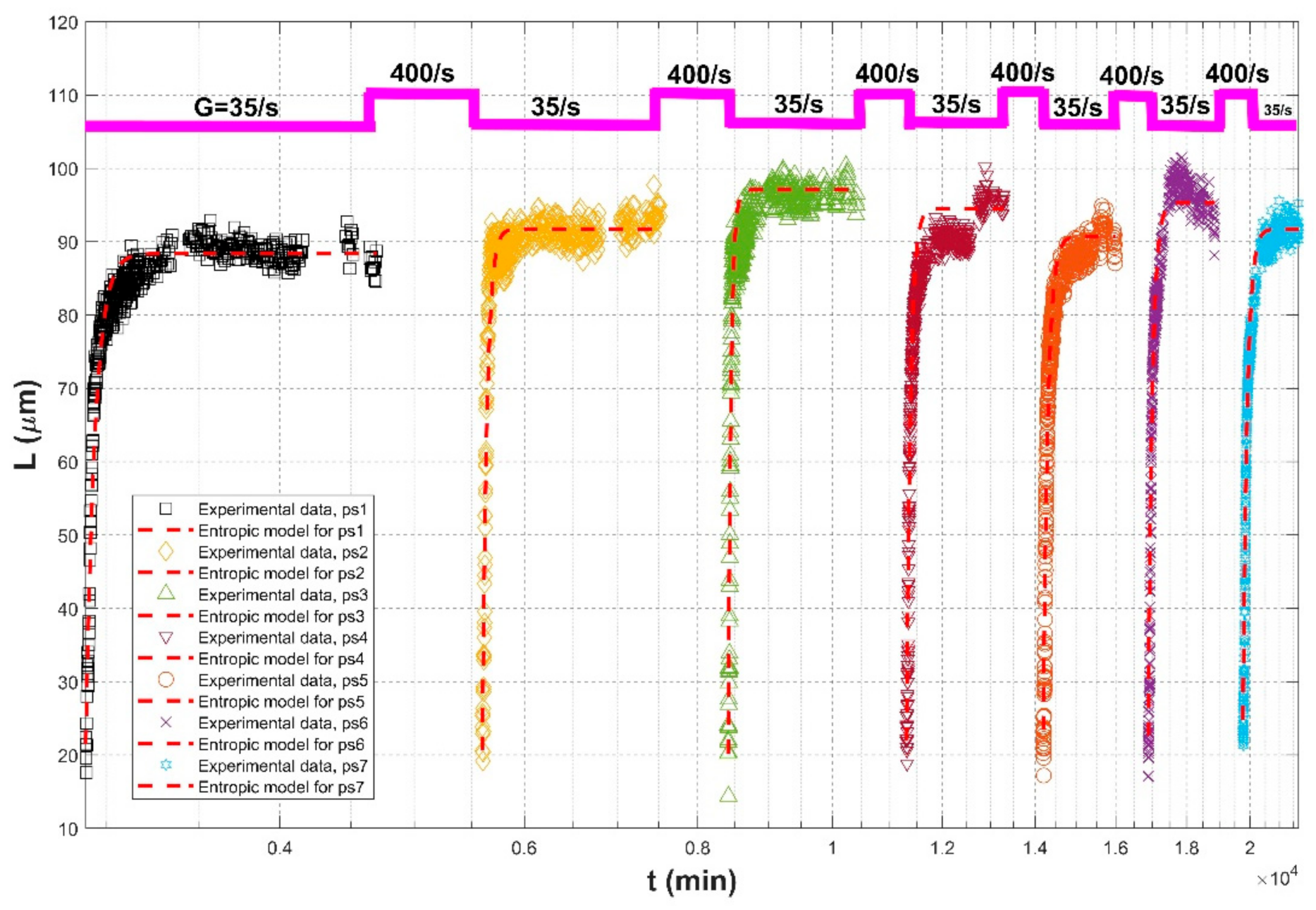
| Keyvani and Strom (2014) [28] | 80% kaolinite and 20% montmorillonite | Mixing chamber with a rotating paddle | G = 35/s for floc growth followed by G = 400/s for 15 h breakup, repeated 7 times. | G = 35/s, ps1 | 21.45 | 88.38 | 2800 | 0.9459 | 0.0349 | 3.1614 |
| G = 35/s, ps2 | 20.60 | 91.74 | 3000 | 0.9401 | 0.0361 | 3.4063 | ||||
| G = 35/s, ps3 | 20.20 | 97.07 | 3000 | 0.9361 | 0.0540 | 4.9519 | ||||
| G = 35/s, ps4 | 21.92 | 94.47 | 5500 | 0.9708 | 0.0633 | 4.8604 | ||||
| G = 35/s, ps5 | 23.40 | 90.69 | 7500 | 0.9728 | 0.0545 | 3.6739 | ||||
| G = 35/s, ps6 | 22.58 | 95.35 | 8500 | 0.9752 | 0.0699 | 4.6352 | ||||
| G = 35/s, ps7 | 24.76 | 91.73 | 9500 | 0.9784 | 0.0626 | 4.1021 | ||||
| In average | 0.9453 | 0.0595 | 2.8560 | |||||||
3.2.2. Water Treatment Field
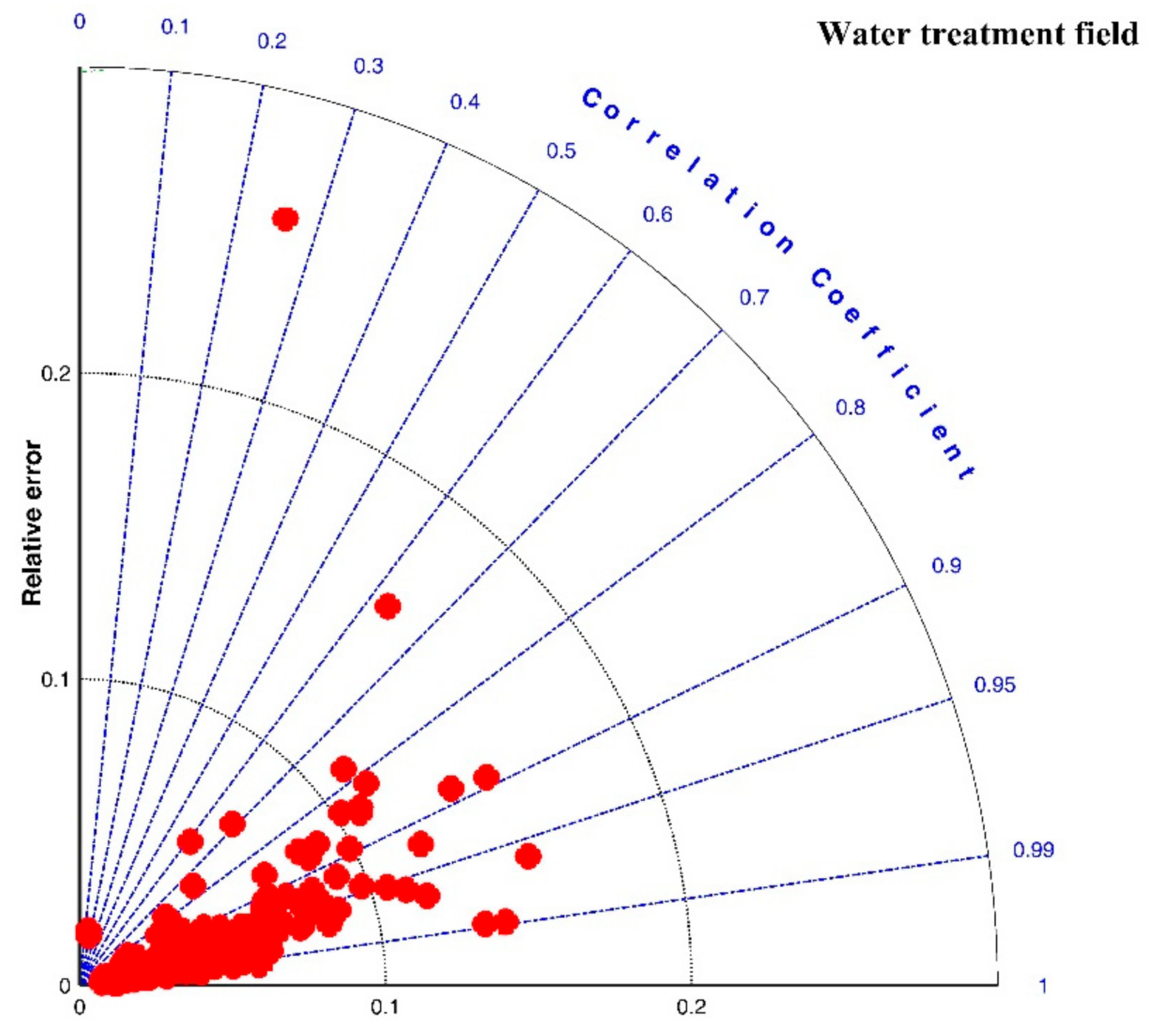
| References | Flocculation Experiment Condition | Fitting Effect | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Material | Flocculation Environment | Flocculation Condition | Flocculation Period | L0 | Ls | M | R2 | RE | RMSE | |
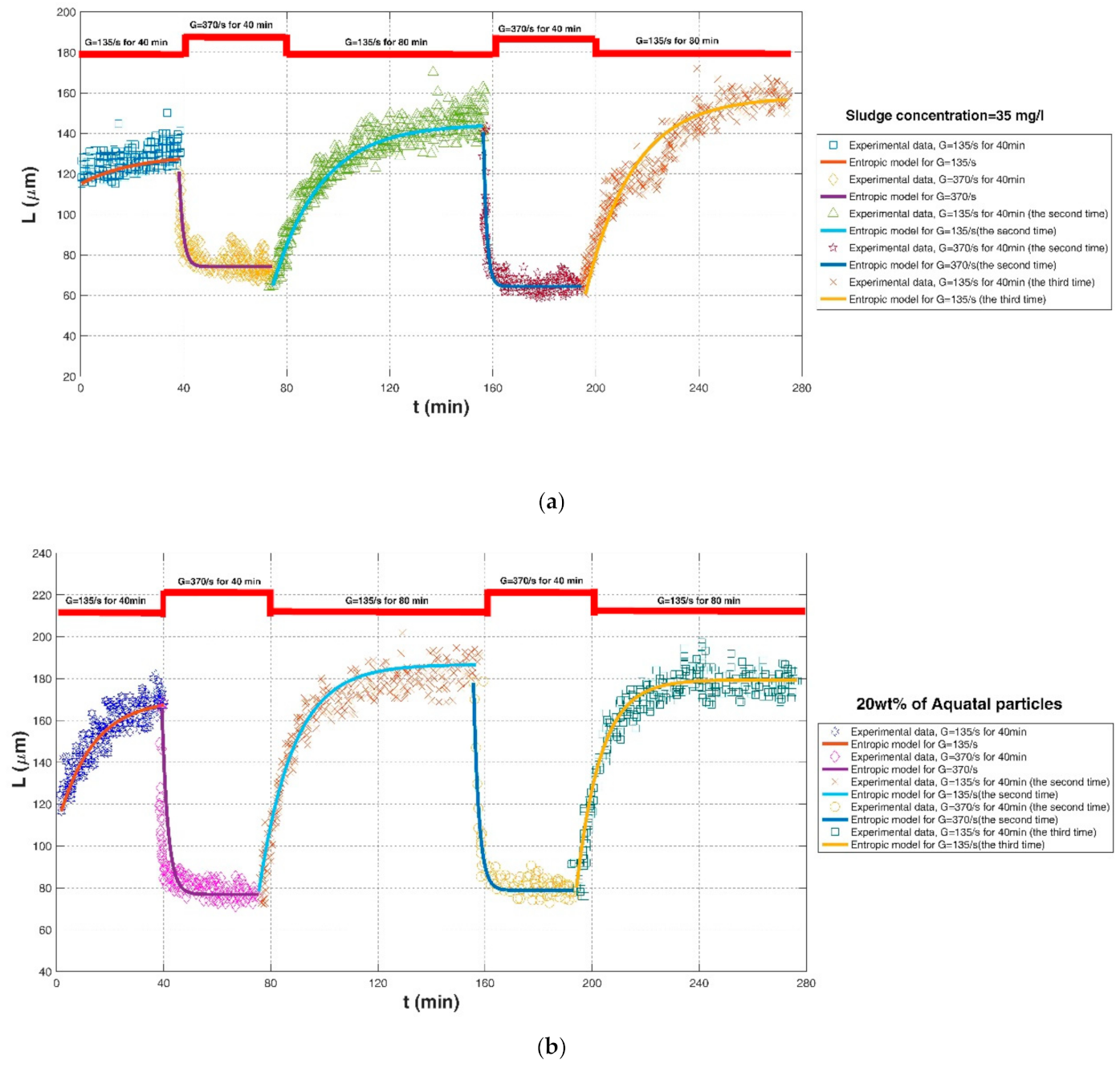
| Chaignon et al. (2002) [27] | Activated sludge | Baffled reactor with a stirring motor | Sludge concentration = 35 mg/L | G = 135/s, for 40 min | 115.30 | 129.64 | 300 | 0.2593 | 0.2593 | 6.5020 |
| G = 370/s, for 40 min breakup | 121.10 | 74.20 | 75 | 0.6102 | 0.0592 | 6.8263 | ||||
| G = 135/s, for 80 min | 64.67 | 144.69 | 1500 | 0.9286 | 0.0411 | 6.4584 | ||||
| G = 370/s, for 40 min breakup | 140.51 | 64.46 | 100 | 0.6875 | 0.0725 | 10.7549 | ||||
| G = 135/s, for 80 min | 60.58 | 158.16 | 1800 | 0.9215 | 0.0651 | 9.0372 | ||||
| Chaignon et al. (2002) [27] | Activated sludge | Baffled reactor with a stirring motor | Activated sludge spiked with 20 wt% of aquatic particles | G = 135/s, for 40 min | 116.56 | 170.20 | 700 | 0.8092 | 0.0361 | 0.0361 |
| G = 370/s, for 40 min breakup | 165.56 | 76.89 | 200 | 0.6312 | 0.1596 | 28.4818 | ||||
| G = 135/s, for 80 min | 78.15 | 186.81 | 1400 | 0.9514 | 0.0433 | 7.6736 | ||||
| G = 370/s, for 40 min breakup | 178.15 | 78.81 | 200 | 0.7514 | 0.0492 | 10.3384 | ||||
| G = 135/s, for 80 min | 80.13 | 179.47 | 900 | 0.9347 | 0.0407 | 7.5324 | ||||
| Biggs et al. (2003) [18] | Activated sludge | Baffled batch vessel with an impeller | E1 | G = 19.4/s | 38.29 | 133.63 | 1150 | 0.9819 | 0.0327 | 4.0838 |
| G = 113 s | 135.42 | 72.07 | 75 | 0.8831 | 0.0380 | 7.5874 | ||||
| G = 19.4/s | 72.14 | 119.85 | 300 | 0.9784 | 0.0185 | 2.5094 | ||||
| E2 | G = 19.4/s | 40.92 | 131.08 | 800 | 0.9852 | 0.0205 | 2.9983 | |||
| G = 113 s | 132.73 | 71.33 | 75 | 0.9946 | 0.0117 | 1.1121 | ||||
| G = 19.4/s | 71.69 | 121.37 | 300 | 0.9852 | 0.0155 | 2.2554 | ||||
| G = 113 s | 122.08 | 72.64 | 60 | 0.8453 | 0.0303 | 6.0052 | ||||
| G = 19.4/s | 73.59 | 117.47 | 250 | 0.9874 | 0.0119 | 1.6873 | ||||
| Gregory (2004) [19] | Kaolin | Modified jar test with different stirring rates | Alum coagulant | G = 11/s | 0.06 (dimensional, hereinafter) | 0.81 (dimensional, hereinafter) | 50 (s, hereinafter) | 0.9921 | 0.0381 | 0.0209 |
| G = 340/s, for 10 s breakup | -- | - | - | - | - | - | ||||
| G = 11/s | 0.39 | 0.65 | 20 | 0.9608 | 0.0153 | 0.0134 | ||||
| XL-1: degrees of neutralization (OH/Al) = 1.9, 5.1 wt.% Al | G = 11/s | 0.05 | 1.42 | 55 | 0.9888 | 0.1341 | 0.0693 | |||
| G = 340/s, for 10 s breakup | - | - | - | - | - | - | ||||
| G = 11/s | 0.61 | 0.99 | 30 | 0.9835 | 0.0133 | 0.0148 | ||||
| XL-9: degrees of neutralization (OH/Al) = 2.1, 4.6 wt.% Al | G = 11/s | 0.05 | 1.98 | 70 | 0.9891 | 0.1407 | 0.1113 | |||
| G = 340/s, for 10 s breakup | - | - | - | - | - | - | ||||
| G = 11/s | 0.83 | 1.31 | 30 | 0.9880 | 0.0113 | 0.0170 | ||||
| Gregory (2004) [19] | Kaolin | Modified jar test with different stirring rates | Alum coagulant | G = 23/s | 0.14 | 0.76 | 45 | 0.9807 | 0.0522 | 0.0297 |
| G = 520/s, for breakup | - | - | - | - | - | - | ||||
| G = 23/s | 0.14 | 0.40 | 15 | 0.9636 | 0.0359 | 0.0151 | ||||
| XL-9: degrees of neutralization (OH/Al) = 2.1, 4.6 wt.% Al | G = 23/s | 0.16 | 1.45 | 80 | 0.9713 | 0.0840 | 0.0833 | |||
| G = 520/s, for breakup | - | - | - | -- | - | - | ||||
| G = 23/s | 0.36 | 0.72 | 20 | 0.9720 | 0.0224 | 0.0175 | ||||
| polyDADMAC coagulant (a high-charge, low-molecular-weight cationic polyelectrolyte) | G = 23/s | 0.17 | 3.14 | 1200 | 0.9082 | 0.0669 | 0.1840 | |||
| G = 520/s, for breakup | - | -- | - | - | - | - | ||||
| G = 23/s | 0.65 | 2.74 | 450 | 0.9060 | 0.0448 | 0.1442 | ||||
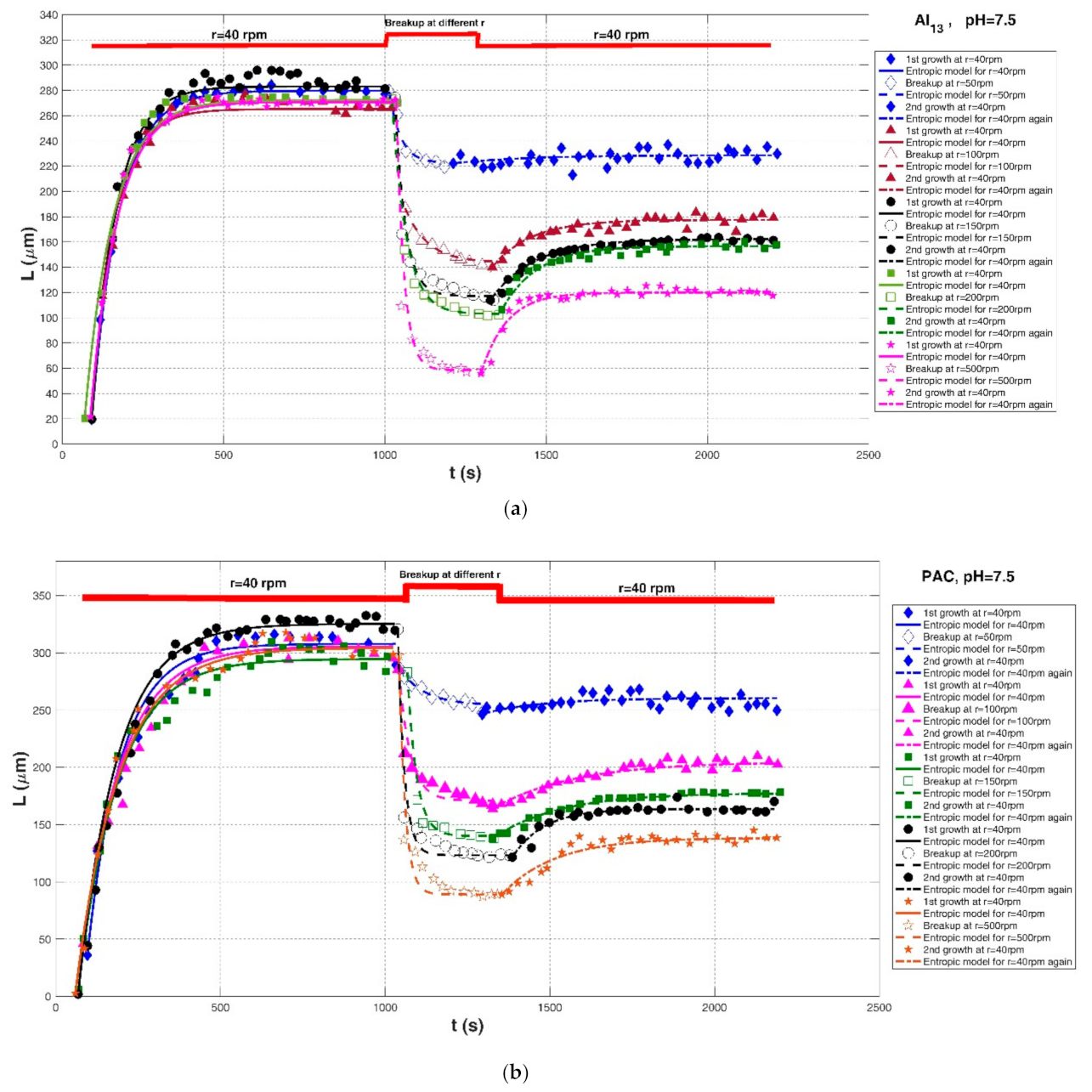
| Xu et al. (2010) [24] | Humic acid (HA). | Jar-test apparatus with a stirrer, Al13 polymer at different shear rate, pH = 7.5 | 50-rpm breakup after 40-rpm growth | r = 40 rpm | 19.63 | 279.74 | , hereinafter) | 0.9984 | 0.0116 | 3.1415 |
| r = 50 rpm | 279.27 | 222.55 | 2000 | 0.9576 | 0.0111 | 4.6007 | ||||
| r = 40 rpm | 222.28 | 228.76 | 1500 | 0.1546 | 0.0181 | 5.0816 | ||||
| 100-rpm breakup after 40-rpm growth | r = 40 rpm | 117.55 | 265.28 | 11,000 | 0.9810 | 0.0230 | 6.6168 | |||
| r = 100 rpm | 186.77 | 143.75 | 3000 | 0.9632 | 0.0165 | 3.1873 | ||||
| r = 40 rpm | 140.01 | 177.50 | 4500 | 0.8730 | 0.0167 | 3.7291 | ||||
| 150-rpm breakup after 40-rpm growth | r = 40 rpm | 19.63 | 283.18 | 20,000 | 0.9901 | 0.0222 | 7.0939 | |||
| r = 150 rpm | 274.46 | 117.26 | 5000 | 0.9556 | 0.0458 | 10.2740 | ||||
| r = 40 rpm | 113.99 | 162.29 | 6500 | 0.9852 | 0.0089 | 1.7233 | ||||
