1. Introduction
Large-scale energy storage is an effective solution for improving and expanding renewable energy systems. It can also be used for the storage of electrical energy and for grid load shifting. Currently, pumped hydro energy storage (PHES) and compressed air energy storage (CAES) are the major large-scale energy storage technologies. PHES is well-developed and efficient, but it is restricted by geological characteristics. Small-scale CAES systems usually use a high-pressure container as the air storage tank [
1], which increases the investment cost and limits the install capacity. In large-scale CAES systems, salt caverns and underground mines can be used for storing the high-pressure air [
2], but here again, the locations of the plants are restricted by geological characteristics.
Apart from PHES and CAES, another promising solution for large-scale energy storage is liquid air energy storage (LAES), which has the notable advantages of high energy-storage density and no geological constraints. The concept of storing energy in liquid air was first proposed by Smith in 1977 [
3], and Highview Power Storage Ltd. designed and established the world’s first LAES pilot plant (350 kW/2.5 MWh) [
4]. Since then, many researchers have studied the performance of standalone LAES and integrated LAES systems with various configurations. Ameel et al. [
5] investigated a thermodynamic cycle for energy storage using liquid air, and a maximum round-trip efficiency (RTE) of 43.3% was attained. Sciacovelli et al. [
6] studied a standalone LAES system with packed bed cold energy storage. The dynamic characteristics of the system were analyzed, and an RTE of 50% was obtained. Xue et al. [
7] presented a thermodynamic analysis of a standalone LAES system, and the influence of key parameters was discussed. Guizzi et al. [
8] investigated an LAES system with cryogenic liquid cold energy storage and assessed its efficiency. An RTE in the range of 54–55% could be obtained with reasonable and conservative design parameters. Howe et al. [
9] presented an energy and exergy analysis for a combined building-scale LAES system. Their analytical approach can be applied to other LAES configurations to identify optimal operating parameters. Tafone et al. [
10] studied an LAES system as a cooling application in hot climates, and an RTE of 45% was obtained.
In the study of integrated LAES systems, Li et al. [
11] proposed a hybrid system, integrating LAES with nuclear power plants and obtaining a high RTE of 70%. Zhang et al. [
12] introduced the cold energy of liquefied natural gas (LNG) into the liquefaction process of LAES, and a higher electrical energy storage efficiency was obtained. Antonelli et al. [
13] analyzed the potential and challenges of hybrid power plants based on LAES; the performance of possible configurations was analyzed and compared. Al-Zareer et al. [
14] studied hybrid LAES systems for district heating and cooling. Brayton, Rankine, and absorption cooling cycles were introduced into the system, and an RTE of about 70% was obtained.
In baseline LAES (B-LAES), the compression heat is surplus because of the low liquefaction ratio. Therefore, the effective utilization of compression heat has a significant influence on the RTE of an LAES system. She et al. [
15] introduced an organic Rankine cycle (ORC) system driven by the surplus compression heat into an LAES system to improve its RTE. In a subsequent study, Peng et al. [
16] compared two configurations of ORC systems with different cold sources: ambient and a low-temperature cold source obtained through an absorption refrigeration cycle. The results indicated that the introduction of an ORC can effectively improve the performance of the system, and the LAES combined with the ambient ORC system had the better performance. In order to further improve the RTE of LAES, Tafone et al. [
17,
18] studied and compared different integrated LAES systems consisting of an ORC and an absorption chiller. The results showed that such integrated energy systems can significantly improve efficiency and reduce the payback period.
Apart from the ORC system, another representative technology for utilizing waste heat to generate electricity is the Kalina cycle (KC). The KC used ammonia–water as the working fluid to realize a good temperature match between the heat source and working fluid due to the variable boiling temperature of ammonia–water [
19,
20]. Numerous investigations have been carried out to study the performance of the KC from the perspective of the first and second law of thermodynamics [
21,
22]. Moreover, the KC also has been considered as both the bottom and topping cycle in the integrated systems. For example, Zhao et al. [
23] presented a thermodynamic analysis of an integrated energy system based on CAES and KC. In another study, Li et al. [
24] compared the performance of KC and ORC in recovering the residual heat of CAES. In this paper, a novel integrated energy system based on LAES and KC (KC-LAES) is proposed and studied. In the discharging process of the proposed system, the stored compression heat is first used to heat the inlet air to the air turbines; then, the surplus portion is used to drive the KC subsystem to generate additional electricity. A mathematical model is developed to analyze the performance of the KC and the integrated energy system. In the analysis of the KC, the influence of the basic concentration of ammonia–water, the evaporating temperature, and the operating pressure is discussed. In the analysis of the KC-LAES, the influence of the liquefaction and expansion pressure is studied. Finally, the calculation results of the KC-LAES with typical operating parameters are presented and discussed.
