The Optimal Evaporation Temperature of Subcritical ORC Based on Second Law Efficiency for Waste Heat Recovery
Abstract
:1. Introduction
2. System Description, Analysis and Method
2.1. System Description and Analysis
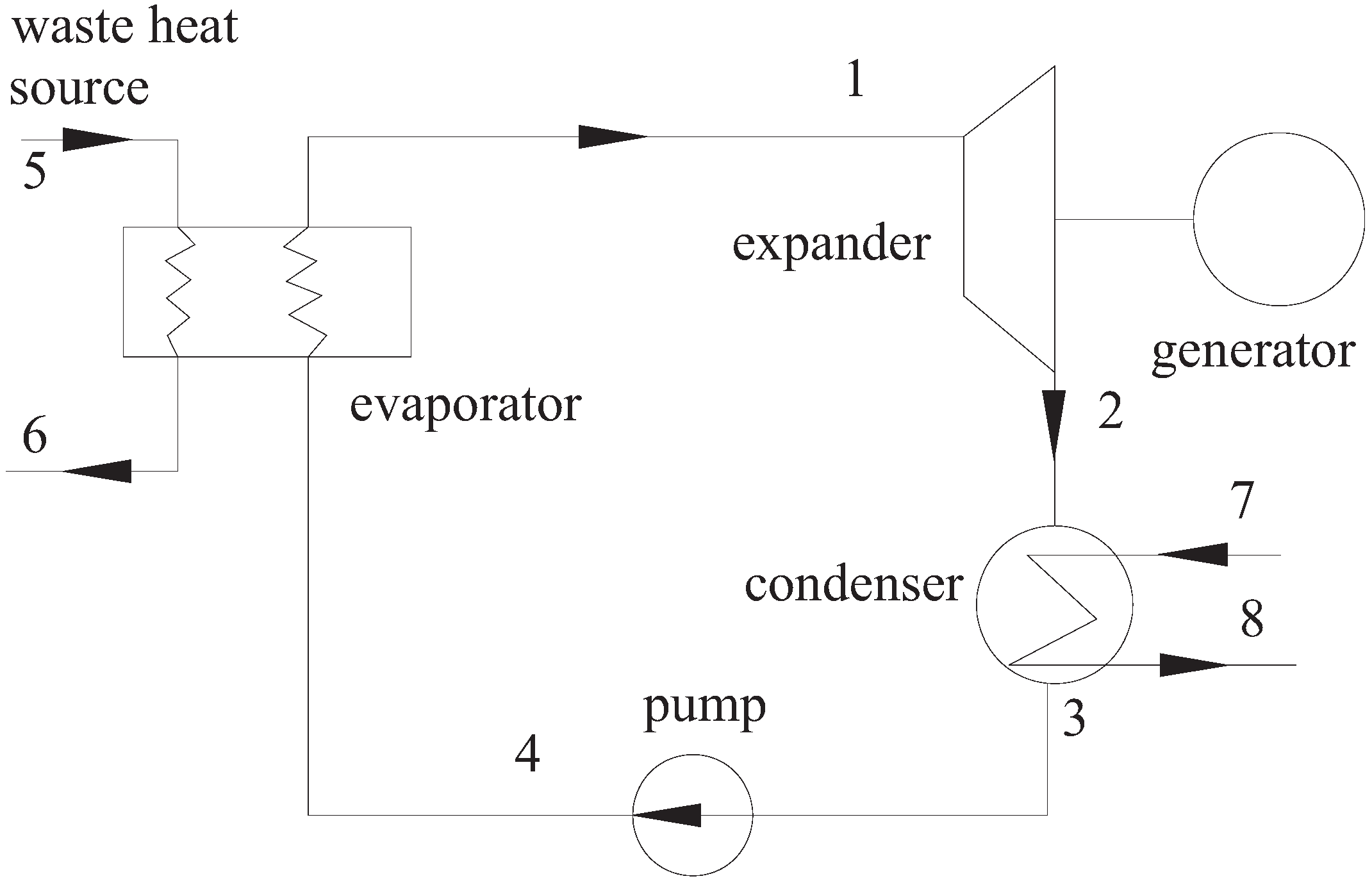

| Description | Data |
|---|---|
| Waste heat source temperature (K) | 358.15–423.15 |
| Mass flow rate of waste heat source (kg/s) | 1 |
| Cooling water temperature (K) | 293.15 |
| Environment temperature (K) | 293.15 |
| Environment pressure (MPa) | 0.1 |
| Pinch temperature difference in the evaporator (K) | 5–20 |
| Pinch temperature difference in the condenser (K) | 5 |
| Isentropic efficiency of the expander (%) | 80 |
| Generator efficiency (%) | 96 |
| Pump isentropic efficiency (%) | 75 |
2.2. Calculation Method
2.3. Choice of Working Fluids
| Working Fluids | Type of Fluids | Molecular Weight (g/mol) | Critical Temperature (K) |