| 200-rpm breakup after 40-rpm growth | r = 40 rpm | 20.33 | 272.50 | 22,500 | 0.9909 | 0.0227 | 6.8536 | |||
| r = 200 rpm | 273.10 | 103.04 | 7500 | 0.9822 | 0.0335 | 6.9715 | ||||
| r = 40 rpm | 106.43 | 156.72 | 5500 | 0.9487 | 0.0171 | 3.2077 | ||||
| 500-rpm breakup after 40-rpm growth | r = 40 rpm | 21.68 | 270.73 | 21,000 | 0.9932 | 0.0138 | 5.3752 | |||
| r = 500 rpm | 270.35 | 58.56 | 5000 | 0.9210 | 0.0911 | 19.5654 | ||||
| r = 40 rpm | 55.79 | 119.96 | 5000 | 0.9489 | 0.0255 | 3.6906 | ||||
| Xu et al. (2010) [24] | Humic acid (HA). | Jar-test apparatus with a stirrer, polyaluminum chloride polymer at different shear rate, pH = 7.5 | 50-rpm breakup after 40-rpm growth | r = 40 rpm | 36.05 | 307.90 | 28,000 | 0.9735 | 0.0439 | 12.3611 |
| r = 50 rpm | 287.22 | 254.27 | 2500 | 0.9447 | 0.0081 | 2.8922 | ||||
| r = 40 rpm | 246.78 | 260.54 | 2500 | 0.1824 | 0.0164 | 5.4085 | ||||
| 100-rpm breakup after 40-rpm growth | r = 40 rpm | 45.80 | 305.79 | 32,000 | 0.9695 | 0.0502 | 14.1697 | |||
| r = 100 rpm | 285.60 | 170.96 | 4000 | 0.9355 | 0.0338 | 9.2723 | ||||
| r = 40 rpm | 163.88 | 204.92 | 10,000 | 0.9456 | 0.0139 | 3.4411 | ||||
| 150-rpm breakup after 40-rpm growth | r = 40 rpm | 5.95 | 294.72 | 35,000 | 0.9781 | 0.0443 | 11.6565 | |||
| r = 150 rpm | 283.18 | 140.06 | 4000 | 0.9728 | 0.0295 | 7.4282 | ||||
| r = 40 rpm | 137.86 | 177.69 | 8000 | 0.9851 | 0.0079 | 1.7108 | ||||
| 200-rpm breakup after 40-rpm growth | r = 40 rpm | 1.88 | 325.50 | 38,000 | 0.9901 | 0.0541 | 11.6801 | |||
| r = 200 rpm | 320.56 | 123.38 | 4000 | 0.9390 | 0.0505 | 13.4764 | ||||
| r = 40 rpm | 121.65 | 163.71 | 4000 | 0.8932 | 0.0156 | 3.8415 | ||||
| 500-rpm breakup after 40-rpm growth | r = 40 rpm | 2.69 | 304.50 | 40,000 | 0.9877 | 0.0479 | 10.4163 | |||
| r = 500 rpm | 297.79 | 89.07 | 4000 | 0.9253 | 0.0821 | 16.7552 | ||||
| r = 40 rpm | 89.10 | 138.51 | 8000 | 0.9207 | 0.0294 | 4.7246 | ||||
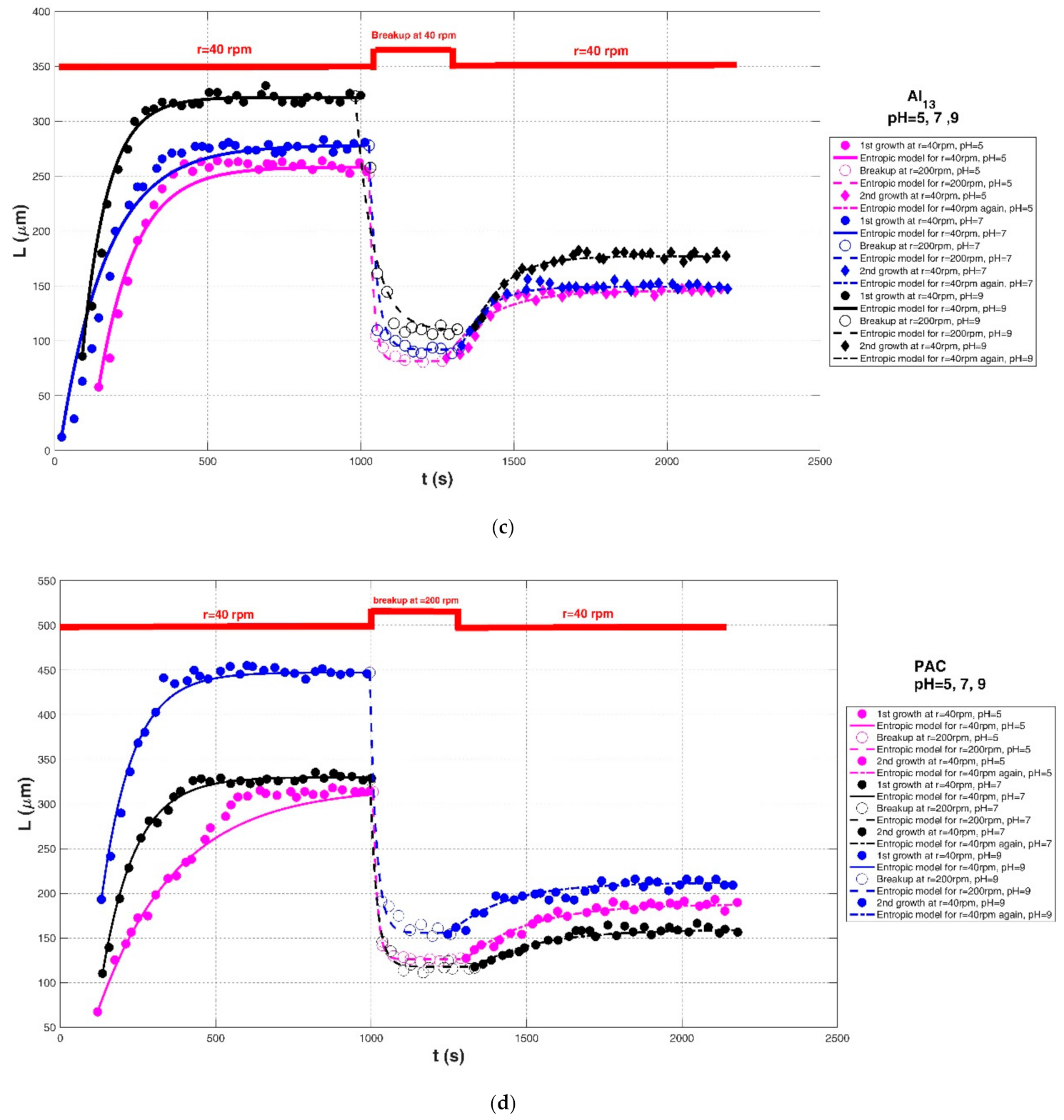
| Xu et al. (2010) [24] | Humic acid (HA). | Jar-test apparatus with a stirrer, Al13 polymer at different shear rate | pH = 5 | r = 40 rpm | 57.89 | 258.33 | 24,000 | 0.9744 | 0.0431 | 10.7588 |
| r = 200 rpm | 252.63 | 81.58 | 2500 | 0.9907 | 0.0351 | 5.7008 | ||||
| r = 40 rpm | 84.21 | 145.61 | 8500 | 0.9329 | 0.0317 | 5.5597 | ||||
| pH = 7 | r = 40 rpm | 12.28 | 278.22 | 40,500 | 0.9685 | 0.1172 | 17.4689 | |||
| r = 200 rpm | 278.07 | 92.11 | 4500 | 0.9459 | 0.0589 | 13.3823 | ||||
| r = 40 rpm | 95.61 | 149.27 | 4500 | 0.9525 | 0.0161 | 3.4224 | ||||
| pH = 9 | r = 40 rpm | 85.96 | 321.78 | 19,500 | 0.9830 | 0.0261 | 9.1763 | |||
| r = 200 rpm | 322.81 | 109.21 | 12,500 | 0.9326 | 0.0667 | 18.3096 | ||||
| r = 40 rpm | 113.16 | 177.19 | 6500 | 0.9730 | 0.0149 | 3.1652 | ||||
| Xu et al. (2010) [24] | Humic acid (HA). | Jar-test apparatus with a stirrer, polyaluminum chloride polymer at different shear rate | pH = 5 | r = 40 rpm | 67.34 | 318.50 | 64,000 | 0.9817 | 0.0393 | 14.2694 |
| r = 200 rpm | 313.70 | 126.08 | 2500 | 0.9987 | 0.0122 | 2.0395 | ||||
| r = 40 rpm | 127.36 | 187.98 | 12,500 | 0.9567 | 0.0191 | 4.1434 | ||||
| pH = 7 | r = 40 rpm | 110.33 | 330.10 | 23,500 | 0.9932 | 0.0143 | 4.9210 | |||
| r = 200 rpm | 326.61 | 117.96 | 4500 | 0.9946 | 0.0287 | 4.3394 | ||||
| r = 40 rpm | 117.65 | 159.39 | 8500 | 0.9259 | 0.0208 | 4.1117 | ||||
| pH = 9 | r = 40 rpm | 193.13 | 447.35 | 25,500 | 0.9846 | 0.0168 | 9.4848 | |||
| r = 200 rpm | 447.04 | 155.76 | 6000 | 0.9921 | 0.0399 | 8.8524 | ||||
| r = 40 rpm | 154.30 | 211.99 | 10,500 | 0.8975 | 0.0209 | 5.3591 | ||||
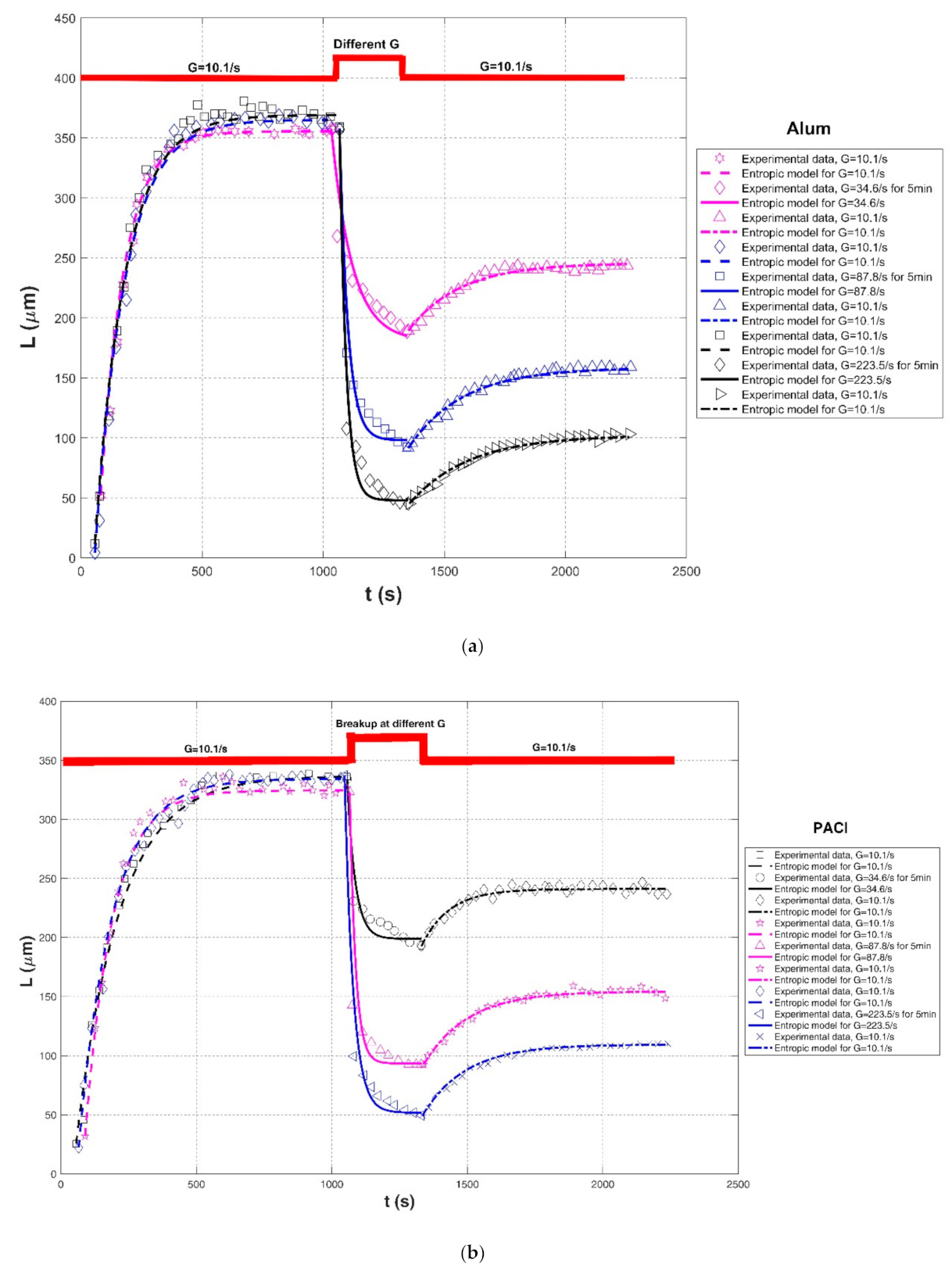
| Xu and Gao (2012) [74] | Humic acid | Jar-test apparatus with different mixing rates | Alum coagulant | G = 10.1/s, for 15 min | 52.13 | 355.51 | , hereinafter) | 0.9437 | 0.0977 | 24.1654 |
| G = 34.6/s, for 5 min breakup | 357.45 | 180.54 | 15,000 | 0.9248 | 0.0496 | 16.5140 | ||||
| G = 10.1/s, for 15 min | 189.36 | 245.60 | 12,000 | 0.9703 | 0.0101 | 2.8881 | ||||
| G = 10.1/s, for 15 min | 4.30 | 365.05 | 45,000 | 0.9937 | 0.0590 | 10.3406 | ||||
| G = 87.8/s, for 5 min breakup | 358.06 | 98.00 | 9000 | 0.9663 | 0.0753 | 14.7623 | ||||
| G = 10.1/s, for 15 min | 91.40 | 158.38 | 15,000 | 0.9882 | 0.0145 | 2.5093 | ||||
| G = 10.1/s, for 15 min | 11.83 | 369.06 | 45,000 | 0.9913 | 0.0351 | 10.6234 | ||||
| G = 223.5/s, for 5 min breakup | 356.99 | 47.95 | 8500 | 0.9611 | 0.1525 | 18.5870 | ||||
| G = 10.1/s, for 15 min | 45.16 | 102.15 | 14,000 | 0.9911 | 0.0196 | 1.9381 | ||||
| Xu and Gao (2012) [74] | Humic acid | Jar-test apparatus with different mixing rates | PACl coagulant | G = 10.1/s, for 15 min | 25.46 | 336.66 | 50,000 | 0.9924 | 0.0506 | 9.5759 |
| G = 34.6/s, for 5 min breakup | 334.06 | 198.91 | 4000 | 0.9263 | 0.0379 | 11.3640 | ||||
| G = 10.1/s, for 15 min | 192.62 | 241.12 | 5000 | 0.9513 | 0.0097 | 2.8908 | ||||
| G = 10.1/s, for 15 min | 31.84 | 324.52 | 30,000 | 0.9944 | 0.0179 | 7.0992 | ||||
| G = 87.8/s, for 5 min breakup | 323.29 | 93.50 | 5000 | 0.8354 | 0.1023 | 32.0393 | ||||
| G = 10.1/s, for 15 min | 92.59 | 154.47 | 10,000 | 0.9837 | 0.0133 | 2.4118 | ||||
| G = 10.1/s, for 15 min | 22.22 | 334.26 | 39,000 | 0.9921 | 0.0211 | 7.2941 | ||||
| G = 223.5/s, for 5 min breakup | 337.31 | 51.82 | 9000 | 0.9598 | 0.1112 | 17.3637 | ||||
| G = 10.1/s, for 15 min | 49.59 | 109.48 | 9500 | 0.9921 | 0.0159 | 1.8107 | ||||