2. System Description
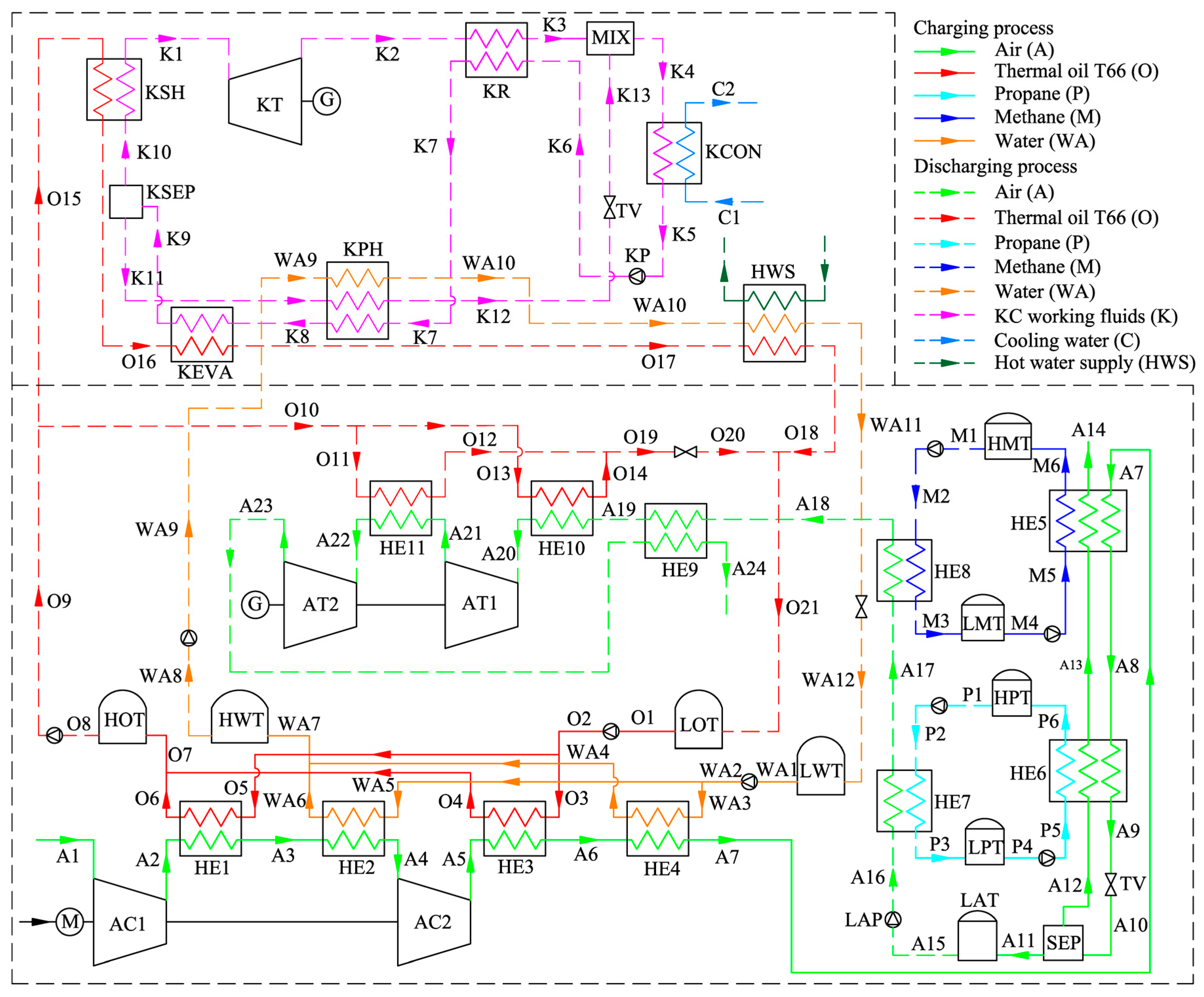
Figure 1 shows the schematic of the proposed KC-LAES. In order to clearly describe and analyze the system, all the streams have been numbered. The bottom part of
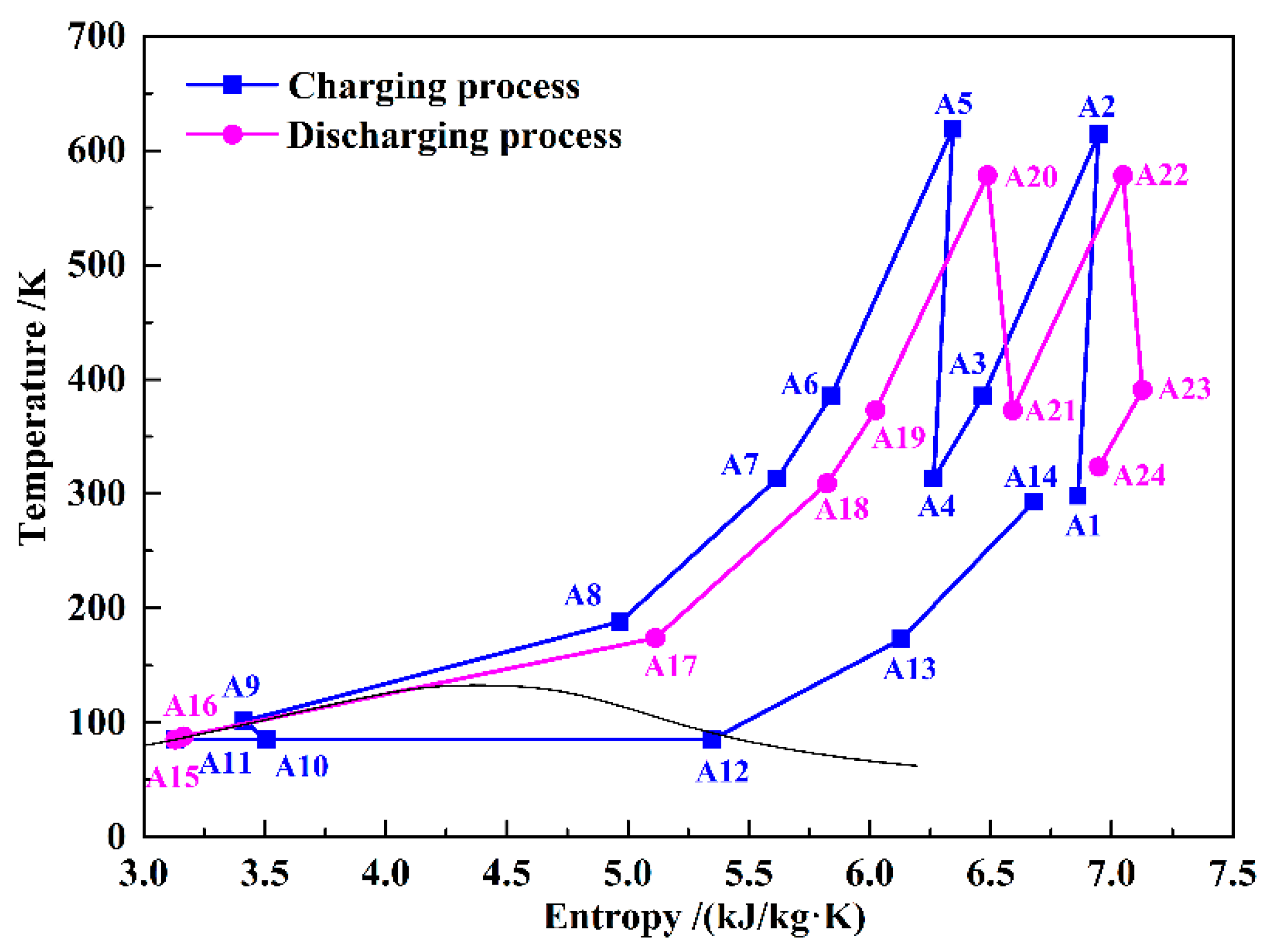
Figure 1 is a typical B-LAES with cryogenic liquid cold energy storage, and
Figure 2 shows its T–s diagram. In the charging process, the air is compressed to a high-pressure state (A7), and then cooled to a liquid state (A9). The high and low temperature compression heat generated in the compression process is stored in thermal oil and water, respectively. The cold energy utilized in cooling the compressed air is harvested in the discharging process. Then, a vapor–liquid mixture (A10) is obtained through an expansion process in the throttle valve (TV). After separation, the