Alterations of Cardiovascular Complexity during Acute Exposure to High Altitude: A Multiscale Entropy Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Data Collection
2.2. Data Preprocessing and Spectral Analysis
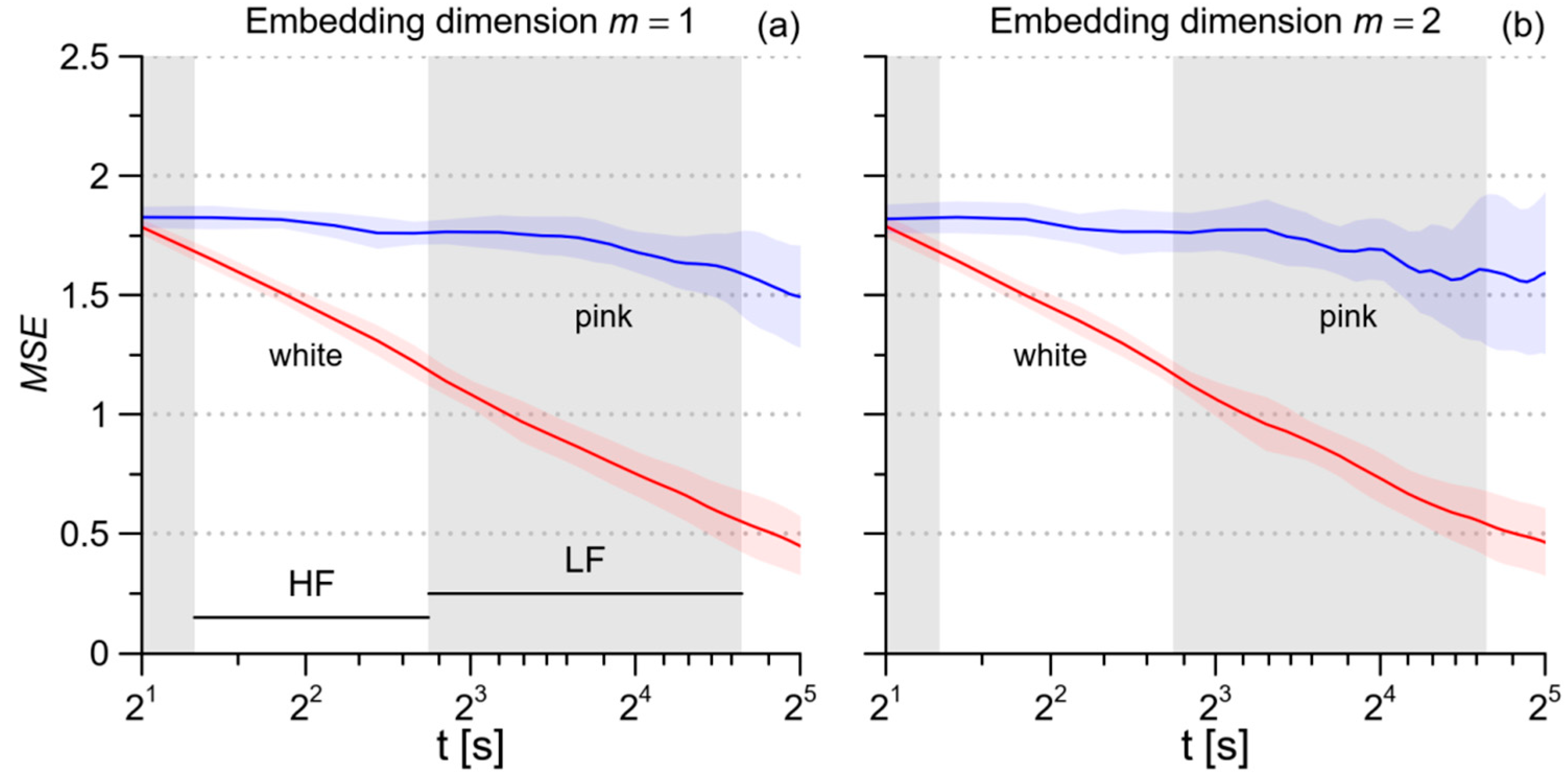
2.3. Multiscale Entropy of RRI, SBP, and DBP
2.4. Multiscale Cross-Entropy between SBP and PI
2.5. Statistical Analysis
3. Results
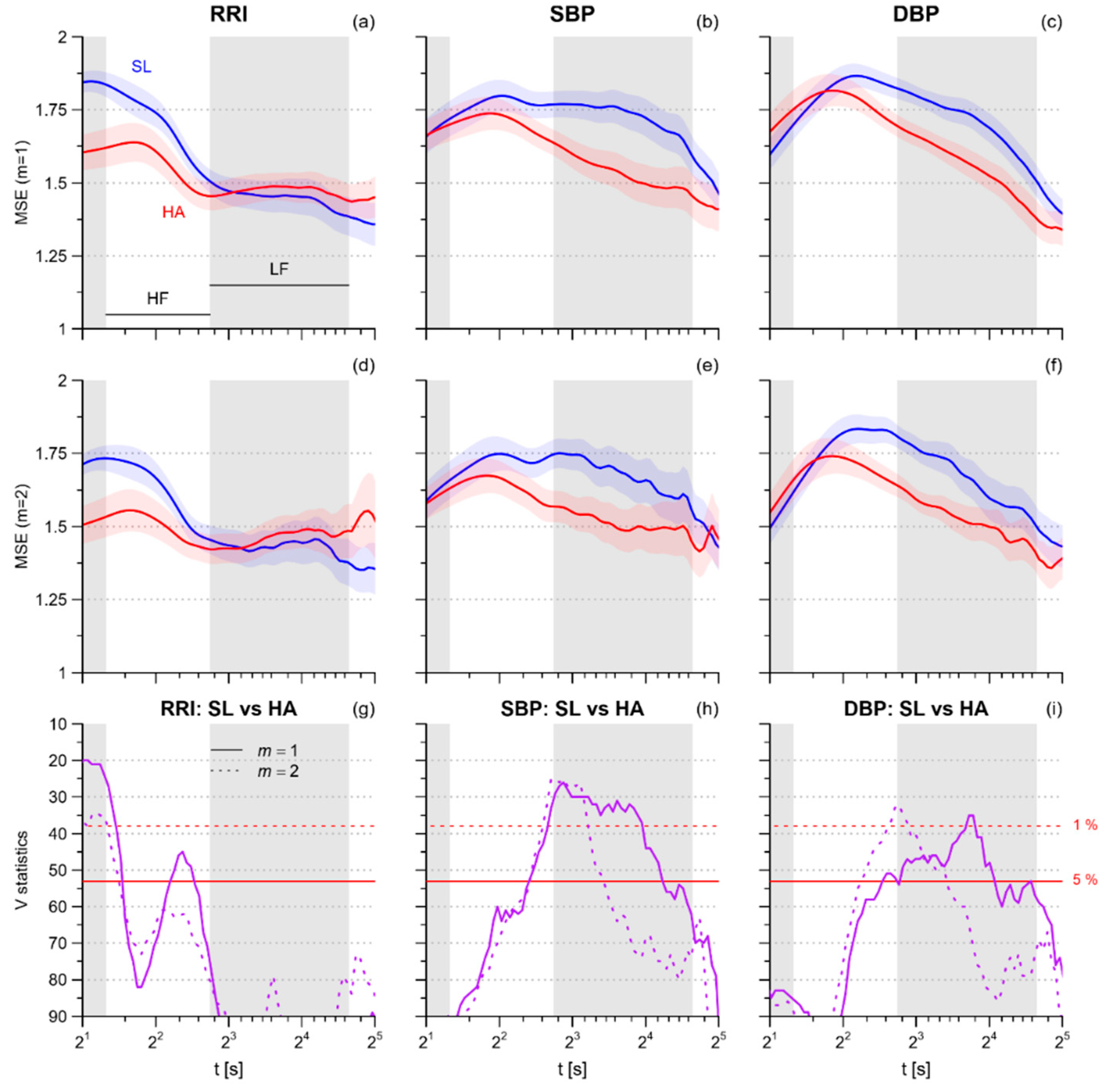
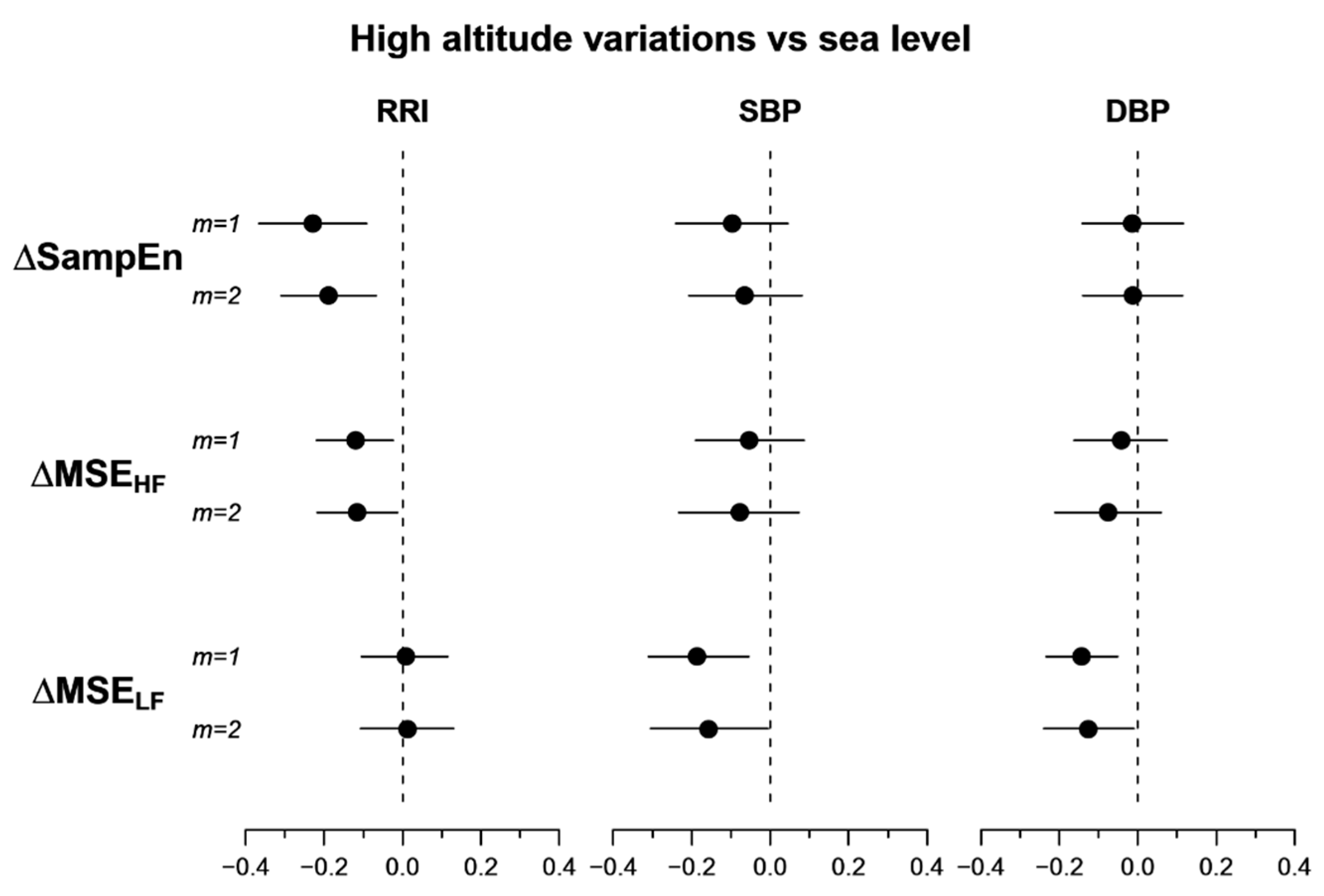
3.1. Multiscale Entropy
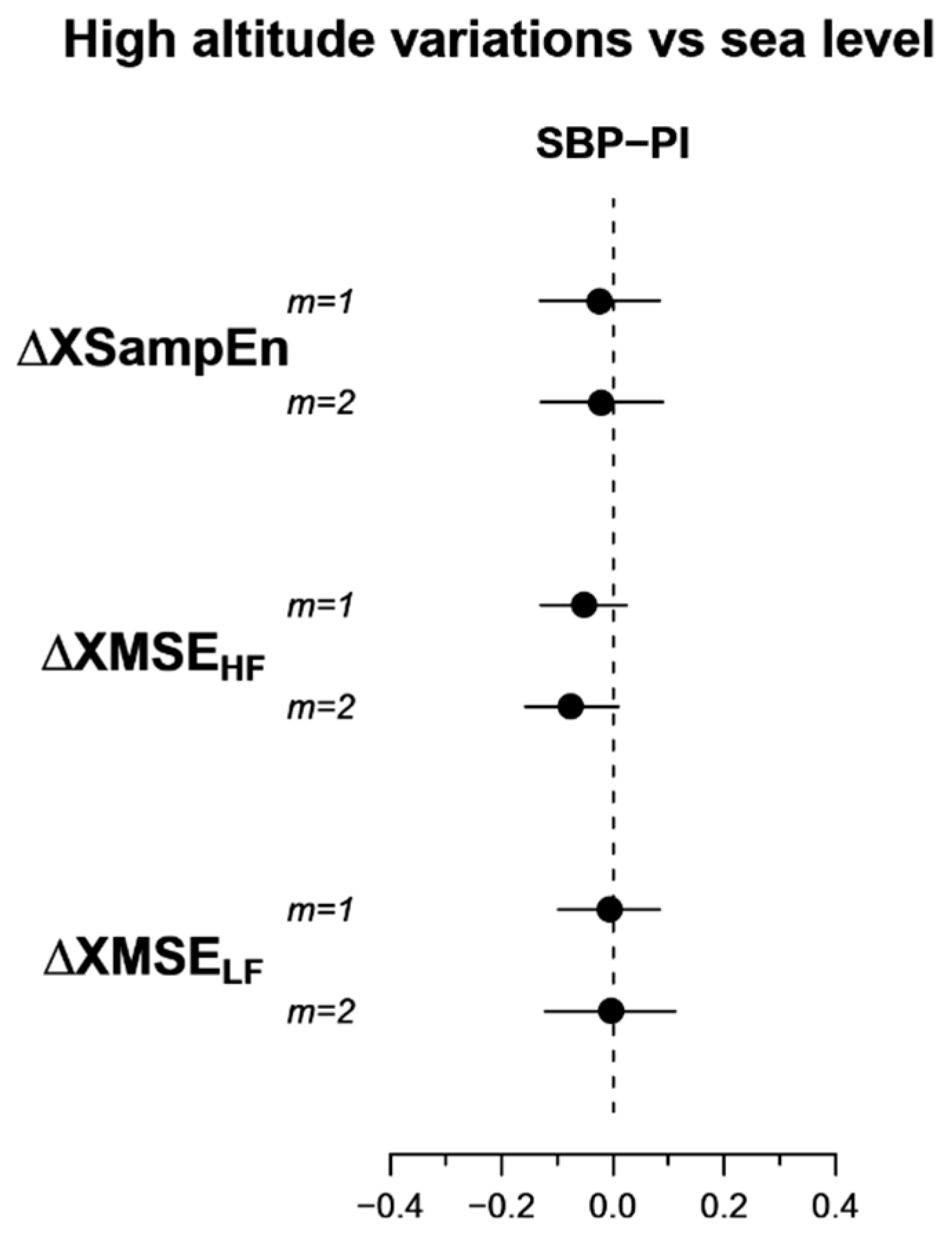
3.2. Multiscale Cross-Entropy
4. Discussion
4.1. Cardiorespiratoy Variables and Spectral Powers at High Altitude
4.2. Heart Rate Multiscale Entropy
4.3. Blood Pressure Multiscale Entropy
4.4. Blood Pressure-Heart Rate Multiscale Cross-Entropy
4.5. Limitations and Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hainsworth, R.; Drinkhill, M.J.; Rivera-Chira, M. The autonomic nervous system at high altitude. Clin. Auton. Res. 2007, 17, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, C.; Meriggi, P.; Agostoni, P.; Faini, A.; Bilo, G.; Revera, M.; Caldara, G.; Di Rienzo, M.; Castiglioni, P.; Maurizio, B.; et al. High-altitude hypoxia and periodic breathing during sleep: Gender-related differences. J. Sleep Res. 2013, 22, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Caravita, S.; Faini, A.; Lombardi, C.; Valentini, M.; Gregorini, F.; Rossi, J.; Meriggi, P.; Di Rienzo, M.; Bilo, G.; Agostoni, P.; et al. Sex and Acetazolamide Effects on Chemoreflex and Periodic Breathing During Sleep at Altitude. Chest 2015, 147, 120–131. [Google Scholar] [CrossRef]
