Identification of the Framingham Risk Score by an Entropy-Based Rule Model for Cardiovascular Disease
Abstract
1. Introduction
1.1. Research Background and Research Problem
1.2. Research Gaps and Motivations
1.3. Study Goals and Research Objective
2. Related Work
2.1. Cardiovascular Disease and Its Identification Applications
- (1)
- Hypertension: Blood pressure refers to the arterial blood pressure based on the vessel wall and is divided into two types: diastolic blood pressure (DBP) and systolic blood pressure (SBP). According to a WHO standard, normal SBP is between 90 and 140 mmHg, and DBP is between 50 and 90 mmHg. With hypertension, the value of SBP/DBP exceeds 140/90 mmHg. Based on a report from the USA entitled “Hypertension prevention, detection, and treatment criteria”, SBP has an average of 140 mmHg, but DBP is over 90 mmHg [18]. In particular, McManus et al. [19] showed by a meta-analysis that the primary prevention of CVD consisted of treating patients with blood pressure under 140/90 mmHg. Thus, hypertension is a risk factor for CVD, and it is important to lower blood pressure.
- (2)
- Hyperlipidemia: Hyperlipidemia refers to a higher-than-normal concentration of cholesterol or triglycerides in the blood [20]. Dyslipidemia for non-normal hyperlipidemia is the major cause of atherosclerosis with high cholesterol, high triglycerides, and their combination, which may increase the probability of coronary and heart diseases, and results in CVD. Moreover, Burkhardt [20] indicated that elevated plasma levels of low-density lipoprotein (LDL) are modifiable major risk factors for atherosclerotic CVD. However, high-density lipoprotein (HDL) is a good factor for identifying CVD. Thus, subsequent researchers should identify and measure crucial factors causing LDL and HDL in Taiwan’s CVD patients.
- (3)
- Tobacco: Tobacco use multiplies the risk of CVD through a number of negative mechanisms [21], such as damaging the endothelium of the blood vessels, increasing fatty deposits of arteries, increasing clotting, raising LDL-cholesterol (LDL-C) and lowering HDL-cholesterol (HDL-C), and promoting coronary artery spasms. Banks et al. [21] noted that tobacco use is a major source of CVD mortality and morbidity. In particular, nicotine, an addictive component of tobacco, raises blood pressure and accelerates the heart rate. According to a report from the World Heart Federation [22], if one started smoking as a child, the potential risk of suffering CVD is many times higher compared to those who began as a legal adult. Furthermore, passive smoking is harmful and increases the risk of CVD; chewing tobacco and taking snuff are also dangerous.
2.2. Framingham Risk Score
2.3. Classification Technology
2.3.1. Rough Set Theory and Its Practical Applications
- (1)
- Defining the problem faced: The first stage in RST is to define the problem to be faced by explaining and forming the decisional attributes by means of condition attributes and creating decisional rule-set units for future use following the function of classification parts.
- (2)
- Preprocessing the data collected: In this step, data values collected from the units of both the condition attributes and decision attributes are preprocessed. RST allows these attributes to represent types of categorical data; therefore, if there are continuous data in the condition attributes of a given dataset, they can be first discretized into a meaningful form by applying various transforming algorithms [41].
- (3)
- Forming the information and decision systems: The decisional information system (DIS) is framed in accordance with the data collected. A DIS is a table that defines the units of condition attributes, and this table is built with the decision attribute in the last column.
- (4)