| Xu and Gao (2012) [74] | Humic acid | Jar-test apparatus with different mixing rates | PACl-Alb coagulant | G = 10.1/s, for 15 min | 16.84 | 292.84 | 30,000 | 0.9859 | 0.0480 | 10.0659 |
| G = 34.6/s, for 5 min breakup | 287.37 | 185.79 | 8000 | 0.9736 | 0.0191 | 4.9168 | ||||
| G = 10.1/s, for 15 min | 183.16 | 231.89 | 8000 | 0.9357 | 0.0144 | 3.8579 | ||||
| G = 10.1/s, for 15 min | 18.95 | 297.14 | 40,000 | 0.9941 | 0.0401 | 7.7517 | ||||
| G = 87.8/s, for 5 min breakup | 298.95 | 125.26 | 7000 | 0.8588 | 0.0703 | 21.0826 | ||||
| G = 10.1/s, for 15 min | 123.16 | 183.03 | 15,000 | 0.9883 | 0.0129 | 2.7313 | ||||
| G = 10.1/s, for 15 min | 26.32 | 301.18 | 40,000 | 0.9941 | 0.0226 | 7.6730 | ||||
| G = 223.5/s, for 5 min breakup | 301.05 | 65.26 | 12,000 | 0.9908 | 0.0445 | 7.0965 | ||||
| G = 10.1/s, for 15 min | 65.26 | 128.87 | 15,000 | 0.9911 | 0.0172 | 2.2243 | ||||
| Xu and Gao (2012) [74] | Humic acid | Jar-test apparatus with different mixing rates | Alum coagulant | G = 10.1/s, for 15 min | 148.96 | 370.24 | 27000 | 0.9770 | 0.0228 | 9.8363 |
| G = 87.8/s, for 10 min breakup | 370.83 | 63.02 | 30,000 | 0.9241 | 0.1206 | 26.1279 | ||||
| G = 10.1/s, for 15 min | 58.33 | 99.66 | 9000 | 0.9813 | 0.0171 | 1.9847 | ||||
| PACl coagulant | G = 10.1/s, for 15 min | 21.88 | 326.04 | 38,000 | 0.9909 | 0.0239 | 8.1788 | |||
| G = 87.8/s, for 10 min breakup | 320.83 | 70.05 | 17,000 | 0.8838 | 0.1374 | 23.0509 | ||||
| G = 10.1/s, for 15 min | 66.67 | 118.71 | 14,500 | 0.9949 | 0.0149 | 1.7564 | ||||
| PACl-Alb coagulant | G = 10.1/s, for 15 min | 28.13 | 299.31 | 36,000 | 0.9885 | 0.0324 | 8.6583 | |||
| G = 87.8/s, for 10 min breakup | 297.92 | 106.25 | 15,000 | 0.8735 | 0.0856 | 19.8588 | ||||
| G = 10.1/s, for 15 min | 104.17 | 152.34 | 10,000 | 0.9931 | 0.0073 | 1.2614 | ||||
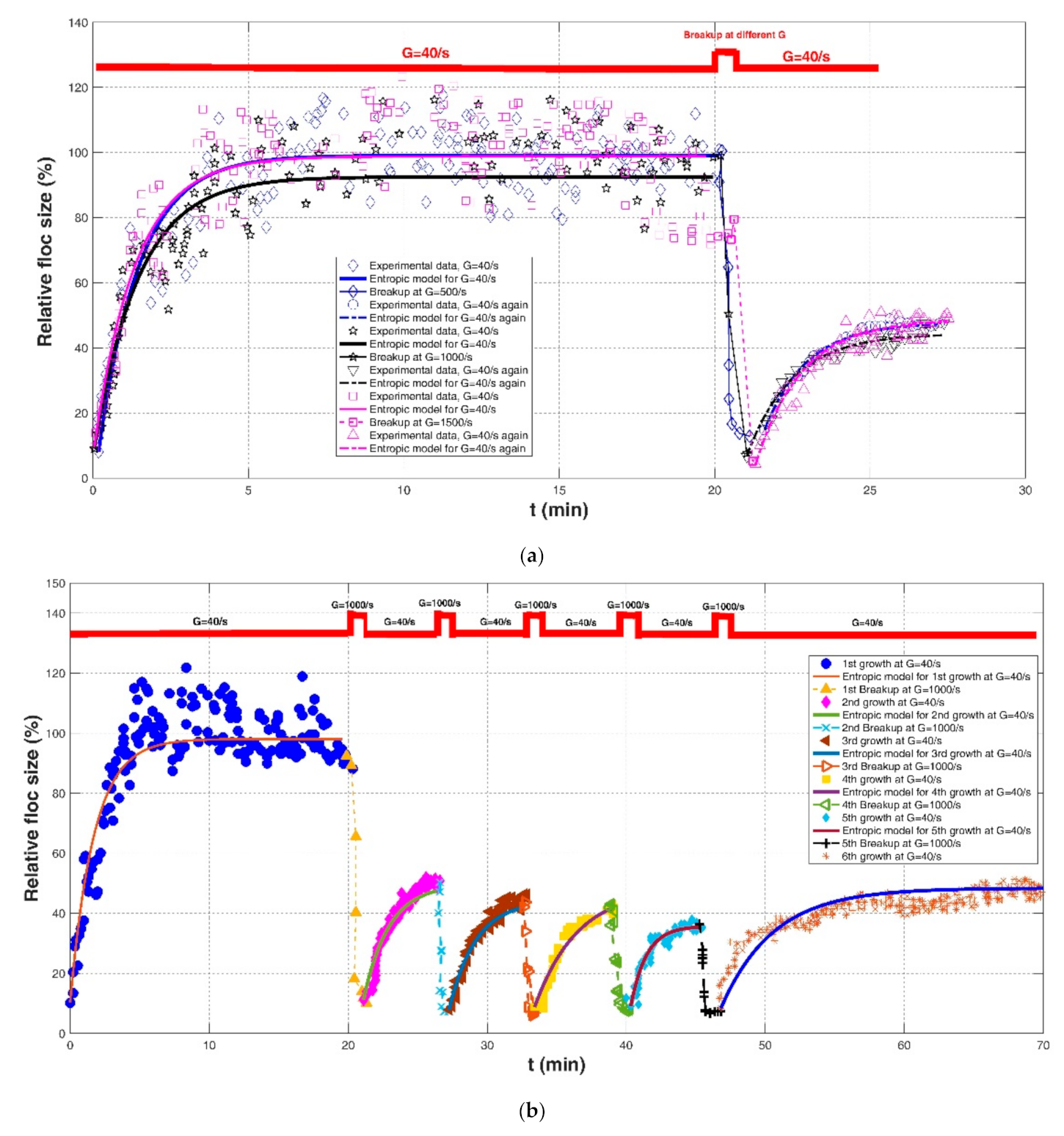
| Slavik et al. (2012) [22] | Raw water in Saxony (Germany) | Jar test with single mixers | pH 6.5 with coagulant dosages of 0.2 mmol/L and G = 40/s for 20 min. | G = 40/s for 20 min | 8.10(%, hereinafter) | 99.13(%, hereinafter) | 120 (%*min, hereinafter) | 0.8447 | 0.1085 | 9.7307 |
| G = 500/s for 1 min breakup | - | - | - | - | - | - | ||||
| G = 40/s | 14.76 | 47.79 | 45 | 0.9863 | 0.0212 | 1.1262 | ||||
| G = 40/s for 20 min | 9.05 | 92.55 | 120 | 0.8519 | 0.1075 | 10.1488 | ||||
| G = 1000/s for 1 min breakup | -- | - | - | - | - | - | ||||
| G = 40/s | 6.67 | 44.39 | 55 | 0.9622 | 0.0562 | 2.0993 | ||||
| G = 40/s for 20 min | 9.52 | 98.83 | 120 | 0.7737 | 0.1114 | 11.5515 | ||||
| G = 1500/s for 1 min breakup | - | - | - | -- | - | - | ||||
| G = 40/s | 4.29 | 48.71 | 65 | 0.9182 | 0.0734 | 3.2751 | ||||
| Slavik et al. (2012) [22] | Raw water in Saxony (Germany) | Jar test with single mixers | pH 6.5 with coagulant dosages of 0.2 mmol/L and repeatedshearing | G = 40/s for 20 min | 10.22 | 98.12 | 150 | 0.8529 | 0.0837 | 8.7473 |
| G = 1000/s for 1 min breakup | - | - | - | - | - | - | ||||
| G = 40/s | 11.26 | 49.62 | 70 | 0.9603 | 0.0880 | 3.1496 | ||||
| G = 1000/s for 1 min breakup | - | - | - | - | -- | |||||
| G = 40/s | 7.87 | 44.26 | 70 | 0.9676 | 0.0745 | 2.6660 | ||||
| G = 1000/s for 1 min breakup | -- | - | - | - | - | - | ||||
| G = 40/s | 8.78 | 48.10 | 120 | 0.9529 | 0.1054 | 2.5735 | ||||
| G = 1000/s for 1 min breakup | - | -- | - | - | - | - | ||||
| G = 40/s | 9.13 | 35.78 | 30 | 0.8923 | 0.0989 | 2.8444 | ||||
| G = 1000/s for 1 min breakup | - | - | - | - | - | - | ||||
| G = 40/s | 8.42 | 48.40 | 150 | 0.8595 | 0.0902 | 4.0423 | ||||
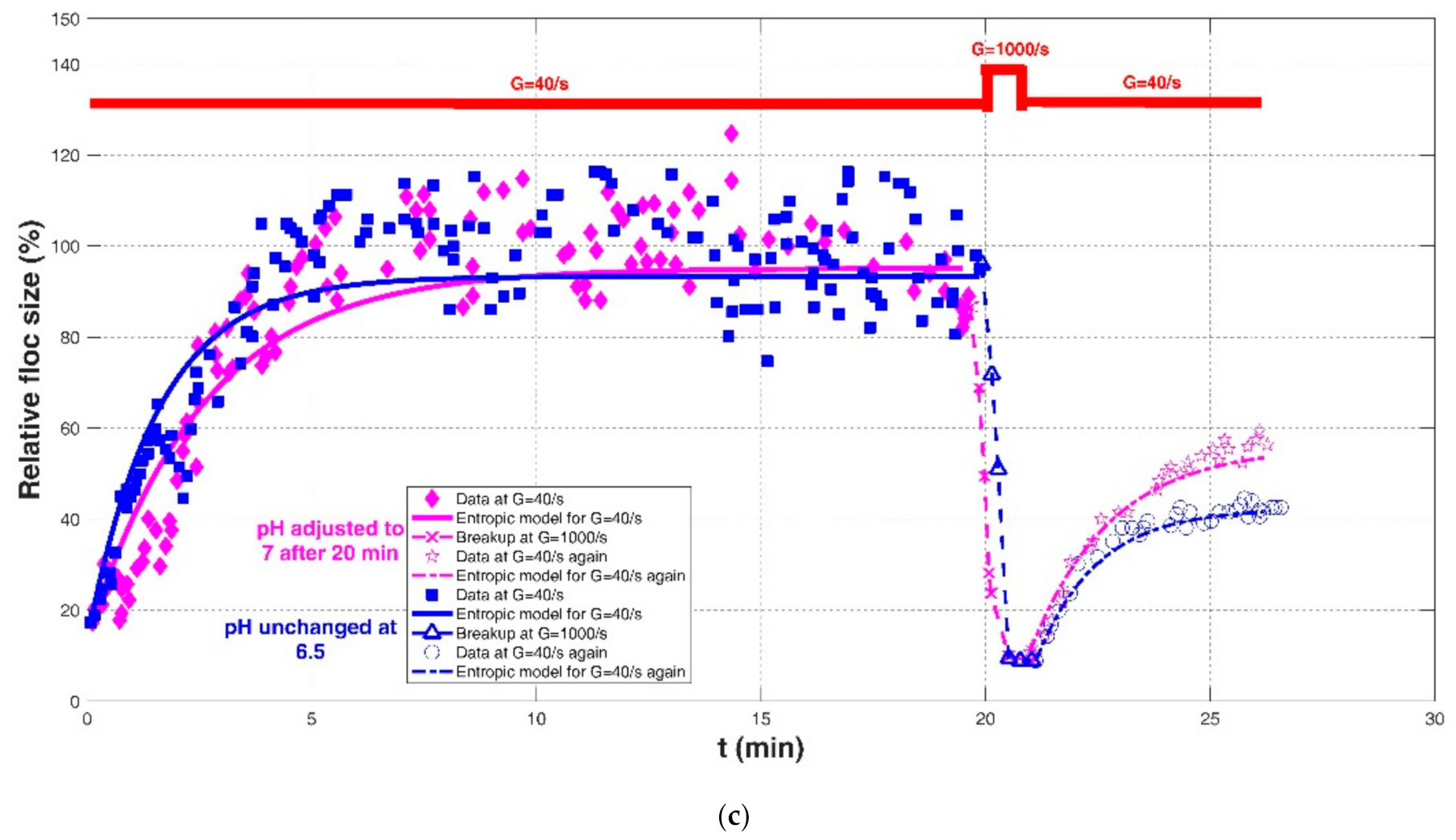
| Slavik et al. (2012) [22] | Raw water in Saxony (Germany) | Jar test with single mixers | pH adjusted to 7 after 20 min | G = 40/s for 20 min | 17.33 | 95.17 | 200 | 0.8906 | 0.1493 | 11.4095 |
| G = 1000/s for 1 min breakup | - | - | - | - | - | - | ||||
| G = 40/s | 10.89 | 55.94 | 80 | 0.9845 | 0.0633 | 2.8540 | ||||
| pH unchanged at 6.5 | G = 40/s for 20 min | 17.33 | 93.36 | 120 | 0.8176 | 0.1145 | 0.1145 | |||
| G = 1000/s for 1 min breakup | - | - | - | - | - | - | ||||
| G = 40/s | 8.91 | 42.35 | 45 | 0.9742 | 0.0368 | 1.7954 | ||||
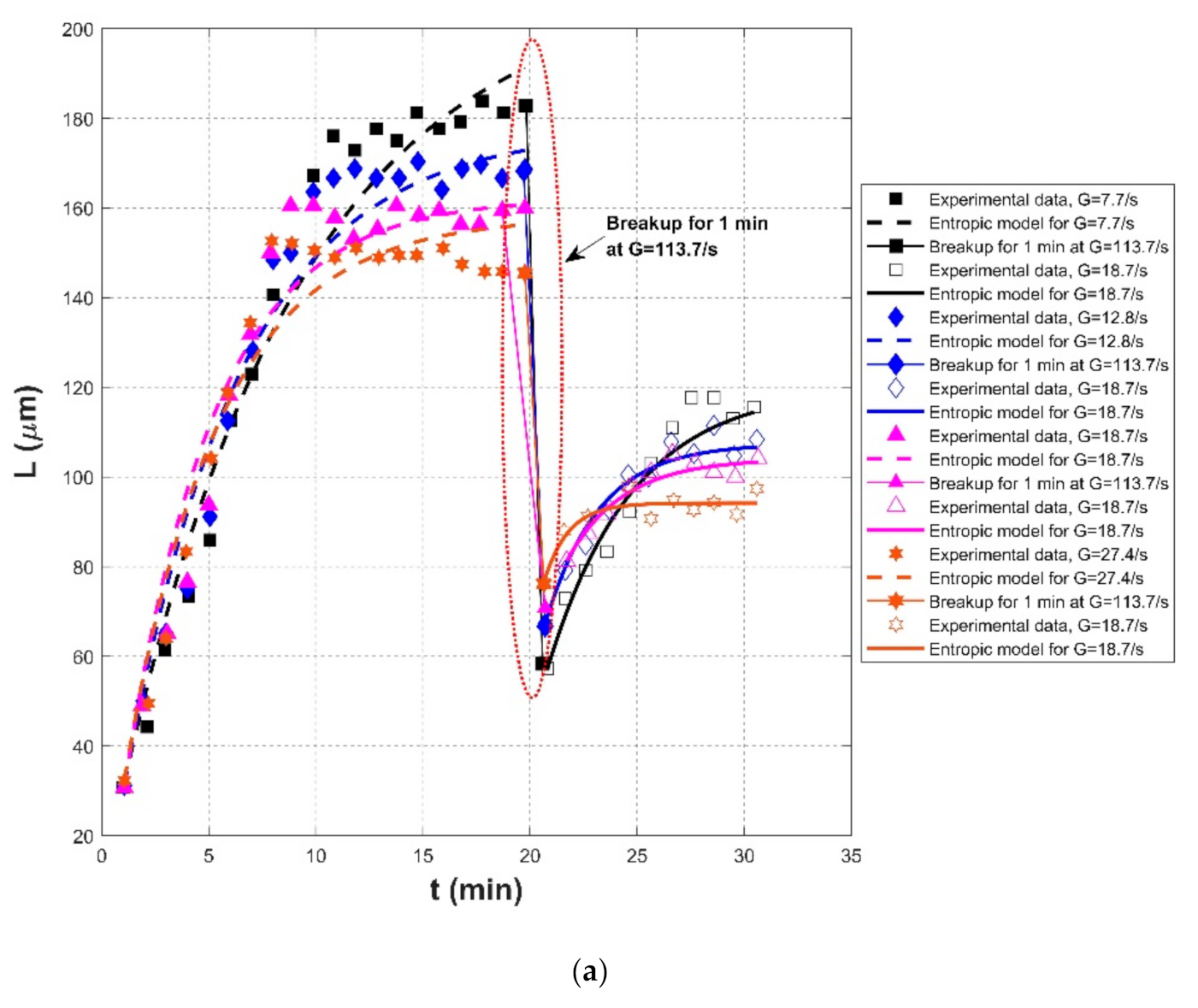
| Nan et al. (2016) [20] | Kaolin clay | Jar test reactor with aR1342-type impeller | Effect of slow mixing before breakage | G = 7.7/s, for 20 min | 30.73 | 210.13 | 1500 | 0.9682 | 0.0661 | 9.7669 |
| G = 113.7/s, for 1 min breakup | - | -- | - | - | - | - | ||||
| G = 18.7/s, for 10 min | 57.29 | 120.02 | 250 | 0.9604 | 0.0346 | 4.5132 | ||||
| G = 12.8/s, for 20 min | 31.25 | 177.60 | 800 | 0.9675 | 0.0603 | 8.8210 | ||||
| G = 113.7/s, for 1 min breakup | - | - | -- | - | - | - | ||||
| G = 18.7/s, for 10 min | 67.19 | 107.42 | 100 | 0.9641 | 0.0214 | 2.7208 | ||||
| G = 18.7/s, for 20 min | 30.73 | 162.25 | 550 | 0.9556 | 0.0644 | 9.6154 | ||||
| G = 113.7/s, for 1 min breakup | - | - | - | - | - | - | ||||
| G = 18.7/s, for 10 min | 81.25 | 104.08 | 60 | 0.9289 | 0.0164 | 2.0539 | ||||
| G = 27.4/s, for 20 min | 32.29 | 157.98 | 550 | 0.9435 | 0.0666 | 9.0538 | ||||
| G = 113.7/s, for 1 min breakup | - | -- | - | - | - | - | ||||
| G = 18.7/s, for 10 min | 76.56 | 94.17 | 20 | 0.8483 | 0.0184 | 2.2616 | ||||
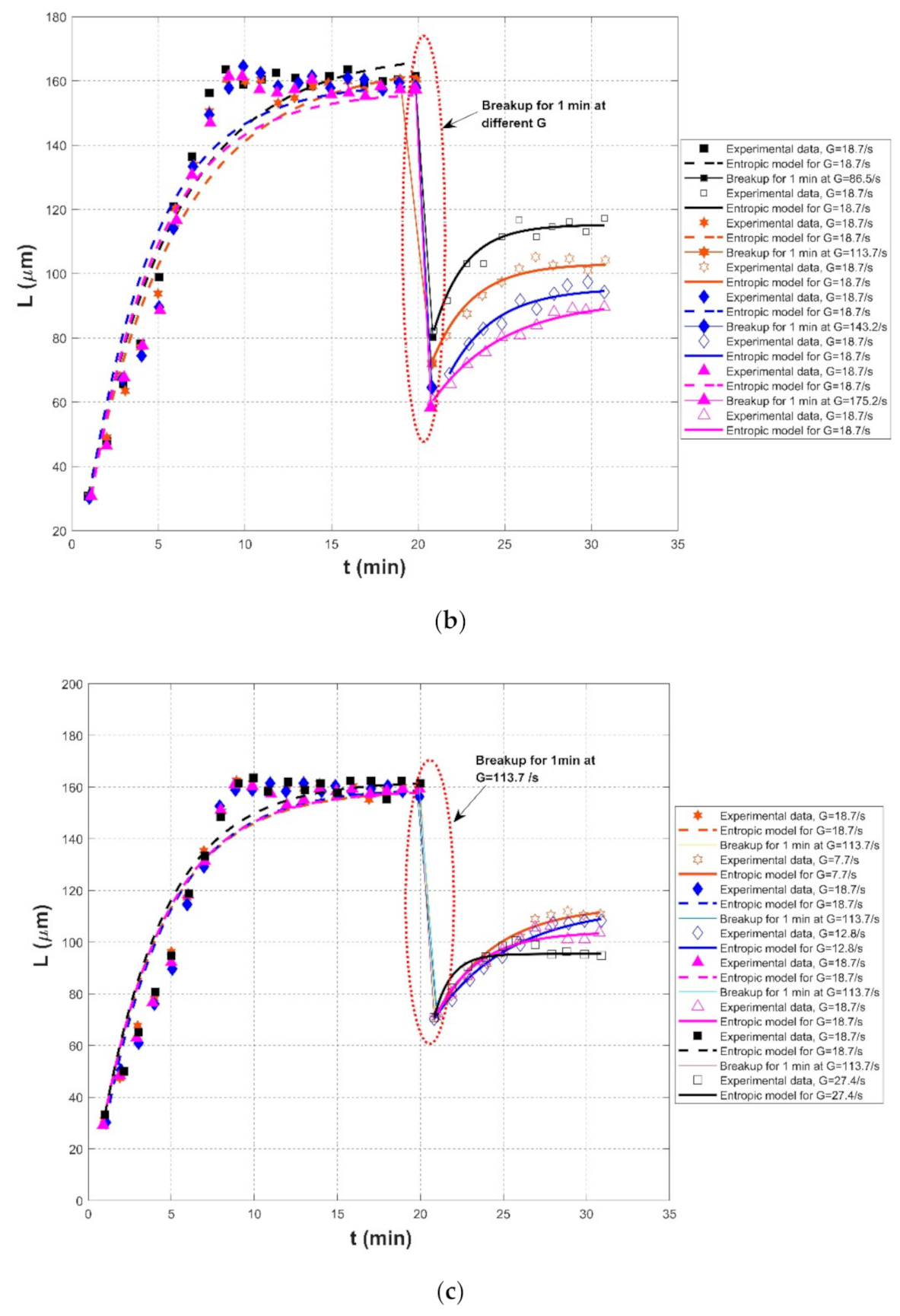
| Nan et al. (2016) [20] | Kaolin clay | Jar-test reactor with a R1342-type impeller | Effect of rapid mixing during breakage | G = 18.7/s, for 20 min | 30.73 | 169.02 | 700 | 0.9501 | 0.0687 | 10.2591 |
| G = 86.5/s, for 1 min breakup | - | -- | - | - | - | - | ||||
| G = 18.7/s, for 10 min | 82.29 | 115.23 | 60 | 0.9558 | 0.0162 | 2.3454 | ||||