- Di Rienzo, M.; Castiglioni, P.; Rizzo, F.; Faini, A.; Mazzoleni, P.; Lombardi, C.; Meriggi, P.; Parati, G. The HIGHCARE investigators Linear and Fractal Heart Rate Dynamics during Sleep at High Altitude: Investigation with Textile Technology. Methods Inf. Med. 2010, 49, 521–525. [Google Scholar] [PubMed]
- Boos, C.J.; Bye, K.; Sevier, L.; Bakker-Dyos, J.; Woods, D.R.; Sullivan, M.; Quinlan, T.; Mellor, A. High Altitude Affects Nocturnal Non-linear Heart Rate Variability: PATCH-HA Study. Front. Physiol. 2018, 9, 390. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, L.; Passino, C.; Spadacini, G.; Calciati, A.; Robergs, R.; Greene, R.; Martignoni, E.; Anand, I.; Appenzeller, O. Cardiovascular autonomic modulation and activity of carotid baroreceptors at altitude. Clin. Sci. 1998, 95, 565–573. [Google Scholar] [CrossRef]
- Bernardi, L. Heart rate and cardiovascular variability at high altitude. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2007, 2007, 6679–6681. [Google Scholar]
- Parati, G.; Revera, M.; Giuliano, A.; Faini, A.; Bilo, G.; Gregorini, F.; Lisi, E.; Salerno, S.; Lombardi, C.; Ramos Becerra, C.G.; et al. Effects of acetazolamide on central blood pressure, peripheral blood pressure, and arterial distensibility at acute high altitude exposure. Eur. Heart J. 2013, 34, 759–766. [Google Scholar] [CrossRef]
- Gizdulich, P.; Prentza, A.; Wesseling, K.H. Models of brachial to finger pulse wave distortion and pressure decrement. Cardiovasc. Res. 1997, 33, 698–705. [Google Scholar] [CrossRef]
