Digital Volume Pulse Measured at the Fingertip as an Indicator of Diabetic Peripheral Neuropathy in the Aged and Diabetic
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Protocol
2.3. Calculation of the Percussion Entropy Index (PEIPPI) Using Synchronized {PPI} and {Amp} Signals
2.3.1. Surrogate Data for Baroreflex: Synchronized {PPI} and {Amp} Signals
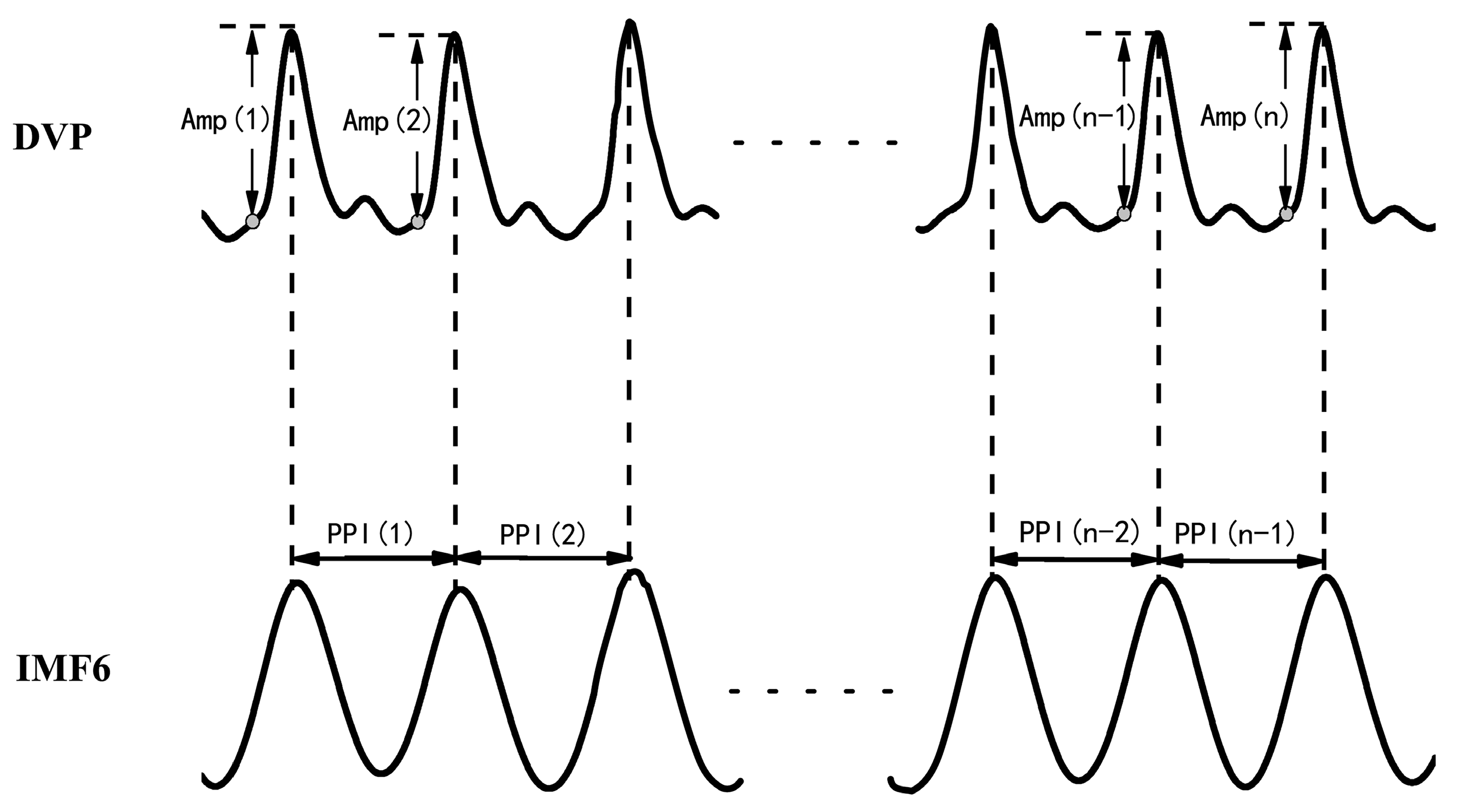
- Synchronized {PPI} and {Amp} signals{Amp} = {Amp(1), Amp(2),…, Amp(n)} for time series of DVP amplitude signals, and {PPI} = {PPI(1), PPI(2),…, PPI(n − 1)} for the PPI of IMF6 after EEMD, were simultaneously synchronized for each subject, as shown in Figure 2.{Amp} = {Amp(1), Amp(2), Amp(3), …, Amp(n)},{PPI} = {PPI(1), PPI(2), PPI(3), …, PPI(n − 1)}.
- Synchronized {BPPI} and {BAmp} signals for fluctuation t patternsBaroreflex sensitivity (BRS) had been shown to be a novel parameter of autonomic function. BRS can quantitatively reflect the matching degree between a change in DVP amplitudes of dominant fingers (i.e., {Amp} in Equation (1)) and a change in the peak-to-peak intervals (PPIs) of IMF6 (i.e., {PPI} in Equation (2)). Therefore, the fluctuations among successive DVP waveform amplitudes and PPIs from IMF6 undergo binary transformation to get two binary sequences (i.e., {BAmp} and {BPPI}, respectively). In that way, {BAmp} and {BPPI} represented the fluctuations of series {Amp} and {PPI}, respectively.
