Optimizing Our Patients’ Entropy Production as Therapy? Hypotheses Originating from the Physics of Physiology
Abstract
1. Introduction
1.1. Introduction to Entropy
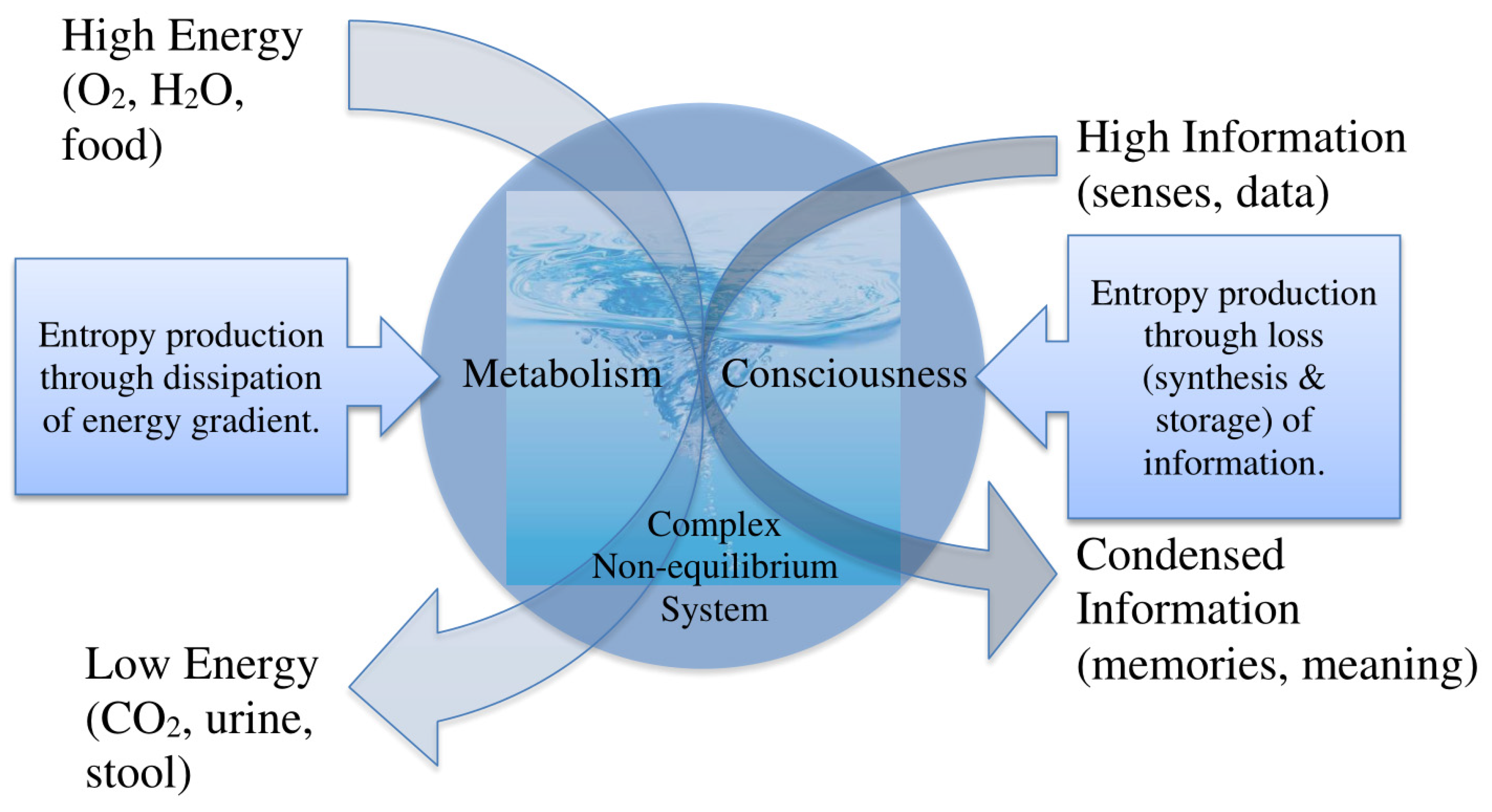
1.2. Entropy Production and Life
2. The Maximum Entropy Production Principle (MEPP)
MEPP and the Origin of Metabolism and Physiological Ordered Structures
3. Interaction between MEPP and Evolution
Impact of Evolution and Maximum Entropy Production on Human Health
4. Informational Entropy Production
5. Evaluation and Implications
Therapeutic Implications
6. Limitations
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Atkins, P. The Second Law; Scientific American Library: New York, NY, USA, 1984. [Google Scholar]
- Lambert, F.L. Disorder—A Cracked Crutch for Supporting Entropy Discussions. J. Chem. Educ. 2002, 79, 187. [Google Scholar] [CrossRef]
- Martyushev, L.M. Entropy and Entropy Production: Old Misconceptions and New Breakthroughs. Entropy 2013, 15, 1152–1170. [Google Scholar] [CrossRef]
- Schneider, E.D.; Sagan, D. Into the Cool: Energy Flow, Thermodynamics and Life; University of Chicago Press Books: Chicago, IL, USA, 2005; p. 378. [Google Scholar]
- Kondepudi, D.P.; Prigogine, I. Thermodynamics Nonequilibrium. In Encyclopedia of Applied Physics, 2nd ed.; Trigg, G.L., Ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Rastogi, R. Introduction to Non-Equilibrium Physical Chemistry; Elsevier: Amsterdam, The Netherlands, 2007; p. 356. [Google Scholar]
- Kleidon, A.; Lorenz, R.D. Non-Equilibrium Thermodynamics and The Production of Entropy: Life, Earth, and Beyond; Springer: Berlin/Heidelberg, Germany, 2005; p. 264. [Google Scholar]
- Aoki, I. Entropy Principle for the Development of Complex. Biotic Systems: Organisms, Ecosystems, the Earth, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012; Chapter 4. [Google Scholar]
- Schrödinger, E. What Is life? Cambridge University Press: Cambridge, UK, 1944. [Google Scholar]
- Annamalai, K.; Nanda, A. Biological Aging and Life Span Based on Entropy Stress via Organ and Mitochondrial Metabolic Loading. Entropy 2017, 19, 566. [Google Scholar] [CrossRef]
- Martyushev, L.M.; Seleznev, V.D. Maximum entropy production principle in physics, chemistry and biology. Phys. Rep. 2006, 426, 1–45. [Google Scholar] [CrossRef]