| G = 18.7/s, for 20 min | 30.73 | 165.11 | 700 | 0.9506 | 0.0664 | 10.6025 | ||||
| G = 113.7/s, for 1 min breakup | - | - | -- | - | - | - | ||||
| G = 18.7/s, for 10 min | 72.40 | 103.13 | 70 | 0.9697 | 0.0168 | 2.0119 | ||||
| G = 18.7/s, for 20 min | 30.21 | 159.27 | 500 | 0.9345 | 0.0762 | 11.4771 | ||||
| G = 143.2 s, for 1 min breakup | - | - | - | - | - | - | ||||
| G = 18.7/s, for 10 min | 68.75 | 95.44 | 70 | 0.9566 | 0.0158 | 1.9016 | ||||
| G = 18.7/s, for 20 min | 30.73 | 156.68 | 500 | 0.9509 | 0.0780 | 11.0099 | ||||
| G = 175.2/s, for 1 min breakup | - | -- | - | - | - | - | ||||
| G = 18.7/s, for 10 min | 60.42 | 91.81 | 130 | 0.9880 | 0.0121 | 1.1794 | ||||
| Nan et al. (2016) [20] | Kaolin clay | Jar-test reactor with a R1342-type impeller | Effect of slow mixing after breakage | G = 18.7/s, for 20 min | 31.25 | 158.75 | 500 | 0.9540 | 0.0798 | 10.7711 |
| G = 113.7/s, for 1 min breakup | - | -- | - | - | - | - | ||||
| G = 7.7/s, for 10 min | 70.83 | 113.94 | 150 | 0.9733 | 0.0210 | 2.4718 | ||||
| G = 18.7/s, for 20 min | 30.21 | 159.59 | 500 | 0.9461 | 0.0835 | 11.3822 | ||||
| G = 113.7/s, for 1 min breakup | - | - | -- | - | - | - | ||||
| G = 12.8/s, for 10 min | 70.31 | 113.81 | 200 | 0.9914 | 0.0119 | 1.3387 | ||||
| G = 18.7/s, for 20 min | 29.17 | 158.75 | 500 | 0.9519 | 0.0791 | 10.8267 | ||||
| G = 113.7/s, for 1 min breakup | - | - | - | - | - | - | ||||
| G = 18.7/s, for 10 min | 72.40 | 103.99 | 80 | 0.9590 | 0.0167 | 2.2380 | ||||
| G = 18.7/s, for 20 min | 33.33 | 162.42 | 500 | 0.9531 | 0.0834 | 10.9332 | ||||
| G = 113.7/s, for 1 min breakup | - | -- | - | - | - | - | ||||
| G = 27.4/s, for 10 min | 70.83 | 95.44 | 25 | 0.9130 | 0.0195 | 2.6405 | ||||
| Wu et al. (2019) [29] | Deionized (DI) water with alum and PACl25 as coagulants | Jar-test equipment with a stirrer | 1-min breakup at 200 rpm | G = 23/s | 0.03 (%, hereinafter) | 1.15 (%, hereinafter) | 3 (%*min, hereinafter) | 0.9399 | 0.0561 | 0.0691 |
| G = 184/s for 1 min breakup | -- | - | - | - | - | - | ||||
| G = 23/s | 0.53 | 0.82 | 0.5 | 0.7810 | 0.0359 | 0.0379 | ||||
| 10-min breakup at 200 rpm | G = 23/s | 0.03 | 1.10 | 1.5 | 0.9534 | 0.0606 | 0.0685 | |||
| G = 184/s for 10 min breakup | 1.05 | 0.36 | 0.5 | 0.9056 | 0.0683 | 0.0575 | ||||
| G = 23/s | 0.33 | 0.62 | 0.4 | 0.9006 | 0.0328 | 0.0250 | ||||
| Wu et al. (2019) [29] | Deionized (DI) water with alum and PACl25 as coagulants | Jar-test equipment with a stirrer | pH = 7 | G = 23/s | 0.04 | 1.16 | 1.8 | 0.9783 | 0.0545 | 0.0535 |
| G = 184/s for 1 min breakup | - | - | - | - | -- | - | ||||
| G = 23/s | 0.32 | 0.75 | 0.6 | 0.9269 | 0.0359 | 0.0315 | ||||
| pH = 5 | G = 23/s | 0.04 | 1.11 | 1.8 | 0.9648 | 0.0534 | 0.0573 | |||
| G = 184/s for 10 min breakup | - | - | - | - | - | - | ||||
| G = 23/s | 0.32 | 1.06 | 1.8 | 0.9506 | 0.0420 | 0.0505 | ||||
| In average | 0.9301 | 0.0475 | 6.6286 | |||||||
3.2.3. Colloidal Science Field
| References | Flocculation Experiment Condition | Fitting Effect | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Material | Flocculation Environment | Flocculation Condition | Flocculation Period | L0 (um) | Ls (um) | M (um * min) | R2 | RE | RMSE | |
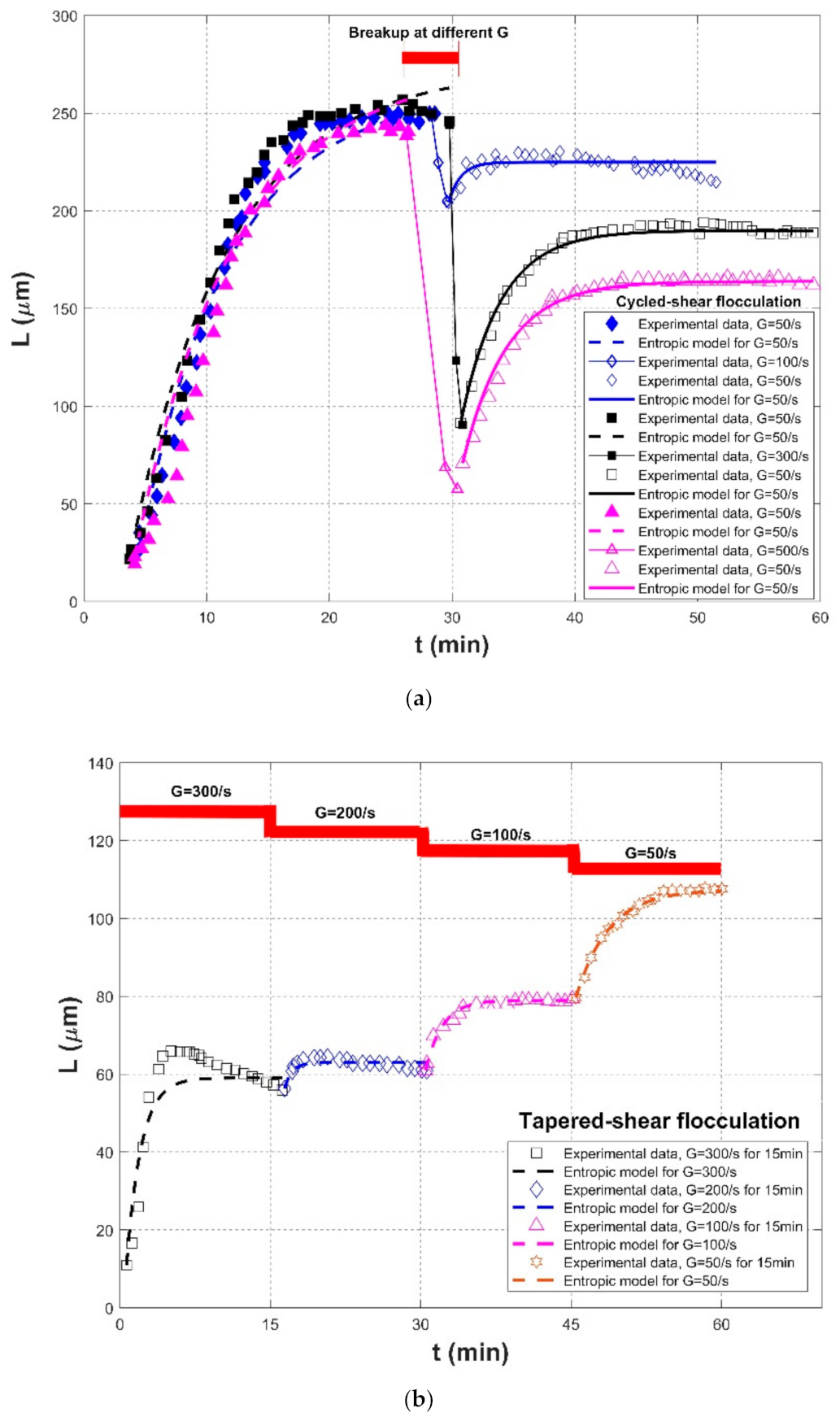
| Spicer et al. (1998) [23] | Polystyrene-alum floc | Stirred tank with a Rushton impeller | Tapered-shear flocculation | G = 300/s, for 15 min | 10.93 | 59.03 | 70 | 0.9064 | 0.1043 | 5.8630 |
| G = 200/s, for 15 min | 56.30 | 63 | 5 | 0.6229 | 0.0146 | 1.0965 | ||||
| G = 100/s, for 15 min | 60.93 | 78.98 | 30 | 0.9834 | 0.0069 | 0.7520 | ||||
| G = 50/s, for 15 min | 79.63 | 107.35 | 90 | 0.9947 | 0.0055 | 0.7335 | ||||
| Cycled-shear flocculation | G = 50/s, for 30 min | 26.66 | 260.42 | 1700 | 0.9782 | 0.0806 | 12.4689 | |||
| G = 100/s, for 1 min | - | - | - | - | -- | |||||
| G = 50/s, for 30 min | 204.28 | 224.91 | 20 | 0.5020 | 0.0151 | 4.2064 | ||||
| G = 50/s, for 30 min | 21.81 | 272.52 | 2000 | 0.9768 | 0.0812 | 12.9654 | ||||
| G = 300/s, for 1 min | - | - | -- | - | -- | - | ||||
| G = 50/s, for 30 min | 91.49 | 189.90 | 320 | 0.9907 | 0.0136 | 2.7977 | ||||
| G = 50/s, for 30 min | 22.80 | 272.52 | 2000 | 0.9732 | 0.1730 | 19.0067 | ||||
| G = 500/s, for 1 min | - | - | - | - | -- | - | ||||
| G = 50/s, for 30 min | 70.73 | 163.88 | 330 | 0.9920 | 0.0152 | 2.8624 | ||||
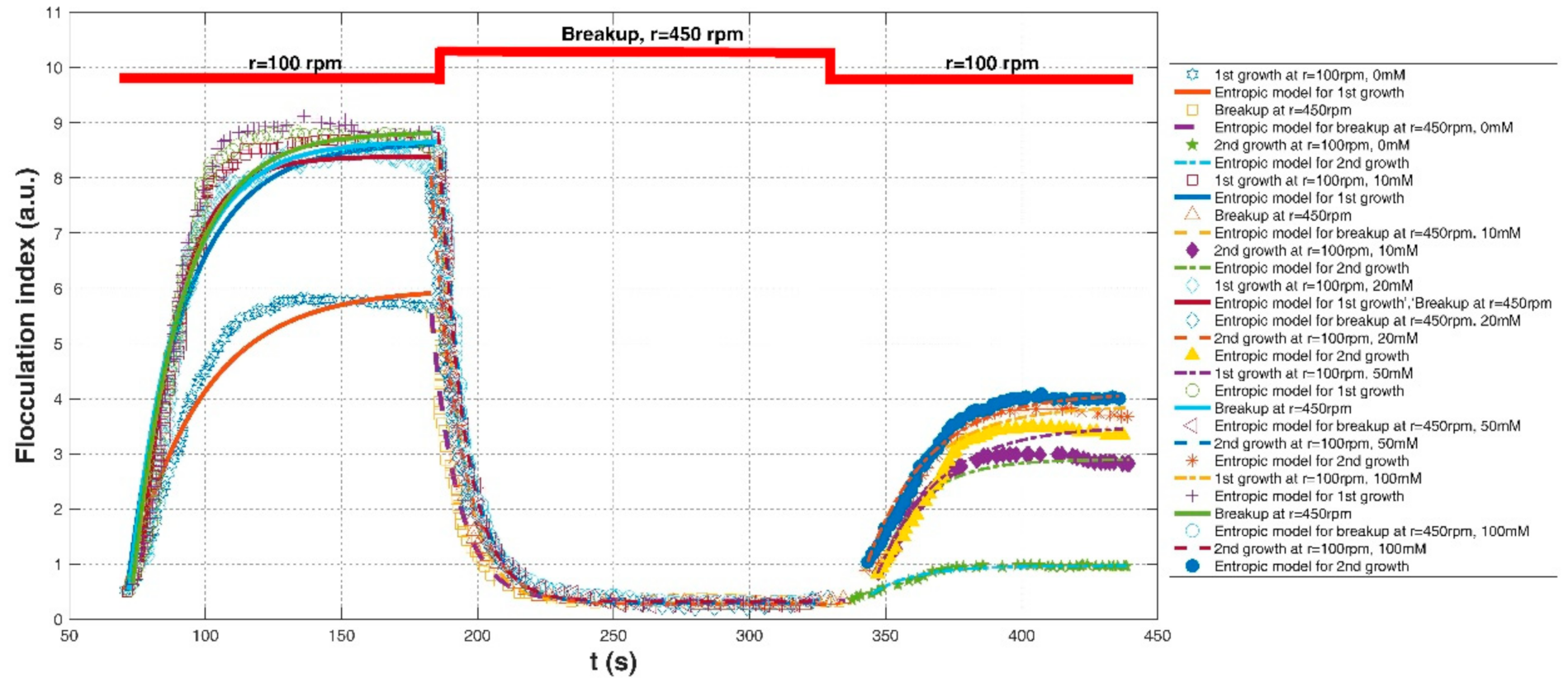
| Wu and Ven (2009) [25] | Thermomechanical pulp (TMP) particles at various CPR (carboxylated phenolic resin) –PEO (poly(ethylene oxide)) ratios | A beaker with a stirrer (the stirring speed from 100 to 450 rpm) | NaCl concentration: 0 mM | r = 100 rpm | 0.52 (a.u. hereinafter) | 6.00 (a.u. hereinafter) | 150 (a.u.*s, hereinafter) | 0.9653 | 0.1247 | 0.3793 |
| r = 450 rpm for breakup | 5.54 | 0.34; | 50 | 0.9715 | 0.0827 | 0.2920 | ||||
| r = 100 rpm | 0.46 | 0.97 | 8 | 0.9702 | 0.0233 | 0.0267 | ||||
| NaCl concentration: 10 mM | r = 100 rpm | 0.51 | 8.64 | 170 | 0.9626 | 0.1816 | 0.7183 | |||
| r = 450 rpm for breakup | 8.37 | 0.29 | 70 | 0.9775 | 0.1095 | 0.4264 | ||||
| r = 100 rpm | 0.85 | 2.9 | 32 | 0.9737 | 0.0456 | 0.1249 | ||||
| NaCl concentration: 20 mM | r = 100 rpm | 1.04 | 8.39 | 100 | 0.9823 | 0.1139 | 0.5077 | |||
| r = 450 rpm for breakup | 8.04 | 0.27 | 85 | 0.9902 | 0.0926 | 0.2762 | ||||
| r = 100 rpm | 0.83 | 3.5 | 60 | 0.9670 | 0.0651 | 0.2028 | ||||
| NaCl concentration: 50 mM | r = 100 rpm | 0.53 | 8.67 | 150 | 0.9612 | 0.1926 | 0.7468 | |||