liquid air (A11) is stored in the liquid air tank (LAT), and the gaseous air (A12) flows black to cool the compressed air. In the discharging process, the liquid air is first pumped to a high-pressure state (A16), and then flows through a two-stage heat exchanger to be gasified. During the gasification process, the cold energy of the liquid air is stored in methane or propane, depending on the temperature, and then utilized in the liquefaction process in the next cycle. Before flowing into the turbines, the air is heated by the high-temperature compression heat stored in the thermal oil. A regenerator is introduced to reduce the temperature of the exhausted gas.
The upper part of
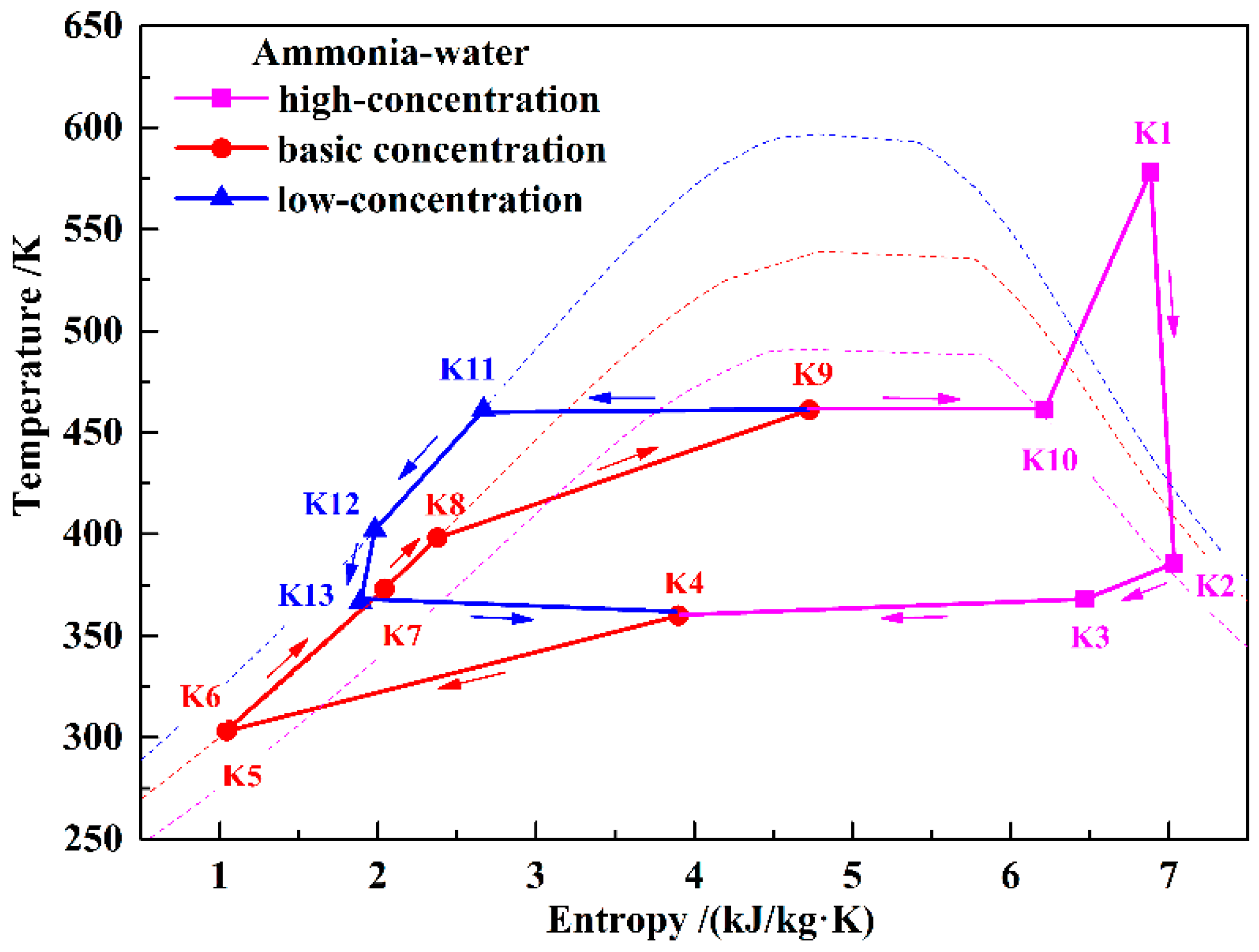
Figure 1 shows a schematic of the KC. In order to simplify the system, a simple regenerative KC is assumed in this paper.
