Entropy Measures in Analysis of Head up Tilt Test Outcome for Diagnosing Vasovagal Syncope
Abstract
1. Introduction
2. Entropy Measures
2.1. Sample Entropy
2.2. Fuzzy Entropy
2.3. Shannon Entropy
2.4. Conditional Entropy
2.5. Permutation Entropy
3. Materials and Methods
3.1. Study Group
3.2. Head up Tilt Test
3.3. Data Analysis and Statistical Methods
4. Results
4.1. Comparisons of Entropies between HUTT Phases in the HUTT(+) and HUTT(−) Group
4.2. Comparisions of Entropies in the HUTT(+) versus HUTT(−) Group in Individual Phases of HUTT
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Graff, B.; Graff, G.; Kaczkowska, A. Entropy measures of heart rate variability for short ECG datasets in patients with congestive heart failure. Acta Phys. Pol. B Proc. Suppl. 2012, 5, 153–158. [Google Scholar] [CrossRef]
- Liu, C.; Liu, C.; Shao, P.; Li, L.; Sun, X.; Wang, X.; Liu, F. Comparison of different threshold values r for approximate entropy: Application to investigate the heart rate variability between heart failure and healthy control groups. Physiol. Meas. 2011, 32, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Sarlabous, L.; Torres, A.; Fiz, J.A.; Gea, J.; Martínez-Llorens, J.M.; Morera, J.; Jané, R. Interpretation of the approximate entropy using fixed tolerance values as a measure of amplitude variations in biomedical signals. In Proceedings of the 32nd Annual International Conference of the IEEE and Engineering in Medicine and Biology Society (EMBS 2010), Buenos Aires, Argentina, 31 August–4 September 2010; pp. 5967–5970. [Google Scholar]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S. Approximate entropy as a complexity measure. Chaos 1995, 5, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Tuzcu, V.; Nas, S.; Börklü, T.; Ugur, A. Decrease in the heart rate complexity prior to the onset of atrial fibrillation. Europace 2006, 8, 98–402. [Google Scholar] [CrossRef] [PubMed]
- Sosnoff, J.J.; Goldman, M.D.; Motl, R.W. Real life walking impairment in multiple sclerosis: Preliminary comparison of four methods for processing accelerometry data. Mult. Scler. 2010, 16, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S.; Huang, W. Approximate entropy-statistical properties and applications. Commun. Stat. Theory Methods 1992, 21, 3061–3077. [Google Scholar] [CrossRef]
- Fleisher, L.A.; Dipietro, J.A.; Johnson, T.R.; Pincus, S. Complementary and noncoincident increases in heart rate variability and irregularity during fetal development. Clin. Sci. 1997, 92, 345–349. [Google Scholar] [CrossRef]
- Fleisher, L.A.; Pincus, S.M.; Rosenbaum, S.H. Approximate entropy of heart rate as a correlate of postoperative ventricular dysfunction. Anesthesiology 1993, 78, 683–692. [Google Scholar] [CrossRef]
- Luo, D.; Pan, W.; Li, Y.; Feng, K.; Liu, G. The interaction analysis between the sympathetic and sarasympathetic systems in CHF by using transfer entropy method. Entropy 2018, 20, 795. [Google Scholar] [CrossRef]
- Xiao, M.-X.; Wei, H.-C.; Xu, Y.-J.; Wu, H.-T.; Sun, C.-K. Combination of R-R interval and crest time in assessing complexity using multiscale cross-approximate entropy in normal and diabetic subjects. Entropy 2018, 20, 497. [Google Scholar] [CrossRef]
- Tang, H.; Jiang, Y.; Li, T.; Wang, X. Identification of pulmonary hypertension using entropy measure analysis of heart sound signal. Entropy 2018, 20, 389. [Google Scholar] [CrossRef]
- Shi, B.; Zhang, Y.; Yuan, C.; Wang, S.; Li, P. Entropy analysis of short-term heartbeat interval time series during regular walking. Entropy 2017, 19, 568. [Google Scholar] [CrossRef]
- Chicote, B.; Irusta, U.; Alcaraz, R.; Rieta, J.J.; Aramendi, E.; Isasi, I.; Alonso, D.; Ibarguren, K. Application of entropy-based features to predict defibrillation outcome in cardiac arrest. Entropy 2016, 18, 313. [Google Scholar] [CrossRef]
- Makowiec, D.; Wejer, D.; Graff, B.; Struzik, Z.R. Dynamical pattern representation of cardiovascular couplings evoked by head-up tilt test. Entropy 2018, 20, 235. [Google Scholar] [CrossRef]