- Defining the indiscernibility relations and setting approximations: First, the data used to address the decisional problems occur in a DIS by determining the relationships of indiscernibility. However, the derived system may encounter larger scales of tables due to excessive attributes or unnecessary attributes that repeat similar parts, which has a negative impact on the classification performance of the model. Thus, this negative effect should be reduced as much as possible against the given data of attributes. Second, defining the condition attributes and the decision attributes with the current properties to a certain class after using the relationships of indiscernibility should not be specific for determining these set approximations. In this case, the unit that completely belongs to a given set or the unit that possibly belongs to that set is defined by the functions of the β-upper and β-lower approximations of spaces.
- (5)
- Defining the sets reduced: Attributes in a DIS are filtered from the table without losing their fundamental properties. We assume that one attribute a belongs to the B set, where B ⊆ A. For the B set, if I(B) = I(B − {a}), then attribute a is unnecessary; however, for a specific case in which the above formula is unfulfilled, attribute a is necessary from the B set [42]. The unnecessary attributes are removed from the subsets, and these are then called the reduced sets [43].
- (6)
- Forming decisional rules: Decisional rules are formed and produced by accounting for the attributes of reduced sets [44], and their propositions highly depend on the values of the condition attributes against the decision attribute. Importantly, each decisional rule-set has an accuracy percentage, and the total accuracy rate can then be acquired.
2.3.2. Decision Tree and Its Real Applications
2.3.3. Random Forest and Its Practical Applications
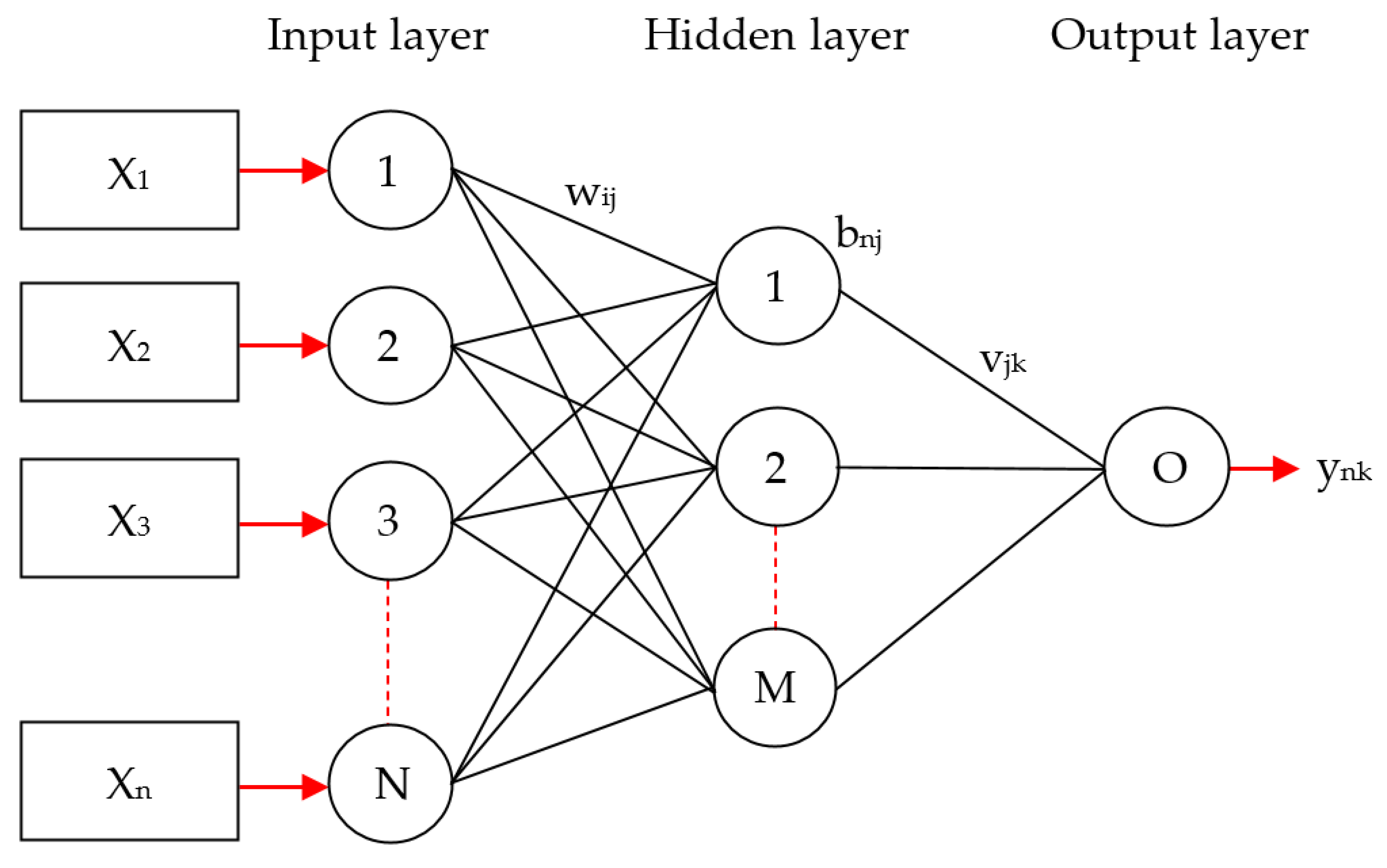
2.3.4. Multilayer Perceptron and Its Applications
2.3.5. Support Vector Machine and Its Industrial Applications
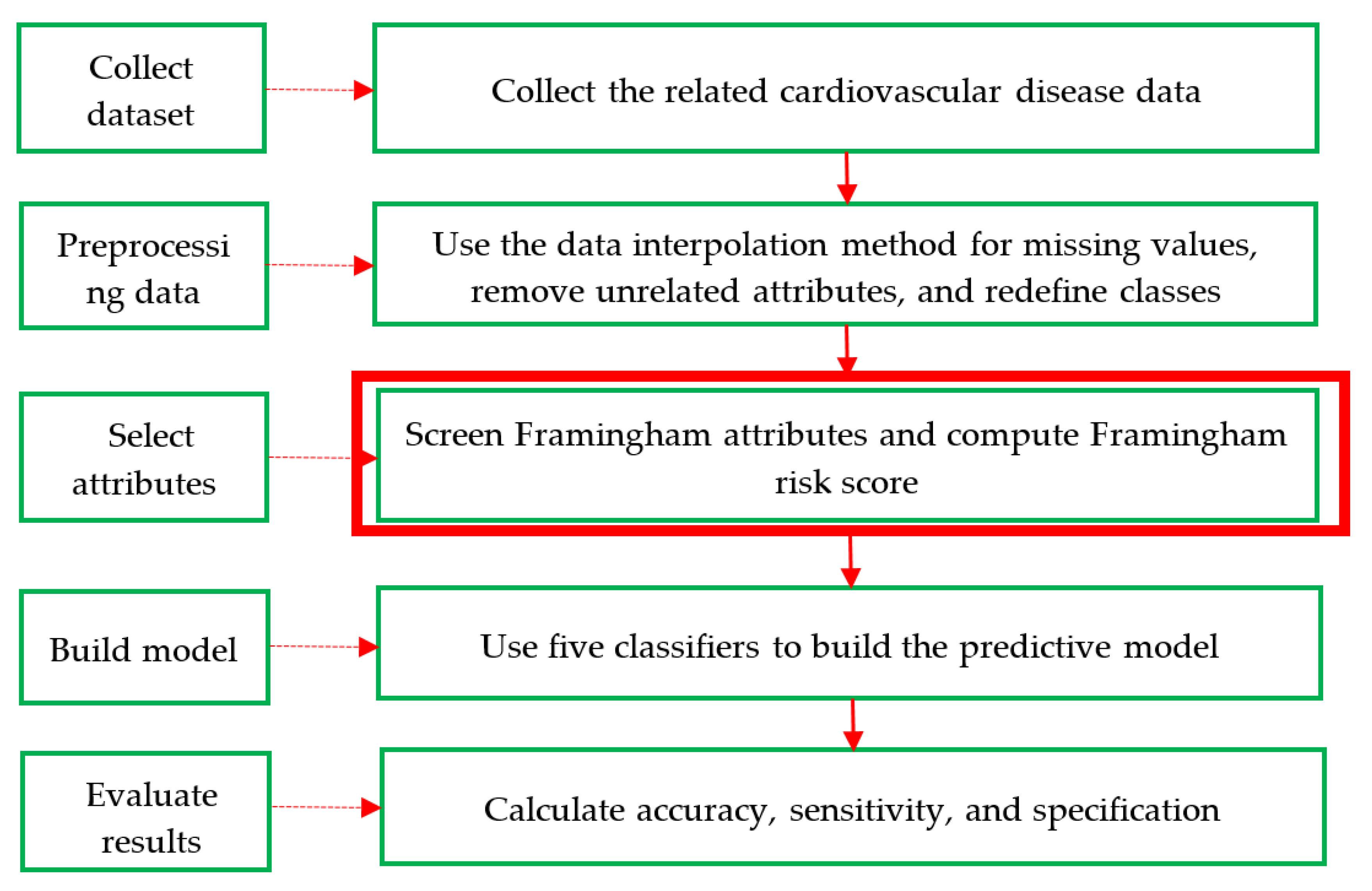
3. Proposed Method
- Step 1: Collect dataset. An adequate dataset is key in the identification of CVD; thus, this study compiled two datasets with CVD data. The first was compiled from a real prevention and treatment plan of CVD in Taiwan’s regional teaching hospital and had 20 attributes with 1190 records (including 551 men and 639 women). It is referred to as the CVD dataset in this study. The second dataset is a Framingham public dataset collected with an original 18 attributes and extracted from the University of Washington (http://courses.washington.edu/b513/datasets/datasets.php?class=513) via a uniform resource locator. It is referred to as the Framingham dataset.
- Step 2: Preprocessing data. In general, real data may have inconsistencies, gaps, errors, inaccuracies, impossible data combinations, and noise compared to the original dataset. Such data are not suitable for use by machine learning to discover hidden information/knowledge from large datasets. However, this step cannot remove these values. The average values of the data interpolation method are filled with missing values, and the dataset is then reformatted appropriately.
- Step 3: Select attributes. First, the eight attributes of the Framingham risk score are age, gender, DBP, SBP, LDL-C, HDL-C, fasting glucose, and smoking, which are named the Framingham attributes. Next, these factors are selected to calculate and transform their score values based on the conversion method of the Framingham risk score discussed in Section 2.2 [28] and shown in Table 2, Table 3 and Table 4 from the CVD dataset. Finally, all 13 attributes of the CVD dataset are selected and called the full attributes, which additionally include body mass index, waistline, red blood cells, and white blood cells.