2.3.2. Surrogate Data for Percussion Entropy in Assessing the Complexity of BRS
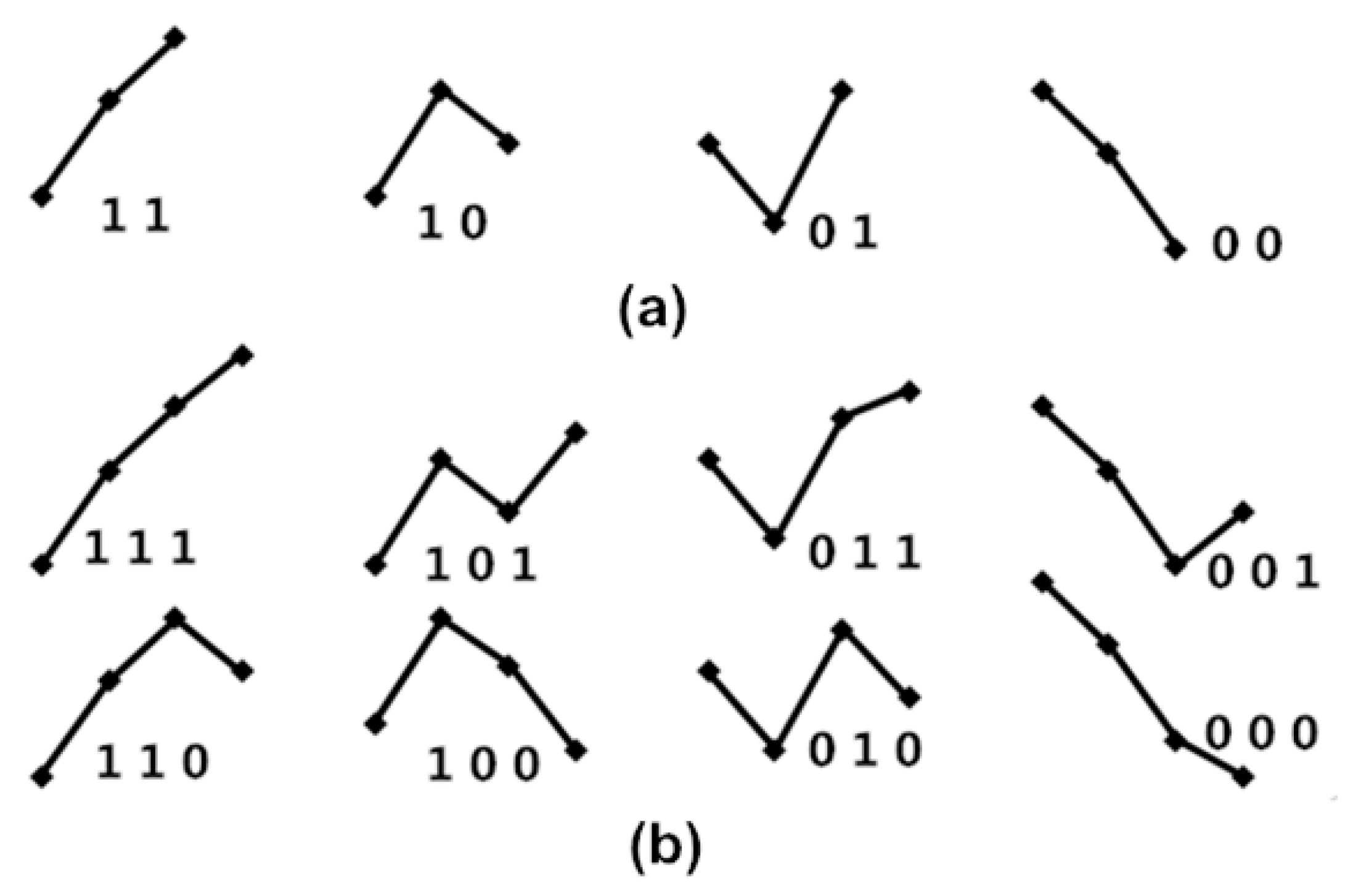
- Fluctuation patterns with length of twoAny two consecutive values [a1 a2] form one of the four fluctuation patterns, not six ordinal patterns, because only “ups and downs” are focused on BRS, as shown in Figure 3a. If one cardiac cycle delay exists between {Amp} and {PPI}, then {a1 a2 a3 ⋯⋯ an} and {p2 p3 p4 ⋯⋯ pn+1} are used to match the obedience in fluctuation tendency with every two dimension counts, and then the percussion number (i.e., the numbers of matches) is acquired and divided by the total number of the two dimensions of fluctuation patterns. This is called percussion frequency with one cardiac cycle delay, and it is expressed by the following equation:If two cardiac cycle delays exist between {Amp} and {PPI}, then {a1 a2 a3 ⋯⋯ an} and {p3 p4 p5 ⋯⋯ pn+2} are used for matching obedience in the fluctuation tendency with every two dimension counts, and then the percussion number (i.e., the number of matches) is acquired and divided by the total number of the two dimensions of fluctuation patterns. This is called percussion frequency with two cardiac cycle delays, expressed by the following equation:Thus, percussion frequency with five cardiac cycle delays between {Amp} and {PPI} is expressed as follows:Hence, the percussion entropy with length two of obedience in the fluctuation tendency can be defined as follows:The higher the value in (8), the higher the BRS.
- Fluctuation patterns with length threeSimilarly, the percussion entropy with length three of obedience in the fluctuation tendency can be expressed as follows:The higher the value in Equation (9), the lower the human biological complexity.