| r = 450 rpm for breakup | 8.35 | 0.29 | 78 | 0.9795 | 0.1021 | 0.4129 | ||||
| r = 100 rpm | 0.88 | 3.87 | 65 | 0.9830 | 0.0714 | 0.1773 | ||||
| NaCl concentration: 100 mM | r = 100 rpm | 0.64 | 8.83 | 150 | 0.9639 | 0.1641 | 0.6959 | |||
| r = 450 rpm for breakup | 8.83 | 0.32 | 80 | 0.9919 | 0.0795 | 0.2579 | ||||
| r = 100 rpm | 1.04 | 4.10 | 70 | 0.9862 | 0.0545 | 0.1632 | ||||
| In average | 0.9419 | 0.0805 | 2.7264 | |||||||

4. Discussion
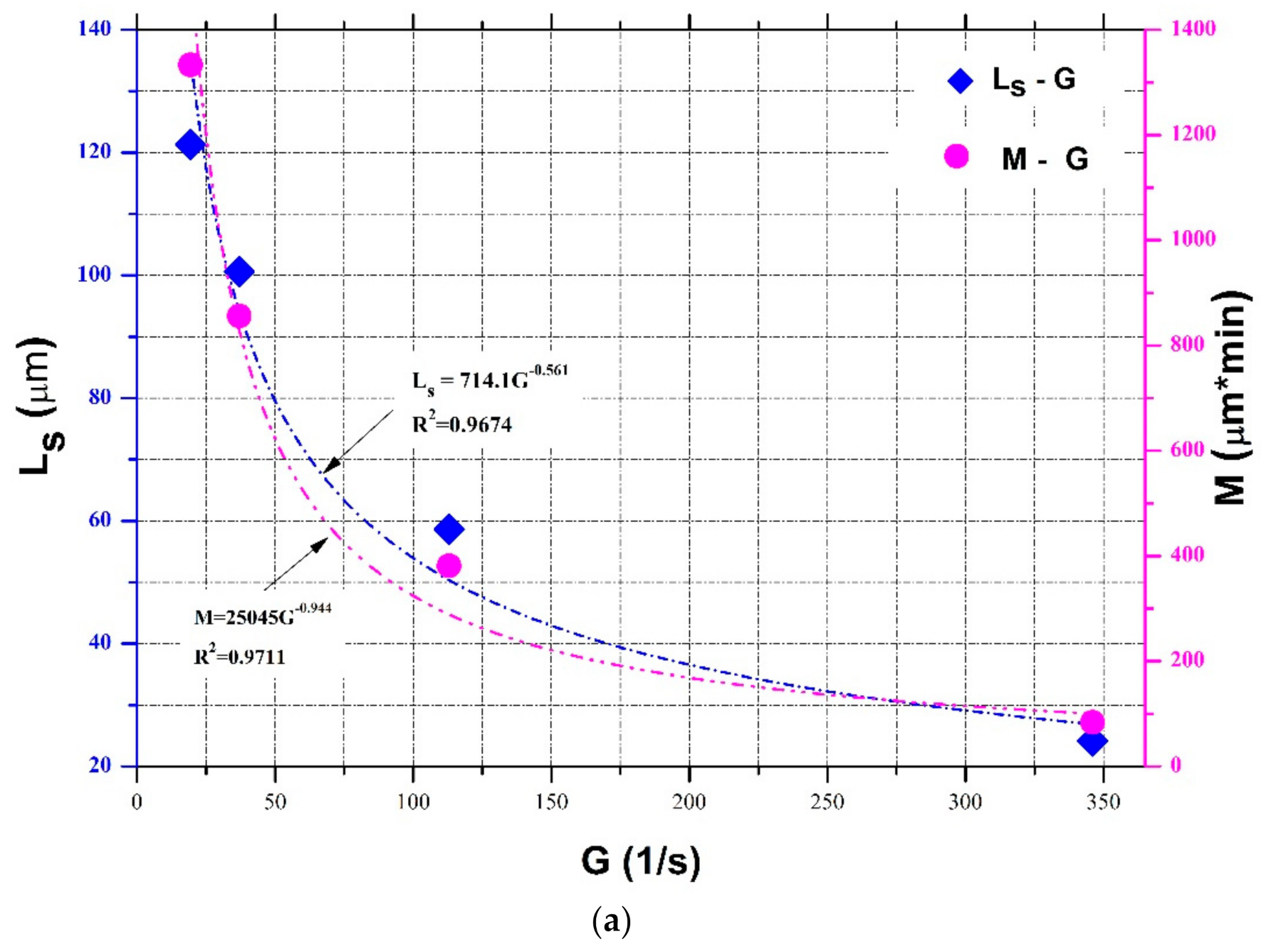
4.1. Parameterization of and

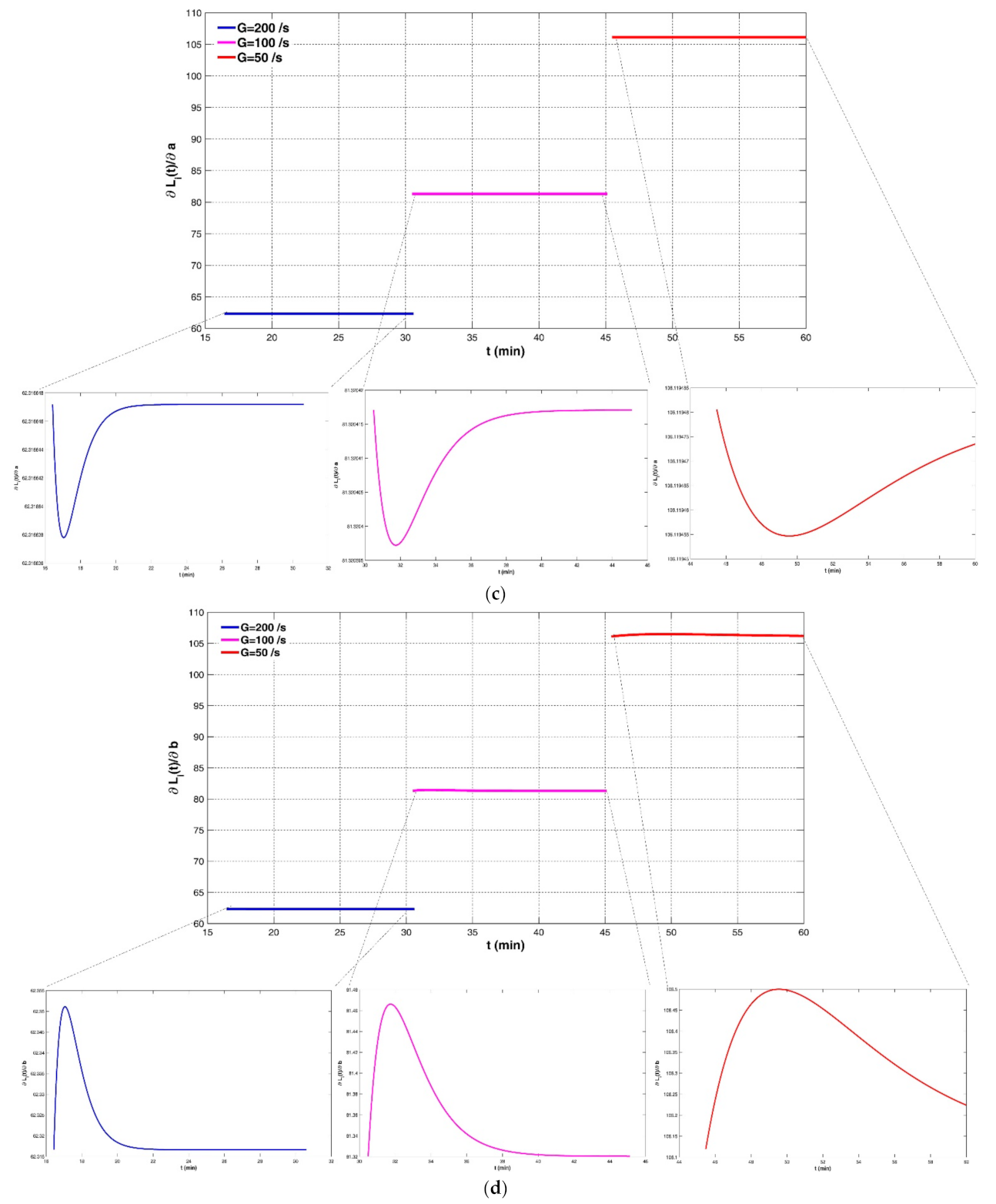
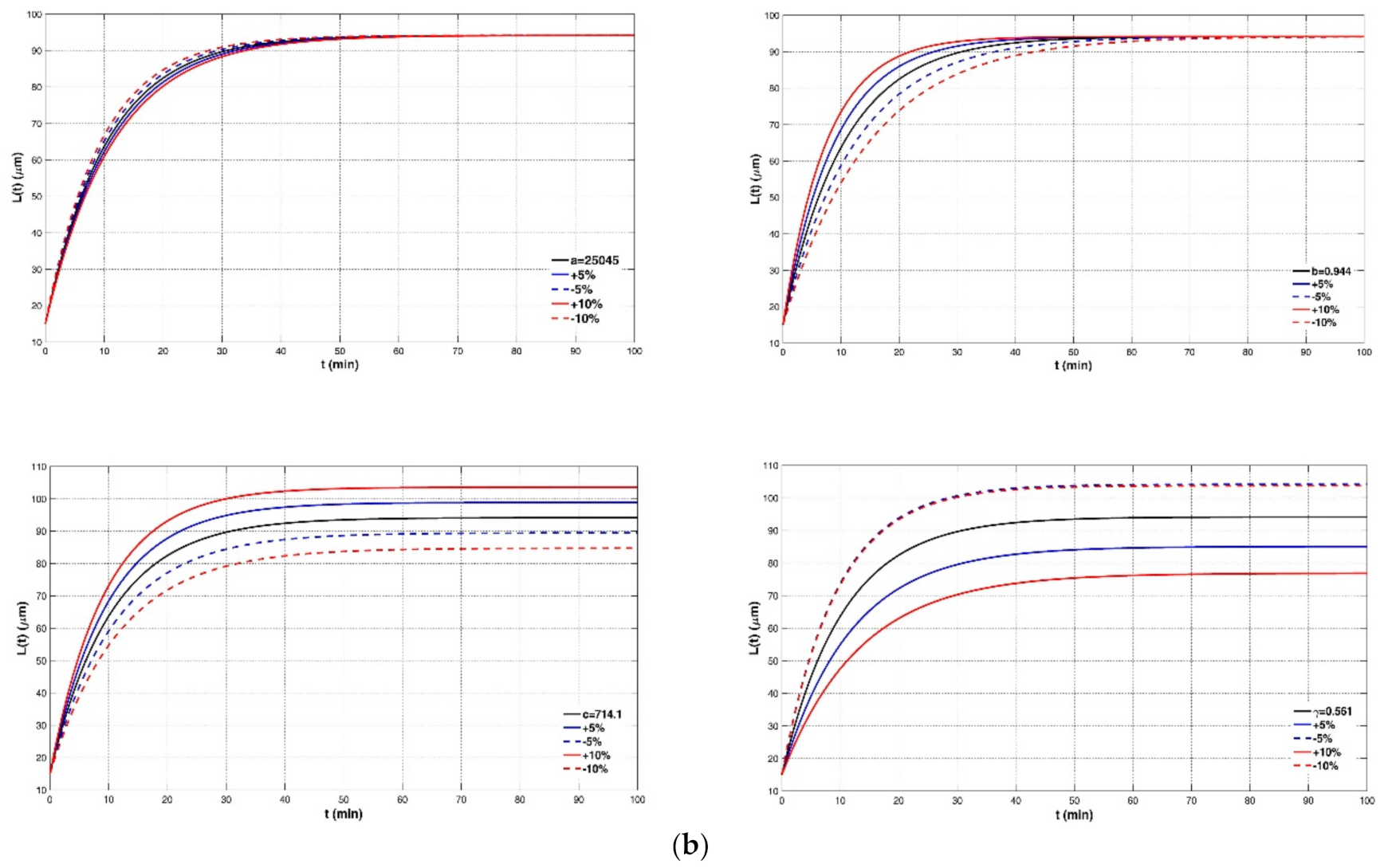
4.2. Sensitivity of the Model to Four Empirical Parameters
4.3. Effect of Repeated Cycles of a Low and High Shear Rate on the Floc Size Growth
4.4. Application of the Entropic Model in Engineering Practices and Its Limitations
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, H.W.; Lai, H.J.; Cheng, W.; Huang, L.; He, G.J. Modeling sediment transport with an integrated view of the biofilm effects. Water Resour. Res. 2017, 53, 7536–7557. [Google Scholar] [CrossRef]
- Son, M.; Hsu, T.J. Flocculation model of cohesive sediment using variable fractal dimension. Environ. Fluid Mech. 2008, 8, 55–71. [Google Scholar] [CrossRef]
- Lai, H.; Fang, H.; Huang, L.; He, G.; Reible, D. A review on sediment bioflocculation: Dynamics, influencing factors and modeling. Sci. Total Environ. 2018, 642, 1184–1200. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F. The settling velocity of mineral, biomineral, and biological particles and aggregates in water. J. Geophys. Res. Ocean. 2013, 118, 2118–2132. [Google Scholar] [CrossRef]
- Maggi, F.; Tang, F.H.M. Analysis of the effect of organic matter content on the architecture and sinking of sediment aggregates. Mar. Geol. 2015, 363, 102–111. [Google Scholar] [CrossRef]
- Mietta, F.; Chassagne, C.; Manning, A.J.; Winterwerp, J.C. Influence of shear rate, organic matter content, pH and salinity on mud flocculation. Ocean Dyn. 2009, 59, 751–763. [Google Scholar] [CrossRef] [Green Version]
- Tang, F.H.M.; Maggi, F. A mesocosm experiment of suspended particulate matter dynamics in nutrient- and biomass-affected waters. Water Res. 2016, 89, 76–86. [Google Scholar] [CrossRef]
- Dyer, K.R. Sediment processes in estuaries: Future research requirements. J. Geophys. Res. 1989, 94, 14327–14339. [Google Scholar] [CrossRef]
- Winterwerp, J.C. A simple model for turbulence induced flocculation of cohesive sediment. J. Hydraul. Res. 1998, 36, 309–326. [Google Scholar] [CrossRef]
- Xu, F.; Wang, D.P.; Riemer, N. Modeling flocculation processes of fine-grained particles using a size-resolved method: Comparison with published laboratory experiments. Cont. Shelf Res. 2008, 28, 2668–2677. [Google Scholar] [CrossRef]
- Ahn, J.H. Size distribution and settling velocities of suspended particles in a tidal embayment. Water Res. 2012, 46, 3219–3228. [Google Scholar] [CrossRef]
- Soulsby, R.L.; Manning, A.J.; Spearman, J.; Whitehouse, R.J.S. Settling velocity and mass settling flux of flocculated estuarine sediments. Mar. Geol. 2013, 339, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Zhang, D.; Xu, D.; Chen, Y. An experimental study of low concentration sludge settling velocity under turbulent condition. Water Res. 2009, 43, 2383–2390. [Google Scholar] [CrossRef]
- Boyd, P.W.; Trull, T.W. Understanding the export of biogenic particles in oceanic waters: Is there consensus? Prog. Oceanogr. 2007, 72, 276–312. [Google Scholar] [CrossRef]
- Gratiot, N.; Bildstein, A.; Anh, T.T.; Thoss, H.; Denis, H.; Michallet, H.; Apel, H. Sediment flocculation in the Mekong River estuary, Vietnam, an important driver of geomorphological changes. Comptes Rendus Geosci. 2017, 349, 260–268. [Google Scholar] [CrossRef]
- Herbert, R.A. Nitrogen cycling in coastal marine ecosystems. FEMS Microbiol. Rev. 1999, 23, 563–590. [Google Scholar] [CrossRef]
- Hill, P.S.; Newgard, J.P.; Law, B.A.; Milligan, T.G. Flocculation on a muddy intertidal flat in Willapa Bay, Washington, Part II: Observations of suspended particle size in a secondary channel and adjacent flat. Cont. Shelf Res. 2013, 60, S145–S156. [Google Scholar] [CrossRef]
- Biggs, C.; Lant, P.; Hounslow, M. Modelling the effect of shear history on activated sludge flocculation. Water Sci. Technol. 2003, 47, 251–257. [Google Scholar] [CrossRef]
- Gregory, J. Monitoring floc formation and breakage. Water Sci. Technol. 2004, 50, 163–170. [Google Scholar] [CrossRef]
- Nan, J.; Wang, Z.; Yao, M.; Yang, Y.; Zhang, X. Characterization of re-grown floc size and structure: Effect of mixing conditions during floc growth, breakage and re-growth process. Environ. Sci. Pollut. Res. 2016, 23, 23750–23757. [Google Scholar] [CrossRef]
- Oles, V. Shear-induced aggregation and breakup of polystyrene latex particles. J. Colloid Interface Sci. 1992, 154, 351–358. [Google Scholar] [CrossRef]
- Slavik, I.; Müller, S.; Mokosch, R.; Azongbilla, J.A.; Uhl, W. Impact of shear stress and pH changes on floc size and removal of dissolved organic matter (DOM). Water Res. 2012, 46, 6543–6553. [Google Scholar] [CrossRef]
- Spicer, P.T.; Pratsinis, S.E.; Raper, J.; Amal, R.; Bushell, G.; Meesters, G. Effect of shear schedule on particle size, density, and structure during flocculation in stirred tanks. Powder Technol. 1998, 97, 26–34. [Google Scholar] [CrossRef]