|---|---|---|---|
| R143a | dry | 84.04 | 345.86 |
| R32 | wet | 52.02 | 351.26 |
| Propylene | wet | 42.08 | 365.57 |
| R22 | wet | 86.47 | 369.3 |
| R290 | wet | 44.1 | 369.89 |
| R134a | wet | 102.03 | 374.21 |
| R227ea | dry | 170.03 | 374.8 |
| R12 | isentropic | 120.91 | 385.12 |
| R152a | wet | 66.05 | 386.41 |
| RC318 | isentropic | 200.03 | 388.38 |
| R124 | isentropic | 136.48 | 395.45 |
| R236fa | isentropic | 152.04 | 398.05 |
| R717 | wet | 17.03 | 405.4 |
| R600a | isentropic | 58.12 | 407.85 |
| R142b | isentropic | 100.5 | 410.26 |
| R114 | isentropic | 170.92 | 418.83 |
| R600 | dry | 58.12 | 425.13 |
| R245fa | dry | 134.05 | 427.2 |
| R123 | dry | 152.93 | 456.83 |
| R601a | dry | 72.15 | 460.4 |
| R601 | dry | 72.15 | 469.7 |
| R11 | isentropic | 137.37 | 471.11 |
| R141b | isentropic | 116.95 | 479.96 |