- Step 4: Build a model. This step designs a hybrid model of five classifiers (i.e., RST, DT, RF, MLP, and SVM) with various attribute components, which include the Framingham score attributes, the Framingham attributes, and the full attributes, to highlight and differentiate the performance of the proposed method based on a commonly used 67–33% training–testing ratio. This step can be divided into five sub-steps and is executed using different software packages. The procedure of the five sub-steps is as follows: First, the selected attributes are used as input variables. Second, for the percentage-split data method, the two training–testing sub-datasets are formed using the common 2:1 ratio to achieve a good and reasonable result in practice. Thus, 67% of the data is used as a training sub-dataset, and the remaining 33% of the data is used as the testing sub-dataset. Third, all of the default parameters are defined to implement each of the above classifiers. Fourth, RST is applied using the rough set exploration system (RSES) [29], and DT, RF, MLP, and SVM are applied separately. Fifth, comprehensive knowledge-based rule sets are created using DT. For further details, the pseudo-code of the construction of the hybrid model is shown in Algorithm 1.
| Algorithm 1: Pseudo Code of Building Hybrid Model |
| Input: SA = selected attributes list, CD = collected data, RSES = rough set exploration system Use attributes from SA; Create 67% of data for training from CD; Create the other 33% of data for testing from CD; Define all the default parameters; Apply RST classifier by RSES; Apply DT, RF, MLP, and SVM classifiers, respectively; Create tree-based rules sets by DT; Output: knowledge(tree)-based rules sets |
- Step 5: Evaluate the results. To evaluate machine learning, most researchers use three common metrics: accuracy, sensitivity, and specificity. These are defined in Table 7 of the confusion matrix and Equations (7)–(9). In Table 7, a true positive (TP) means the truth is positive, and the prediction is positive; a false positive (FP) means the truth is negative, but the prediction is positive; a false negative (FN) means the truth is positive, but the prediction is negative, and a true negative (TN) means the truth is negative, and the prediction is negative.
4. Experiment Result and Comparisons
4.1. Introduction and Preprocessing of the Two Medical Datasets
4.2. Experimental Results
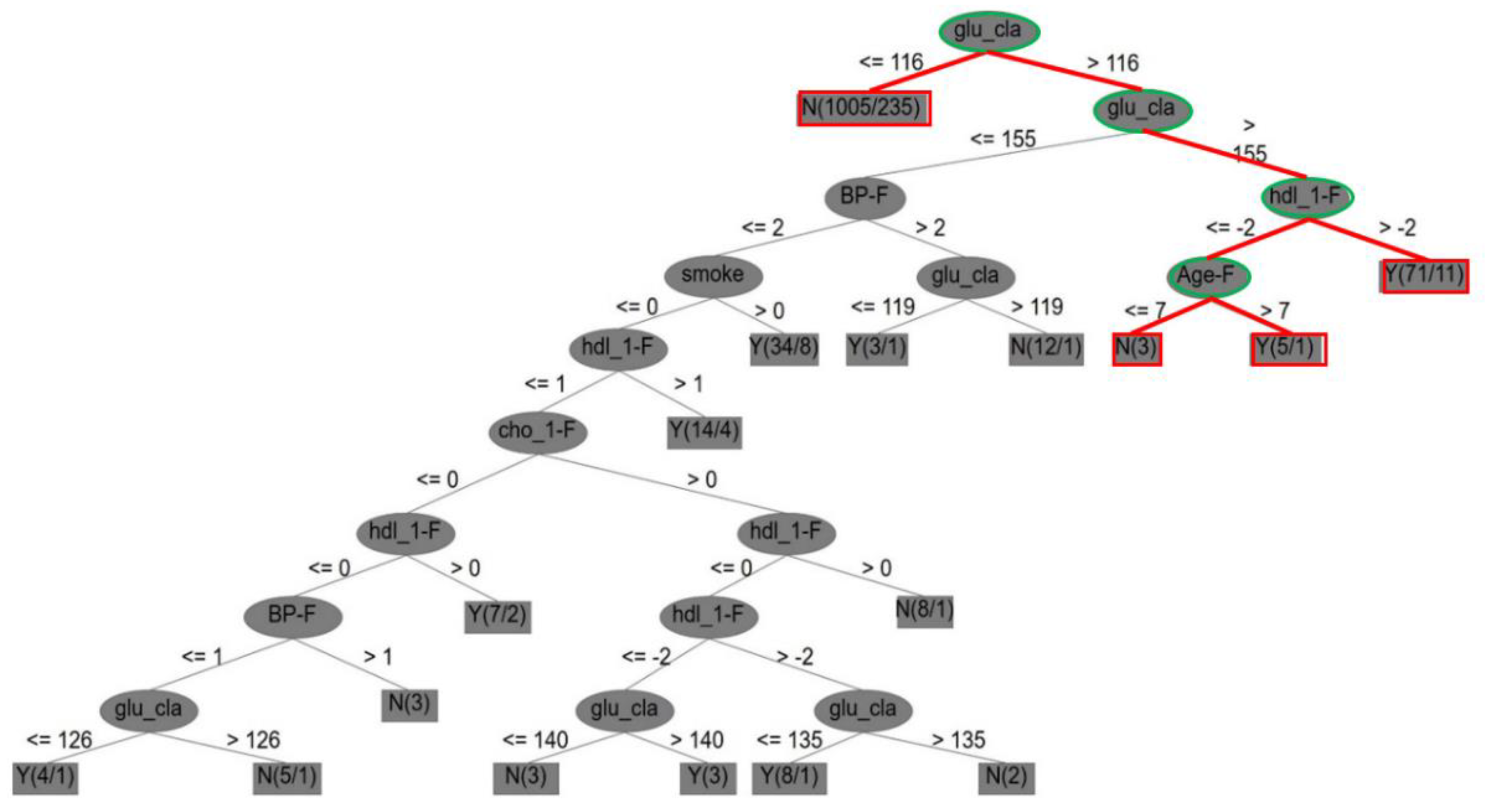
4.2.1. The CVD Dataset
- (1)
- First, it is indicated that, if the score measurement of glu_cla (i.e., diabetes) is higher than 116, glu_cla is also higher than 155, else if glu_cla is less than 116, there is no CVD case; if the score measurement of hdl_1-F (i.e., Framingham high-density cholesterol) is higher than −2, then there is a CVD case; else if Age-F (score of Framingham age) is higher than 7, there is a CVD case, but if it is less than or equal to 7, there is no CVD case.
- (2)
- Second, the core attributes were obtained by dynamic reduction through discrete tables, and seven core attributes for Framingham risks were identified, including sex (i.e., gender), age_F (i.e., age), cho_1-F, hdl_1-F, BP_F, glu_cla, and smoke (i.e., smoking).