- Xu, W.; Gao, B.; Yue, Q.; Wang, Y. Effect of shear force and solution pH on flocs breakage and re-growth formed by nano-Al13 polymer. Water Res. 2010, 44, 1893–1899. [Google Scholar] [CrossRef]
- Wu, M.R.; van de Ven, T.G.M. Flocculation and reflocculation: Interplay between the adsorption behavior of the components of a dual flocculant. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 341, 40–45. [Google Scholar] [CrossRef]
- Biggs, C.A.; Lant, P.A. Activated sludge flocculation: On-line determination of floc size and the effect of shear. Water Res. 2000, 34, 2542–2550. [Google Scholar] [CrossRef]
- Chaignon, V.; Lartiges, B.S.; El Samrani, A.; Mustin, C. Evolution of size distribution and transfer of mineral particles between flocs in activated sludges: An insight into floc exchange dynamics. Water Res. 2002, 36, 676–684. [Google Scholar] [CrossRef]
- Keyvani, A.; Strom, K. Influence of cycles of high and low turbulent shear on the growth rate and equilibrium size of mud flocs. Mar. Geol. 2014, 354, 1–14. [Google Scholar] [CrossRef]
- Wu, M.; Yu, W.; Qu, J.; Gregory, J. The variation of flocs activity during floc breakage and aging, adsorbing phosphate, humic acid and clay particles. Water Res. 2019, 155, 131–141. [Google Scholar] [CrossRef]
- Son, M.; Hsu, T.J. The effect of variable yield strength and variable fractal dimension on flocculation of cohesive sediment. Water Res. 2009, 43, 3582–3592. [Google Scholar] [CrossRef]
- Xu, C.; Dong, P. A Dynamic Model for Coastal Mud Flocs with Distributed Fractal Dimension. J. Coast. Res. 2017, 33, 218–225. [Google Scholar] [CrossRef]
- Kuprenas, R.; Tran, D.; Strom, K. A Shear-Limited Flocculation Model for Dynamically. J. Geophys. Res. Oceans 2018, 123, 6736–6752. [Google Scholar] [CrossRef]
- Kranenburg, C. Effects of floc strength on viscosity and deposition of cohesive sediment suspensions. Cont. Shelf Res. 1999, 19, 1665–1680. [Google Scholar] [CrossRef]
- Li, D.; Li, Z.; Gao, Z. Quadrature-based moment methods for the population balance equation: An algorithm review. Chin. J. Chem. Eng. 2019, 27, 483–500. [Google Scholar] [CrossRef]
- Maggi, F. Effect of variable fractal dimension on the floc size distribution of suspended cohesive sediment. J. Hydrol. 2007, 343, 43–55. [Google Scholar] [CrossRef]
- Shen, X.; Maa, J.P.Y. Numerical simulations of particle size distributions: Comparison with analytical solutions and kaolinite flocculation experiments. Mar. Geol. 2016, 379, 84–99. [Google Scholar] [CrossRef]
- Shen, X.; Maa, J.P.Y. Modeling floc size distribution of suspended cohesive sediments using quadrature method of moments. Mar. Geol. 2015, 359, 106–119. [Google Scholar] [CrossRef]
- Shen, X.; Toorman, E.A.; Fettweis, M.; Lee, B.J.; He, Q. Simulating multimodal floc size distributions of suspended cohesive sediments with lognormal subordinates: Comparison with mixing jar and settling column experiments. Coast. Eng. 2019, 148, 36–48. [Google Scholar] [CrossRef]
- Zhang, J.F.; Zhang, Q.H. Lattice boltzmann simulation of the flocculation process of cohesive sediment due to differential settling. Cont. Shelf Res. 2011, 31, S94–S105. [Google Scholar] [CrossRef]
- Tran, D.; Kuprenas, R.; Strom, K. How do changes in suspended sediment concentration alone influence the size of mud flocs under steady turbulent shearing? Cont. Shelf Res. 2018, 158, 1–14. [Google Scholar] [CrossRef]
- Vlieghe, M.; Coufort-Saudejaud, C.; Liné, A.; Frances, C. QMOM-based population balance model involving a fractal dimension for the flocculation of latex particles. Chem. Eng. Sci. 2016, 155, 65–82. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.N.; Judd, S.J.; Fawcett, N. Flocculation modelling: A review. Water Res. 1999, 33, 1579–1592. [Google Scholar] [CrossRef]
- Sporleder, F.; Borka, Z.; Solsvik, J.; Jakobsen, H.A. On the population balance equation. Rev. Chem. Eng. 2012, 28, 149–169. [Google Scholar] [CrossRef]
- Su, J.; Gu, Z.; Xu, X.Y. Advances in numerical methods for the solution of population balance equations for disperse phase systems. Sci. China Ser. B Chem. 2009, 52, 1063–1079. [Google Scholar] [CrossRef]
- Zhang, J.F.; Zhang, Q.H.; Maa, J.P.Y.; Qiao, G.Q. Lattice Boltzmann simulation of turbulence-induced flocculation of cohesive sediment Topical Collection on the 11th International Conference on Cohesive Sediment Transport. Ocean Dyn. 2013, 63, 1123–1135. [Google Scholar] [CrossRef]
- Maggi, F. Stochastic flocculation of cohesive sediment: Analysis of floc mobility within the floc size spectrum. Water Resour. Res. 2008, 44, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.J.; Son, M.; Lee, G.H. Stochastic flocculation model for cohesive sediment suspended in water. Water 2015, 7, 2527–2541. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Lin, M.; Zhu, Y.; Ha, H.K.; Fettweis, M.; Hou, T.; Toorman, E.A.; Maa, J.P.Y.; Zhang, J. A quasi-Monte Carlo based flocculation model for fine-grained cohesive sediments in aquatic environments. Water Res. 2021, 194, 116953. [Google Scholar] [CrossRef]
- Bubakova, P.; Pivokonsky, M.; Filip, P. Effect of shear rate on aggregate size and structure in the process of aggregation and at steady state. Powder Technol. 2013, 235, 540–549. [Google Scholar] [CrossRef]
- Burban, P.Y.; Lick, W.; Lick, J. The flocculation of fine-grained sediments in estuarine waters. J. Geophys. Res. 1989, 94, 8323–8330. [Google Scholar] [CrossRef]
- Stone, M.; Krishnappan, B.G. Floc morphology and size distributions of cohesive sediment in steady-state flow. Water Res. 2003, 37, 2739–2747. [Google Scholar] [CrossRef]