- Zivieri, R.; Pacini, N.; Finocchio, G.; Carpentieri, M. Rate of entropy model for irreversible processes in living systems. Sci. Rep. 2017, 7, 9134. [Google Scholar] [CrossRef]
- Aoki, I. Entropy production in human life span: A thermodynamical measure for aging. Age 1994, 17, 29–31. [Google Scholar] [CrossRef]
- Silva, C.; Annamalai, K. Entropy Generation and Human Aging: Lifespan Entropy and Effect of Physical Activity Level. Entropy 2008, 10, 100–123. [Google Scholar] [CrossRef]
- Brown, S.J.; Ryan, H.J.; Brown, J.A. Age-Associated Changes In VO2 and Power Output—A Cross-Sectional Study of Endurance Trained New Zealand Cyclists. J. Sports Sci. Med. 2007, 6, 477–483. [Google Scholar]
- Hawkins, S.A.; Wiswell, R.A. Rate and Mechanism of Maximal Oxygen Consumption Decline with Aging: Implications for Exercise Training. Sports Med. 2003, 33, 877–888. [Google Scholar] [CrossRef]
- Plowman, S.; Smith, D. Cardiovascular_Responses to Exercise. In Exercise Physiology for Health, Fitness, and Performance, 2nd ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2008. [Google Scholar]
- Kleidon, A.; Malhi, Y.; Cox, P.M. Maximum entropy production in environmental and ecological systems. Philos. Trans. R Soc. Lond B Biol. Sci. 2010, 365, 1297–1302. [Google Scholar] [CrossRef]
- Swenson, R. Emergent Evolution and the Global Attractor: The Evolutionary Epistemology of Entropy Production Maximization. In Proceedings of the 33rd Annual Meeting of the International Society for the Systems Sciences, Edinburgh, UK, 2–7 July 1989; pp. 46–53. [Google Scholar]
- Swenson, R. Autocatakinetics, Evolution, and the Law of Maximum Entropy Production: A Principled Foundation Toward the Study of Human Ecology. Adv. Hum. Ecol. 1997, 6, 1–46. [Google Scholar]
- Morel, R.E.; Fleck, G. A Fourth Law of Thermodynamics. Chemistry 2006, 15, 305–310. [Google Scholar]
- Beretta, G.P. The fourth law of thermodynamics: Steepest entropy ascent. Philos. Trans. A Math. Phys. Eng. Sci. 2020, 378, 20190168. [Google Scholar] [CrossRef]
- Bejan, A. The Physics of Life: Evolution of Everything; St. Martin’s Press: New York, NY, USA, 2016. [Google Scholar]
- Chaisson, E.J. Cosmic Evolution: State of Science; NASA SP-2009-4802: Washington, DC, USA, 2009.