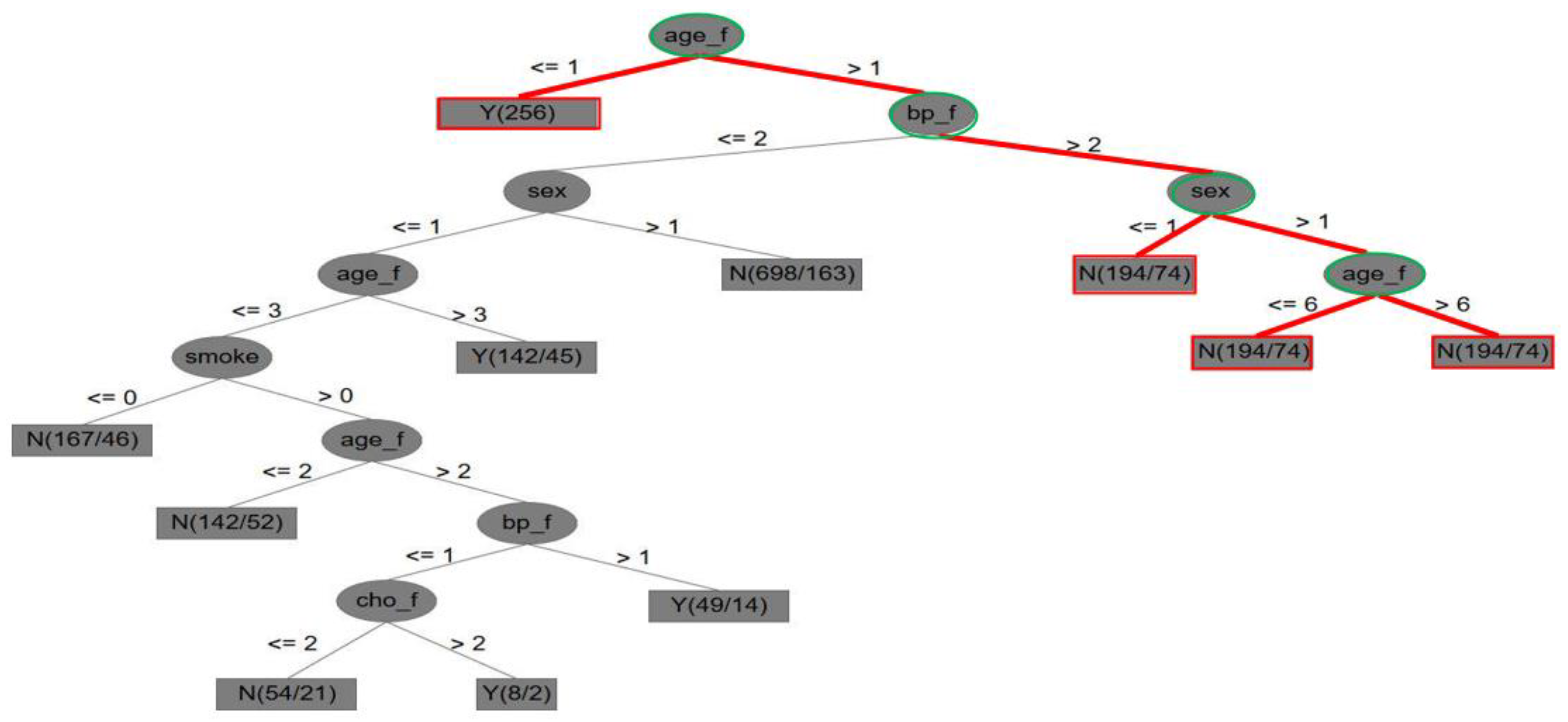
4.2.2. The Framingham Dataset
- (1)
- In Figure 4, it is indicated that, if the score measurement of the Framingham attribute of age (age_f) is higher than 1, else if age is less than 1, there is a CVD case; if the score measurement of the Framingham attribute of blood pressure (bp_f) is higher than 2, and the sex (gender) score is higher than 1, then the age (age_f) score is higher than 6, and there is a CVD case; else if the score of Framingham age is less than or equal to 6, then there is no CVD case.
- (2)
- Five key attributes were also obtained by dynamic reduction through discrete tables: sex, age_f (i.e., age), cho_f, bp_f, and smoke (i.e., smoking).
4.3. Findings
- (1)
- The advantage of Framingham attributes with classification techniques: Although previous studies have used Framingham attributes and statistical methods to process CVD, they did not use a hybrid model to integrate Framingham score attributes and the five noted classification techniques for the identification of CVD or differentiation of various classifier performances. This study closes these gaps, and the proposed method not only aids understanding of comprehensive CVD tree-like entropy-based rules but also helps to prevent or even solve CVD problems. In addition, the score use of Framingham attributes can be an effective clinical reference for doctors and health care workers to improve the identification of CVD. Thus, a key specificity and novelty in this study are that the Framingham risk attribute scores can be calculated and used to produce entropy-based decision rules. This has not been undertaken in previous research based on our limited literature review.
- (2)
- The identification of key attributes for CVD: The key attributes of the original Framingham risk data were identified by Dr. Rupert Payne, University of Edinburgh, and were uniformly compared with those of the first and second medical datasets in the experiments of this study. Table 11 shows their comparative results from the following three perspectives for the CVD issue: (a) Table 11 shows that the key attributes of the Framingham risk, the CVD dataset, and the Framingham dataset are in the order of 9, 7, and 5, respectively, for identifying CVD. It was found that the number of Framingham key attributes significantly and positively affects the overall classification accuracy, which can be proven from the accuracy rates in Table 9 and Table 10. (b) The two datasets are missing key attributes, resulting in poor classification performance. If sufficient key attributes are addressed in the future, overall accuracy can improve. (c) The importance of the key attributes for identifying CVD is listed in a top-down order, which may provide helpful information.
- (3)
- The technical implications of the classifiers used: Based on the experimental results of the two medical datasets, no classifier or model is suitable for all practical datasets used in different applications. Thus, an appropriate classifier must be first found and defined to address specific data in the machine learning community.
- (4)
- The management implications of healthcare issues: Regarding management implications, a set of standards was provided from the entropy-based decision rules of a tree-like structure (e.g., Figure 3 and Figure 4) to help prevent or solve CVD problems in advance. (a) First, this can indirectly remind CVD patients how to self-manage. (b) Second, the experimental results allow doctors to provide patients with managerial suggestions, such as changes in eating habits, self-measurement and self-control of blood pressure, cessation of smoking, and increased exercise. (c) Finally, the Framingham risk attributes used to identify CVD with the classification techniques listed in this study can be regarded as an effective prediction model for processing CVD. The analytical results can be stored for doctors and health personnel as clinical references.
5. Conclusions
- (1)
- According to the empirical results, the CVD and Framingham datasets were processed into useful entropy-based tree information/knowledge by applying five classifiers to knowledge discovery in the databases. Furthermore, machine learning tools were found to be useful in medical applications with the integration of Framingham risk attributes. Regarding the experimental results, the support vector machine method showed a better performance using Framingham score attributes and five classifier techniques in terms of accuracy, sensitivity, and specificity in the CVD dataset. However, the rough set method outperformed the other classifiers in the Framingham dataset. In addition, through the entropy-based decision rule’s visualization of trees, the if–then–else control statement provides an understanding of how all decision items with a class status of “Y” or “N” (i.e., Yes or No) are identified in the two medical datasets to address CVD problems.