- Tseng, L.; Tang, S.C.; Chang, C.Y.; Lin, Y.C.; Abbod, M.F.; Shieh, J.S. Nonlinear and Conventional Biosignal Analyses Applied to Tilt Table Test for Evaluating Autonomic Nervous System and Autoregulation. Open Biomed. Eng. J. 2013, 7, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Faes, L. Investigating the mechanisms of cardiovascular and cerebrovascular regulation in orthostatic syncope through an information decomposition strategy. Auton. Neurosci. Basic Clin. 2013, 178, 76–82. [Google Scholar] [CrossRef]
- Graff, B.; Wejer, D.; Faes, L.; Graff, G.; Makowiec, D.; Narkiewicz, K. The use of Transfer Entropy method for the assessment of cardiovascular regulation during head-up tilt test. Auton. Neurosci. Basic Clin. 2015, 192, 101–102. [Google Scholar] [CrossRef]
- Wejer, D.; Graff, B.; Makowiec, D.; Budrejko, S.; Struzik, Z.R. Complexity of cardiovascular rhythms during head-up tilt test by entropy of patterns. Physiol. Meas. 2017, 38, 819–832. [Google Scholar] [CrossRef]
- Reulecke, S.; Charleston-Villalobos, S.; Voss, A.; González-Camarena, R.; González-Hermosillo, J.A.; Gaitán-González, M.J.; Hernández-Pacheco, G.; Schroeder, R.; Aljama-Corrales, T. Temporal Analysis of Cardiovascular and Respiratory Complexity by Multiscale Entropy Based on Symbolic Dynamics. IEEE J. Biomed. Health Inform. 2018, 22, 1046–1058. [Google Scholar] [CrossRef]
- Bari, V.; De Maria, B.; Mazzucco, C.E.; Rossato, G.; Tonon, D.; Nollo, G.; Faes, L.; Porta, A. Cerebrovascular and cardiovascular variability interactions investigated through conditional joint transfer entropy in subjects prone to postural syncope. Physiol. Meas. 2017, 38, 976. [Google Scholar] [CrossRef] [PubMed]
- Reulecke, S.; Charleston-Villalobos, S.; González-Hermosillo, J.; González-Camarena, R.; Voss, A.; Gaitán-González, M.; Hernández-Pacheco, G.; Schroeder, R.; Aljama-Corrales, T. Study of impaired cardiovascular and respiratory coupling during orthostatic stress based on joint symbolic dynamics. Med. Eng. Phys. 2018, 61, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Reulecke, S.; Charleston-Villalobos, S.; Voss, A.; González-Camarena, R.; González-Hermosillo, J.; Gaitan-Gonzalez, M.J.; Hernández-Pacheco, G.; Schroeder, R.; Aljama-Corrales, T. Orthostatic stress causes immediately increasedblood pressure variability in women withvasovagal syncope. Comput. Methods Programs Biomed. 2016, 127, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S.M. Approximate entropy as a measure of system complexity. Proc. Natl. Acad. Sci. USA 1991, 88, 2297–2301. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.; Scilingo, E.P.; Valenza, G. Complexity and Nonlinearity in Cardiovascular Signals; Springer: Basel, Switzerland, 2017. [Google Scholar]
- Graff, B.; Graff, G.; Makowiec, D.; Kaczkowska, A.; Wejer, D.; Budrejko, S.; Kozłowski, D.; Narkiewicz, K. Entropy measures in the assessment of heart rate variability in patients with Cardiodepressive Vasovagal Syncope. Entropy 2015, 17, 1007–1022. [Google Scholar] [CrossRef]
- Buszko, K.; Piątkowska, A.; Koźluk, E.; Opolski, G. Entropy in investigation of vasovagal syndrome in passive head up tilt test. Entropy 2017, 19, 236. [Google Scholar] [CrossRef]
- Buszko, K.; Piatkowska, A.; Koźluk, E.; Fabiszak, T.; Opolski, G. The complexity of hemodynamic response to the tilt test with and without nitroglycerine provocation in patients with vasovagal syncope. Sci. Rep. 2018, 8, 15554. [Google Scholar] [CrossRef]
- Brignole, M.; Moya, A.; de Lange, F.; Deharo, J.-C.; Elliot, P.M.; Faniculli, A. Guidelines for the diagnosis and management of syncope. Eur. Heart J. 2018, 30, 2631–2671. [Google Scholar]
- Parry, S.W.; Reeve, P.; Lawson, J.; Shaw, F.E.; Davison, J.; Norton, M.; Frearson, R.; Kerr, S.; Newton, J.L. The Newcastle Protocols 2008: An update on head-up tilt table testing and the management of vasovagal syncope and related disorders. Heart 2009, 95, 416–420. [Google Scholar] [CrossRef]
- Task Force® Monitor. Available online: https://www.cnsystems.com/products/task-force-monitor (accessed on 10 November 2018).