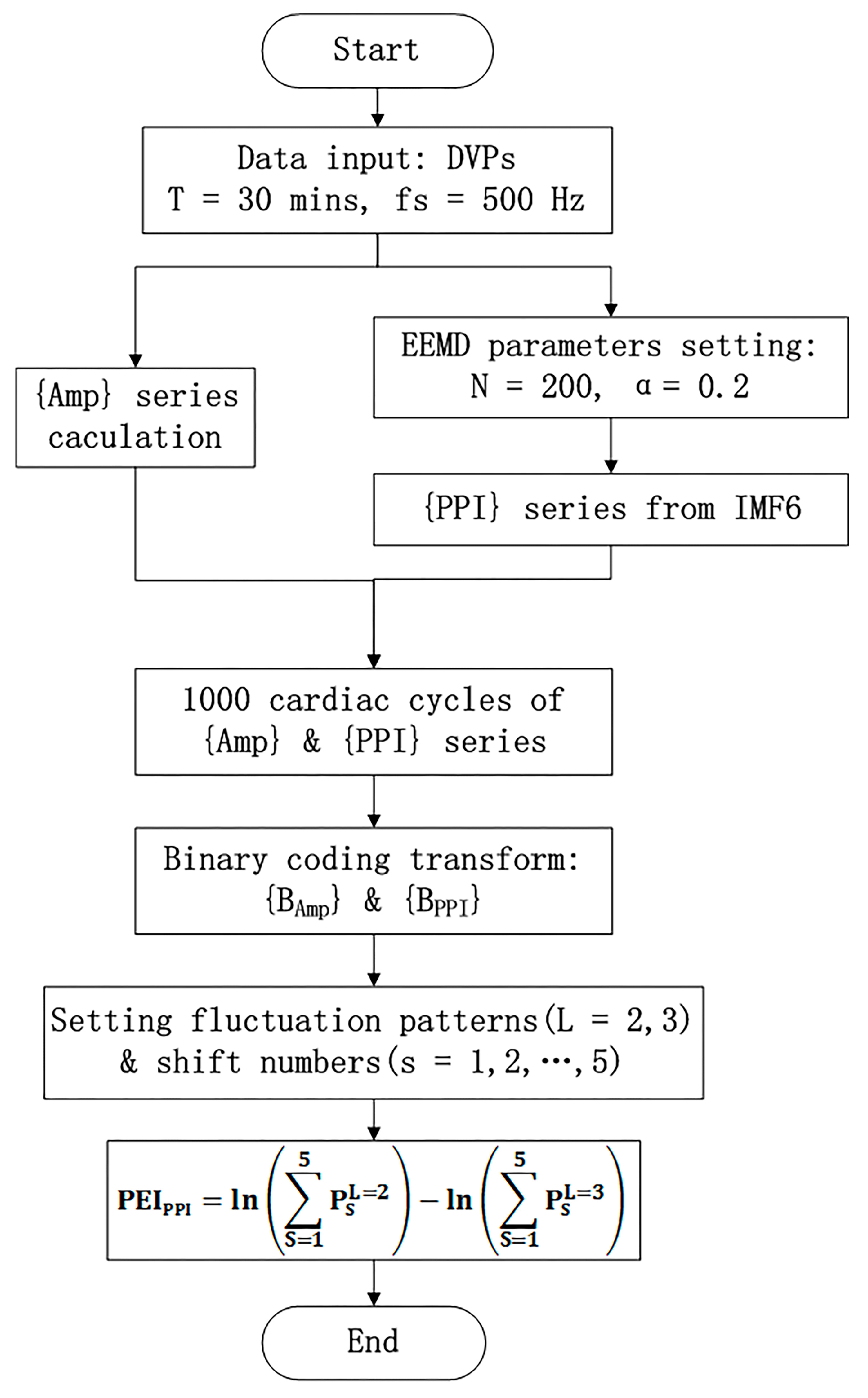
- PEIPPI calculationThe calculation of the new percussion entropy index (PEIPPI) comprises the flow chart shown in Figure 4. Compared with PEIPPI, PPG and ECG signals were synchronized and sampled on the same system. The previous parameter (i.e., PEIRRI) was then computed from {Amp} and {RRI} for every subject. In addition, LHRRRI and MEIRRI were computed with only the RRI dataset used for comparison.
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Age-Controlled Healthy and Diabetic Subjects
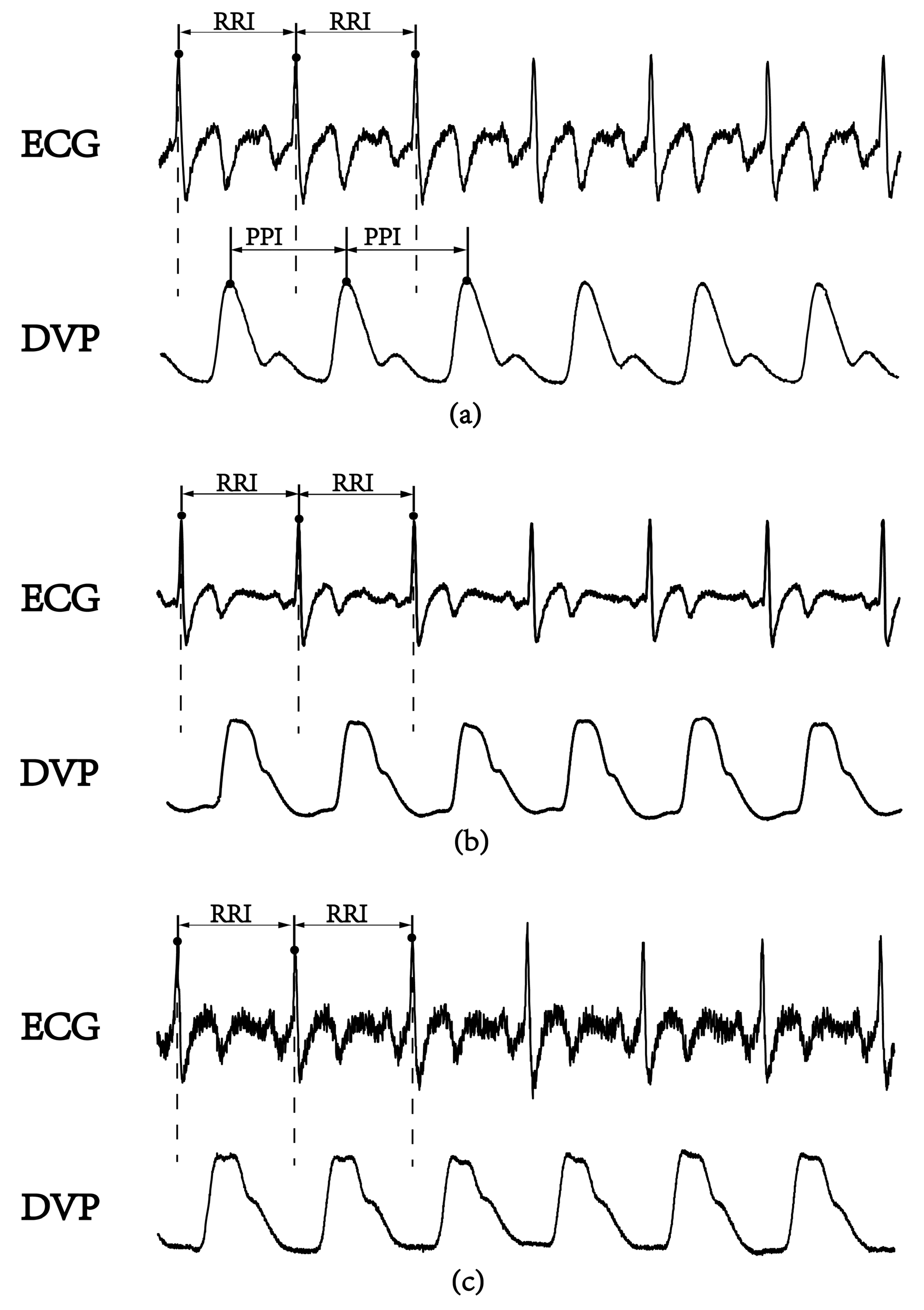
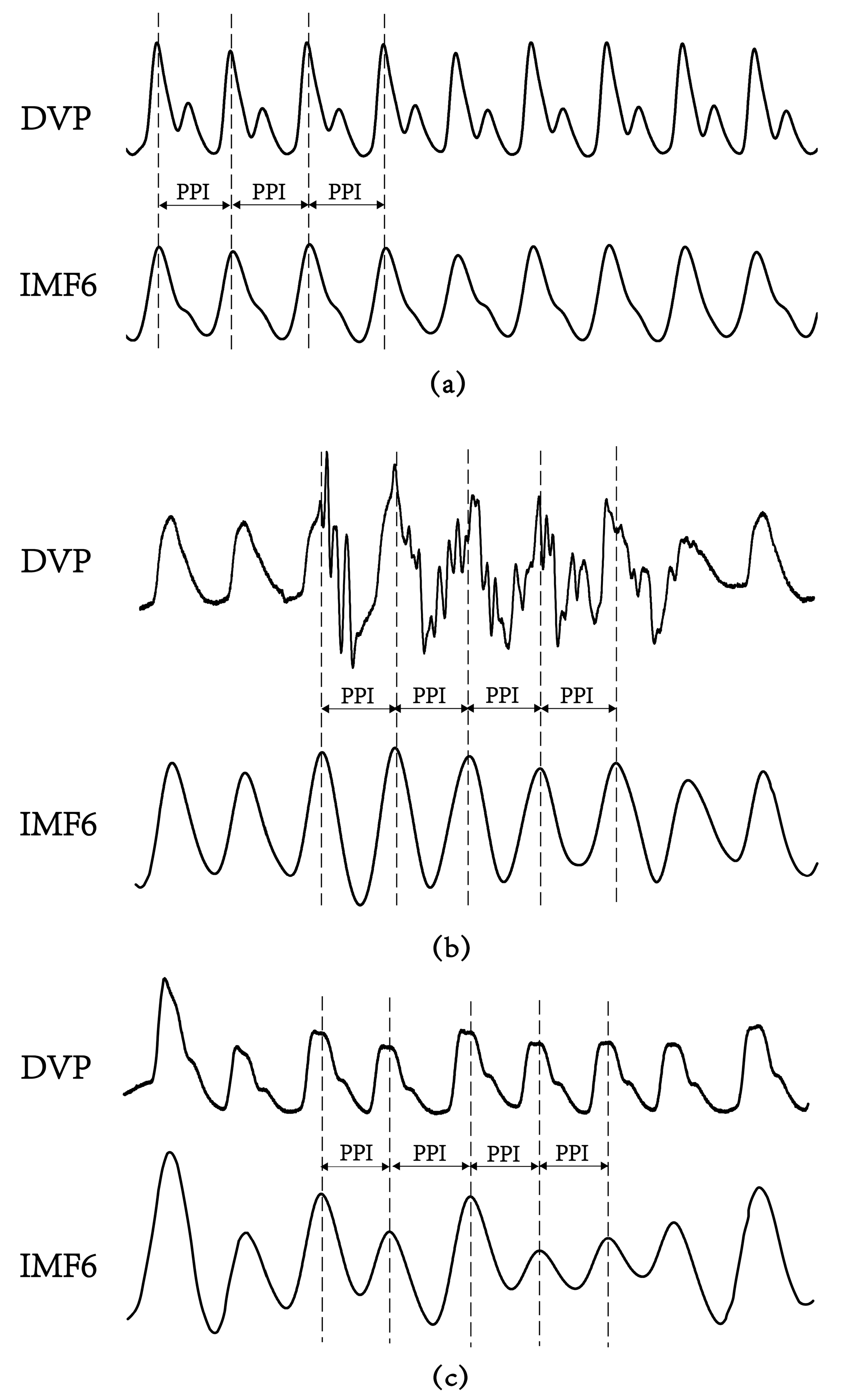
3.2. Failure of DVPs to Detect Peak-to-Peak Intervals in an Aged Subject and a Diabetic Subject
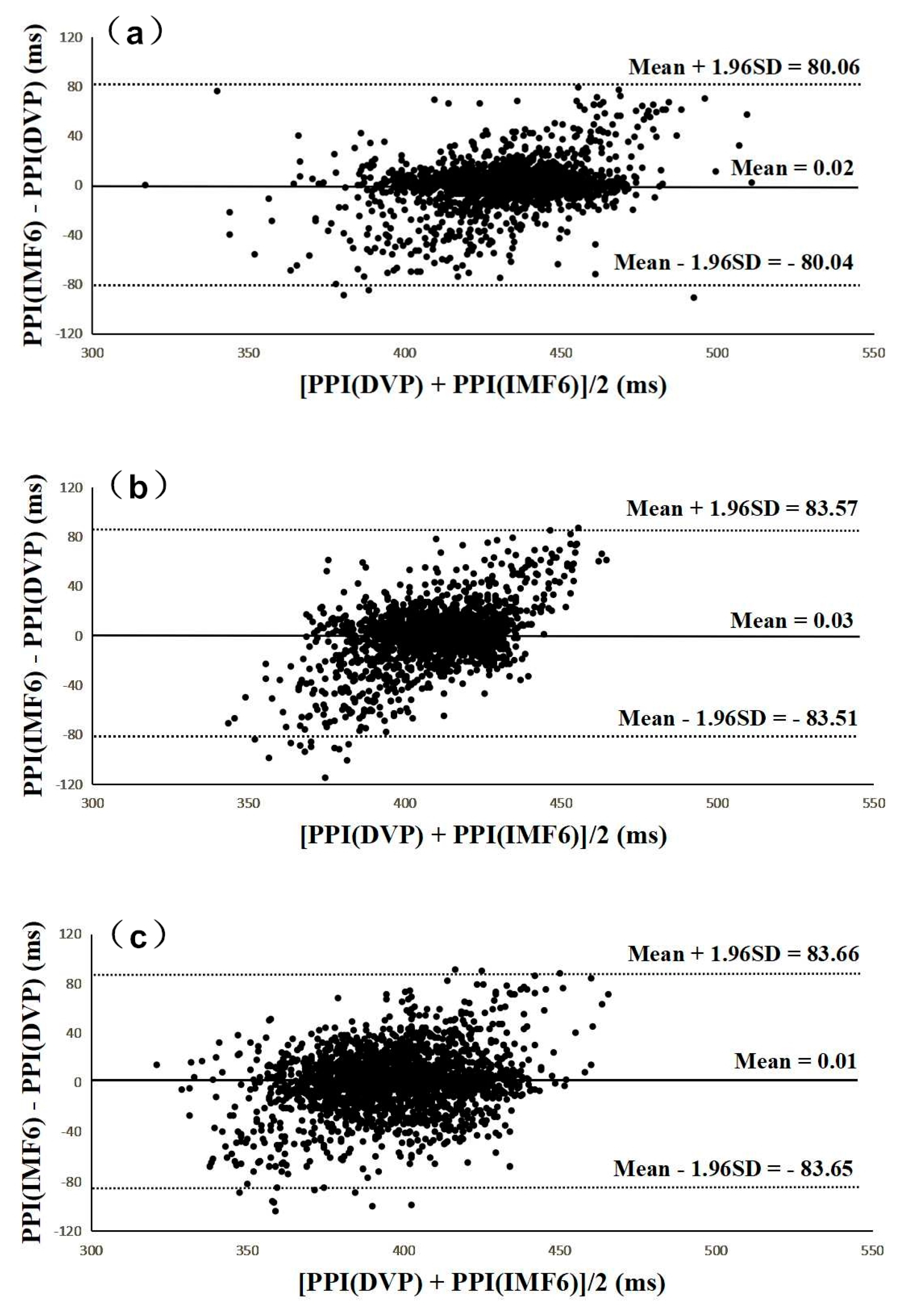
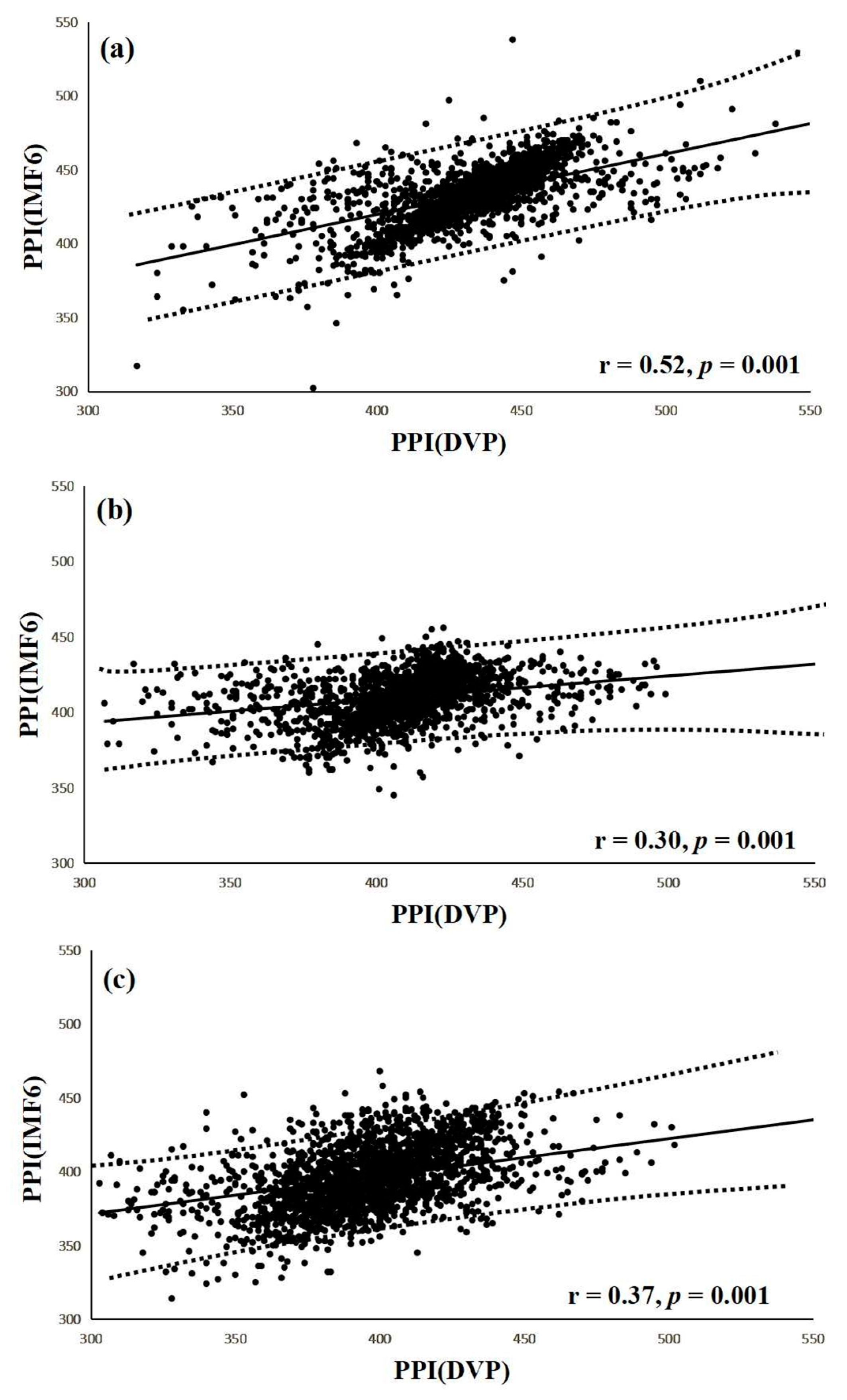
3.3. Agreement Assessment between PPI (DVP) and PPI (IMF6) for the Three Groups
3.4. Performance Compared among PEIPPI, PEIRRI, MEIRRI, and LHRRRI to Differentiate Future Peripheral Neuropathy from Type 2 Diabetic Patients
3.5. Correlations of Risk factors with PEIPPI, PEIRRI, MEIRRI, and LHRRRI
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| BRS | Baroreflex Sensitivity |
| DBP | Diastolic Blood Pressure |
| DVP | Digital Volume Pulse |
| DPN | Diabetic Peripheral Neuropathy |
| ECG | Electrocardiography |
| EEMD | Ensemble Empirical Mode Decomposition |
| FBS | Fasting Blood sugar |
| HbA1c | Glycosylated hemoglobin |
| HDL | High-Density Lipoprotein cholesterol |
| HFP | High Frequency Power |
| HRV | Heart Rate Variability |
| IMF6 | The 6th decomposed Intrinsic Mode Function |
| LDL | Low-Density Lipoprotein cholesterol |
| LFP | Low Frequency Power |
| LHRRRI | Low-/High-frequency power Ratio (LFP/HFP, LHR) using the RRI dataset |
| MEIRRI | Multiscale Entropy Index using the RRI dataset only |
| PC | Personal Computer |
| PEIPPI | Percussion Entropy Index using synchronized {PPI} and {Amp} signals |
| PEIRRI | Percussion Entropy Index using synchronized {RRI} and {Amp} signals |
| PP | Pulse Pressure |
| PPG | Photoplethysmography |
| PPI | Peak-to-Peak Interval |
| RRI | R-R Interval of ECG |
| SBP | Systolic Blood Pressure |
| SPSS | Statistical Package for the Social Sciences |
| TG | Triglyceride |
| WC | Waist Circumference |
References