- Meier, D.; Collet, T.-H.; Locatelli, I.; Cornuz, J.; Kayser, B.; Simel, D.L.; Sartori, C. Does This Patient Have Acute Mountain Sickness? The Rational Clinical Examination Systematic Review. JAMA 2017, 318, 1810. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology. The North American Society of Pacing Electrophysiology. Heart Rate Variability: Standards of Measurement, Physiological Interpretation, and Clinical Use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Castiglioni, P.; Parati, G.; Faini, A. Information-Domain Analysis of Cardiovascular Complexity: Night and Day Modulations of Entropy and the Effects of Hypertension. Entropy 2019, 21, 550. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.-K. Multiscale Entropy Analysis of Complex Physiologic Time Series. Phys. Rev. Lett. 2002, 89, 068102. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, P.; Coruzzi, P.; Bini, M.; Parati, G.; Faini, A. Multiscale Sample Entropy of Cardiovascular Signals: Does the Choice between Fixed- or Varying-Tolerance among Scales Influence Its Evaluation and Interpretation? Entropy 2017, 19, 590. [Google Scholar] [CrossRef]
- Wu, S.-D.; Wu, C.-W.; Lee, K.-Y.; Lin, S.-G. Modified multiscale entropy for short-term time series analysis. Phys. A Stat. Mech. Its Appl. 2013, 392, 5865–5873. [Google Scholar] [CrossRef]
- Valencia, J.F.; Porta, A.; Vallverdu, M.; Claria, F.; Baranowski, R.; Orlowska-Baranowska, E.; Caminal, P. Refined Multiscale Entropy: Application to 24-h Holter Recordings of Heart Period Variability in Healthy and Aortic Stenosis Subjects. IEEE Trans. Biomed. Eng. 2009, 56, 2202–2213. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Ser. B (Methodol.) 1964, 26, 211–252. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.-K. Multiscale entropy analysis of biological signals. Phys. Rev. E 2005, 71, 021906. [Google Scholar] [CrossRef]
