Entropy and Its Correlations with Other Related Quantities
Abstract
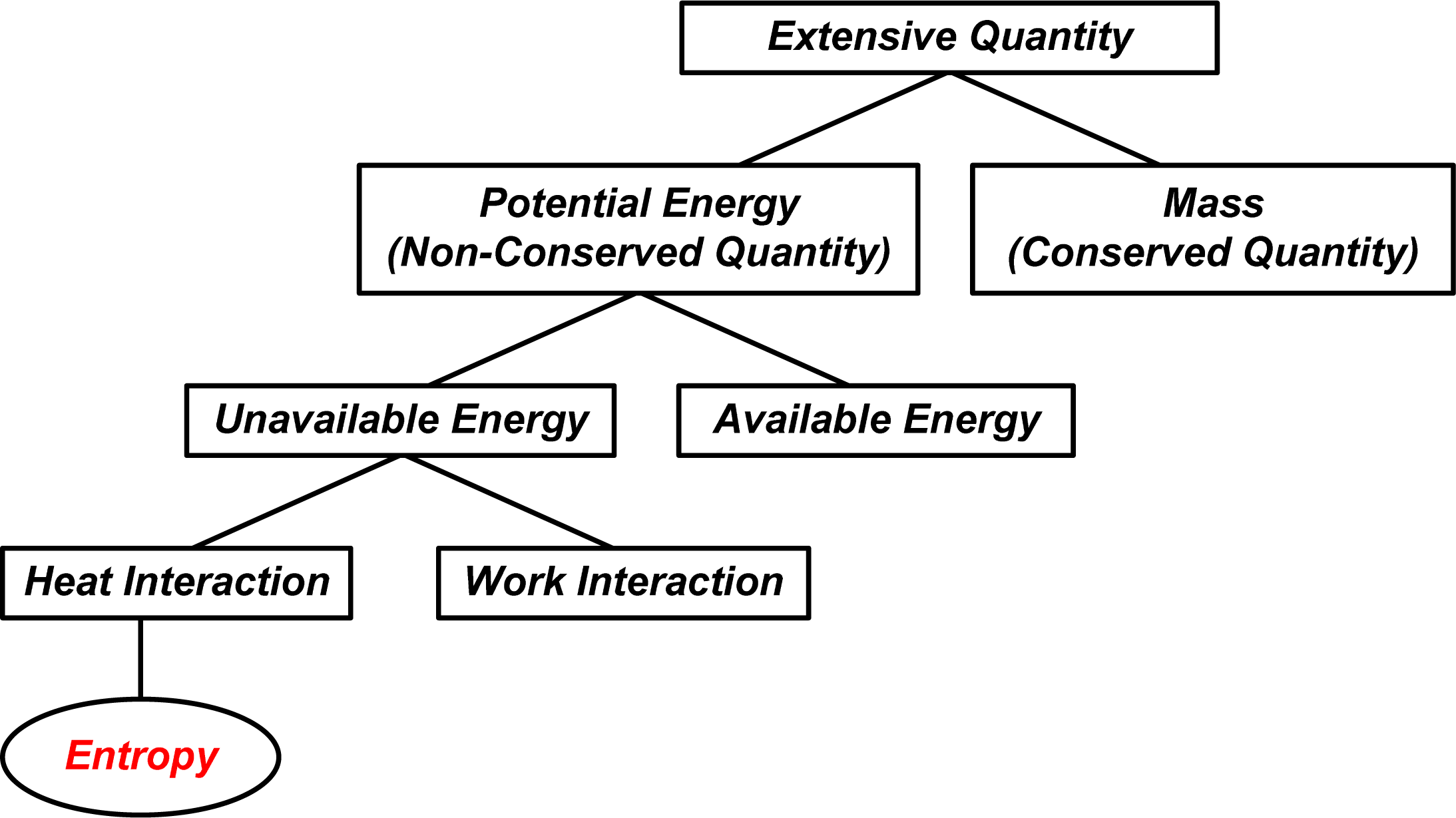
: In order to find more correlations between entropy and other related quantities, an analogical analysis is conducted between thermal science and other branches of physics. Potential energy in various forms is the product of a conserved extensive quantity (for example, mass or electric charge) and an intensive quantity which is its potential (for example, gravitational potential or electrical voltage), while energy in specific form is a dissipative quantity during irreversible transfer process (for example mechanical or electrical energy will be dissipated as thermal energy). However, it has been shown that heat or thermal energy, like mass or electric charge, is conserved during heat transfer processes. When a heat transfer process is for object heating or cooling, the potential of internal energy U is the temperature T and its potential “energy” is UT/2 (called entransy and it is the simplified expression of thermomass potential energy); when a heat transfer process is for heat-work conversion, the potential of internal energy U is (1 − T0/T), and the available potential energy of a system in reversible heat interaction with the environment is U − U0 − T0(S − S0), then T0/T and T0(S − S0) are the unavailable potential and the unavailable potential energy of a system respectively. Hence, entropy is related to the unavailable potential energy per unit environmental temperature for heat-work conversion during reversible heat interaction between the system and its environment. Entropy transfer, like other forms of potential energy transfer, is the product of the heat and its potential, the reciprocal of temperature, although it is in form of the quotient of the heat and the temperature. Thus, the physical essence of entropy transfer is the unavailable potential energy transfer per unit environmental temperature. Entropy is a non-conserved, extensive, state quantity of a system, and entropy generation in an irreversible heat transfer process is proportional to the destruction of available potential energy.1. Introduction