- (2)
- Based on the literature review, machine learning and knowledge discovery using medical databases have attracted a significant amount of interest from academia and other fields. Given this interest in CVD applications, this study provides insights into data-mining techniques of machine learning and statistical methods with industry databases.
- (3)
- This study concerns practical CVD applications and is a trial of machine learning techniques. It involves the extraction of helpful entropy-based decision rules, the anticipation of decision-making challenges for real-life applications of knowledge discovery, understanding of current results and insights, and exploration of future research directions.
- (4)
- In Taiwan, the number of CVD patients has increased during the past 10 years due to changes in lifestyle and eating habits. Furthermore, CVD, cerebrovascular disease, diabetes, and hypertension were ranked second, third, fifth, and tenth among the top causes of death. It is clear that CVD is a serious issue; thus, early diagnosis and prevention strategies of CVD are important for healthcare and to decrease the use of medical resources.
- (5)
- Several issues can be examined in subsequent research: (a) to further evaluate the proposed method, more attributes of Framingham risk can be collected to better predict CVD; (b) more classification techniques can be used and measured to predict CVD; (c) an alternative to the proposed method can be constructed with a variety of evaluation standards; and (d) more knowledge-based decision rules, which are not based on a variation of DT entropy, such as RSES of the rough set theory, can be provided for medical applications.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hojat, M.; Jahromi, M.K.; Koshkaki, S.R.; Rahmanian, M. Comparison of risk factors of cardiovascular diseases in male and female nurses. J. Educ. Health Promot. 2019, 8, 19. [Google Scholar] [CrossRef]
- Suárez, C. Baseline characteristics of patients with cerebrovascular disease in the REACH registry: The Spanish contribution. Cerebrovasc. Dis. 2007, 24, 89–95. [Google Scholar] [CrossRef]
- Jahromi, M.K.; Hojat, M.; Koshkaki, S.R.; Nazari, F.; Ragibnejad, M. Risk factors of heart disease in nurses. Iran. J. Nurs. Midwifery Res. 2017, 22, 332–337. [Google Scholar]
- World Health Organization. Cardiovascular Diseases (CVDs). Available online: http://www.who.int/ mediacentre/factsheets/fs317/en/ (accessed on 1 May 2020).
- Ministry of Health and Welfare, Executive Yuan, Taiwan. Available online: http://www.mohw.gov.tw/CHT/ DOS/Statistic.aspx?f_list_no=312&fod_list_no=6201 (accessed on 1 May 2020).
- McPherson, R.; Frohlich, J.; Fodor, G.; Genest, J. Canadian Cardiovascular Society position statement–recommendations for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease. Can. J. Cardiol. 2006, 22, 913–927. [Google Scholar] [CrossRef]
- Simsekler, M.C.E.; Qazi, A.; Alalami, M.; Ellahham, S.; Ozonoff, A. Evaluation of patient safety culture using a random forest algorithm. Reliab. Eng. Syst. Saf. 2020, 204, 107186. [Google Scholar] [CrossRef]
- Sri, M.N.; Priyanka, J.H.; Sailaja, D.; Murthy, M.R. A comparative analysis of breast cancer data set using different classification methods. In Smart Intelligent Computing and Applications; Satapathy, S., Bhateja, V., Das, S., Eds.; Springer: Singapore, 2019; Volume 104. [Google Scholar]
- Amin, M.S.; Chiam, Y.K.; Varathan, K.D. Identification of significant features and data mining techniques in predicting heart disease. Telemat. Inform. 2019, 36, 82–93. [Google Scholar] [CrossRef]
- Nilashi, M.; Ahmadi, N.; Samad, S.; Shahmoradi, L.; Ahmadi, H.; Ibrahim, O.; Asadi, S.; Abdullah, R.; Abumalloh, R.A.; Yadegaridehkordi, E.; et al. Disease diagnosis using machine learning techniques: A review and classification. J. Soft Comput. Decis. Support Syst. 2020, 7, 19–30. [Google Scholar]
- Boursalie, O.; Samavi, R.; Doyle, T.E. M4CVD: Mobile machine learning model for monitoring cardiovascular disease. Proc. Comput. Sci. 2015, 63, 384–391. [Google Scholar] [CrossRef]
- Santos, A.S.A.C.; Rodrigues, A.P.S.; Rosa, L.P.S.; Sarrafzadegan, N.; Silveira, E.A. Cardiometabolic risk factors and Framingham risk score in severely obese patients: Baseline data from DieTBra trial. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A.K.; Colak, C.; Sarihan, M.E. Different medical data mining approaches based prediction of ischemic stroke. Comput. Methods Prog. Biomed. 2016, 130, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Servadio, J.L.; Convertino, M. Optimal information networks: Application for data-driven integrated health in populations. Sci. Adv. 2018, 4, 1701088. [Google Scholar] [CrossRef] [PubMed]
- Mayosi, B.M.; Cupido, B.; Lawrenson, J. 2—Cardiovascular diseases. In Hunter’s Tropical Medicine and Emerging Infectious Diseases, 10th ed.; Ryan, E.T., Hill, D.R., Solomon, T., Endy, T.P., Aronson, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 8–15. [Google Scholar] [CrossRef]
- Yeh, D.Y.; Cheng, C.H.; Chen, Y.W. A predictive model for cerebrovascular disease using data mining. Expert Sys. Apps. 2011, 38, 8970–8977. [Google Scholar] [CrossRef]
- Henriksson, H.; Henriksson, P.; Tynelius, P.; Ekstedt, M.; Berglind, D.; Labayen, I.; Ruiz, J.R.; Lavie, C.J.; Ortega, F.B. Cardiorespiratory fitness, muscular strength, and obesity in adolescence and later chronic disability due to cardiovascular disease: A cohort study of 1 million men. Eur. Heart J. 2020, 41, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Flack, J.M.; Adekola, B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc. Med. 2020, 30, 160–164. [Google Scholar] [CrossRef] [PubMed]
- McManus, R.; Constanti, M.; Floyd, C.N.; Glover, M.; Wierzbicki, A.S. Managing cardiovascular disease risk in hypertension. Lancet 2020, 395, 869–870. [Google Scholar] [CrossRef]
- Burkhardt, R. Hyperlipidemia and cardiovascular disease: New insights on lipoprotein (a). Curr. Opin. Lipidol. 2019, 30, 260–261. [Google Scholar] [CrossRef]
- Banks, E.; Joshy, G.; Korda, R.J.; Stavreski, B.; Soga, K.; Egger, S.J.; Day, C.; Clarke, N.; Lewington, S.; Lopez, A.D.; et al. Tobacco smoking and risk of 36 cardiovascular disease subtypes: Fatal and non-fatal outcomes in a large prospective Australian study. BMC Med. 2019, 17, 128. [Google Scholar] [CrossRef]
- World Heart Federation. Cardiovascular Disease Risk Factors. Available online: http://www.world-heart-federation.org/cardiovascular-health/cardiovascular-disease-risk-factors/tobacco/ (accessed on 1 May 2020).
- Framingham Heart Study (FHS). Framingham Risk Functions. Available online: https://www. framinghamheartstudy.org/index.php (accessed on 2 May 2020).