- Ross, J.; Corlan, A.D.; Muller, S.C. Proposed principles of maximum local entropy production. J. Phys. Chem. B 2012, 116, 7858–7865. [Google Scholar] [CrossRef] [PubMed]
- Vellela, M.; Qian, H. Stochastic dynamics and non-equilibrium thermodynamics of a bistable chemical system: The Schlogl model revisited. J. R Soc. Interface 2009, 6, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Nicolis, C. Stochastic resonance in multistable systems: The role of intermediate states. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2010, 82 Pt 1, 011139. [Google Scholar] [CrossRef]
- Meysman, F.J.; Bruers, S. Ecosystem functioning and maximum entropy production: A quantitative test of hypotheses. Philos. Trans. R Soc. Lond B Biol. Sci. 2010, 365, 1405–1416. [Google Scholar] [CrossRef]
- Polettini, M. Fact-Checking Ziegler’s Maximum Entropy Production Principle beyond the Linear Regime and towards Steady States. Entropy 2013, 15, 2570–2584. [Google Scholar] [CrossRef]
- Andresen, B.; Zimmermann, E.C.; Ross, J. Objections to a proposal on the rate of entropy production in systems far from equilibrium. J. Chem. Phys. 1984, 81, 4676–4677. [Google Scholar] [CrossRef]
- Martyushev, L.M.; Seleznev, V.D. The restrictions of the maximum entropy production principle. Phys. A Stat. Mech. Its Appl. 2014, 4410, 17–21. [Google Scholar] [CrossRef]
- Kleidon, A.; Lorenz, R.D. Entropy production by earth system processes. In In Non-Equilibrium Thermodynamics and The Production of Entropy: Life, Earth, and Beyond; Kleidon, A., Lorenz, R.D., Eds.; Springer: Berlin, Germany, 2005. [Google Scholar]
- Ozawa, H.; Ohmura, A.; Lorenz, R.D.; Pujol, T. The second law of thermodynamics and the global climate system: A review of the maximum entropy production principle. Rev. Geophys. 2003, 41, 1018–1042. [Google Scholar] [CrossRef]
- Paltridge, G.W. Climate and thermodynamic systems of maximum dissipation. Nature 1979, 279, 630–631. [Google Scholar] [CrossRef]
- Koch, L.G.; Britton, S.L. Aerobic metabolism underlies complexity and capacity. J. Physiol 2008, 586, 83–95. [Google Scholar] [CrossRef]
- Lucia, U.; Maino, G. Entropy generation in biophysical systems. EPL (Eur. Lett.) 2017, 101, 56002. [Google Scholar] [CrossRef]
- Unrean, P.; Srienc, F. Metabolic networks evolve towards states of maximum entropy production. Metab. Eng. 2011, 13, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Chiasson, E.J. Cosmic Evolution: The Rise of Complexity in Nature; Harvard University Press: Cambridge, UK, 2001; p. 274. [Google Scholar]
- Niven, R.K. Steady state of a dissipative flow-controlled system and the maximum entropy production principle. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 2009, 80 Pt 1, 021113. [Google Scholar] [CrossRef]
- Seely, A.J.; Macklem, P. Fractal variability: An emergent property of complex dissipative systems. Chaos Woodbury Ny 2012, 22, 013108. [Google Scholar] [CrossRef]
- Boser, S.R.; Park, H.; Perry, S.F.; Menache, M.G.; Green, F.H. Fractal geometry of airway remodeling in human asthma. Am. J. Respir. Crit. Care Med. 2005, 172, 817–823. [Google Scholar] [CrossRef]
- Gupta, S.; Hartley, R.; Khan, U.T.; Singapuri, A.; Hargadon, B.; Monteiro, W.; Pavord, I.D.; Sousa, A.R.; Marshall, R.P.; Subramanian, D.; et al. Quantitative computed tomography-derived clusters: Redefining airway remodeling in asthmatic patients. J. Allergy Clin. Immunol. 2014, 133, 729–738.e18. [Google Scholar] [CrossRef]
- Doubal, F.N.; MacGillivray, T.J.; Patton, N.; Dhillon, B.; Dennis, M.S.; Wardlaw, J.M. Fractal analysis of retinal vessels suggests that a distinct vasculopathy causes lacunar stroke. Neurology 2010, 74, 1102–1107. [Google Scholar] [CrossRef]