It is well known that entropy has a wide use in the second-law analysis of engineering devices. However, its macroscopic physical meaning is thought to be difficult to understand, even for Prigogine, the Nobel Prize winner, who has indicated that [1] “...entropy is a very strange concept without hoping to achieve a complete description...”. People are so confused about this concept that the macroscopic physical meaning of entropy is rarely discussed both in textbooks and the literature. By now, an answer to the physical meaning of entropy is mostly derived by considering the microscopic nature of matter, that is, entropy can be viewed as a measure of molecular disorder [2]. The more disordered a system becomes, the more entropy of this system is. However, the microscopic interpretation is not adequate to show the essence of entropy for engineers and researchers who are more interested in the macroscopic significance of entropy related to the efficiency of heat-work conversion of a heat engine or/and the heat transfer irreversibility etc.
This paper aims to further clarify more correlations between entropy and other related quantities within the framework of classical thermodynamics in terms of introducing the potential and potential energy of heat based on the analogy of thermodynamics/heat transfer with other branches of physics. It is hoped that the discussion will shed some light on the macroscopic physical meaning of entropy.
2. Exergy and Entropy
The macroscopic physical meaning of available energy (exergy) is easier to be understood compared with that of entropy. Under the environmental conditions of T0 and p0, the available energy transfer accompanying heat transfer is the maximal possible useful work produced from heat, while the available energy of a system is the maximum useful work obtainable during a process that brings the system into equilibrium with the environment. The concept of entropy is more easily appreciated to some extent with the help of the concept of available energy. For instance, the product of entropy generation and the environmental temperature is the available energy destruction during an irreversible process with regard to environmental dead state, and thus the entropy generation may represent the available energy destruction per unit environmental temperature.