- Mosley, J.D.; Gupta, D.K.; Tan, J.; Yao, J.; Wells, Q.S.; Shaffer, C.M.; Kundu, S.; Robinson-Cohen, C.; Psaty, B.M.; Rich, S.S.; et al. Predictive accuracy of a polygenic risk score compared with a clinical risk score for incident coronary heart disease. JAMA 2020, 323, 627–635. [Google Scholar] [CrossRef]
- Sultani, R.; Tong, D.C.; Peverelle, M.; Lee, Y.S.; Baradi, A.; Wilson, A.M. Elevated triglycerides to high-density lipoprotein cholesterol (TG/HDL-C) ratio predicts long-term mortality in high-risk patients. Heart Lung Circ. 2020, 29, 414–421. [Google Scholar] [CrossRef]
- Patterson, K.A.E.; Ferrar, K.; Gall, S.L.; Venn, A.J.; Blizzard, L.; Dwyer, T.; Cleland, V.J. Cluster patterns of behavioural risk factors among children: Longitudinal associations with adult cardio-metabolic risk factors. Prev. Med. 2020, 130, 105861. [Google Scholar] [CrossRef]
- O’Connor, S.D.; Graffy, P.M.; Zea, R.; Pickhardt, P.J. Does nonenhanced CT-based quantification of abdominal aortic calcification outperform the Framingham risk score in predicting cardiovascular events in asymptomatic adults? Radiology 2019, 290, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, Z. Rough sets. Int. J. Comput. Inf. Sci. 1982, 11, 341–356. [Google Scholar] [CrossRef]
- Acharjya, D.P. A hybrid scheme for heart disease diagnosis using rough set and cuckoo search technique. J. Med. Syst. 2020, 44, 27. [Google Scholar]
- Liu, J.; Bai, M.; Jiang, N.; Yu, D. Structural risk minimization of rough set-based classifier. Soft Comput. 2020, 24, 2049–2066. [Google Scholar] [CrossRef]
- Huang, Q.; Li, T.; Huang, Y.; Yang, X.; Fujita, H. Dynamic dominance rough set approach for processing composite ordered data. Knowl. Based Syst. 2020, 187, 104829. [Google Scholar] [CrossRef]
- Nabwey, H.A. An intelligent mining model for medical diagnosis of heart disease based on rough set data analysis. Int. J. Eng. Res. Technol. 2020, 13, 355–363. [Google Scholar] [CrossRef]
- Jain, K.; Kulkarni, S. Multi-reduct rough set classifier for computer-aided diagnosis in medical data. In Advancement of Machine Intelligence in Interactive Medical Image Analysis; Verma, O., Roy, S., Pandey, S., Mittal, M., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Abdolrazzagh-Nezhad, M.; Radgohar, H.; Salimian, S.N. Enhanced cultural algorithm to solve multi-objective attribute reduction based on rough set theory. Math. Comput. Simul. 2020, 170, 332–350. [Google Scholar] [CrossRef]
- Song, W.; Li, J.; Li, H.; Ming, X. Human factors risk assessment: An integrated method for improving safety in clinical use of medical devices. Appl. Soft Comput. 2020, 86, 105918. [Google Scholar] [CrossRef]
- Cekik, R.; Uysal, A.K. A novel filter feature selection method using rough set for short text data. Expert Sys. Apps. 2020, 160, 113691. [Google Scholar] [CrossRef]
- Bhuvaneshwarri, I.; Tamilarasi, A. Optimization of big data using rough set theory and data mining for textile applications. In Artificial Intelligence and Evolutionary Computations in Engineering Systems; Dash, S., Lakshmi, C., Das, S., Panigrahi, B., Eds.; Springer: Singapore, 2020; Volume 1056. [Google Scholar] [CrossRef]
- Mehdizadeh, M. Integrating ABC analysis and rough set theory to control the inventories of distributor in the supply chain of auto spare parts. Comput. Ind. Eng. 2020, 139, 105673. [Google Scholar] [CrossRef]
- Lazim, Y.M.; Rahman, M.N.A.; Mohamed, F. Clustering model of multimedia data by using rough sets theory. In Proceedings of the International Conference, Computer & Information Science (ICCIS), Kuala Lumpur, Malaysia, 12–14 June 2012; pp. 336–340. [Google Scholar]
- Cheng, C.H. A DWPT domain transform and COM statistics method combined with rough set for images classification. Multimed. Tools Appl. 2020, 79, 29845–29864. [Google Scholar] [CrossRef]
- Abosuliman, S.S.; Abdullah, S.; Qiyas, M. Three-way decisions making using covering based fractional Orthotriple fuzzy rough set model. Mathematics 2020, 8, 1121. [Google Scholar] [CrossRef]
- Tharwat, A.; Darwish, A.; Hassanien, A.E. Rough sets and social ski-driver optimization for drug toxicity analysis. Comput. Methods Prog. Biomed. 2020, 197, 105702. [Google Scholar] [CrossRef]
- Wu, S.; Yang, S.; Wang, Q. Classification of open pit iron mine rock mass blastability based on concept lattice and rough set. Geotech. Geol. Eng. 2020, 38, 449–458. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, B.; Tan, R.; Tseng, M.L.; Lin, R.C.W.; Lim, M.K. Using neighborhood rough set theory to address the smart elderly care in multi-level attributes. Symmetry 2020, 12, 297. [Google Scholar] [CrossRef]
- Yang, S.B.; Chen, T.L. Uncertain decision tree for bank marketing classification. J. Comput. Appl. Math. 2020, 371, 112710. [Google Scholar] [CrossRef]
- Bhargavi, M.V.; Mudunuru, V.R.; Veeramachaneni, S. Colon cancer stage classification using decision trees. In Data Engineering and Communication Technology; Raju, K., Senkerik, R., Lanka, S., Rajagopal, V., Eds.; Springer: Singapore, 2020; Volume 1079. [Google Scholar]
- Abdelsalam, A.M.; Elsheikh, A.; Chidambaram, S.; David, J.P.; Langlois, J.M.P. POLYBiNN: Binary inference engine for neural networks using decision trees. J. Signal Process. Syst. 2020, 92, 95–107. [Google Scholar] [CrossRef]
- Musharraf, M.; Smith, J.; Khan, F.; Veitch, B. Identifying route selection strategies in offshore emergency situations using decision trees. Reliab. Eng. Syst. Saf. 2020, 194, 106179. [Google Scholar] [CrossRef]