- Valencia, W.M.; Florez, H. How to prevent the microvascular complications of type 2 diabetes beyond glucose control. BMJ 2017, 356, i6505. [Google Scholar] [CrossRef] [PubMed]
- Gubitosi-Klug, R.A.; DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: Summary and future directions. Diabetes Care 2014, 37, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Juster-Switlyk, K.; Smith, A.G. Updates in diabetic peripheral neuropathy. F1000Res. 2016, 5. F1000 Faculty Rev-738. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Azmi, S.; Yadav, R.; Ferdousi, M.; Kumar, M.; Cuthbertson, D.J.; Lim, J.; Malik, R.A.; Alam, U. Diabetic peripheral neuropathy: Epidemiology, diagnosis, and pharmacotherapy. Clin. Ther. 2018, 40, 828–849. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, V.; Gangan, N.; Sheehan, J. Impact of cardiovascular complications among patients with Type 2 diabetes mellitus: A systematic review. Expert Rev. Pharmacoecon. Outcomes Res. 2015, 15, 487–497. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Pumper, G.M.; Higano, S.T.; Holmes, D.R., Jr.; Kuvin, J.T.; Lerman, A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J. Am. Coll. Cardiol. 2004, 44, 2137–2141. [Google Scholar] [CrossRef]
- Grover-Paez, F.; Zavalza-Gomez, A.B. Endothelial dysfunction and cardiovascular risk factors. Diabetes Res. Clin. Pract. 2009, 84, 1–10. [Google Scholar] [CrossRef]
- Quattrini, C.; Jeziorska, M.; Boulton, A.J.; Malik, R.A. Reduced vascular endothelial growth factor expression and intra-epidermal nerve fiber loss in human diabetic neuropathy. Diabetes Care 2008, 31, 140–145. [Google Scholar] [CrossRef]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Dalla Pozza, R.; Bechtold, S.; Bonfig, W.; Putzker, S.; Kozlik-Feldmann, R.; Schwarz, H.-P.; Netz, H. Impaired short-term blood pressure regulation and autonomic dysbalance in children with type 1 diabetes mellitus. Diabetologia 2007, 50, 2417–2423. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rosengard-Barlund, M.; Bernardi, L.; Fagerudd, J.; Mantysaari, M.; Af Bjorkesten, C.G.; Lindholm, H.; Forsblom, C.; Waden, J.; Groop, P.H. Early autonomic dysfunction in type 1 diabetes: A reversible disorder? Diabetologia 2009, 52, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Merati, G.; Di Rienzo, M.; Parati, G.; Veicsteinas, A.; Castiglioni, P. Assessment