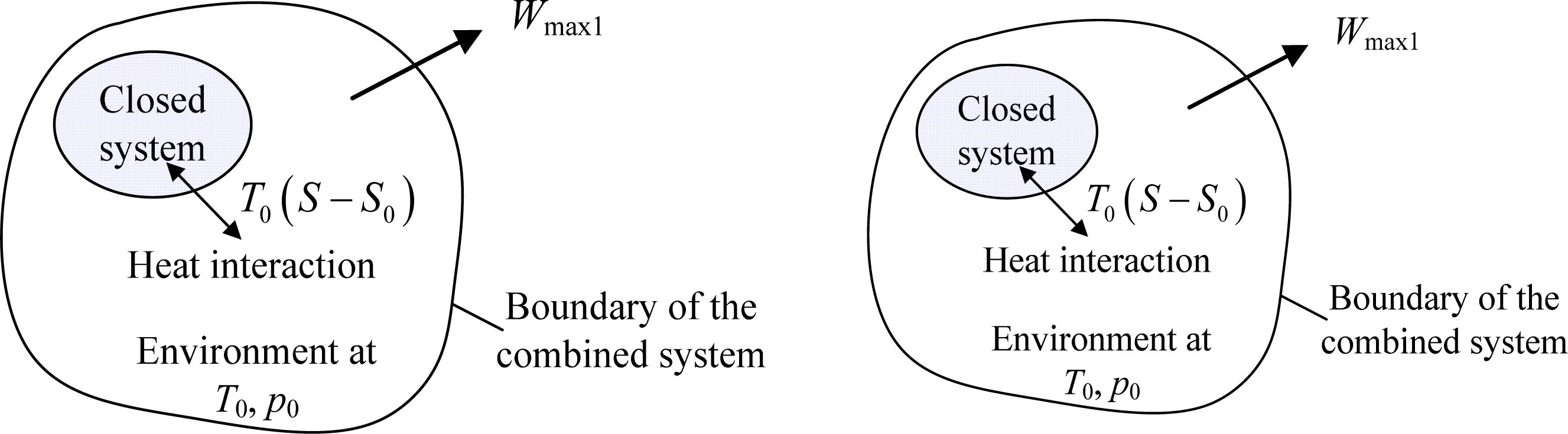
In the previous studies, there is a generally adopted notion that entropy is a measure of the unavailability of a system [3–5]. However, it has been manifested that the notion holds only when the volume of a system is kept constant [6]. By separating the reversible interactions between a closed system and the environment into two forms, i.e., the reversible heat interaction and work interaction [7], as shown in Figure 1, the available energy of the system, Ex, can be expressed as [6]:
It can be clearly seen from the term A1 in Equation (1) that the influence of entropy on the unavailability of the closed system displays only in the reversible process of heat interaction. That is, entropy is related to the unavailability of a system during reversible heat interaction between the system and the environment. In general, the value of T0 is taken as typical value, such as 25 °C. In addition, for a closed system with designated composition, its entropy at the dead state, S0, is a fixed value. Under this condition, the more entropy of a closed system is, the more unavailable energy will be through reversible heat interaction only. Particularly, for a system with constant volume (for instance, an incompressible substance), there is no work interaction, that is, A2 = 0, and then T0 (S − S0) is the unavailable energy of the system. It is shown that the environmental temperature or the available energy serves as the role of an auxiliary line as in solving geometrical problems to help clarify the macroscopic physical meaning of entropy.
4. Conclusions
The analogies between thermodynamics/heat transfer and fluid mechanics, electrics show that the potential and the potential energy transfer accompanying heat transfer are the temperature, T, and the product of the heat transfer and temperature, QT, respectively as the transferred heat is for object heating or cooling, while the potential and the potential energy (available energy) transfer accompanying heat transfer are 1−T0/T and Q(1−T0/T), respectively, as the transferred heat is for heat-work conversion, where QT0 / T is the unavailable energy transfer. Thus it can be seen that, Q / T, usually referred to as entropy transfer, is the unavailable potential energy transfer per unit environmental temperature. Therefore, though the entropy transfer is in form of the quotient of the heat transfer and the temperature, its physical essence, like other forms of potential energy transfer, is the product of the extensive quantity, i.e., the heat transfer Q and its unavailable potential, the reciprocal of temperature 1/T.
For an incompressible system for doing work through the reversible heat interaction only its potential energy (available energy) is U − U0 − T0 (S − S0), where T0 (S − S0) is the unavailable potential energy of system and S − S0 is the unavailable potential energy per unit environmental temperature. Therefore, the entropy of the system can be understood as the unavailable potential energy of a system per unit environmental temperature for heat-work conversion under the reversible heat interaction only between the system and its environment.
The analogies between thermodynamics/heat transfer and fluid mechanics, electrics show that the unavailable energy of heat or a system is essentially due to the non-zero reference point of the potential, that is, non-zero environment temperature, T0 ≠ 0. Hence, the environmental temperature can help clarify the macroscopic physical meaning of entropy, like an auxiliary line added to a diagram to help in a proof of geometrical problem. For instance, entropy generation is the available energy destruction per unit environmental temperature and the entropy transfer is the unavailable potential energy transfer per unit environmental temperature and so on.
