Bivariate Entropy Analysis of Electrocardiographic RR–QT Time Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Data and Subjects
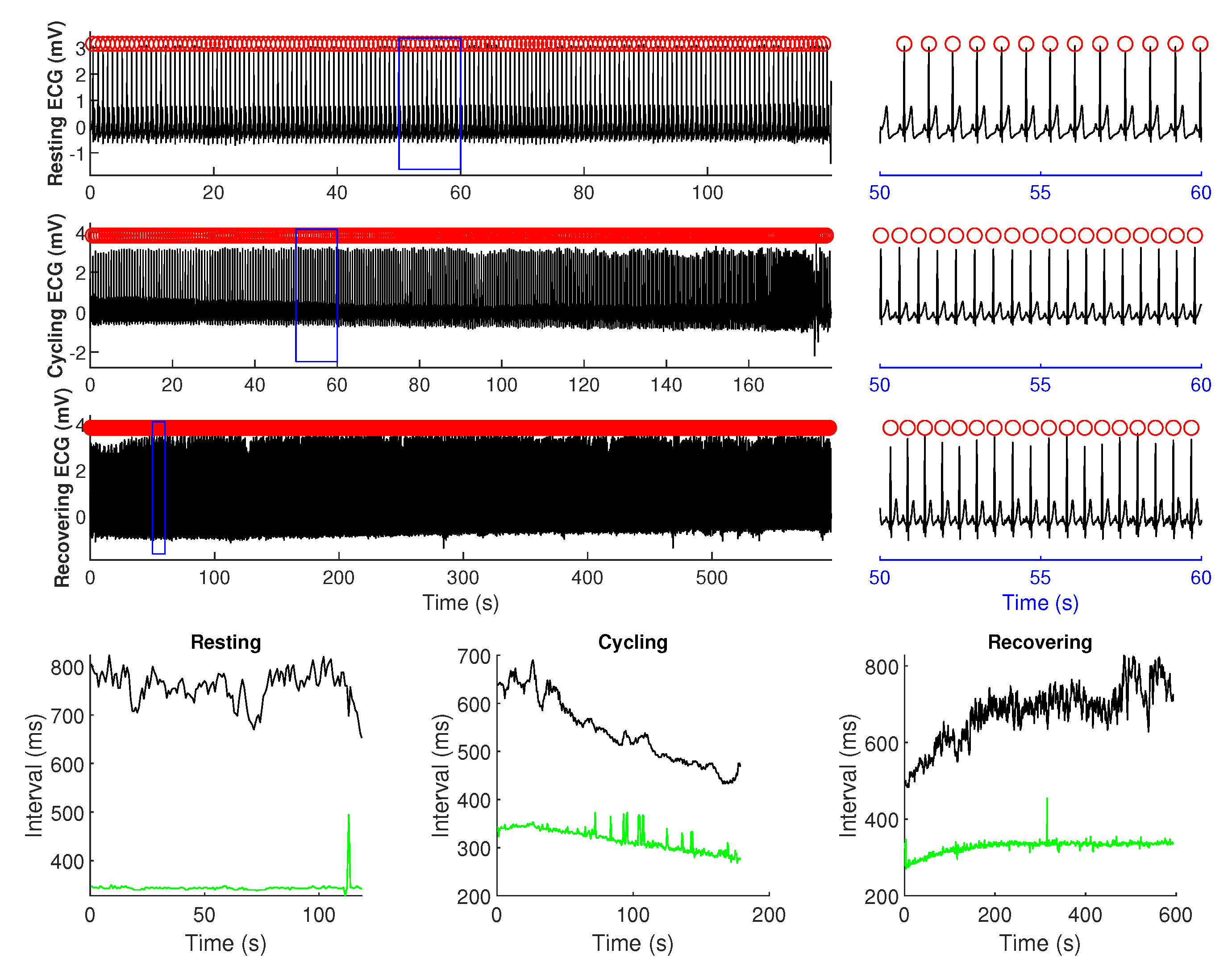
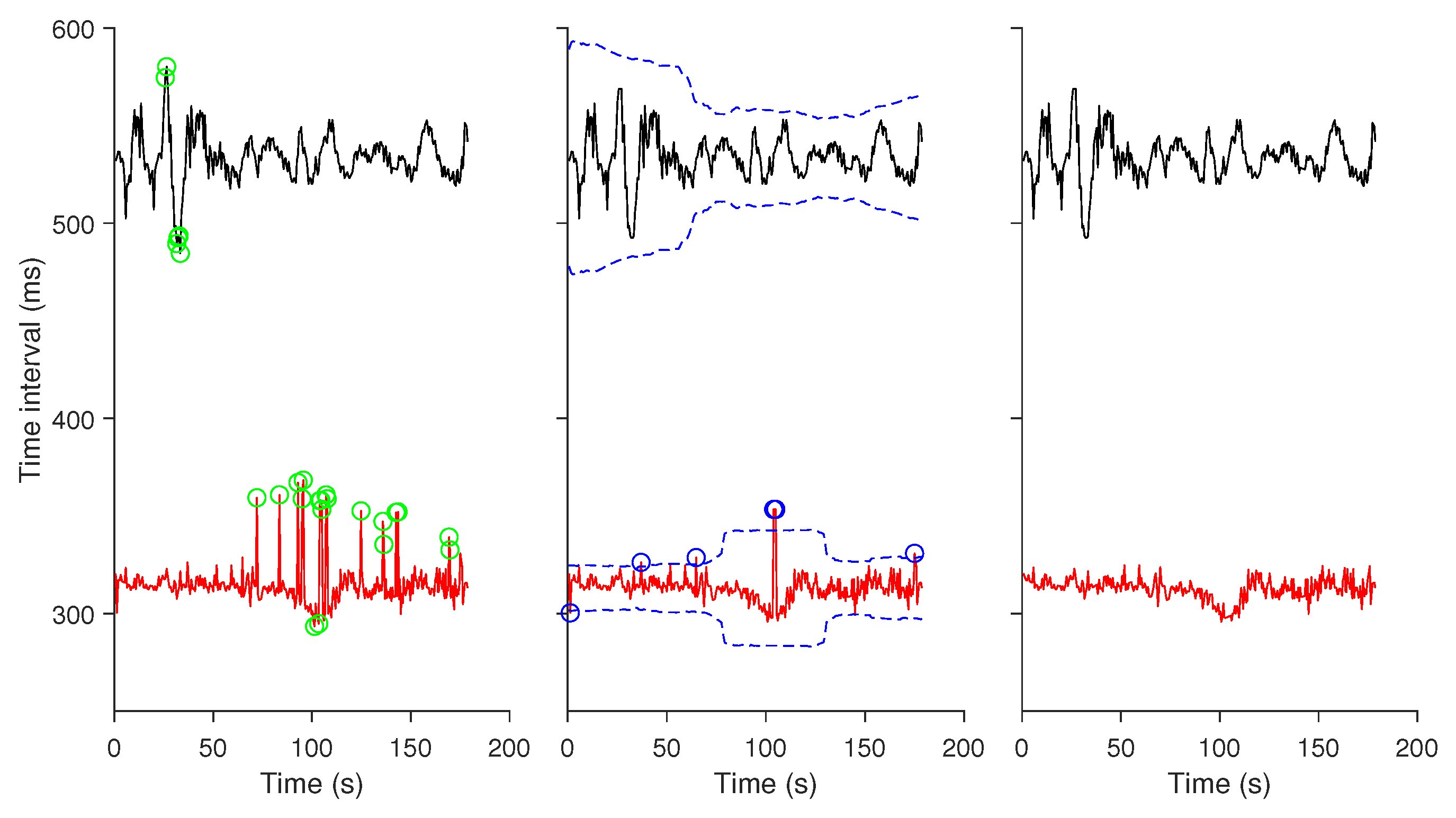
2.2. Data Preprocessing
2.3. Bivariate Entropy Analysis of RR–QT Intervals Time Series
2.3.1. Cross Sample Entropy (XSampEn)
2.3.2. Cross Fuzzy Entropy (XFuzzyEn)
2.3.3. Cross Conditional Entropy (XCE)
2.3.4. Joint Distribution Entropy (JDistEn)
2.3.5. Parameter Selection
2.4. Statistical Analysis
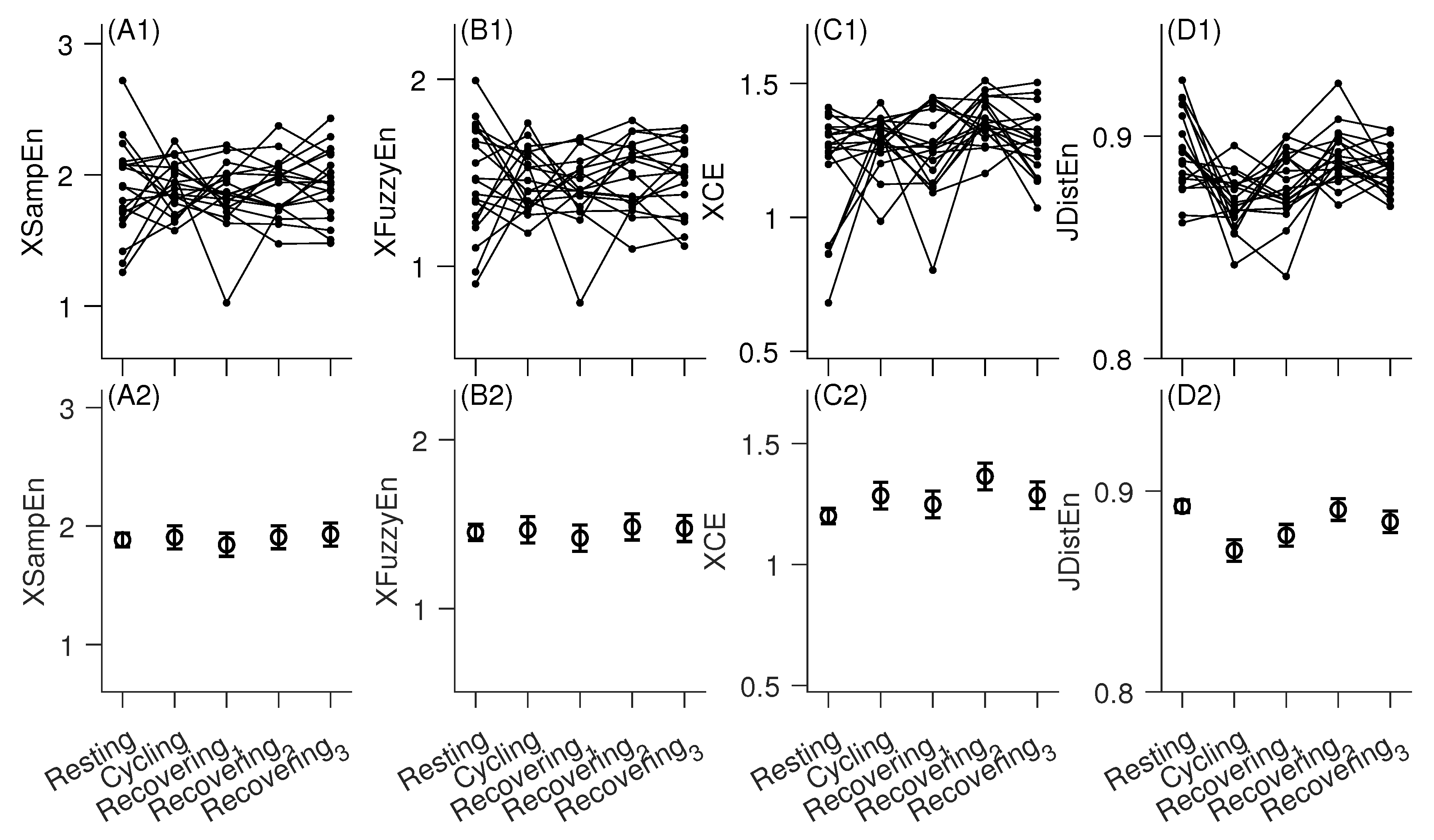
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Atiga, W.L.; Calkins, H.; Lawrence, J.H.; Tomaselli, G.F.; Smith, J.M.; Berger, R.D. Beat-to-beat repolarization lability identifies patients at risk for sudden cardiac death. J. Cardiovasc. Electrophysiol. 1998, 9, 899–908. [Google Scholar] [CrossRef]
- Thomsen, M.B.; Volders, P.G.; Beekman, J.D.; Matz, J.; Vos, M.A. Beat-to-beat variability of repolarization determines proarrhythmic outcome in dogs susceptible to drug-induced torsades de pointes. J. Am. Coll. Cardiol. 2006, 48, 1268–1276. [Google Scholar] [CrossRef]
- Baumert, M.; Porta, A.; Vos, M.A.; Malik, M.; Couderc, J.P.; Laguna, P.; Piccirillo, G.; Smith, G.L.; Tereshchenko, L.G.; Volders, P.G. QT interval variability in body surface ECG: Measurement, physiological basis, and clinical value: Position statement and consensus guidance endorsed by the European Heart Rhythm Association jointly with the ESC Working Group on Cardiac Cellular Electrophysiology. Europace 2016, 18, 925–944. [Google Scholar]