of the autonomic control of heart rate variability in healthy and spinal-cord injured subjects: Contribution of different complexity-based estimators. IEEE Trans. Biomed. Eng. 2006, 53, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Lerma, C.; Infante, O.; Perez-Grovas, H.; Jose, M.V. Poincare plot indexes of heart rate variability capture dynamic adaptations after haemodialysis in chronic renal failure patients. Clin. Physiol. Funct. Imag. 2003, 23, 72–80. [Google Scholar]
- Pan, W.Y.; Su, M.C.; Wu, H.T.; Su, T.J.; Lin, M.C.; Sun, C.K. Multiscale entropic assessment of autonomic dysfunction in patients with obstructive sleep apnea and therapeutic impact of continuous positive airway pressure treatment. Sleep Med. 2016, 20, 12–17. [Google Scholar] [CrossRef]
- Wei, H.C.; Xiao, M.X.; Ta, N.; Wu, H.T.; Sun, C.K. Assessment of diabetic autonomic nervous dysfunction with a novel percussion entropy approach. Complexity 2019, 2019, 6469853. [Google Scholar] [CrossRef]
- Xiao, M.X.; Lu, C.H.; Ta, N.; Jiang, W.W.; Tang, X.J.; Wu, H.T. Application of a Speedy Modified Entropy Method in Assessing the Complexity of Baroreflex Sensitivity for Age-Controlled Healthy and Diabetic Subjects. Entropy 2019, 21, 894. [Google Scholar] [CrossRef]
- Sun, C.K.; Liu, C.C.; Liu, W.M.; Wu, H.T.; Huang, R.M.; Liu, A.B. Compatibility of pulse–pulse intervals with R–R intervals in assessing cardiac autonomic function and its relation to risks of atherosclerosis. Tzu Chi Med. J. 2019. [Google Scholar] [CrossRef]
- Selvaraj, N.; Jaryal, A.; Santhosh, J.; Deepak, K.K.; Anand, S. Assessment of heart rate variability derived from finger-tip photoplethysmography as compared to electrocardiography. J. Med. Eng. Tech. 2008, 32, 479–484. [Google Scholar] [CrossRef]
- Lin, S.-T.; Chen, W.-H.; Lin, Y.-H. A Pulse Rate Detection Method for Mouse Application Based on Multi-PPG Sensors. Sensors 2017, 17, 1628. [Google Scholar]
- Song, J.; Li, D.; Ma, X.; Teng, G.; Wei, J. A Robust Dynamic Heart-Rate Detection Algorithm Framework During Intense Physical Activities Using Photoplethysmographic Signals. Sensors 2017, 17, 2450. [Google Scholar] [CrossRef] [PubMed]