- Ghasemi, E.; Gholizadeh, H.; Adoko, A.C. Evaluation of rockburst occurrence and intensity in underground structures using decision tree approach. Engine. Comput. 2020, 36, 213–225. [Google Scholar] [CrossRef]
- Höppner, S.; Stripling, E.; Baesens, B.; Van den Broucke, S.; Verdonck, T. Profit driven decision trees for churn prediction. Eur. J. Oper. Res. 2020, 284, 920–933. [Google Scholar] [CrossRef]
- Golbayani, P.; Florescu, I.; Chatterjee, R. A comparative study of forecasting corporate credit ratings using neural networks, support vector machines, and decision trees. N. Am. J. Econ. Financ. 2020, 54, 101251. [Google Scholar] [CrossRef]
- Alam, F.; Mehmood, R.; Katib, I. Comparison of decision trees and deep learning for object classification in autonomous driving. In Smart Infrastructure and Applications; Mehmood, R., See, S., Katib, I., Chlamtac, I., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Khan, Z.; Gul, A.; Perperoglou, A.; Miftahuddin, M.; Mahmoud, O.; Adler, W.; Lausen, B. Ensemble of optimal trees, random forest and random projection ensemble classification. Adv. Data Anal. Classif. 2020, 14, 97–116. [Google Scholar] [CrossRef]
- Xu, Z.; Shen, D.; Nie, T.; Kou, Y. A hybrid sampling algorithm combining M-SMOTE and ENN based on random forest for medical imbalanced data. J. Biomed. Inform. 2020, 107, 103465. [Google Scholar] [CrossRef]
- Struye, J.; Latré, S. Hierarchical temporal memory and recurrent neural networks for time series prediction: An empirical validation and reduction to multilayer perceptrons. Neurocomputing 2020, 396, 291–301. [Google Scholar] [CrossRef]
- Jung, S.; Moon, J.; Park, S.; Rho, S.; Baik, S.W.; Hwang, E. Bagging ensemble of multilayer perceptrons for missing electricity consumption data imputation. Sensors 2020, 20, 1772. [Google Scholar] [CrossRef]
- Heidari, A.A.; Faris, H.; Mirjalili, S.; Aljarah, I.; Mafarja, M. Ant lion optimizer: Theory, literature review, and application in multi-layer perceptron neural networks. In Nature-Inspired Optimizers; Mirjalili, S., Song, D.J., Lewis, A., Eds.; Springer: Cham, Switzerland, 2020; Volume 811. [Google Scholar] [CrossRef]
- Rather, S.A.; Bala, P.S. A hybrid constriction coefficient-based particle swarm optimization and gravitational search algorithm for training multi-layer perceptron. Int. J. Intell. Comput. Cybern. 2020, 13, 129–165. [Google Scholar] [CrossRef]
- Lorencin, I.; Anđelić, N.; Španjol, J.; Car, Z. Using multi-layer perceptron with Laplacian edge detector for bladder cancer diagnosis. Artif. Intell. Med. 2020, 102, 101746. [Google Scholar] [CrossRef]
- Vapnik, V. The Nature of Statistical Learning Theory; Springer: New York, NY, USA, 1995. [Google Scholar]
- Dhara, S.; Dang, T.; Parial, K.; Lu, X.X. Accounting for uncertainty and reconstruction of flooding patterns based on multi-satellite imagery and support vector machine technique: A case study of Can Tho City, Vietnam. Water 2020, 12, 1543. [Google Scholar] [CrossRef]
- Ezzahar, J.; Ouaadi, N.; Zribi, M.; Elfarkh, J.; Aouade, G.; Khabba, S.; Er-Raki, S.; Chehbouni, A.; Jarlan, L. Evaluation of backscattering models and support vector machine for the retrieval of bare soil moisture from sentinel-1 data. Remote Sens. 2020, 12, 72. [Google Scholar] [CrossRef]
- Li, L.L.; Zhao, X.; Tseng, M.L.; Tan, R.R. Short-term wind power forecasting based on support vector machine with improved dragonfly algorithm. J. Clean. Prod. 2020, 242, 118447. [Google Scholar] [CrossRef]
- Richhariya, B.; Tanveer, M. A reduced universum twin support vector machine for class imbalance learning. Pattern Recognit. 2020, 102, 107150. [Google Scholar] [CrossRef]
- Wang, M.; Chen, H. Chaotic multi-swarm whale optimizer boosted support vector machine for medical diagnosis. Appl. Soft Comput. 2020, 88, 105946. [Google Scholar] [CrossRef]
- Simsek, S.; Kursuncu, U.; Kibis, E.; AnisAbdellatif, M.; Dag, A. A hybrid data mining approach for identifying the temporal effects of variables associated with breast cancer survival. Expert Sys. Apps. 2020, 139, 112863. [Google Scholar] [CrossRef]
| Rank | Origins of Death | Mortality |
|---|---|---|
| 1 | Malignant neoplasm | 46,829 |
| 2 | Cardiovascular disease | 19,202 |
| 3 | Cerebrovascular disease | 11,169 |
| 4 | Pneumonia | 10,761 |
| 5 | Diabetes mellitus | 9530 |
| Age | Cholesterol | ||||
|---|---|---|---|---|---|
| Years | Man | Woman | (mg/dL) | Man | Woman |
| 30–34 | −1 | −9 | <160 | −3 | −2 |
| 35–39 | 0 | −4 | 160–199 | 0 | 0 |
| 40–44 | 1 | 0 | 200–239 | 1 | 1 |
| 45–49 | 2 | 3 | 240–279 | 2 | 1 |
| 50–54 | 3 | 6 | ≥280 | 3 | 3 |
| 55–59 | 4 | 7 | |||
| 60–64 | 5 | 8 | |||
| 65–69 | 6 | 8 | |||
| 70–74 | 7 | 8 | |||
| HDL-C | Blood Pressure | ||||
|---|---|---|---|---|---|
| (mg/dL) | Man | Woman | Systolic/Diastolic (mmHg) | Man | Woman |
| <35 | 2 | 5 | <120/80 | 0 | −3 |
| 35–44 | 1 | 2 | 120–129/80–84 | 0 | 0 |
| 45–49 | 0 | 1 | 130–139/85–89 | 1 | 0 |
| 50–59 | 0 | 0 | 140–159/90–99 | 2 | 2 |
| ≥60 | −2 | −3 | ≥160/100 | 3 | 3 |
| Framingham Risk | Diabetes | Smoking | ||
|---|---|---|---|---|
| Man | Woman | Man | Woman | |
| Yes | 2 | 4 | 2 | 2 |
| No | 0 | 0 | 0 | 0 |
| Physical Exam | Blood Test | Class | |||
|---|---|---|---|---|---|
| Attribute | Code | Attribute | Code | CVD Name | Code |
| Age | Age | Cholesterol | cho_1 | Arrhythmia | arr |