| R113 | dry | 187.38 | 487.21 |
| n-Hexane | dry | 86.17 | 507.4 |
| Methanol | wet | 32.04 | 513.4 |
| Ethanol | wet | 46.07 | 513.9 |
| Toluene | isentropic | 92.14 | 591.75 |
3. Results and Discussion
3.1. Influence of Low-Grade Waste Heat Temperature on the OET
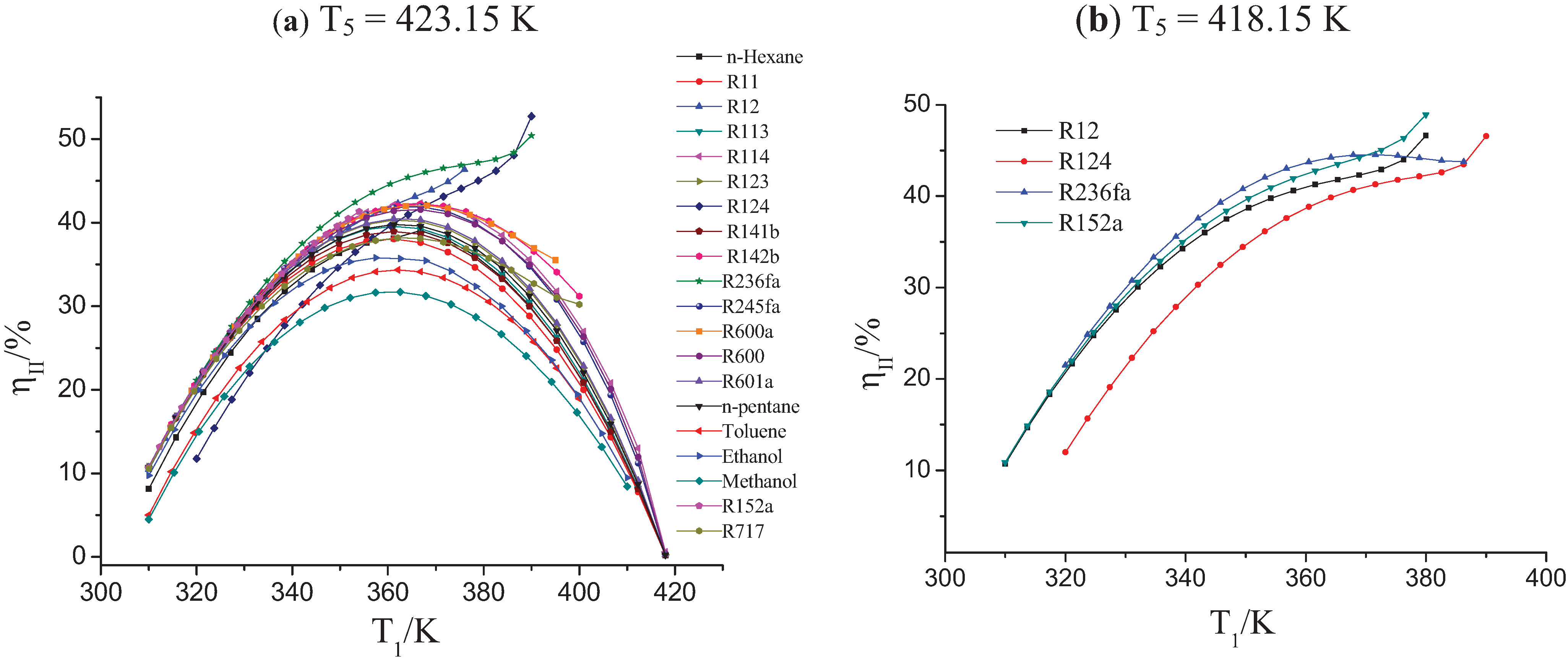
| Working Fluids | T5 = 423.15 K | T5 = 358.15 K | ||||
|---|---|---|---|---|---|---|
| ∆Tc/K | Tb/K | ηII/% | ∆Tc/K | Tb/K | ηII/% | |
| R143a | −77.29 | / | / | −12.29 | 330.9 | 29.04 |
| R32 | −71.89 | / | / | −6.89 | 328.7 | 27.27 |
| Propylene | −57.58 | / | / | 7.42 | 328.3 | 27.42 |
| R22 | −53.85 | / | / | 11.15 | 327.9 | 27.34 |
| R290 | −53.26 | / | / | 11.74 | 328.2 | 27.61 |
| R134a | −48.94 | / | / | 16.06 | 328.1 | 27.91 |
| R227ea | −48.35 | / | / | 16.65 | 333.2 | 20.07 |
| R12 | −38.03 | / | / | 26.97 | 327.5 | 26.91 |