- Tejedor, J.; García, C.A.; Márquez, D.G.; Raya, R.; Otero, A. Multiple Physiological Signals Fusion Techniques for Improving Heartbeat Detection: A Review. Sensors 2019, 19, 4708. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Maeda, Y.; Sekine, M.; Yoshida, M. Wearable Photoplethysmographic Sensors—Past and Present. Electronics 2014, 3, 282–302. [Google Scholar] [CrossRef]
- Lee, J.; Yang, S.; Lee, S.; Kim, H.C. Analysis of Pulse Arrival Time as an Indicator of Blood Pressure in a Large Surgical Biosignal Database: Recommendations for Developing Ubiquitous Blood Pressure Monitoring Methods. J. Clin. Med. 2019, 8, 1773. [Google Scholar] [CrossRef]
- Baldoumas, G.; Peschos, D.; Tatsis, G.; Chronopoulos, S.K.; Christofilakis, V.; Kostarakis, P.; Varotsos, P.; Sarlis, N.V.; Skordas, E.S.; Bechlioulis, A.; et al. A Prototype Photoplethysmography Electronic Device that Distinguishes Congestive Heart Failure from Healthy Individuals by Applying Natural Time Analysis. Electronics 2019, 8, 1288. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Z.; Ward, R.; Elgendi, M. Hypertension Assessment Using Photoplethysmography: A Risk Stratification Approach. J. Clin. Med. 2019, 8, 12. [Google Scholar] [CrossRef]
- Wu, H.T.; Hsu, P.C.; Lin, C.F.; Wang, H.J.; Sun, C.K.; Liu, A.B.; Lo, M.T.; Tang, C.J. Multiscale entropy analysis of pulse wave velocity for assessing atherosclerosis in the aged and diabetic. IEEE Trans. Biomed. Eng. 2011, 58, 2978–2981. [Google Scholar]
- Elgendi, M. On the analysis of fingertip photoplethysmogram signals. Curr. Cardiol. Rev. 2012, 8, 14–25. [Google Scholar] [CrossRef]
- Chawla, A.; Chawla, R.; Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J. Endocrinol. Metab. 2016, 20, 4–546. [Google Scholar] [CrossRef]
- Lin, I.W.; Chang, H.H.; Lee, Y.H.; Wu, Y.C.; Lu, C.W.; Huang, K.C. Blood sugar control among type 2 diabetic patients who travel abroad: A cross sectional study. Medicine (Baltimore) 2019, 98, e14946. [Google Scholar] [CrossRef]
- Sohaila, C.; Patrick, M.; Zirie, M.; Jayyousi, A.; Alrouh, H.; Amit Abraham, A.; Al-Samraye, S.; Mahfoud, Z.; Al-Janahi, I.M.; Ibrahim, B.; et al. Risk factors for microvascular complications of diabetes in a high-risk middle east population. J. Diabetes Res. 2018, 2018, 8964027. [Google Scholar]