- Guo, C.; He, Q.; Guo, L.; Winterwerp, J.C. A study of in-situ sediment flocculation in the turbidity maxima of the Yangtze Estuary. Estuar. Coast. Shelf Sci. 2017, 191, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; He, Q. Freshwater flocculation of suspended sediments in the Yangtze River, China. Ocean Dyn. 2011, 61, 371–386. [Google Scholar] [CrossRef]
- Lee, B.J.; Toorman, E.; Fettweis, M. Multimodal particle size distributions of fine-grained sediments: Mathematical modeling and field investigation. Ocean Dyn. 2014, 64, 429–441. [Google Scholar] [CrossRef]
- Lefebvre, J.P.; Ouillon, S.; Vinh, V.D.; Arfi, R.; Panché, J.Y.; Mari, X.; van Thuoc, C.; Torréton, J.P. Seasonal variability of cohesive sediment aggregation in the Bach Dang-Cam Estuary, Haiphong (Vietnam). Geo-Marine Lett. 2012, 32, 103–121. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, Z.; Cai, H.; Tang, H. Suspended sediment dynamics in a well-mixed estuary: The role of high suspended sediment concentration (SSC) from the adjacent sea area. Estuar. Coast. Shelf Sci. 2018, 209, 191–204. [Google Scholar] [CrossRef]
- Li, B.G.; Eisma, D.; Xie, Q.C.; Kalf, J.; Li, Y.; Xia, X. Concentration, clay mineral composition and Coulter counter size distribution of suspended sediment in the turbidity maximum of the Jiaojiang river estuary, Zhejiang, China. J. Sea Res. 1999, 42, 105–116. [Google Scholar] [CrossRef]
- Eisma, D.; Li, A. Changes in suspended-matter floc size during the tidal cycle in the Dollard estuary. Netherlands J. Sea Res. 1993, 31, 107–117. [Google Scholar] [CrossRef]
- Braithwaite, K.M.; Bowers, D.G.; Nimmo Smith, W.A.M.; Graham, G.W. Controls on floc growth in an energetic tidal channel. J. Geophys. Res. Ocean. 2012, 117, 1–12. [Google Scholar] [CrossRef]
- Chang, T.S.; Joerdel, O.; Flemming, B.W.; Bartholomä, A. The role of particle aggregation/disaggregation in muddy sediment dynamics and seasonal sediment turnover in a back-barrier tidal basin, East Frisian Wadden Sea, southern North Sea. Mar. Geol. 2006, 235, 49–61. [Google Scholar] [CrossRef]
- Singh, V.P.; Sivakumar, B.; Cui, H. Tsallis entropy theory for modeling in water engineering: A review. Entropy 2017, 19, 641. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.L. Entropy and probability concepts in hydraulics. J. Hydraul. Eng. 1987, 113, 583–599. [Google Scholar] [CrossRef]
- Luo, H.; Singh, V.; Schmidt, A. Comparative study of 1D entropy-based and conventional deterministic velocity distribution equations for open channel flows. J. Hydrol. 2018, 563, 679–693. [Google Scholar] [CrossRef]
- Mihailović, D.; Mimić, G.; Gualtieri, P.; Arsenić, I.; Gualtieri, C. Randomness representation of turbulence in canopy flows using Kolmogorov complexity measures. Entropy 2017, 19, 519. [Google Scholar] [CrossRef] [Green Version]
- Kundu, S. Derivation of different suspension equations in sediment-laden flow from Shannon entropy. Stoch. Environ. Res. Risk Assess. 2018, 32, 563–576. [Google Scholar] [CrossRef]
- Khozani, Z.S.; Wan Mohtar, W.H.M. Investigation of new Tsallis-based equation to predict shear stress distribution in circular and trapezoidal channels. Entropy 2019, 21, 1046. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.P. Entropy theory for movement of moisture in soils. Water Resour. Res. 2010, 46, 1–12. [Google Scholar] [CrossRef]
- Singh, V.P.; Byrd, A.; Cui, H. Flow Duration Curve Using Entropy Theory. J. Hydrol. Eng. 2014, 19, 1340–1348. [Google Scholar] [CrossRef]
- Singh, V.P.; Cui, H.; Byrd, A. Sediment Graphs Based on Entropy Theory. J. Hydrol. Eng. 2015, 20, 1–10. [Google Scholar] [CrossRef]
- Singh, V.P.; Cui, H.; Byrd, A.R. Derivation of rating curve by the Tsallis entropy. J. Hydrol. 2014, 513, 342–352. [Google Scholar] [CrossRef]
- Zhu, Z.; Hei, P.; Dou, J.; Peng, D. Evaluating different methods for determining the velocity-dip position over the entire cross section and at the centerline of a rectangular open channel. Entropy 2020, 22, 605. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Vowinckel, B.; Hsu, T.J.; Köllner, T.; Bai, B.; Meiburg, E. An efficient cellular flow model for cohesive particle flocculation in turbulence. J. Fluid Mech. 2020, 889, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z. A simple explicit expression for the flocculation dynamics modeling of cohesive sediment based on entropy considerations. Entropy 2018, 20, 845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Gao, B. Effect of shear conditions on floc properties and membrane fouling in coagulation/ultrafiltration hybrid process-The significance of Al b species. J. Memb. Sci. 2012, 415–416, 153–160. [Google Scholar] [CrossRef]
- Wu, B.; Zheng, S.; Thorne, C.R. A general framework for using the rate law to simulate morphological response to disturbance in the fluvial system. Prog. Phys. Geogr. 2012, 36, 575–597. [Google Scholar] [CrossRef]
- Jing, H.; Zhong, D.; Zhang, H.; Shi, X.; Wang, Y. The accumulation phenomenon and stochastic model in fluvial processes. Acta Geogr. Sin. 2020, 75, 1079–1094. [Google Scholar] [CrossRef]
- Tsai, C.H.; Iacobellis, S.; Lick, W. Flocculation of Fine-Grained Lake Sediments Due to a Uniform Shear Stress. J. Great Lakes Res. 1987, 13, 135–146. [Google Scholar] [CrossRef]
- Colomer, J.; Peters, F.; Marrasé, C. Experimental analysis of coagulation of particles under low-shear flow. Water Res. 2005, 39, 2994–3000. [Google Scholar] [CrossRef] [Green Version]