- Cavallari, M.; Falco, T.; Frontali, M.; Romano, S.; Bagnato, F.; Orzi, F. Fractal analysis reveals reduced complexity of retinal vessels in CADASIL. PLoS ONE 2011, 6, e19150. [Google Scholar] [CrossRef] [PubMed]
- Seely, A.; Newman, K.D.; Herry, C. Fractal Structure and Entropy Production within the Central Nervous System. Entropy 2014, 16, 4497–4520. [Google Scholar] [CrossRef]
- Seely, A.J.; Macklem, P.T. Complex systems and the technology of variability analysis. Crit. Care 2004, 8, R367–R384. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of complex physiologic time series. Phys. Rev. Lett. 2002, 89, 068102. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, E215–E220. [Google Scholar] [CrossRef]
- Bravi, A.; Longtin, A.; Seely, A.J. Review and classification of variability analysis techniques with clinical applications. Biomed. Eng. Online 2011, 10, 90. [Google Scholar] [CrossRef]
- Axelrod, S.; Lishner, M.; Oz, O.; Bernheim, J.; Ravid, M. Spectral analysis of fluctuations in heart rate: An objective evaluation of autonomic nervous control in chronic renal failure. Nephron 1987, 45, 202–206. [Google Scholar] [CrossRef]
- van de Borne, P.; Montano, N.; Pagani, M.; Oren, R.; Somers, V.K. Absence of low-frequency variability of sympathetic nerve activity in severe heart failure. Circulation 1997, 95, 1449–1454. [Google Scholar] [CrossRef]
- Bonaduce, D.; Petretta, M.; Marciano, F.; Vicario, M.L.; Apicella, C.; Rao, M.A.; Nicolai, E.; Volpe, M. Independent and incremental prognostic value of heart rate variability in patients with chronic heart failure. Am. Heart J. 1999, 138 Pt 1, 273–284. [Google Scholar] [CrossRef]
- Guzzetti, S.; Mezzetti, S.; Magatelli, R.; Porta, A.; De Angelis, G.; Rovelli, G.; Malliani, A. Linear and non-linear 24 h heart rate variability in chronic heart failure. Auton Neurosci. 2000, 86, 114–119. [Google Scholar] [CrossRef]
- Huang, J.; Sopher, S.M.; Leatham, E.; Redwood, S.; Camm, A.J.; Kaski, J.C. Heart rate variability depression in patients with unstable angina. Am. Heart J. 1995, 130, 772–779. [Google Scholar] [CrossRef]
- Lishner, M.; Akselrod, S.; Avi, V.M.; Oz, O.; Divon, M.; Ravid, M. Spectral analysis of heart rate fluctuations. A non-invasive, sensitive method for the early diagnosis of autonomic neuropathy in diabetes mellitus. J. Auton. Nerv. Syst. 1987, 19, 119–125. [Google Scholar] [CrossRef]
- Mussalo, H.; Vanninen, E.; Ikaheimo, R.; Laitinen, T.; Laakso, M.; Lansimies, E.; Hartikainen, J. Heart rate variability and its determinants in patients with severe or mild essential hypertension. Clin. Physiol. 2001, 21, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, S.H.; Jensen, S.E.; Moller, J.E.; Egstrup, K. Prognostic value of left ventricular diastolic function and association with heart rate variability after a first acute myocardial infarction. Heart 2001, 86, 376–380. [Google Scholar] [CrossRef][Green Version]
- Odemuyiwa, O.; Malik, M.; Farrell, T.; Bashir, Y.; Poloniecki, J.; Camm, J. Comparison of the predictive characteristics of heart rate variability index and left ventricular ejection fraction for all-cause mortality, arrhythmic events and sudden death after acute myocardial infarction. Am. J. Cardiol. 1991, 68, 434–439. [Google Scholar] [CrossRef]
- van Boven, A.J.; Jukema, J.W.; Haaksma, J.; Zwinderman, A.H.; Crijns, H.J.; Lie, K.I. Depressed heart rate variability is associated with events in patients with stable coronary artery disease and preserved left ventricular function. REGRESS Study Group. Am. Heart J. 1998, 135, 571–576. [Google Scholar] [CrossRef]