- van Duijvenboden, S.; Ramírez, J.; Young, W.J.; Mifsud, B.; Orini, M.; Tinker, A.; Munroe, P.B.; Lambiase, P.D. Genetic Basis and Prognostic Value of Exercise QT Dynamics. Circ. Genom. Precis. Med. 2020. [Google Scholar] [CrossRef]
- Susmano, A. Effect of heart rate and autonomic tone on the QT interval. Circulation 1982, 66, 478. [Google Scholar] [CrossRef]
- Baumert, M.; Schlaich, M.P.; Nalivaiko, E.; Lambert, E.; Sari, C.I.; Kaye, D.M.; Elser, M.D.; Sanders, P.; Lambert, G. Relation between QT interval variability and cardiac sympathetic activity in hypertension. Am. J. Physiol.-Heart Circ. Physiol. 2011, 300, H1412–H1417. [Google Scholar] [CrossRef]
- Acharya, U.R.; Joseph, K.P.; Kannathal, N.; Min, L.C.; Suri, J.S. Heart rate variability. In Advances in Cardiac Signal Processing; Springer: Berlin/Heidelberg, Germany, 2007; pp. 121–165. [Google Scholar]
- Buccelletti, E.; Gilardi, E.; Scaini, E.; Galiuto, L.; Persiani, R.; Biondi, A.; Basile, F.; Silveri, N.G. Heart rate variability and myocardial infarction: Systematic literature review and metanalysis. Eur. Rev. Med. Pharmacol. Sci. 2009, 13, 299–307. [Google Scholar]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J.F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010, 141, 122–131. [Google Scholar] [CrossRef]
- Scully, C.G.; Lee, J.; Meyer, J.; Gorbach, A.M.; Granquist-Fraser, D.; Mendelson, Y.; Chon, K.H. Physiological parameter monitoring from optical recordings with a mobile phone. IEEE Trans. Biomed. Eng. 2012, 59, 303–306. [Google Scholar] [CrossRef]