- Forster, H.V.; Smith, C.A. Contributions of central and peripheral chemoreceptors to the ventilatory response to CO2/H+. J. Appl. Physiol. 2010, 108, 989–994. [Google Scholar] [CrossRef]
- Fletcher, E.C. Invited Review: Physiological consequences of intermittent hypoxia: Systemic blood pressure. J. Appl. Physiol. 2001, 90, 1600–1605. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Pilowsky, P.M.; Fong, A.Y. Mechanism of Sympathetic Activation and Blood Pressure Elevation in Humans and Animals Following Acute Intermittent Hypoxia. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 209, pp. 131–146. ISBN 978-0-444-63274-6. [Google Scholar]
- Chen, Y.-C.; Lin, F.-C.; Shiao, G.-M.; Chang, S.-C. Effect of rapid ascent to high altitude on autonomic cardiovascular modulation. Am. J. Med. Sci. 2008, 336, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Hughson, R.L.; Yamamoto, Y.; McCullough, R.E.; Sutton, J.R.; Reeves, J.T. Sympathetic and parasympathetic indicators of heart rate control at altitude studied by spectral analysis. J. Appl. Physiol. 1994, 77, 2537–2542. [Google Scholar] [CrossRef] [PubMed]
- Perini, R.; Milesi, S.; Biancardi, L.; Veicsteinas, A. Effects of high altitude acclimatization on heart rate variability in resting humans. Eur. J. Appl. Physiol. 1996, 73, 521–528. [Google Scholar] [CrossRef]
- Saito, S.; Tanobe, K.; Yamada, M.; Nishihara, F. Relationship between arterial oxygen saturation and heart rate variability at high altitudes. Am. J. Emerg. Med. 2005, 23, 8–12. [Google Scholar] [CrossRef]
- Zhang, D.; She, J.; Yang, J.; Yu, M. Linear and nonlinear dynamics of heart rate variability in the process of exposure to 3600 m in 10 min. Australas. Phys. Eng. Sci. Med. 2015, 38, 263–270. [Google Scholar] [CrossRef]