Figure 3 shows the T–s diagram of the KC system. During the discharging process, the KC turbine (KT) and the air turbines work simultaneously to generate electricity. Ammonia–water with a certain concentration has been chosen as the working fluid. In the KC system, the low-temperature basic concentration ammonia–water (BCAW) is first pumped to a high-pressure state (K6). Then, before flowing into the KC evaporator (KEVA), the BCAW is gradually heated by the exhausted gas in the KC regenerator (KR) and the low-concentration ammonia–water (LCAW) in the KC preheater (KPH). The low-temperature compression heat stored in water can also be used to preheat the BCAW in the KPH if the heat of the LCAW is insufficient. In the KEVA, the BCAW is heated by the high-temperature compression heat stored in thermal oil, and a liquid–vapor mixture is obtained (K9). In the KC separator (KSEP), the mixture is separated into a high-concentration ammonia–water (HCAW) stream (K10) and an LCAW stream (K11). The HCAW is then heated to a superheated state (K1) by the thermal oil and expanded in the KT to generate electricity. The expanded gas (K2) flows through the KR to heat the BCAW and mixes with the throttled LCAW in the mixer (MIX). Finally, the liquid–vapor mixture (K4) is cooled to a liquid state (K5) by the cooling water in the KC condenser (KCON). Before flowing back to the low-temperature storage tank, the thermal oil and water are cooled, and a hot water supply can be obtained. In this paper, only the power generation is considered in analyzing the performance of the proposed system; the heating supply is neglected.
5. Conclusions
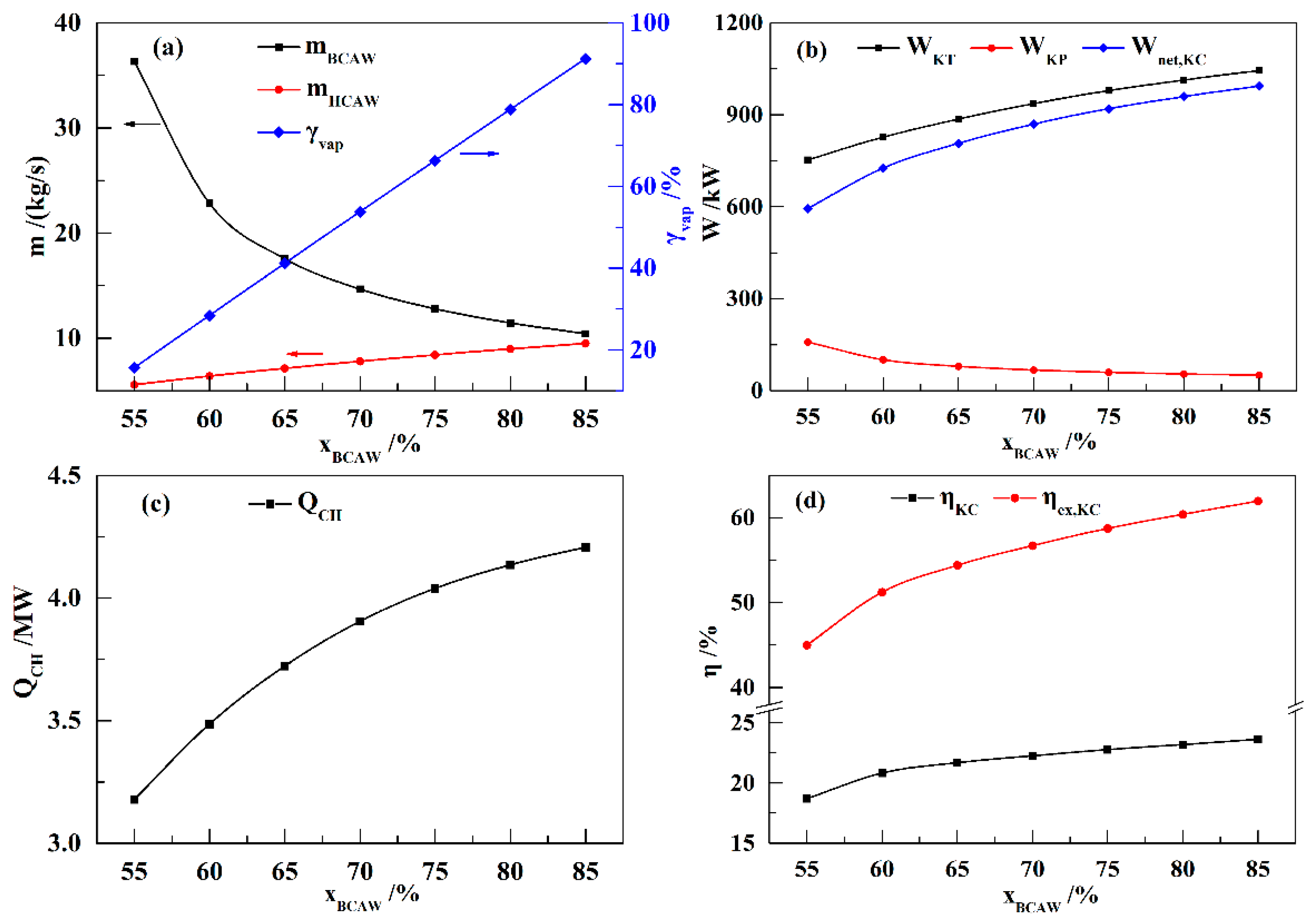
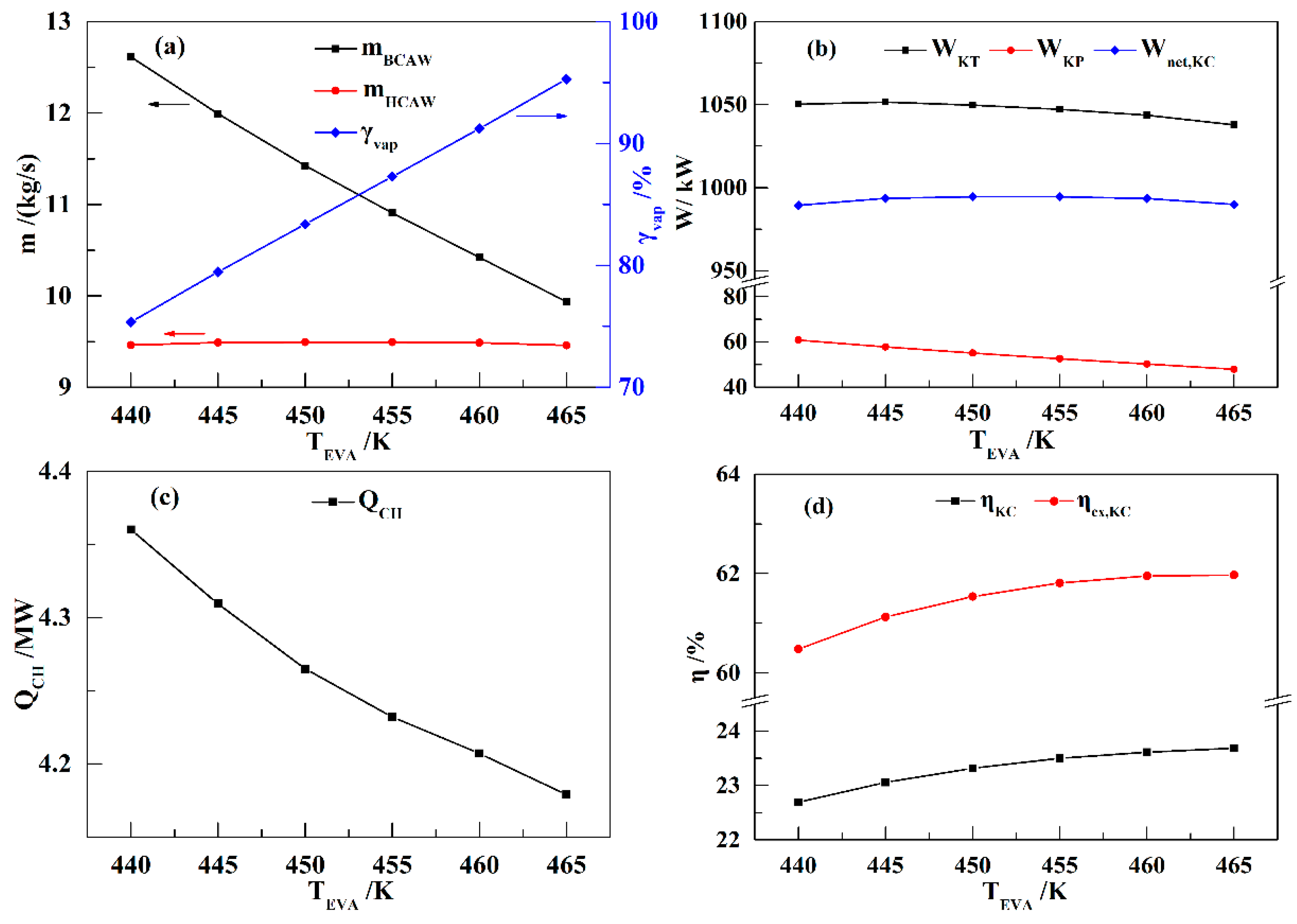
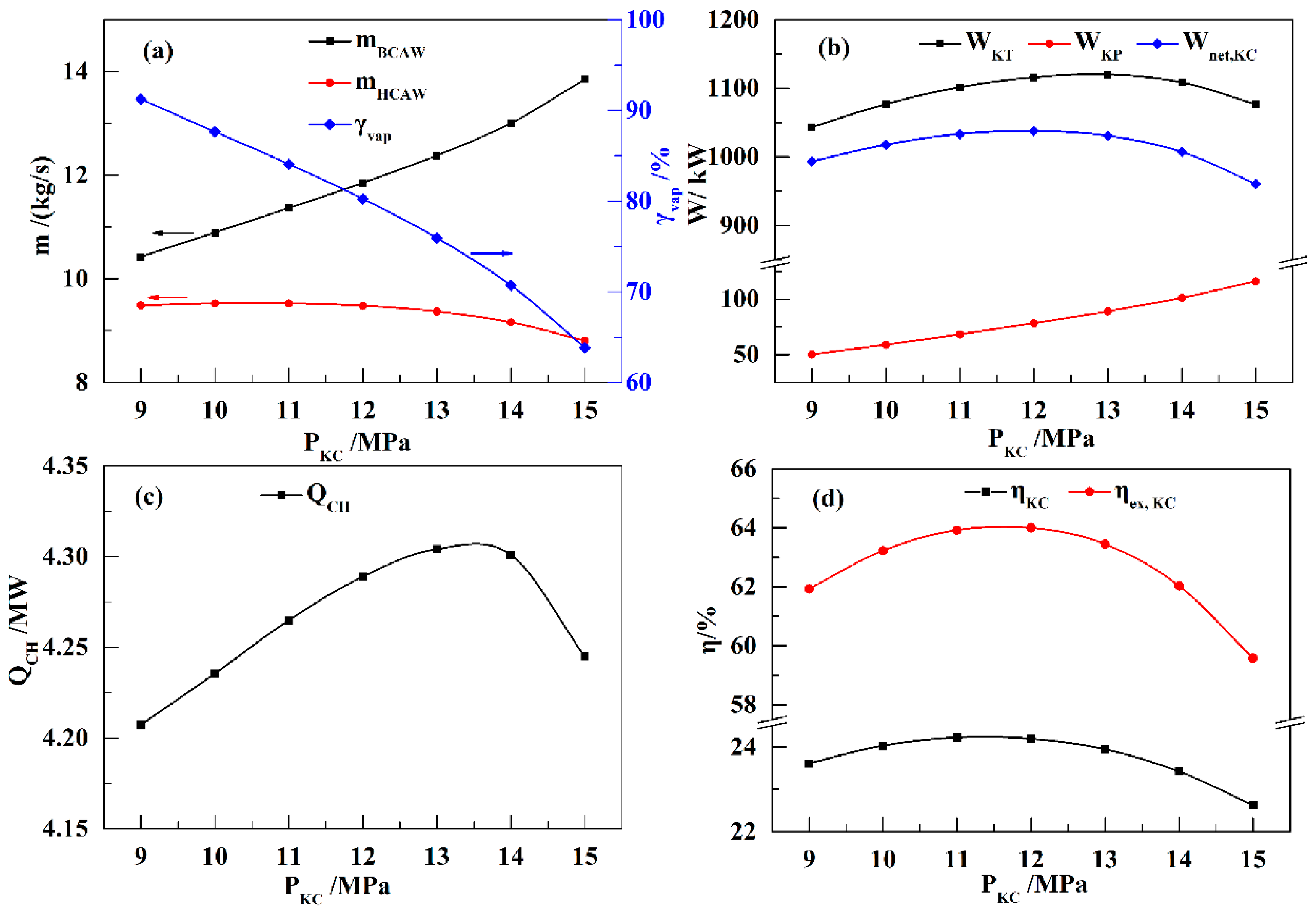
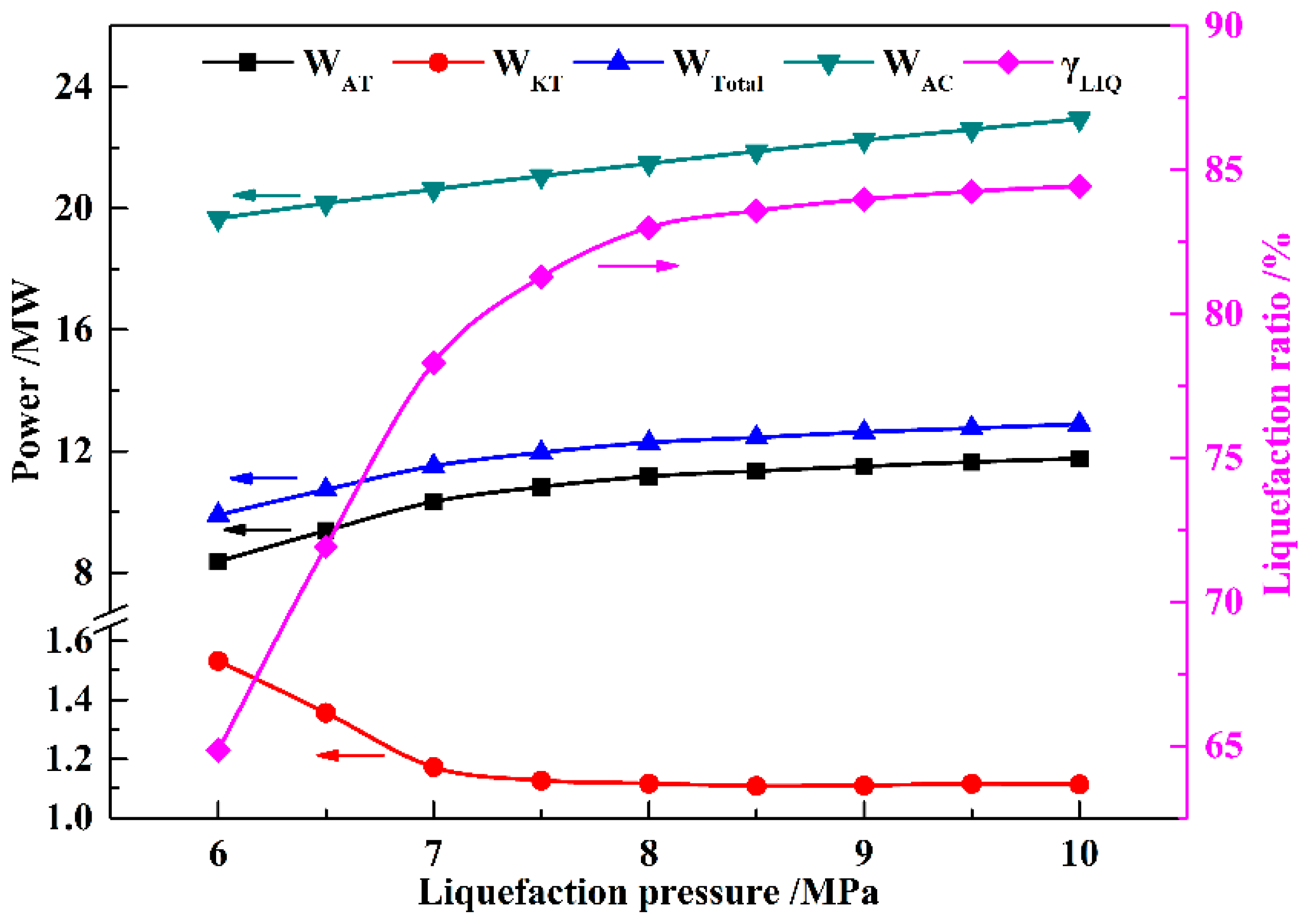
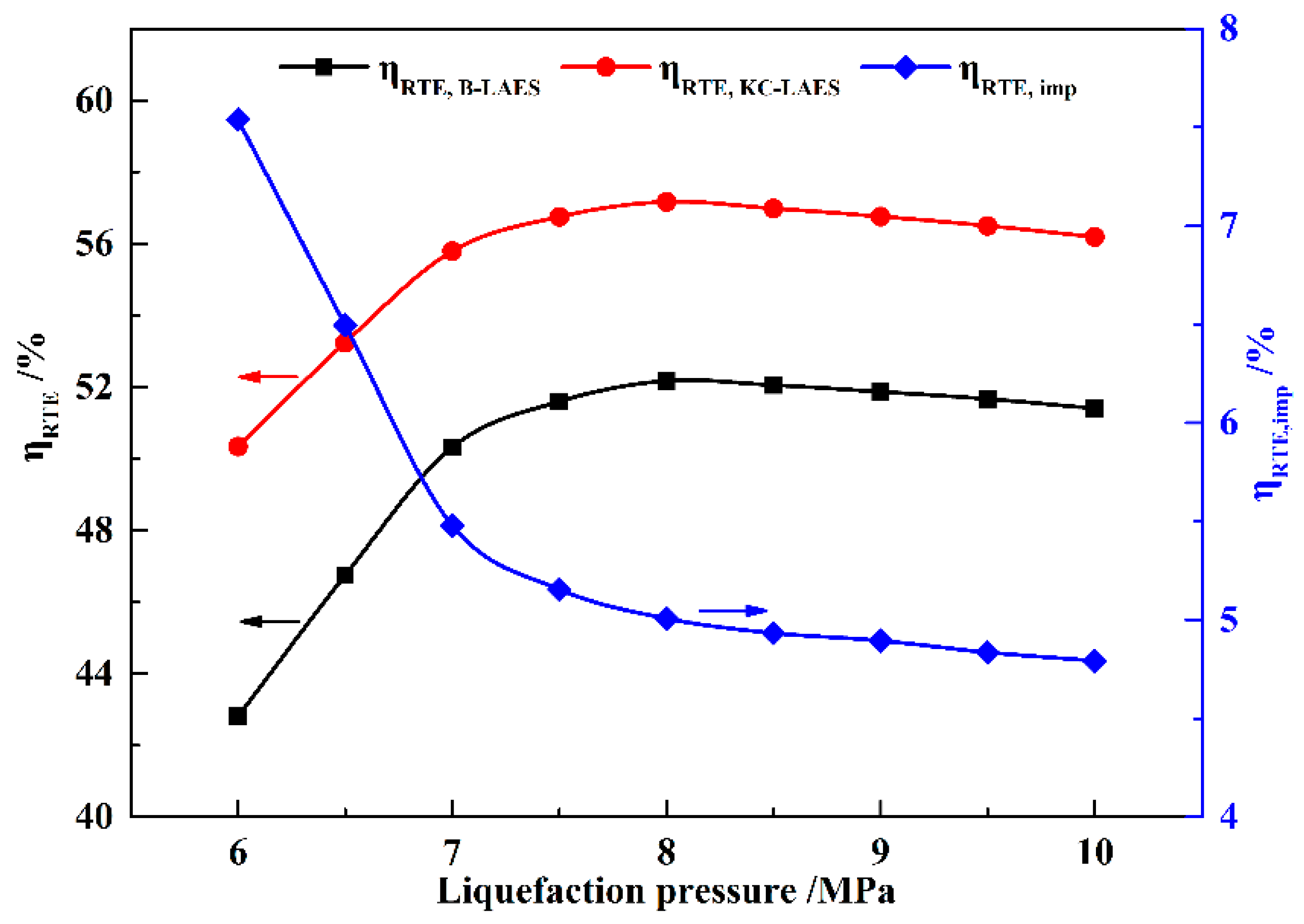
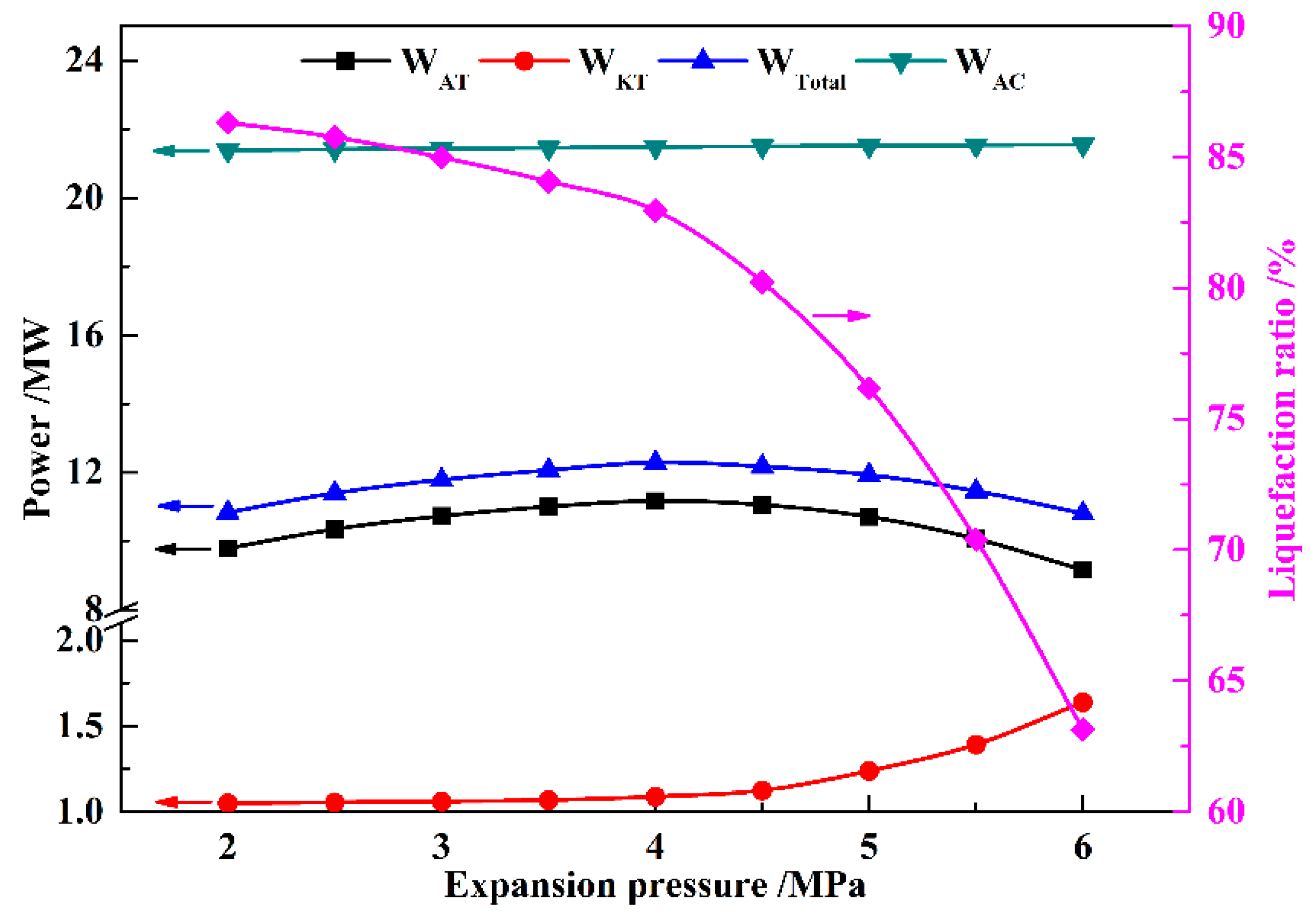
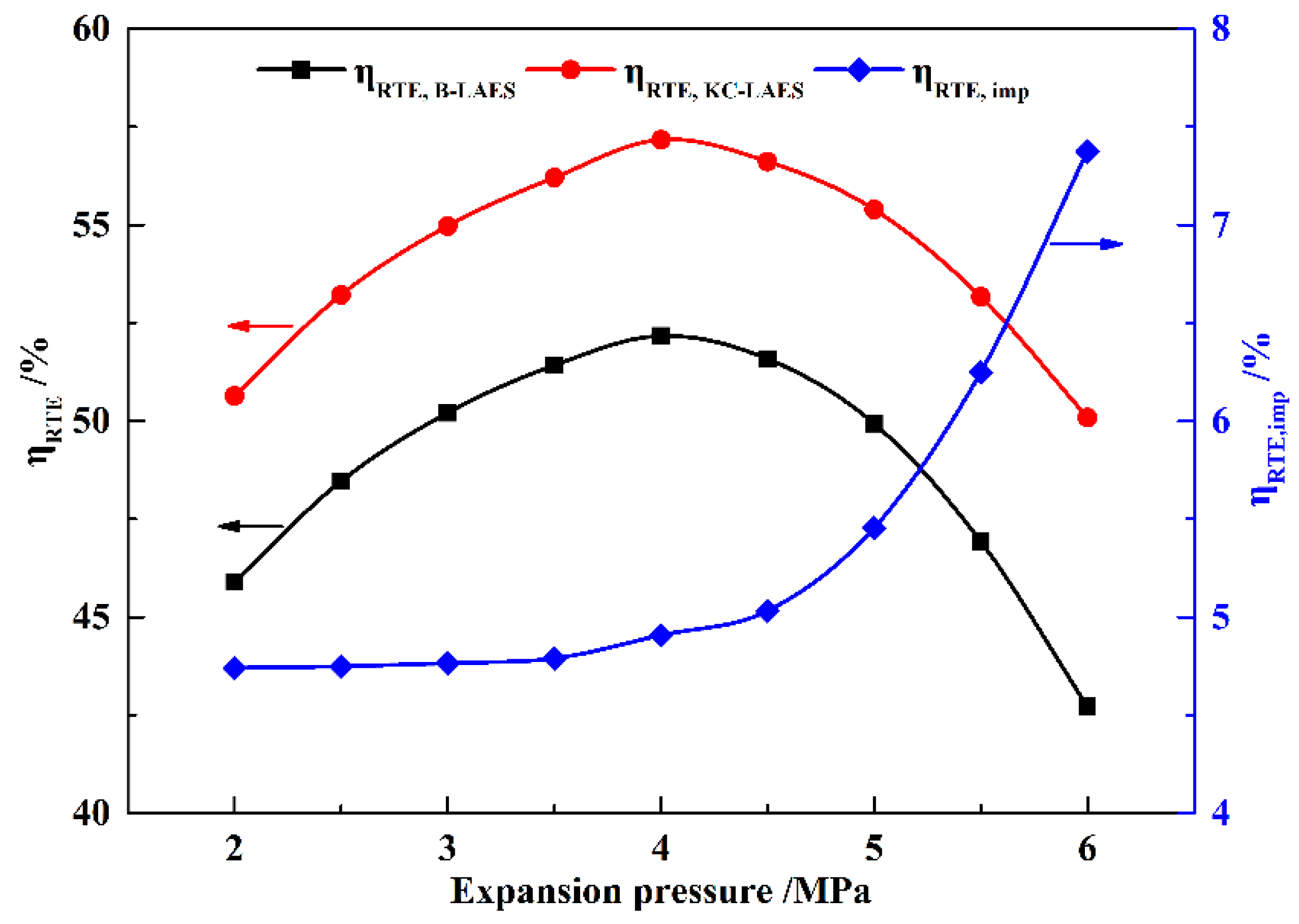
In this paper, a novel integrated system based on liquid air energy storage (LAES) and Kalina cycle (KC), called KC-LAES, has been proposed and analyzed. In baseline LAES (B-LAES), the compression heat is surplus because of the low liquefaction ratio. Therefore, a KC system is introduced into the LAES to utilize the surplus compression heat to generate additional electricity. An energetic model was developed to assess the performance of the proposed system. In the analysis of the KC, the influence of the working fluid concentration, evaporating temperature, and operating pressure was discussed. The power generation of the KC turbine, efficiency, and exergy efficiency for each calculation case was presented. According to the calculation results, the evaporating temperature has less influence on the performance of the KC, and the optimal working fluid concentration and operating pressure are 85% and 12 MPa, respectively. In the analysis of the KC-LAES, the influence of liquefaction and of expansion pressure was presented. The calculation results indicate that the introduction of the KC can notably improve the performance of LAES. The RTE of the KC-LAES is 57.18%, compared with that of B-LAES, 52.16%, with a liquefaction pressure value of eight MPa and an expansion pressure value of four MPa.