- Balkau, B.; Calvi-Gries, F.; Freemantle, N.; Vincent, M.; Pilorget, V.; Home, P.D. Predictors of HbA1c over 4 years in people with type 2 diabetes starting insulin therapies: The CREDIT study. Diabetes Res. Clin. Pract. 2015, 108, 432–440. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Francesco, G.; Home, P.D.; Tuomilehto, J. Glucose control and vascular outcomes in Type 2 diabetes: Is the picture clear? Diabetes Care 2016, 39 (Suppl. 2), S187–S195. [Google Scholar]
- Ruiz, J.; Monbaron, D.; Parati, G.; Perret, S.; Haesler, E.; Danzeisen, C.; Hayoz, D. Diabetic neuropathy is a more important determinant of baroreflex sensitivity than carotid elasticity in type 2 diabetes. Hypertension 2005, 46, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N. Prediabetes diagnosis and treatment: A review. World J. Diabetes 2015, 6, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.J.; Echouffo-Tcheugui, J.B.; Golden, S.H.; Chen, H.; Jenny, N.S.; Carnethon, M.R.; Jacobs, D., Jr.; Burke, G.L.; Vaidya, D.; Ouyang, P.; et al. Physical activity, sedentary behaviors and the incidence of type 2 diabetes mellitus: The Multi-Ethnic Study of Atherosclerosis. BMJ Open Diabetes Res. Care 2016, 4, e000185. [Google Scholar] [CrossRef]
- Papanas, N.; Ziegler, D. Risk Factors and Comorbidities in Diabetic Neuropathy: An Update 2015. RDS 2015, 12, 48–62. [Google Scholar] [CrossRef]
- Martín-Timón, I.; Sevillano-Collantes, C.; Segura-Galindo, A.; Del Cañizo-Gómez, F.J. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J. Diabetes 2014, 5, 444–470. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.; DeFronzo, R.A.; Del Prato, S.; Chilton, R.; Singh, R.; Ryder, R.E. Cardiovascular disease and type 2 diabetes: Has the dawn of a new era arrived? Diabetes Care 2017, 40, 813–820. [Google Scholar] [CrossRef]
| Parameters | Group 1 | Group 2 | Group 3 |
|---|---|---|---|
| Number: 34 | Number: 42 | Number: 24 | |
| Female/Male | Female/Male | Female/Male | |
| (15/19) | (14/28) | (10/14) | |
| Age, year | 59.78 ± 9.74 | 65.90 ± 11.10 | 62.26 ± 8.67 |
| Body height, cm Body weight, kg WC, cm BMI, kg/m2 | 160.95 ± 7.16 61.51 ± 10.02 82.56 ± 10.57 23.59 ± 3056 | 159.58 ± 7.97 68.74 ± 10.31 ** 93.85 ± 8.99 27.02 ± 3.93 ** | 164.35 ± 9.67 71.98 ± 7.54 95.44 ± 6.88 26.85 ± 3.94 |
| SBP, mmHg DBP, mmHg PP, mmHg HDL, mg/dL LDL, mg/dL Cholesterol, mg/dL | 123.88 ± 18.69 75.01 ± 8.99 48.88 ± 14.41 51.76 ± 21.05 113.23 ± 30.25 190.08 ± 48.99 | 128.80 ± 16.61 75.66 ± 10.35 51.88 ± 16.05 45.14 ± 17.82 124.11 ± 47.08 174.53 ± 50.79 | 125.09 ± 31.64 72.01 ± 18.04 53.09 ± 18.76 39.32 ± 6.18 106.23 ± 25.08 179.78 ± 31.38 |