- Zhang, D.; She, J.; Zhang, Z.; Yu, M. Effects of acute hypoxia on heart rate variability, sample entropy and cardiorespiratory phase synchronization. Biomed. Eng. Online 2014, 13, 73. [Google Scholar] [CrossRef]
- Porta, A.; Castiglioni, P.; Bari, V.; Bassani, T.; Marchi, A.; Cividjian, A.; Quintin, L.; Di Rienzo, M. K-nearest-neighbor conditional entropy approach for the assessment of the short-term complexity of cardiovascular control. Physiol. Meas. 2013, 34, 17–33. [Google Scholar] [CrossRef]
- Porta, A.; Bari, V.; Marchi, A.; De Maria, B.; Castiglioni, P.; di Rienzo, M.; Guzzetti, S.; Cividjian, A.; Quintin, L. Limits of permutation-based entropies in assessing complexity of short heart period variability. Physiol. Meas. 2015, 36, 755–765. [Google Scholar] [CrossRef]
- Vigo, D.E.; Pérez Lloret, S.; Videla, A.J.; Pérez Chada, D.; Hünicken, H.M.; Mercuri, J.; Romero, R.; Nicola Siri, L.C.; Cardinali, D.P. Heart Rate Nonlinear Dynamics During Sudden Hypoxia at 8230 m Simulated Altitude. Wilderness Environ. Med. 2010, 21, 4–10. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Pesek, C.A.; van de Borne, P.J.; Kato, M.; Somers, V.K. Enhanced sympathetic and ventilatory responses to central chemoreflex activation in heart failure. Circulation 1999, 100, 262–267. [Google Scholar] [CrossRef]
- Chao, H.-H.; Yeh, C.-W.; Hsu, C.F.; Hsu, L.; Chi, S. Multiscale Entropy Analysis with Low-Dimensional Exhaustive Search for Detecting Heart Failure. Appl. Sci. 2019, 9, 3496. [Google Scholar] [CrossRef]
- Boos, C.J.; Vincent, E.; Mellor, A.; O’Hara, J.; Newman, C.; Cruttenden, R.; Scott, P.; Cooke, M.; Matu, J.; Woods, D.R. The Effect of Sex on Heart Rate Variability at High Altitude. Med. Sci. Sports Exerc. 2017, 49, 2562–2569. [Google Scholar] [CrossRef]