- Verheyden, B.; Ector, H.; Aubert, A.E.; Reybrouck, T. Tilt training increases the vasoconstrictor reserve in patients with neurally mediated syncope evoked by head-up tilt testing. Eur. Heart J. 2008, 29, 1523–1530. [Google Scholar] [CrossRef]
- Schwalm, T. Modern Tilt Table Testing and Non-Invasive Monitoring; ABW Wissenschftsverlag GmbH: Berlin, Germany, 2007. [Google Scholar]
- Clausius, R. The Mechanical Theory of Heat—With its Applications to the Steam Engine and to Physical Properties of Bodies; J. Van Voorst: London, UK, 1867. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Liu, C.Y.; Li, K.; Zhao, L.; Liu, F.; Zheng, D.C.; Liu, C.C.; Liu, S.T. Analysis of heart rate variability using fuzzy measure entropy. Comput. Biol. Med. 2013, 43, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Porta, A.; Baselli, G.; Liberati, D.; Monatno, N.; Cogliati, C.; Gnecchi-Ruscone, T.; Malliani, A.; Cerutti, S. Measuring regularity by means of corrected conditional entropy in sympathetic outflow. Biol. Cybern. 1998, 78, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Brandt, C.; Pompe, B. Permutation entropy: A natural complexity measure for time series. Phys. Rev. Lett. 2002, 88, 174102. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, J.T.; Kochi, N.; Stergiou, N. Nonlinear analysis of ambulatory activity patterns in community-dwelling older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.T.; Zhuang, J.; Yu, W.X.; Wang, Z.Z. Measuring complexity using FuzzyEn, ApEn and SampEn. Med. Eng. Phys. 2009, 31, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Menozzi, C.; Del Rosso, A.; Costa, S.; Gaggioli, G.; Bottoni, N.; Bartoli, P.; Sutton, R. New classification of hemodynamics of vasovagal syncope: beyond the VASIS classification. Analysis of the pre-syncopal phase of the tilt test without and with nitroglycerin challenge. Vasovagal Syncope International Study. Europace 2000, 2, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, A.P.; Theodorakis, G.; Vardas, P.; Sutton, R. Methodology of head-up tilt testing in patients with unexplained syncope. J. Am. Coll. Cardiol. 