| Body mass index | bmi_1 | Diastolic pressure | dp_1a | Cardiogenic shock | car |
| Gender | sex | High-density cholesterol | hdl_1 | Diabetes mellitus | dm |
| Smoking habit | smoke | Fast glucose | glu_c1a | High uric acid | uric |
| Waistline | wai_1 | Red blood cells | rbc_1 | Hyperlipidemia | lip |
| Systolic pressure | sp_1a | Myocardial infarction | mi | ||
| White blood cells | wbc_1 | Narrow heart disease | hd | ||
| Peripheral vein disease | pns | ||||
| Characteristic | CVD Dataset | Framingham Dataset |
|---|---|---|
| No. of instances | 1190 | 2019 |
| Class | Cardiovascular (Y: 358) Non-cardiovascular (N: 832) | Cardiovascular (Y: 983) Non-cardiovascular (N: 1036) |
| Total attributes | 20 | 8 |
| Full attributes | 13 | 8 |
| Framingham score attributes | 8 | 6 |
| Framingham attributes | 8 | 7 |
| Predicted Class | |||
|---|---|---|---|
| Positive | Negative | ||
| Actual Class | Positive | True-positive | False-negative |
| Negative | False-positive | True-negative | |
| Feature | Value | Number | Ratio (%) | p-Value | Note |
|---|---|---|---|---|---|
| Sex | Man | 551 | 46 | 0.0743 | Nominal |
| Woman | 639 | 54 | |||
| Age | 40–44 | 19 | 2 | 0.0021 ** | Numeric |
| 45–49 | 160 | 13 | |||
| 50–54 | 190 | 16 | |||
| 55–59 | 205 | 17 | |||
| 60–64 | 189 | 16 | |||
| 65–69 | 158 | 13 | |||
| 70–74 | 269 | 23 | |||
| Cholesterol | <160 | 189 | 16 | 0.3806 | Numeric |
| 160–199 | 468 | 39 | |||
| 200–239 | 369 | 31 | |||
| 240–279 | 130 | 11 | |||
| >=280 | 34 | 3 | |||
| HDL-cholesterol | <35 | 84 | 7 | 0.0121 * | Numeric |
| 35–44 | 364 | 30 | |||
| 45–49 | 222 | 19 | |||
| 50–59 | 306 | 26 | |||
| >=60 | 214 | 18 | |||
| Systolic blood pressure | <120 | 330 | 28 | 0.0436 * | Numeric |
| 120–129 | 254 | 21 | |||
| 130–139 | 244 | 21 | |||
| 140–159 | 287 | 24 | |||
| >=160 | 75 | 6 | |||
| Diastolic blood pressure | <80 | 451 | 38 | 0.9508 | Numeric |
| 80–84 | 343 | 29 | |||
| 85–89 | 62 | 5 | |||
| 90–99 | 257 | 22 | |||
| >=100 | 77 | 6 | |||
| Diabetes mellitus | No | 1056 | 89 | <0.001 *** | Nominal |
| Yes | 134 | 11 | |||
| Smoking | No | 846 | 71 | <0.001 *** | Nominal |
| Yes | 344 | 29 |
| Evaluation Indicator | RS (%) | DT (%) | RF (%) | MLP (%) | SVM (%) | |
|---|---|---|---|---|---|---|
| Accuracy | F score | 80.81 (2.25) | 82.49 (1.32) | 79.87 (1.53) | 72.54 (1.48) | 91.23 (0.92) |
| F attributes | 75.75 (1.59) | 83.00 (2.03) | 81.95 (1.61) | 72.54 (1.57) | 99.67 (0.17) | |
| Full attributes | 74.04 (4.19) | 79.07 (2.34) | 81.74 (0.65) | 70.12 (3.54) | 99.14 (0.11) | |
| Sensitivity | F score | 15.93 (9.46) | 91.26 (3.02) | 85.40 (2.29) | 90.57 (3.52) | 94.47 (0.89) |
| F attributes | 26.18 (6.92) | 91.30 (2.57) | 90.35 (1.96) | 92.54 (3.51) | 99.93 (0.20) | |
| Full attributes | 21.16 (5.40) | 87.29 (3.97) | 91.96 (1.73) | 85.95 (7.62) | 99.10 (0.21) | |
| Specificity | F score | 95.07 (4.19) | 71.15 (5.62) | 66.24 (2.72) | 69.36 (5.64) | 87.99 (2.89) |
| F attributes | 91.31 (4.29) | 72.85 (6.09) | 70.81 (4.61) | 73.93 (4.49) | 99.71 (0.11) | |
| Full attributes | 91.92 (4.09) | 69.97 (5.26) | 71.91 (3.77) | 66.67 (8.65) | 99.01 (0.41) | |
| Evaluation Indicator | RS (%) | DT (%) | RF (%) | MLP (%) | SVM (%) | |
|---|---|---|---|---|---|---|
| Accuracy | F Score | 75.63 (0.9) | 74.34 (1.53) | 72.23 (1.44) | 74.91 (1.38) | 74.19 (1.35) |
| F attributes | 85.11 (2.27) | 72.59 (1.21) | 71.37 (1.06) | 73.93 (2.93) | 49.70 (0.88) | |
| Full attributes | 76.79 (1.12) | 72.35 (1.37) | 71.73 (1.29) | 73.61 (1.75) | 63.33 (1.54) | |
| Sensitivity | F Score | 64.87 (3.18) | 83.77 (2.81) | 80.18 (1.92) | 84.53 (3.23) | 80.32 (3.39) |
| F attributes | 86.06 (3.07) | 65.16 (5.29) | 66.05 (2.23) | 69.88 (7.07) | 11.16 (1.79) | |
| Full attributes | 77.74 (4.58) | 82.31 (3.08) | 77.31 (2.55) | 82.19 (8.22) | 61.29 (2.88) | |
| Specificity | F Score | 85.19 (2.76) | 35.59 (2.45) | 36.15 (2.87) | 35.23 (3.69) | 32.26 (3.15) |
| F attributes | 83.38 (4.61) | 20.36 (5.07) | 23.57 (1.27) | 22.23 (10.25) | 13.75 (2.21) | |
| Full attributes | 74.80 (5.25) | 38.15 (4.00) | 34.15 (2.69) | 35.44 (7.96) | 34.51 (1.35) | |
| Framingham Risk (9 1) | CVD Dataset (7) | Framingham Dataset (5) |
|---|---|---|
| Family history of premature | Sex | Age |
| Sex | Diabetes | Sex |
| Age | Blood pressure | Blood pressure |
| Blood pressure | HDL-cholesterol | Smoking |
| Smoking | Smoking | Cholesterol |
| HDL-cholesterol | Age | |
| Diabetes | Cholesterol | |
| Cholesterol | ||
| Left ventricular hypertrophy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-S.; Cheng, C.-H.; Chen, S.-F.; Jhuang, J.-Y. Identification of the Framingham Risk Score by an Entropy-Based Rule Model for Cardiovascular Disease. Entropy 2020, 22, 1406. https://doi.org/10.3390/e22121406
Chen Y-S, Cheng C-H, Chen S-F, Jhuang J-Y. Identification of the Framingham Risk Score by an Entropy-Based Rule Model for Cardiovascular Disease. Entropy. 2020; 22(12):1406. https://doi.org/10.3390/e22121406
Chicago/Turabian StyleChen, You-Shyang, Ching-Hsue Cheng, Su-Fen Chen, and Jhe-You Jhuang. 2020. "Identification of the Framingham Risk Score by an Entropy-Based Rule Model for Cardiovascular Disease" Entropy 22, no. 12: 1406. https://doi.org/10.3390/e22121406
APA StyleChen, Y.-S., Cheng, C.-H., Chen, S.-F., & Jhuang, J.-Y. (2020). Identification of the Framingham Risk Score by an Entropy-Based Rule Model for Cardiovascular Disease. Entropy, 22(12), 1406. https://doi.org/10.3390/e22121406