- Mairer, K.; Wille, M.; Burtscher, M. The Prevalence of and Risk Factors for Acute Mountain Sickness in the Eastern and Western Alps. High Alt. Med. Biol. 2010, 11, 343–348. [Google Scholar] [CrossRef]
- Zhuang, J.; Zhu, H.; Zhou, Z. Reserved Higher Vagal Tone under Acute Hypoxia in Tibetan Adolescents with Long-Term Migration to Sea Level. JJP 2002, 52, 51–56. [Google Scholar] [CrossRef]
- Zhuang, J.; Droma, T.; Sutton, J.R.; McCullough, R.E.; McCullough, R.G.; Groves, B.M.; Rapmund, G.; Janes, C.; Sun, S.; Moore, L.G. Autonomic regulation of heart rate response to exercise in Tibetan and Han residents of Lhasa (3658 m). J. Appl. Physiol. 1993, 75, 1968–1973. [Google Scholar] [CrossRef]
- Dhar, P.; Sharma, V.K.; Das, S.K.; Barhwal, K.; Hota, S.K.; Singh, S.B. Differential responses of autonomic function in sea level residents, acclimatized lowlanders at >3500 m and Himalayan high altitude natives at >3500 m: A cross-sectional study. Respir. Physiol. Neurobiol. 2018, 254, 40–48. [Google Scholar] [CrossRef]
- Schwarz, E.I.; Latshang, T.D.; Furian, M.; Flück, D.; Segitz, S.; Müller-Mottet, S.; Ulrich, S.; Bloch, K.E.; Kohler, M. Blood pressure response to exposure to moderate altitude in patients with COPD. COPD 2019, 14, 659–666. [Google Scholar] [CrossRef]

| Sea Level | High Altitude | p Value | |
|---|---|---|---|
| Respiration | |||
| breathing rate (bpm) | 12.1 (4.4) | 16.9 (6.3) ** | <0.001 |
| minute ventilation (L/min) | 6.7 (1.4) | 11.0 (2.7) ** | <0.001 |
| oxygen saturation (%) | 97.6 (1.1) | 77.4 (6.7) ** | <0.001 |
| RRI | |||