| TG, mg/dL | 94.81 ± 36.16 | 148.95 ± 67.84 ** | 164.18 ± 68.05 |
| HbA1c, % | 5.92 ± 0.32 | 7.99 ± 1.68 ** | 8.48 ± 1.58 |
| FBS, mg/dL | 100.42 ± 26.80 | 150.73 ± 48.52 ** | 162.69 ± 58.24 |
| Parameters | Group 1 (n = 34) | Group 2 (n = 42) | Group 3 (n = 24) |
|---|---|---|---|
| LHRRRI | 1.59 ± 1.03 | 2.09 ± 2.09 | 2.25 ± 2.38 |
| MEIRRI | 0.49 ± 0.16 | 0.36 ± 0.20 ** | 0.37 ± 0.19 |
| PEIRRI | 0.73 ± 0.47 | 0.60 ± 0.11 ** | 0.56 ± 0.10 † |
| PEIPPI | 0.69 ± 0.03 | 0.66 ± 0.04 ** | 0.63 ± 0.06 † |
| PEIPPI | PEIRRI | MEIRRI | LHRRRI | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| BW, kg | −0.20 | 0.04 | −0.19 | 0.06 | −0.07 | 0.53 | −0.07 | 0.53 |
| WC, cm | −0.20 | 0.05 | −0.31 | 0.00 | −0.02 | 0.85 | −0.01 | 0.95 |
| TG, mg/dL | −0.27 | 0.01 | −0.33 | 0.00 | −0.09 | 0.39 | 0.05 | 0.64 |
| HbA1c, % | −0.38 | 0.00 | −0.43 | 0.00 | −0.26 | 0.01 | −0.14 | 0.17 |
| FBS, mg/dL | −0.23 | 0.03 | −0.40 | 0.00 | −0.28 | 0.01 | −0.05 | 0.63 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.-C.; Ta, N.; Hu, W.-R.; Xiao, M.-X.; Tang, X.-J.; Haryadi, B.; Liou, J.J.; Wu, H.-T. Digital Volume Pulse Measured at the Fingertip as an Indicator of Diabetic Peripheral Neuropathy in the Aged and Diabetic. Entropy 2019, 21, 1229. https://doi.org/10.3390/e21121229
Wei H-C, Ta N, Hu W-R, Xiao M-X, Tang X-J, Haryadi B, Liou JJ, Wu H-T. Digital Volume Pulse Measured at the Fingertip as an Indicator of Diabetic Peripheral Neuropathy in the Aged and Diabetic. Entropy. 2019; 21(12):1229. https://doi.org/10.3390/e21121229
Chicago/Turabian StyleWei, Hai-Cheng, Na Ta, Wen-Rui Hu, Ming-Xia Xiao, Xiao-Jing Tang, Bagus Haryadi, Juin J. Liou, and Hsien-Tsai Wu. 2019. "Digital Volume Pulse Measured at the Fingertip as an Indicator of Diabetic Peripheral Neuropathy in the Aged and Diabetic" Entropy 21, no. 12: 1229. https://doi.org/10.3390/e21121229
APA StyleWei, H.-C., Ta, N., Hu, W.-R., Xiao, M.-X., Tang, X.-J., Haryadi, B., Liou, J. J., & Wu, H.-T. (2019). Digital Volume Pulse Measured at the Fingertip as an Indicator of Diabetic Peripheral Neuropathy in the Aged and Diabetic. Entropy, 21(12), 1229. https://doi.org/10.3390/e21121229