- Ahmad, S.; Ramsay, T.; Huebsch, L.; Flanagan, S.; McDiarmid, S.; Batkin, I.; McIntyre, L.; Sundaresan, S.R.; Maziak, D.E.; Shamji, F.M.; et al. Continuous multi-parameter heart rate variability analysis heralds onset of sepsis in adults. PLoS ONE 2009, 4, e6642. [Google Scholar] [CrossRef]
- Buchan, C.A.; Bravi, A.; Seely, A.J. Variability Analysis and the Diagnosis, Management, and Treatment of Sepsis. Curr. Infect. Dis. Rep. 2012, 14, 512–521. [Google Scholar] [CrossRef]
- Bravi, A.; Green, G.; Longtin, A.; Seely, A.J. Monitoring and identification of sepsis development through a composite measure of heart rate variability. PLoS ONE 2012, 7, e45666. [Google Scholar] [CrossRef]
- Green, G.C.; Bradley, B.; Bravi, A.; Seely, A.J. Continuous multiorgan variability analysis to track severity of organ failure in critically ill patients. J. Crit Care 2013, 28, 879.e1–e11. [Google Scholar] [CrossRef]
- Brack, T.; Jubran, A.; Tobin, M.J. Dyspnea and decreased variability of breathing in patients with restrictive lung disease. Am. J. Respir. Crit. Care Med. 2002, 165, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Seely, A.J.; Bravi, A.; Herry, C.; Green, G.; Longtin, A.; Ramsay, T.; Fergusson, D.; McIntyre, L.; Kubelik, D.; Maziak, D.E.; et al. Do heart and respiratory rate variability improve prediction of extubation outcomes in critically ill patients? Crit. Care 2014, 18, R65. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, V.E.; Chouvarda, I.; Maglaveras, N.; Dragoumanis, C.; Pneumatikos, I. Changes of heart and respiratory rate dynamics during weaning from mechanical ventilation: A study of physiologic complexity in surgical critically ill patients. J. Crit Care 2011, 26, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Sapoval, B.; Baldassarri, A.; Gabrielli, A. Self-stabilized fractality of seacoasts through damped erosion. Phys. Rev. Lett 2004, 93, 098501. [Google Scholar] [CrossRef] [PubMed]
- Skene, K.R. Life’s a Gas: A Thermodynamic Theory of Biological Evolution. Entropy 2015, 17, 5522–5548. [Google Scholar] [CrossRef]
- Zotin, A.I.; Lamprecht, I. Aspects of bioenergetics and civilization. J. Biol. 1996, 180, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Lotka, A.J. Contribution to the Energetics of Evolution. Proc. Natl. Acad. Sci. USA 1922, 8, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Schols, A.M.; Fredrix, E.W.; Soeters, P.B.; Westerterp, K.R.; Wouters, E.F. Resting energy expenditure in patients with chronic obstructive pulmonary disease. Am. J. Clin. Nutr. 1991, 54, 983–987. [Google Scholar] [CrossRef]
- Frankenfield, D.C.; Wiles, C.E., 3rd; Bagley, S.; Siegel, J.H. Relationships between resting and total energy expenditure in injured and septic patients. Crit Care Med. 1994, 22, 1796–1804. [Google Scholar] [CrossRef]
- Jin, K. Modern Biological Theories of Aging. Aging Dis. 2010, 1, 72–74. [Google Scholar]
- Lints, F.A. The rate of living theory revisited. Gerontology 1989, 35, 36–57. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.N.; Raven, P.B.; Snell, P.G.; Stray-Gundersen, J.; Levine, B.D. Maximal oxygen uptake as a parametric measure of cardiorespiratory capacity. Med. Sci. Sports Exerc. 2007, 39, 103–107. [Google Scholar]
- Parrondo, J.M.R.; Horowitz, J.M.; Sagawa, T. Thermodynamics of information. Nat. Phys. 2015, 11, 131–139. [Google Scholar] [CrossRef]
- Landauer, R. Irreversibility and Heat Generation in the Computing Process. Ibm J. Res. Dev. 1961, 5, 183–191. [Google Scholar] [CrossRef]
- Anonymous. Standardization of spirometry–1987 update:statement of the American Thoracic Society. Am. Rev. Respir Dis. 1987, 136, 1285–1298. [Google Scholar]