1991, 17, 125–130. [Google Scholar] [CrossRef]
- PhysioToolkit. Available online: https://www.physionet.org/physiotools/ (accessed on 15 January 2017).
- A Set of Entropy Measures for Temporal Series (1D Signals). Available online: https://www.mathworks.com/matlabcentral/fileexchange/50289-a-set-of-entropy-measures-for-temporal-series-1d-signals (assesed on 10 November 2018).
- Virag, N.; Sutton, R.; Vetter, R.; Markowitz, T.; Erickson, M. Prediction of vasovagal syncope from heart rate and blood pressure trend and variability: Experience in 1155 patients. HeartRythm 2007, 4, 1375–1382. [Google Scholar] [CrossRef]
- Duplyakov, D.; Golovina, G.; Sysuenkova, E.; Garkina, S. Can the result of a tilt test be predicted in the first five minutes? Cardiol. J. 2011, 18, 521–526. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Arhi, C.; Chandra, N.; Franzen-McManus, A.C.; Meyer, A.; Sutton, R. Blood pressure oscillations during tilt testing as a predictive marker of vasovagal syncope. Europace 2009, 11, 1696–1701. [Google Scholar] [CrossRef]
- Gimeno-Blanes, F.J.; Rojo-Álvarez, J.L.; Caamaño, A.J.; Flores-Yepes, J.A.; García-Alberola, A. On the feasibility of tilt test outcome early prediction using ECG and pressure parameters. EURASIP J. Adv. Signal Process. 2011, 2011, 33. [Google Scholar] [CrossRef]