- Malik, M.; Färbom, P.; Batchvarov, V.; Hnatkova, K.; Camm, A. Relation between QT and RR intervals is highly individual among healthy subjects: Implications for heart rate correction of the QT interval. Heart 2002, 87, 220–228. [Google Scholar] [CrossRef]
- Batchvarov, V.N.; Ghuran, A.; Smetana, P.; Hnatkova, K.; Harries, M.; Dilaveris, P.; Camm, A.J.; Malik, M. QT-RR relationship in healthy subjects exhibits substantial intersubject variability and high intrasubject stability. Am. J. Physiol.-Heart Circ. Physiol. 2002, 282, H2356–H2363. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Li, L.; Desta, Z.; Malik, M.; Flockhart, D. Variability of heart rate correction methods for the QT interval. Br. J. Clin. Pharmacol. 2003, 55, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Halamek, J.; Jurak, P.; Villa, M.; Novak, M.; Vondra, V.; Soucek, M.; Frana, P.; Somers, V.; Kara, T. Dynamic QT/RR coupling in patients with pacemakers. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 919–922. [Google Scholar]
- Halamek, J.; Couderc, J.P.; Jurak, P.; Vondra, V.; Zareba, W.; Viscor, I.; Leinveber, P. Measure of the QT–RR dynamic coupling in patients with the long QT syndrome. Ann. Noninvasive Electrocardiol. 2012, 17, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Zhang, Y.; Yuan, C.; Wang, S.; Li, P. Entropy analysis of short-term heartbeat interval time series during regular walking. Entropy 2017, 19, 568. [Google Scholar] [CrossRef]