- Serra, T.; Casamitjana, X. Structure of the aggregates during the process of aggregation and breakup under a shear flow. J. Colloid Interface Sci. 1998, 206, 505–511. [Google Scholar] [CrossRef]
- Serra, T.; Colomer, J.; Casamitjana, X. Aggregation and breakup of particles in a shear flow. J. Colloid Interface Sci. 1997, 187, 466–473. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhao, M.; Yang, T.S. Review on experimental research on effect of shear flow on flocculation of cohesive sediment. J. Sedi. Res. 2010, 4, 3–10. [Google Scholar] [CrossRef]
- Rau, M.J.; Ackleson, S.G.; Smith, G.B. Effects of turbulent aggregation on clay floc breakup and implications for the oceanic environment. PLoS ONE 2018, 13, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Verney, R.; Lafite, R.; Brun-cottan, J.C.; Le, P. Behaviour of a floc population during a tidal cycle: Laboratory experiments and numerical modelling. Cont. Shelf Res. 2011, 31, S64–S83. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Wang, D.; Riemer, N. An idealized model study of flocculation on sediment trapping in an estuarine turbidity maximum. Cont. Shelf Res. 2010, 30, 1314–1323. [Google Scholar] [CrossRef]
- Choi, S.M.; Seo, J.Y.; Ha, H.K.; Lee, G. hong Estimating effective density of cohesive sediment using shape factors from holographic images. Estuar. Coast. Shelf Sci. 2018, 215, 144–151. [Google Scholar] [CrossRef]
- Li, W.; Yang, S.; Hu, J.; Fu, X.; Zhang, P. Field measurements of settling velocities of fine sediments in Three Gorges Reservoir using ADV. Int. J. Sediment Res. 2016, 31, 237–243. [Google Scholar] [CrossRef]
- Liu, W.C. Modeling the influence of settling velocity on cohesive sediment transport in Tanshui River estuary. Environ. Geol. 2005, 47, 535–546. [Google Scholar] [CrossRef]
- Sherwood, C.R.; Aretxabaleta, A.L.; Harris, C.K.; Rinehimer, J.P.; Verney, R.; Sherwood, C.C.R. Cohesive and mixed sediment in the Regional Ocean Modeling System (ROMS v3. 6) implemented in the Coupled Ocean—Atmosphere—Wave—Sediment Transport Modeling System (COAWST r1234). Geosci. Model Dev. 2018, 11, 1849–1871. [Google Scholar] [CrossRef] [Green Version]
- Forsberg, P.L.; Skinnebach, K.H.; Becker, M.; Ernstsen, V.B.; Kroon, A.; Andersen, T.J. The influence of aggregation on cohesive sediment erosion and settling. Cont. Shelf Res. 2018, 171, 52–62. [Google Scholar] [CrossRef]
- Manning, A.J.; Bass, S.J. Variability in cohesive sediment settling fluxes: Observations under different estuarine tidal conditions. Mar. Geol. 2006, 235, 177–192. [Google Scholar] [CrossRef]
- Shang, Q.Q.; Fang, H.W.; Zhao, H.M.; He, G.J.; Cui, Z.H. Biofilm effects on size gradation, drag coefficient and settling velocity of sediment particles. Int. J. Sediment Res. 2014, 29, 471–480. [Google Scholar] [CrossRef]
- Strom, K.; Keyvani, A. An Explicit Full-Range Settling Velocity Equation for Mud Flocs. J. Sediment. Res. 2011, 81, 921–934. [Google Scholar] [CrossRef]
- Maerz, J.; Verney, R.; Wirtz, K.; Feudel, U. Modeling flocculation processes: Intercomparison of a size class-based model and a distribution-based model. Cont. Shelf Res. 2011, 31, S84–S93. [Google Scholar] [CrossRef] [Green Version]
- Mietta, F.; Chassagne, C.; Verney, R.; Winterwerp, J.C. On the behavior of mud floc size distribution: Model calibration and model behavior. Ocean Dyn. 2011, 61, 257–271. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Lee, B.J.; Fettweis, M.; Toorman, E.A. A tri-modal flocculation model coupled with TELEMAC for estuarine muds both in the laboratory and in the field. Water Res. 2018, 145, 473–486. [Google Scholar] [CrossRef]
- Keyvani, A.; Strom, K. Flocculation in a decaying shear field and its applications for mud removal in near-field river mouth discharges. J. Geophys. Res. Ocean 2016, 121, 2142–2162. [Google Scholar] [CrossRef]
- Badireddy, A.R.; Chellam, S.; Gassman, P.L.; Engelhard, M.H.; Lea, A.S.; Rosso, K.M. Role of extracellular polymeric substances in bioflocculation of activated sludge microorganisms under glucose-controlled conditions. Water Res. 2010, 44, 4505–4516. [Google Scholar] [CrossRef]
- Deng, Z.; He, Q.; Safar, Z.; Chassagne, C. The role of algae in fine sediment flocculation: In-situ and laboratory measurements. Mar. Geol. 2019, 413, 71–84. [Google Scholar] [CrossRef]
- Fang, H.; Chen, Y.; Huang, L.; He, G. Biofilm growth on cohesive sediment deposits: Laboratory experiment and model validation. Hydrobiologia 2017, 799, 261–274. [Google Scholar] [CrossRef]
- Shen, X.; Toorman, E.A.; Lee, B.J.; Fettweis, M. Biophysical flocculation of suspended particulate matters in Belgian coastal zones. J. Hydrol. 2018, 567, 238–252. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Dou, J. An Extended Entropic Model for Cohesive Sediment Flocculation in a Piecewise Varied Shear Environment. Entropy 2021, 23, 1263. https://doi.org/10.3390/e23101263
Zhu Z, Dou J. An Extended Entropic Model for Cohesive Sediment Flocculation in a Piecewise Varied Shear Environment. Entropy. 2021; 23(10):1263. https://doi.org/10.3390/e23101263
Chicago/Turabian StyleZhu, Zhongfan, and Jie Dou. 2021. "An Extended Entropic Model for Cohesive Sediment Flocculation in a Piecewise Varied Shear Environment" Entropy 23, no. 10: 1263. https://doi.org/10.3390/e23101263
APA StyleZhu, Z., & Dou, J. (2021). An Extended Entropic Model for Cohesive Sediment Flocculation in a Piecewise Varied Shear Environment. Entropy, 23(10), 1263. https://doi.org/10.3390/e23101263