- Mallat, Z.; Vicaut, E.; Sangaré, A.; Verschueren, J.; Fontaine, G.; Frank, R. Prediction of head-up tilt test result by analysis of early heart rate variations. Circulation 1997, 96, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-Blanes, F.-J.; Rojo-Álvarez, J.-L.; García-Alberola, A.; Gimeno-Blanes, J.-R.; Rodríguez-Martínez, A.; Mocci, A.; Flores-Yepes, J.-A. Early prediction of tilt test outcome, with support vector machine non linear classifier, using ECG, pressure and impedance signals. Comput. Cardiol. 2011, 38, 101–104. [Google Scholar]
- Kouakam, C.; Lacroix, D.; Zghal, N.; Logier, R.; Klug, D.; Le Franc, P.; Jarwe, M.; Kacet, S. Inadequate sympathovagal balance in response to orthostatism in patients with unexplained syncope and a positive head up tilt test. Heart 1999, 82, 312–318. [Google Scholar] [CrossRef]
- Mehlsen, J.; Kaijer, M.N.; Mehlsen, A.B. Autonomicand electrocardiographicchanges in cardioinhibitory syncope. Europace 2008, 10, 91–95. [Google Scholar] [CrossRef]
- Evrengul, H.; Tavli, H.V.; Evrengul, H.; Tavli, T.; Dursunoglu, D. Spectral and time-domain analysis of heart-rate variability during head-upright tilt-table testing in children with neurally mediated syncope. Pediatr. Cardiol. 2006, 27, 670–678. [Google Scholar] [CrossRef]
- Salameh, E.; Kadri, Z.; Neemtallah, R.; Azar, R.; Badaoui, G.; Jaoude, S.A.; Kassab, R. Heart ratevariability and vasovagal syncope. Ann. Cardiol. Angeiol. 2007, 56, 88–91. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Niskanen, J.P.; Lipponen, J.A.; Ranta-Aho, P.O.; Karjalainen, P.A. Heart rate variability analysis. Open Heart. 2014, 113, 210–220. [Google Scholar]
- Porta, A.; Gnecchi-Ruscone, T.; Tobaldini, E.; Guzzetti, S.; Furlan, R.; Montano, N. Progressive decrease of heart period variability entropy-based complexity during graded head-up tilt. J. Appl. Physiol. 2007, 103, 1143–1149. [Google Scholar] [CrossRef]
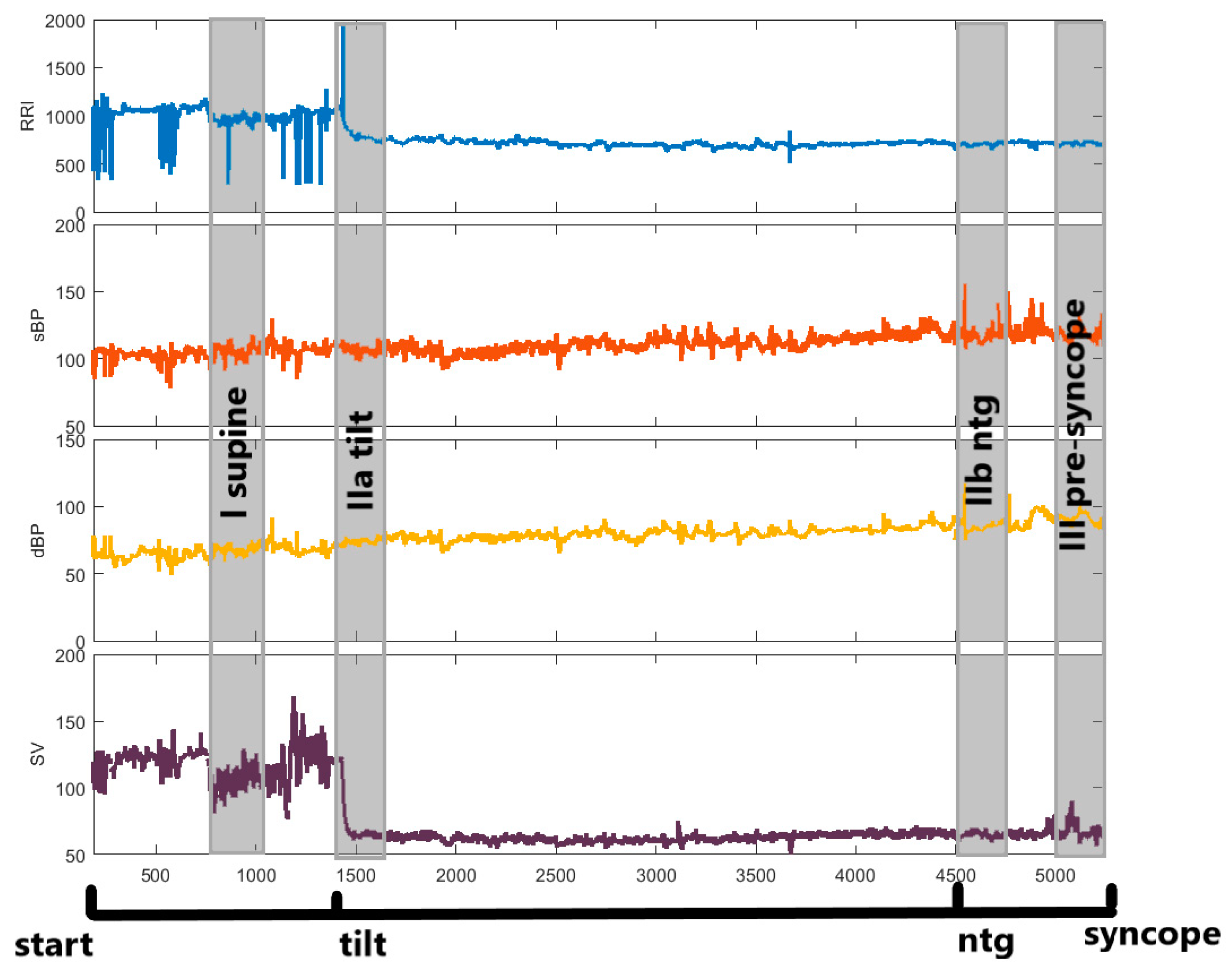
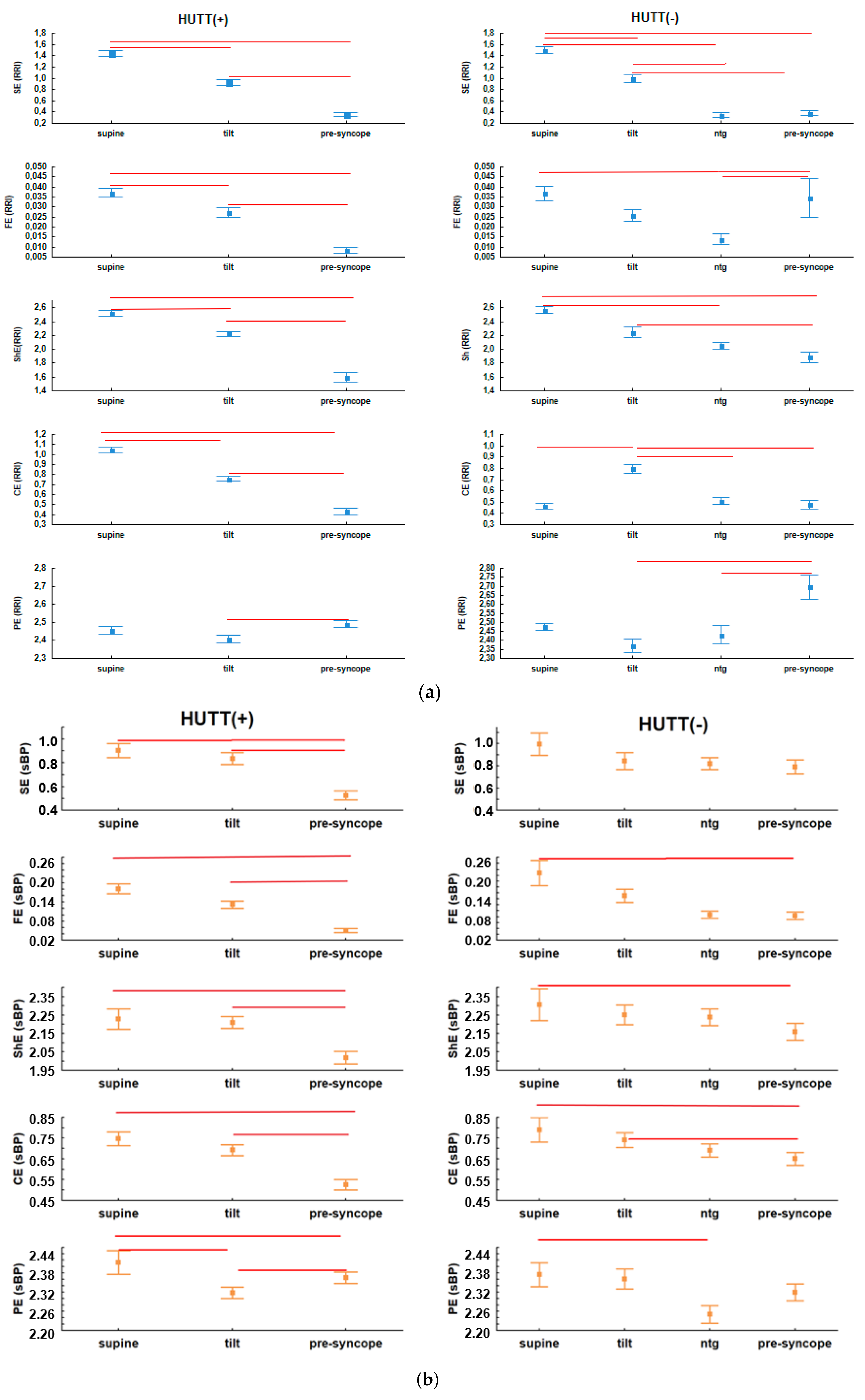
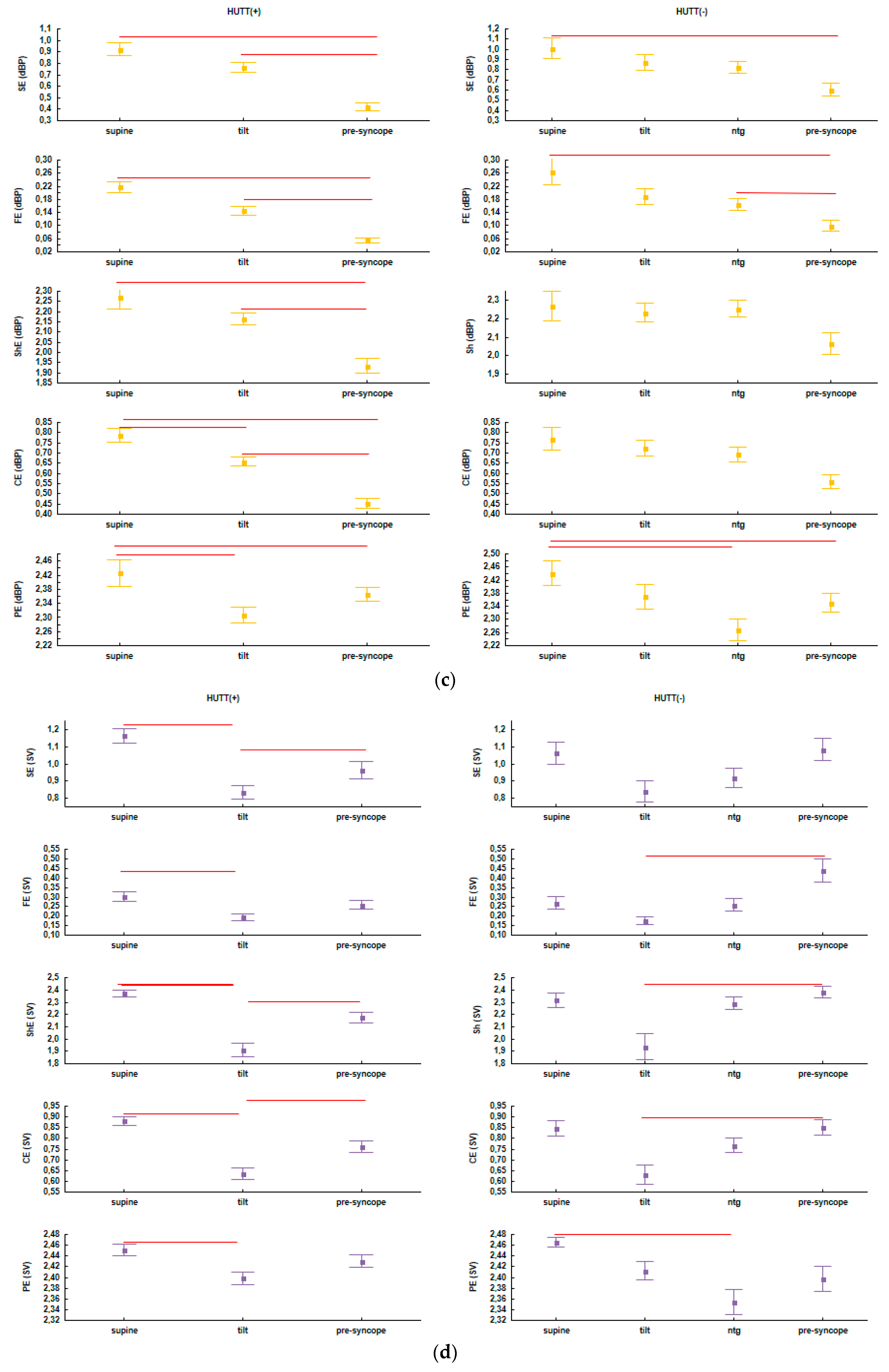
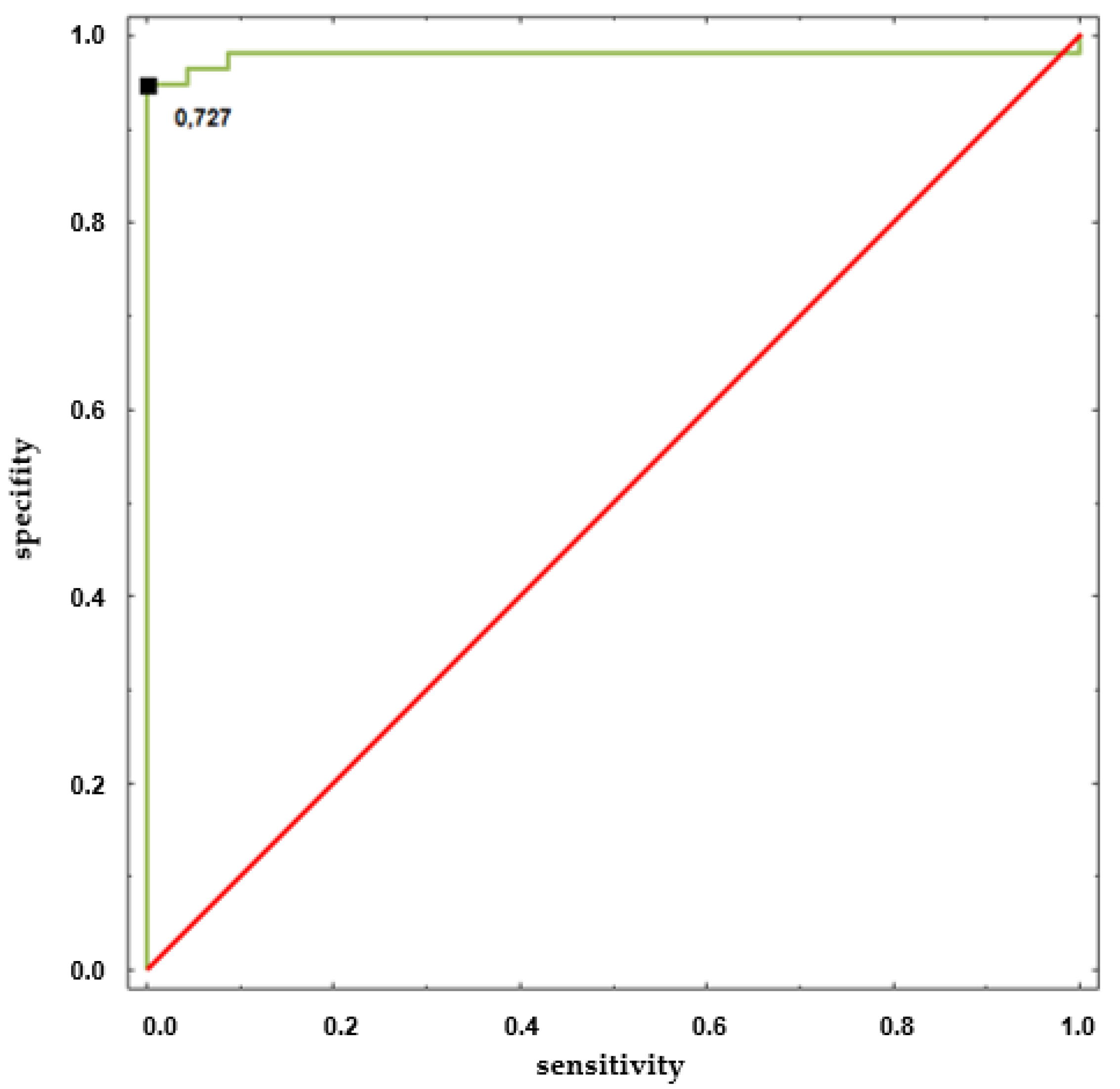