| R152a | −36.74 | --- | --- | 28.26 | 327.6 | 27.36 |
| RC318 | −34.77 | / | / | 30.23 | 328.2 | 28.7 |
| R124 | −27.7 | --- | --- | 37.3 | 332 | 20.1 |
| R236fa | −25.1 | --- | --- | 39.9 | 327.9 | 28.1 |
| R717 | −17.75 | 363.7 | 38.21 | 47.25 | 327 | 26.52 |
| R600a | −15.3 | 366.2 | 42.08 | 49.7 | 327.6 | 27.63 |
| R142b | −12.89 | 366.7 | 42.23 | 52.11 | 327.5 | 27.75 |
| R114 | −4.32 | 365.6 | 42.35 | 60.68 | 327.6 | 27.79 |
| R600 | 1.98 | 365.3 | 41.6 | 66.98 | 327.5 | 27.59 |
| R245fa | 4.05 | 365 | 41.96 | 69.05 | 327.6 | 27.77 |
| R123 | 33.68 | 362.6 | 40.33 | 98.68 | 327.3 | 27.57 |
| R601a | 37.25 | 362.9 | 40.51 | 102.25 | 327.4 | 27.56 |
| R601 | 46.55 | 362.4 | 39.81 | 111.55 | 327.3 | 27.3 |
| R11 | 47.96 | 360.4 | 38.05 | 112.96 | 327 | 26.94 |
| R141b | 56.81 | 361.4 | 38.93 | 121.81 | 327.2 | 27.13 |
| R113 | 64.06 | 361.6 | 39.58 | 129.06 | 327.3 | 27.41 |