Entropy, as a state function of a system, is an extensive, non-conserved quantity that related to the unavailable part of the internal energy during reversible heat interaction between the system and the environment with T0 ≠ 0.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (51206079).
References
- Prigogine, I. What is entropy? Naturwissenschaften 1989, 76, 1–8. [Google Scholar]
- Schwabl, F.; Brewer, W. Statistical Mechanics, 2nd ed.; Springer: Berlin, Germany, 2006; p. 36. [Google Scholar]
- Kirwan, A.D. Mother Nature’s Two Laws; World Scientific: Singapore, Singapore, 2000; pp. 37–39. [Google Scholar]
- Simpson, J.A.; Weiner, E.S.C. The Oxford English Dictionary, 2th ed; Clarendon Press: Oxford, UK, 1989; Volume 5, p. 308. [Google Scholar]
- Wang, L.Q. Minimum heat to environment and entropy. Int. J. Heat Mass Transfer 1998, 41, 1869–1871. [Google Scholar]
- Wu, J.; Guo, Z.Y. An exploration for the macroscopic physical meaning of entropy. Sci. China Tech. Sci 2010, 53, 1809–1816. [Google Scholar]
- Moran, M.J. Availability Analysis: A Guide to Efficient Energy Use; Prentice-Hall, Inc: Upper Saddle River, NJ, USA, 1982; pp. 46–48. [Google Scholar]
- Harman, P.M. Energy, Force, and Matter: The Conceptual Development of Nineteenth-Century Physics; Cambridge University Press: New York, NY, USA, 1982; p. 59. [Google Scholar]
- Hubbert, M.K. Darcy’s law and the field equations of the flow of underground fluids. Trans. AIME 1956, 207, 222–239. [Google Scholar]
- Assis, A.K.T. Circuit theory in weber electrodynamics. Eur. J. Phys 1997, 18, 241–246. [Google Scholar]
- Cheng, Y.C. Macroscopic and Statistical Thermodynamics; World Scientific Publishing Co. Pte. Ltd.: Singapore, Singapore, 2006; pp. 117–118. [Google Scholar]
- Incropera, F.P.; Dewitt, D.P.; Bergman, T.L.; Lavine, A.S. Fundamentals of Heat and Mass Transfer, 6th ed.; John Wiley & Sons, Inc: New York, NY, USA, 2007; p. 98. [Google Scholar]
- Rathore, M.M.; Kapuno, R.R. Engineering Heat Transfer, 2nd ed; Jones & Bartlett Learning: Sudbury, ON, Canada, 2011; p. 77. [Google Scholar]
- Chen, Q.; Wu, J.; Wang, M.R.; Pan, N.; Guo, Z.Y. A comparison of optimization theories for energy conservation in heat exchager groups. Chin. Sci. Bull 2011, 56, 449–454. [Google Scholar]
- Chen, Q.; Zhu, H.Y.; Pan, N.; Guo, Z.Y. An alternative criterion in heat transfer optimization. Proc. R. Soc. A 2011, 467, 1012–1028. [Google Scholar]
- Guo, Z.Y.; Zhu, H.Y.; Liang, X.G. Entransy-A physical quantity describing heat transfer ability. Int. J. Heat Mass Transfer 2007, 50, 2545–2556. [Google Scholar]
- Chen, Q.; Liang, X.G.; Guo, Z.Y. Entransy theory for the optimization of heat transfer–A review and update. Int. J. Heat Mass Transfer 2013, 63, 65–81. [Google Scholar]
- Liu, X.B.; Meng, J.A.; Guo, Z.Y. Entropy generation extremum and entransy dissipation extremum for heat exchanger optimization. Chin. Sci. Bull 2009, 54, 943–947. [Google Scholar]
- Wu, J.; Liang, X.G. Application of entransy dissipation extremum principle in radiative heat transfer optimization. Sci. China E 2008, 51, 1306–1314. [Google Scholar]