| Baseline | HUTT(+) (n = 57: F = 43, M = 14) | HUTT(−) (n = 23: F = 17, M = 6) |
|---|---|---|
| Female-age (year) | 35.6 ± 16 | 32.3 ± 12 |
| Male-age (year) | 41.7 ± 15.6 | 43 ± 15 |
| HR (bpm) | 72.06 ± 9.98 | 70.46 ± 11.35 |
| sBP (mmHg) | 108.6 ± 25.4 | 102.6 ± 20.4 |
| dBP(mmHg) | 68.0 ± 19.7 | 66.1 ± 16.8 |
| Hypertension | 3 | 0 |
| Diabetes | 0 | 0 |
| Medication | 2 | 4 |
| Entropy | Embedding Dimension (m) | Threshold (r) | Time Delay () |
|---|---|---|---|
| SE | 2 | 1 | |
| FE | 2 | 1 | |
| ShE | 2 | - | 1 |
| CE 1 | 2 | - | 1 |
| PE | 3 | - | 1 |
| Parameter | Phase I (supine) | Phase IIa (tilt) | Phase III (pre-syncope) |
|---|---|---|---|
| Sample Entropy [29] | |||
| SE (RRI) | 1.35 ± 0.30 | 0.88 ± 0.30 | 0.34 ± 0.20 |
| SE (sBP) | 0.88 ± 0.44 | 0.82 ± 0.30 | 0.51 ± 0.30 |
| SE (dBP) | 0.91 ± 0.42 | 0.76 ± 0.31 | 0.41 ± 0.30 |
| SE (SV) | 1.16 ± 0.32 | 0.83 ± 0.30 | 0.95 ± 0.40 |
| Fuzzy Entropy | |||
| FE (RRI) | 0.037 ± 0.017 | 0.027 ± 0.017 | 0.008 ± 0.01 |
| FE (sBP) | 0.18 ± 0.12 | 0.13 ± 0.083 | 0.05 ± 0.046 |
| FE (dBP) | 0.22 ± 0.13 | 0.14 ± 0.096 | 0.05 ± 0.06 |
| FE (SV) | 0.30 ± 0.18 | 0.19 ± 0.13 | 0.26 ± 0.17 |
| Shannon Entropy | |||
| Sh (RRI) | 2.52 ± 0.30 | 2.22 ± 0.25 | 1.59 ± 0.56 |
| Sh (sBP) | 2.23 ± 0.41 | 2.20 ± 0.23 | 2.02 ± 0.25 |
| Sh (dBP) | 2.27 ± 0.42 | 2.16 ± 0.23 | 1.93 ± 0.27 |
| Sh (SV) | 2.37 ± 0.21 | 1.91 ± 0.43 | 2.17 ± 0.32 |
| Conditional Entropy | |||
| CE (RRI) | 1.05 ± 0.22 | 0.76 ± 0.18 | 0.43 ± 0.26 |
| CE (sBP) | 0.75 ± 0.25 | 0.70 ± 0.20 | 0.52 ± 0.18 |
| CE (dBP) | 0.78 ± 0.26 | 0.66 ± 0.18 | 0.45 ± 0.19 |
| CE (SV) | 0.88 ± 0.15 | 0.64 ± 0.20 | 0.76 ± 0.20 |
| Permutation Entropy | |||
| PE (RRI) | 2.46 ± 0.15 | 2.40 ± 0.16 | 2.49 ± 0.15 |
| PE (sBP) | 2.41 ± 0.28 | 2.32 ± 0.14 | 2.36 ± 0.14 |
| PE (dBP) | 2.43 ± 0.28 | 2.30 ± 0.17 | 2.36 ± 0.15 |
| PE (SV) | 2.45 ± 0.08 | 2.4 ± 0.09 | 2.43 ± 0.09 |