- Li, P. EZ Entropy: A software application for the entropy analysis of physiological time-series. Biomed. Eng. Online 2019, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Porta, A.; Baselli, G.; Lombardi, F.; Montano, N.; Malliani, A.; Cerutti, S. Conditional entropy approach for the evaluation of the coupling strength. Biol. Cybern. 1999, 81, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol.-Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef]
- Xie, H.B.; Zheng, Y.P.; Guo, J.Y.; Chen, X. Cross-fuzzy entropy: A new method to test pattern synchrony of bivariate time series. Inf. Sci. 2010, 180, 1715–1724. [Google Scholar] [CrossRef]
- Li, P.; Li, K.; Liu, C.; Zheng, D.; Li, Z.M.; Liu, C. Detection of coupling in short physiological series by a joint distribution entropy method. IEEE Trans. Biomed. Eng. 2016, 63, 2231–2242. [Google Scholar] [CrossRef]
- Li, P.; Liu, C.; Zhang, M.; Che, W.; Li, J. A Real-Time QRS Complex Detection Method. Acta Biophys. Sin. 2011, 27, 222–230. [Google Scholar] [CrossRef]
- Berger, R.D.; Kasper, E.K.; Baughman, K.L.; Marban, E.; Calkins, H.; Tomaselli, G.F. Beat-to-beat QT interval variability: Novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation 1997, 96, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, C.; Shi, B.; Liu, C.; Karmakar, C.; Li, P. Does the Temporal Asymmetry of Short-Term Heart Rate Variability Change during Regular Walking? A Pilot Study of Healthy Young Subjects. Comput. Math. Methods Med. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- McNames, J.; Thong, T.; Aboy, M. Impulse rejection filter for artifact removal in spectral analysis of biomedical signals. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004; Volume 1, pp. 145–148. [Google Scholar]