- Berut, A.; Arakelyan, A.; Petrosyan, A.; Ciliberto, S.; Dillenschneider, R.; Lutz, E. Experimental verification of Landauer’s principle linking information and thermodynamics. Nature 2012, 483, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.; Gavrilov, M.; Bechhoefer, J. High-precision test of Landauer’s principle in a feedback trap. Phys. Rev. Lett. 2014, 113, 190601. [Google Scholar] [CrossRef]
- Hong, J.; Lambson, B.; Dhuey, S.; Bokor, J. Experimental test of Landauer’s principle in single-bit operations on nanomagnetic memory bits. Sci. Adv. 2016, 2, e1501492. [Google Scholar] [CrossRef]
- Duncan, T.L.; Semura, J.S. Information Loss as a Foundational Principle for the Second Law of Thermodynamics. Found. Phys. 2007, 37, 1767–1773. [Google Scholar] [CrossRef]
- Aoki, I. Entropy production in living systems: From organisms to ecosystems. Acta 1995, 250, 359–370. [Google Scholar] [CrossRef]
- Westerterp, K.R. Control of energy expenditure in humans. Eur. J. Clin. Nutr. 2017, 71, 340–344. [Google Scholar] [CrossRef]
- Frankenfield, D.C.; Smith, J.S., Jr.; Cooney, R.N.; Blosser, S.A.; Sarson, G.Y. Relative association of fever and injury with hypermetabolism in critically ill patients. Injury 1997, 28, 617–621. [Google Scholar] [CrossRef]
- Manthous, C.A.; Hall, J.B.; Olson, D.; Singh, M.; Chatila, W.; Pohlman, A.; Kushner, R.; Schmidt, G.A.; Wood, L.D. Effect of cooling on oxygen consumption in febrile critically ill patients. Am. J. Respir. Crit. Care Med. 1995, 151, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Haupt, M.T.; Rackow, E.C. Adverse effects of febrile state on cardiac performance. Am. Heart J. 1983, 105, 763–768. [Google Scholar] [CrossRef]
- Nahas, G.G.; Tannieres, M.L.; Lennon, J.F. Direct measurement of leukocyte motility: Effects of pH and temperature. Proc. Soc. Exp. Biol. Med. 1971, 138, 350–352. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Bernheim, H.A.; Duff, G.W.; Le, H.V.; Nagabhushan, T.L.; Hamilton, N.C.; Coceani, F. Mechanisms of fever induced by recombinant human interferon. J. Clin. Investig. 1984, 74, 906–913. [Google Scholar] [CrossRef]
- Sugimura, T.; Fujimoto, T.; Motoyama, H.; Maruoka, T.; Korematu, S.; Asakuno, Y.; Hayakawa, H. Risks of antipyretics in young children with fever due to infectious disease. Acta Paediatr. Jpn. 1994, 36, 375–378. [Google Scholar] [CrossRef]
- Graham, N.M.; Burrell, C.J.; Douglas, R.M.; Debelle, P.; Davies, L. Adverse effects of aspirin, acetaminophen, and ibuprofen on immune function, viral shedding, and clinical status in rhinovirus-infected volunteers. J. Infect. Dis. 1990, 162, 1277–1282. [Google Scholar] [CrossRef]
- Drewry, A.M.; Ablordeppey, E.A.; Murray, E.T.; Stoll, C.R.T.; Izadi, S.R.; Dalton, C.M.; Hardi, A.C.; Fowler, S.A.; Fuller, B.M.; Colditz, G.A. Antipyretic Therapy in Critically Ill Septic Patients: A Systematic Review and Meta-Analysis. Crit Care Med. 2017, 45, 806–813. [Google Scholar] [CrossRef]
- Arrich, J.; Holzer, M.; Havel, C.; Müllner, M.; Herkner, H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst. Rev. 2016, 2, CD004128. [Google Scholar] [CrossRef]
- Adie, S.; Kwan, A.; Naylor, J.M.; Harris, I.A.; Mittal, R. Cryotherapy following total knee replacement. Cochrane Database Syst. Rev. 2012, 9, CD007911. [Google Scholar]
- Goldberger, A.L. Fractal variability versus pathologic periodicity: Complexity loss and stereotypy in disease. Perspect Biol. Med. 1997, 40, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, A.L. Nonlinear Dynamics, Fractals, and Chaos Theory: Implications for Neuroautonomic Heart Rate Control. in Health and Disease; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Goldberger, A.L.; Peng, C.K.; Lipsitz, L.A. What is physiologic complexity and how does it change with aging and disease? Neurobiol. Aging 2002, 23, 23–26. [Google Scholar] [CrossRef]