| Parameter | Phase I (supine) | Phase IIa (tilt) | Phase IIb (ntg) | Phase III (pre-syncope) |
|---|---|---|---|---|
| Sample Entropy [29] | ||||
| SE (RRI) | 1.50 ± 0.30 | 1.0 ± 0.3 | 0.34 ± 20 | 0.38 ± 0.24 |
| SE (sBP) | 1.0 ± 0.50 | 0.84 ± 0.36 | 0.81 ± 0.25 | 0.80 ± 0.30 |
| SE (dBP) | 1.01 ± 0.50 | 0.86 ± 0.36 | 0.82 ± 0.26 | 0.60 ± 0.30 |
| SE (SV) | 1.06 ± 0.30 | 0.84 ± 0.30 | 0.92 ± 0.30 | 1.08 ± 0.30 |
| Fuzzy Entropy | ||||
| FE (RRI) | 0.037 ± 0.017 | 0.026 ± 0.013 | 0.014 ± 0.012 | 0.034 ± 0.046 |
| FE (sBP) | 0.25 ± 0.20 | 0.17 ± 0.10 | 0.10 ± 0.05 | 0.10 ± 0.06 |
| FE (dBP) | 0.26 ± 0.20 | 0.19 ± 0.12 | 0.16 ± 0.85 | 0.10 ± 0.07 |
| FE (SV) | 0.27 ± 0.15 | 0.18 ± 0.10 | 0.26 ± 0.15 | 0.44 ± 0.29 |
| Shannon Entropy | ||||
| Sh (RRI) | 2.56 ± 0.23 | 2.23 ± 0.35 | 2.05 ± 0.25 | 1.88 ± 0.36 |
| Sh (sBP) | 2.30 ± 0.42 | 2.24 ± 0.25 | 2.24 ± 0.22 | 2.16 ± 0.22 |
| Sh (dBP) | 2.27 ± 0.39 | 2.24 ± 0.25 | 2.24 ± 0.22 | 2.07 ± 0.29 |
| Sh (SV) | 2.32 ± 0.28 | 1.92 ± 0.28 | 2.32 ± 0.18 | 2.38 ± 0.24 |
| Conditional Entropy | ||||
| CE (RRI) | 0.46 ± 0.13 | 0.80 ± 0.19 | 0.51 ± 0.15 | 0.48 ± 0.20 |
| CE (sBP) | 0.79 ± 0.29 | 0.74 ± 0.17 | 0.70 ± 0.15 | 0.65 ± 0.14 |
| CE (dBP) | 0.77 ± 0.27 | 0.72 ± 0.19 | 0.69 ± 0.17 | 0.56 ± 0.17 |
| CE (SV) | 0.85 ± 0.17 | 0.63 ± 0.21 | 0.77 ± 0.15 | 0.85 ± 0.17 |
| Permutation Entropy | ||||
| PE (RRI) | 2.47 ± 0.09 | 2.37 ± 0.18 | 2.43 ± 0.24 | 2.70 ± 0.32 |
| PE (sBP) | 2.37 ± 0.18 | 2.36 ± 0.15 | 2.25 ± 0.13 | 2.32 ± 0.13 |
| PE (dBP) | 2.44 ± 0.17 | 2.37 ± 0.18 | 2.27 ± 0.16 | 2.35 ± 0.13 |
| PE (SV) | 2.46 ± 0.05 | 2.41 ± 0.08 | 2.35 ± 0.11 | 2.40 ± 0.11 |
| Parameter | HUTT(−) vs. HUTT(+) | |||||
|---|---|---|---|---|---|---|
| Phase I (supine) | p | Phase IIa (tilt) | p | Phase III (pre-syncope) | p | |
| Sample Entropy [21] | ||||||
| SE (RRI) | 0.46 | 0.48 | 0.52 | |||