- Xu, Y.C.; Chen, Q. Minimization of mass for heat exchanger networks in spacecrafts based on the entransy dissipation theory. Int. J. Heat Mass Transfer 2012, 55, 5148–5156. [Google Scholar]
- Demirel, Y. Nonequilibrium Thermodynamics: Transport and Rate Processes in Physical, Chemical and Biological Systems, 2nd ed.; Elsevier: Oxford, UK, 2007; p. 131. [Google Scholar]
- Kondepudi, D.; Prigogine, I. Modern Thermodynamics: From Heat Engines to Dissipative Structures; John Wiley & Sons Ltd: Chichester, UK, 1998; p. 346. [Google Scholar]
- Jou, D.; Casas-Vazquez, J.; Lebon, G. Extended Irreversible Thermodynamics, 4th ed; Springer Science+Business Media B.V.: Dordrecht, The Netherlands, 2010; pp. 16–19. [Google Scholar]
- Cao, B.Y.; Guo, Z.Y. Equation of motion of a phonon gas and non- Fourier heat conduction. J. Appl. Phys 2007, 102, 053503. [Google Scholar]
- Guo, Z.Y.; Hou, Q.W. Thermal wave based on the thermomass model. J. Heat Transfer 2010, 132, 072403. [Google Scholar]
- Wang, H.D.; Cao, B.Y.; Guo, Z.Y. Heat flow choking in carbon nanotubes. Int. J. Heat Mass Transfer 2010, 53, 1796–1800. [Google Scholar]
- Tzou, D.Y.; Guo, Z.Y. Nonlocal behavior in thermal lagging. Int. J. Therm. Sci 2010, 49, 1133–1137. [Google Scholar]
- Wang, M.; Guo, Z.Y. Understanding of temperature and size dependences of effective thermal conductivity of nanotubes. Phys. Lett. A 2010, 374, 4312–4315. [Google Scholar]
- Wang, M.; Yang, N.; Guo, Z.Y. Non-Fourier heat conduction in nanomaterials. J. Appl. Phys 2011, 110, 064310. [Google Scholar]
| Subject | Transferred quantity (conserved, extensive) | Transferred quantity (in the rate form) | Potential of transferred quantity (intensive) | Potential energy transfer | Potential energy transfer (in the rate form) | ||
|---|---|---|---|---|---|---|---|
| Fluid Mechanics | M | Ṁ | gH | MgH | ṀgH | ||
| Electrics | Qe | Q̇e | Ve | QeVe | Q̇eVe | ||
| Heat transfer | Purpose | Object heating or cooling | Qh | Q̇h | T | QhT | Q̇hT |
| Heat-work conversion | |||||||
| Subject | Quantity stored in the system (or capacitor) | Differential potential energy of a system | Potential energy of a system (non-conserved, extensive) | Transfer law (a balance between driving force and resistive force) | Dissipation (or generation) rate of potential energy | ||
|---|---|---|---|---|---|---|---|
| Fluid Mechanics | M = CmH | dM · gH | |||||
| Electrics | Qe = CeVe | dQe · Ve | |||||
| Heat transfer | Purpose | Object heating or cooling | U = CvT | dU·T | Q̇h = −kA∇T Fourier's law | k(∇T)2 | |
| Heat-work conversion | |||||||
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, J.; Guo, Z. Entropy and Its Correlations with Other Related Quantities. Entropy 2014, 16, 1089-1100. https://doi.org/10.3390/e16021089
Wu J, Guo Z. Entropy and Its Correlations with Other Related Quantities. Entropy. 2014; 16(2):1089-1100. https://doi.org/10.3390/e16021089
Chicago/Turabian StyleWu, Jing, and Zengyuan Guo. 2014. "Entropy and Its Correlations with Other Related Quantities" Entropy 16, no. 2: 1089-1100. https://doi.org/10.3390/e16021089
APA StyleWu, J., & Guo, Z. (2014). Entropy and Its Correlations with Other Related Quantities. Entropy, 16(2), 1089-1100. https://doi.org/10.3390/e16021089