- West, B.J. Physiology in fractal dimensions: Error tolerance. Ann. Biomed. Eng. 1990, 18, 135–149. [Google Scholar] [CrossRef]
- Barnaby, D.P.; Fernando, S.M.; Herry, C.L.; Scales, N.B.; Gallagher, E.J.; Seely, A.J.E. Heart Rate Variability, Clinical and Laboratory Measures to Predict Future Deterioration in Patients Presenting with Sepsis. Shock 2018, 51, 416–422. [Google Scholar] [CrossRef]
- Samsudin, M.I.; Liu, N.; Prabhakar, S.M.; Chong, S.L.; Kit Lye, W.; Koh, Z.X.; Guo, D.; Rajesh, R.; Ho, A.F.W.; Ong, M.E.H. A novel heart rate variability based risk prediction model for septic patients presenting to the emergency department. Medicine 2018, 97, e10866. [Google Scholar] [CrossRef]
- Mutch, W.A.; Lefevre, G.R.; Thiessen, D.B.; Girling, L.G.; Warrian, R.K. Computer-controlled cardiopulmonary bypass increases jugular venous oxygen saturation during rewarming. Ann. Thorac. Surg. 1998, 65, 59–65. [Google Scholar] [CrossRef]
- Mutch, W.A.; Warrian, R.K.; Eschun, G.M.; Girling, L.G.; Doiron, L.; Cheang, M.S.; Lefevre, G.R. Biologically variable pulsation improves jugular venous oxygen saturation during rewarming. Ann. Thorac. Surg. 2000, 69, 491–497. [Google Scholar] [CrossRef]
- McMullen, M.C.; Girling, L.G.; Graham, M.R.; Mutch, W.A. Biologically variable ventilation improves oxygenation and respiratory mechanics during one-lung ventilation. Anesthesiology 2006, 105, 91–97. [Google Scholar] [CrossRef]
- Mutch, W.A.; Buchman, T.G.; Girling, L.G.; Walker, E.K.; McManus, B.M.; Graham, M.R. Biologically variable ventilation improves gas exchange and respiratory mechanics in a model of severe bronchospasm. Crit. Care Med. 2007, 35, 1749–1755. [Google Scholar] [CrossRef]
- Barr, J.; Fraser, G.L.; Puntillo, K.; Ely, E.W.; Gelinas, C.; Dasta, J.F.; Davidson, J.E.; Devlin, J.W.; Kress, J.P.; Joffe, A.M.; et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit. Care Med. 2013, 41, 263–306. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Burry, L.; Cook, D.; Fergusson, D.; Steinberg, M.; Granton, J.; Herridge, M.; Ferguson, N.; Devlin, J.; Tanios, M.; et al. Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol: A randomized controlled trial. JAMA 2012, 308, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Strom, T.; Martinussen, T.; Toft, P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: A randomised trial. Lancet 2010, 375, 475–480. [Google Scholar] [CrossRef]
| Entropy Production |
|
| Physical Structures |
|
| Impact of Evolution |
|
| Informational Entropy Production |
|
| Health |
|
| Illness |
|
| Therapeutic Implications |
|
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seely, A.J.E. Optimizing Our Patients’ Entropy Production as Therapy? Hypotheses Originating from the Physics of Physiology. Entropy 2020, 22, 1095. https://doi.org/10.3390/e22101095
Seely AJE. Optimizing Our Patients’ Entropy Production as Therapy? Hypotheses Originating from the Physics of Physiology. Entropy. 2020; 22(10):1095. https://doi.org/10.3390/e22101095
Chicago/Turabian StyleSeely, Andrew J. E. 2020. "Optimizing Our Patients’ Entropy Production as Therapy? Hypotheses Originating from the Physics of Physiology" Entropy 22, no. 10: 1095. https://doi.org/10.3390/e22101095
APA StyleSeely, A. J. E. (2020). Optimizing Our Patients’ Entropy Production as Therapy? Hypotheses Originating from the Physics of Physiology. Entropy, 22(10), 1095. https://doi.org/10.3390/e22101095