| mean (ms) | 956.4 (120.4) | 770.2 (95.2) ** | <0.001 |
| Total power (ms2) | 5490 (5309) | 2114 (1631) ** | <0.001 |
| VLF power (ms2) | 2146 (2265) | 929 (736) ** | 0.001 |
| LF power (ms2) | 1711 (1546) | 576 (453) ** | <0.001 |
| HF power (ms2) | 1233 (1270) | 403 (516) ** | <0.001 |
| LF/HF powers ratio | 2.11 (1.68) | 3.11 (3.03) * | 0.05 |
| SBP | |||
| mean (mmHg) | 109.5 (13.7) | 120.1 (10.3) ** | 0.002 |
| Total power (mmHg2) | 39.82 (22.60) | 26.12 (16.57) | 0.10 |
| VLF power (mmHg2) | 24.06 (16.96) | 12.16 (8.46) | 0.06 |
| LF power (mmHg2) | 11.01 (6.10) | 8.82 (4.78) | 0.40 |
| HF power (mmHg2) | 2.36 (1.17) | 3.28 (4.45) | 0.40 |
| DBP | |||
| mean (mmHg) | 74.8 (9.4) | 80.7 (9.9) ** | 0.001 |
| Total power (mmHg2) | 18.60 (13.17) | 13.77 (13.77) | 0.10 |
| VLF power (mmHg2) | 10.52 (9.28) | 6.21 (6.95) * | 0.040 |
| LF power (mmHg2) | 6.23 (4.02) | 5.65 (4.68) | 0.30 |
| HF power (mmHg2) | 0.84 (0.56) | 0.89 (1.23) | 0.50 |
| Sea Level | High Altitude | p Value | |
|---|---|---|---|
| RRI | |||
| m = 1 | 1.57 (0.18) | 1.34 (0.37) | 0.06 |
| m = 2 | 1.39 (0.23) | 1.21 (0.35) ** | 0.007 |
| SBP | |||
| m = 1 | 1.35 (0.28) | 1.25 (0.25) | 0.2 |
| m = 2 | 1.22 (0.28) | 1.15 (0.25) | 0.4 |
| DBP | |||
| m = 1 | 1.28 (0.25) | 1.26 (0.25) | 0.8 |
| m = 2 | 1.19 (0.25) | 1.17 (0.24) | 0.9 |
| MSEHF | MSELF | |||||
|---|---|---|---|---|---|---|
| Sea Level | High Altitude | p Value | Sea Level | High Altitude | p Value | |
| RRI | ||||||
| m = 1 | 1.69 (0.20) | 1.57 (0.25) * | 0.031 | 1.46 (0.24) | 1.47 (0.21) | 0.9 |
| m = 2 | 1.62 (0.22) | 1.50 (0.26) * | 0.043 | 1.45 (0.28) | 1.46 (0.24) | 0.8 |
| SBP | ||||||