| SE (sBP) | 0.43 | 0.99 | 0.0006 | |||
| SE (dBP) | 0.35 | 0.15 | 0.0001 | |||
| SE (SV) | 0.13 | 0.73 | 0.24 | |||
| Fuzzy Entropy | ||||||
| FE (RRI) | 0.98 | 0.85 | 0.000007 | |||
| FE (sBP) | 0.30 | 0.14 | 0.00004 | |||
| FE (dBP) | 0.49 | 0.13 | 0.0007 | |||
| FE (SV) | 0.40 | 0.71 | 0.007 | |||
| Shannon Entropy | ||||||
| Sh (RRI) | 0.50 | 0.57 | 0.07 | |||
| Sh (sBP) | 0.23 | 0.48 | 0.01 | |||
| Sh (dBP) | 0.88 | 0.24 | 0.055 | |||
| Sh (SV) | 0.52 | 0.86 | 0.01 | |||
| Conditional Entropy | ||||||
| CE (RRI) | 0.00001 | 0.36 | 0.33 | |||
| CE (sBP) | 0.26 | 0.29 | 0.003 | |||
| CE (dBP) | 0.89 | 0.11 | 0.012 | |||
| CE (SV) | 0.36 | 0.92 | 0.09 | |||
| Permutation Entropy | ||||||
| PE (RRI) | 0.88 | 0.32 | 0.002 | |||
| PE (sBP) | 0.12 | 0.27 | 0.18 | |||
| PE (dBP) | 0.46 | 0.26 | 0.57 | |||
| PE (SV) | 0.81 | 0.58 | 0.22 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buszko, K.; Piątkowska, A.; Koźluk, E.; Fabiszak, T.; Opolski, G. Entropy Measures in Analysis of Head up Tilt Test Outcome for Diagnosing Vasovagal Syncope. Entropy 2018, 20, 976. https://doi.org/10.3390/e20120976
Buszko K, Piątkowska A, Koźluk E, Fabiszak T, Opolski G. Entropy Measures in Analysis of Head up Tilt Test Outcome for Diagnosing Vasovagal Syncope. Entropy. 2018; 20(12):976. https://doi.org/10.3390/e20120976
Chicago/Turabian StyleBuszko, Katarzyna, Agnieszka Piątkowska, Edward Koźluk, Tomasz Fabiszak, and Grzegorz Opolski. 2018. "Entropy Measures in Analysis of Head up Tilt Test Outcome for Diagnosing Vasovagal Syncope" Entropy 20, no. 12: 976. https://doi.org/10.3390/e20120976
APA StyleBuszko, K., Piątkowska, A., Koźluk, E., Fabiszak, T., & Opolski, G. (2018). Entropy Measures in Analysis of Head up Tilt Test Outcome for Diagnosing Vasovagal Syncope. Entropy, 20(12), 976. https://doi.org/10.3390/e20120976