- Xie, H.B.; He, W.X.; Liu, H. Measuring time series regularity using nonlinear similarity-based sample entropy. Phys. Lett. A 2008, 372, 7140–7146. [Google Scholar] [CrossRef]
- Chen, W.; Zhuang, J.; Yu, W.; Wang, Z. Measuring complexity using fuzzyen, apen, and sampen. Med. Eng. Phys. 2009, 31, 61–68. [Google Scholar] [CrossRef]
- Xie, H.B.; Guo, J.Y.; Zheng, Y.P. Fuzzy approximate entropy analysis of chaotic and natural complex systems: Detecting muscle fatigue using electromyography signals. Ann. Biomed. Eng. 2010, 38, 1483–1496. [Google Scholar] [CrossRef]
- Lau, C.P.; Freedman, A.R.; Fleming, S.; Malik, M.; Camm, A.J.; Ward, D.E. Hysteresis of the ventricular paced QT interval in response to abrupt changes in pacing rate. Cardiovasc. Res. 1988, 22, 67–72. [Google Scholar] [CrossRef]
- Magnano, A.R.; Holleran, S.; Ramakrishnan, R.; Reiffel, J.A.; Bloomfield, D.M. Autonomic nervous system influences on QT interval in normal subjects. J. Am. Coll. Cardiol. 2002, 39, 1820–1826. [Google Scholar] [CrossRef]
- Lewis, M.J.; Rassi, D.; Short, A. Analysis of the QT interval and its variability in healthy adults during rest and exercise. Physiol. Meas. 2006, 27, 1211. [Google Scholar] [CrossRef]
- Hasan, M.A.; Abbott, D.; Baumert, M. Relation between Beat-to-Beat QT Interval Variability and T-Wave Amplitude in Healthy Subjects. Ann. Noninvasive Electrocardiol. 2012, 17, 195–203. [Google Scholar] [CrossRef]
| XSampEn | XFuzzyEn | XCE | JDistEn | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | Mean ± SE | p | Mean ± SE | p | Mean± SE | p | Mean ± SE | p |