| m = 1 | 1.76 (0.25) | 1.71 (0.22) | 0.08 | 1.72 (0.26) | 1.53 (0.25) * | 0.014 |
| m = 2 | 1.71 (0.26) | 1.64 (0.25) | 0.09 | 1.67 (0.31) | 1.51 (0.28) | 0.06 |
| DBP | ||||||
| m = 1 | 1.82 (0.21) | 1.78 (0.21) | 0.5 | 1.70 (0.20) | 1.56 (0.17) ** | 0.009 |
| m = 2 | 1.78 (0.23) | 1.71 (0.23) | 0.3 | 1.65 (0.26) | 1.52 (0.19) | 0.06 |
| Sea Level | High Altitude | p Value | |
|---|---|---|---|
| XSampEn | |||
| m = 1 | 1.57 (0.19) | 1.54 (0.26) | 0.7 |
| m = 2 | 1.50 (0.23) | 1.47 (0.25) | 0.7 |
| XMSEHF | |||
| m = 1 | 1.82 (0.16) | 1.77 (0.16) | 0.2 |
| m = 2 | 1.79 (0.16) | 1.72 (0.19) | 0.10 |
| XMSELF | |||
| m = 1 | 1.63 (0.16) | 1.63 (0.15) | >0.9 |
| m = 2 | 1.63 (0.19) | 1.62 (0.19) | >0.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faini, A.; Caravita, S.; Parati, G.; Castiglioni, P. Alterations of Cardiovascular Complexity during Acute Exposure to High Altitude: A Multiscale Entropy Approach. Entropy 2019, 21, 1224. https://doi.org/10.3390/e21121224
Faini A, Caravita S, Parati G, Castiglioni P. Alterations of Cardiovascular Complexity during Acute Exposure to High Altitude: A Multiscale Entropy Approach. Entropy. 2019; 21(12):1224. https://doi.org/10.3390/e21121224
Chicago/Turabian StyleFaini, Andrea, Sergio Caravita, Gianfranco Parati, and Paolo Castiglioni. 2019. "Alterations of Cardiovascular Complexity during Acute Exposure to High Altitude: A Multiscale Entropy Approach" Entropy 21, no. 12: 1224. https://doi.org/10.3390/e21121224
APA StyleFaini, A., Caravita, S., Parati, G., & Castiglioni, P. (2019). Alterations of Cardiovascular Complexity during Acute Exposure to High Altitude: A Multiscale Entropy Approach. Entropy, 21(12), 1224. https://doi.org/10.3390/e21121224