| Intercept | 1.91 ± 0.07 | - | 1.47 ± 0.05 | - | 1.19 ± 0.04 | - | 0.892 ± 0.003 | - |
| Sex (male) | −0.05 ± 0.07 | 0.51 | −0.04 ± 0.06 | 0.49 | 0.03 ± 0.03 | 0.44 | 0.002 ± 0.003 | 0.60 |
| Age | −0.02 ± 0.03 | 0.62 | −0.01 ± 0.03 | 0.61 | 0.00 ± 0.01 | 0.83 | −0.003 ± 0.001 | 0.01 |
| Cycling | 0.02 ± 0.08 | 0.78 | 0.01 ± 0.06 | 0.82 | 0.08 ± 0.04 | 0.07 | −0.022 ± 0.004 | <0.0001 |
| Recovering phase 1 | −0.04 ± 0.08 | 0.60 | −0.03 ± 0.06 | 0.58 | 0.05 ± 0.04 | 0.28 | −0.014 ±0.004 | 0.001 |
| Recovering phase 2 | 0.02 ± 0.08 | 0.77 | 0.03 ± 0.06 | 0.60 | 0.16 ± 0.04 | 0.0004 | −0.002 ± 0.04 | 0.70 |
| Recovering phase 3 | 0.05 ± 0.08 | 0.55 | 0.02 ± 0.06 | 0.71 | 0.08 ± 0.04 | 0.06 | −0.008 ± 0.004 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, B.; Motin, M.A.; Wang, X.; Karmakar, C.; Li, P. Bivariate Entropy Analysis of Electrocardiographic RR–QT Time Series. Entropy 2020, 22, 1439. https://doi.org/10.3390/e22121439
Shi B, Motin MA, Wang X, Karmakar C, Li P. Bivariate Entropy Analysis of Electrocardiographic RR–QT Time Series. Entropy. 2020; 22(12):1439. https://doi.org/10.3390/e22121439
Chicago/Turabian StyleShi, Bo, Mohammod Abdul Motin, Xinpei Wang, Chandan Karmakar, and Peng Li. 2020. "Bivariate Entropy Analysis of Electrocardiographic RR–QT Time Series" Entropy 22, no. 12: 1439. https://doi.org/10.3390/e22121439
APA StyleShi, B., Motin, M. A., Wang, X., Karmakar, C., & Li, P. (2020). Bivariate Entropy Analysis of Electrocardiographic RR–QT Time Series. Entropy, 22(12), 1439. https://doi.org/10.3390/e22121439