| n-Hexane | 84.25 | 362.3 | 38.13 | 149.25 | 328.3 | 25.21 |
| Methanol | 90.25 | 360.6 | 31.73 | 155.25 | 329.2 | 20.88 |
| Ethanol | 90.75 | 359.2 | 35.83 | 155.75 | 327.1 | 25.68 |
| Toluene | 168.6 | 362.1 | 34.35 | 233.6 | 329.3 | 22.03 |
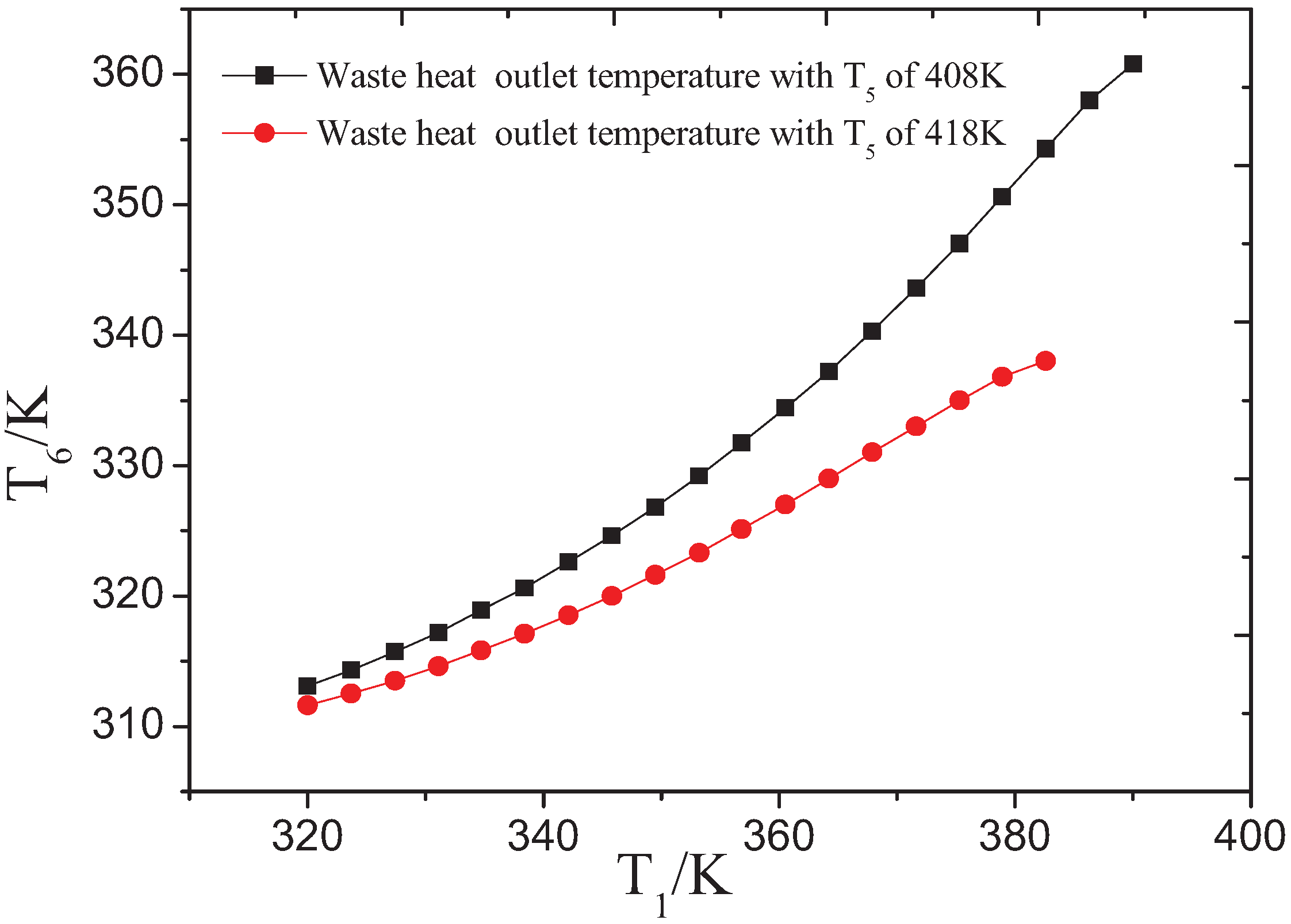
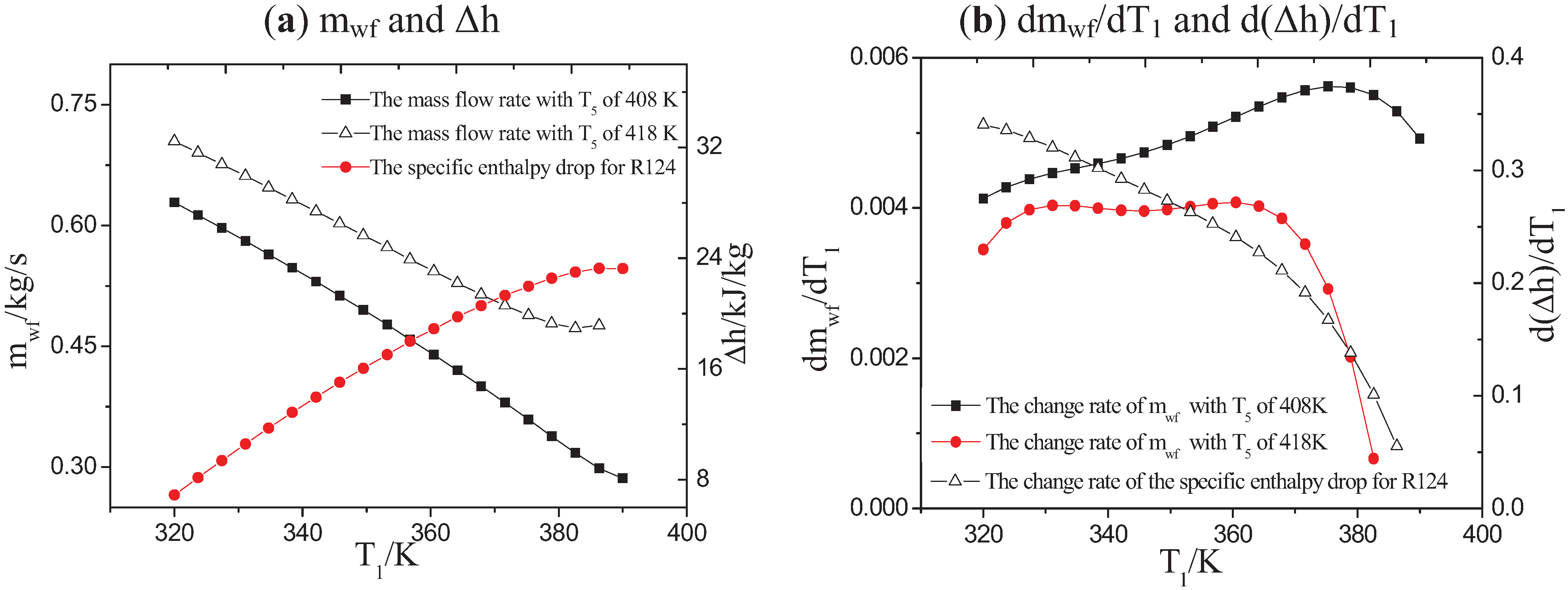
| Working Fluids | ∆Tc/K | Tb/K | ηII/% |
|---|---|---|---|
| R143a | −12.29 | 330.9 | 29.04 |
| R32 | −16.89 | 336.6 | 30.68 |
| Propylene | −17.58 | 347 | 35.29 |
| R22 | −18.85 | 348.6 | 35.71 |
| R290 | −18.26 | 350.6 | 37 |
| R134a | −18.94 | 353.8 | 38.59 |
| R227ea | −13.35 | 356.07 | 33.47 |
| R12 | −23.03 | 359.9 | 38.68 |
| R152a | −21.74 | 362.2 | 40.06 |
| RC318 | −14.77 | 360.2 | 42.76 |
| R124 | −17.7 | 372.2 | 39.09 |
| R236fa | −20.1 | 370.7 | 44.54 |
3.2. The Effect of the Pinch Temperature Difference on the OET
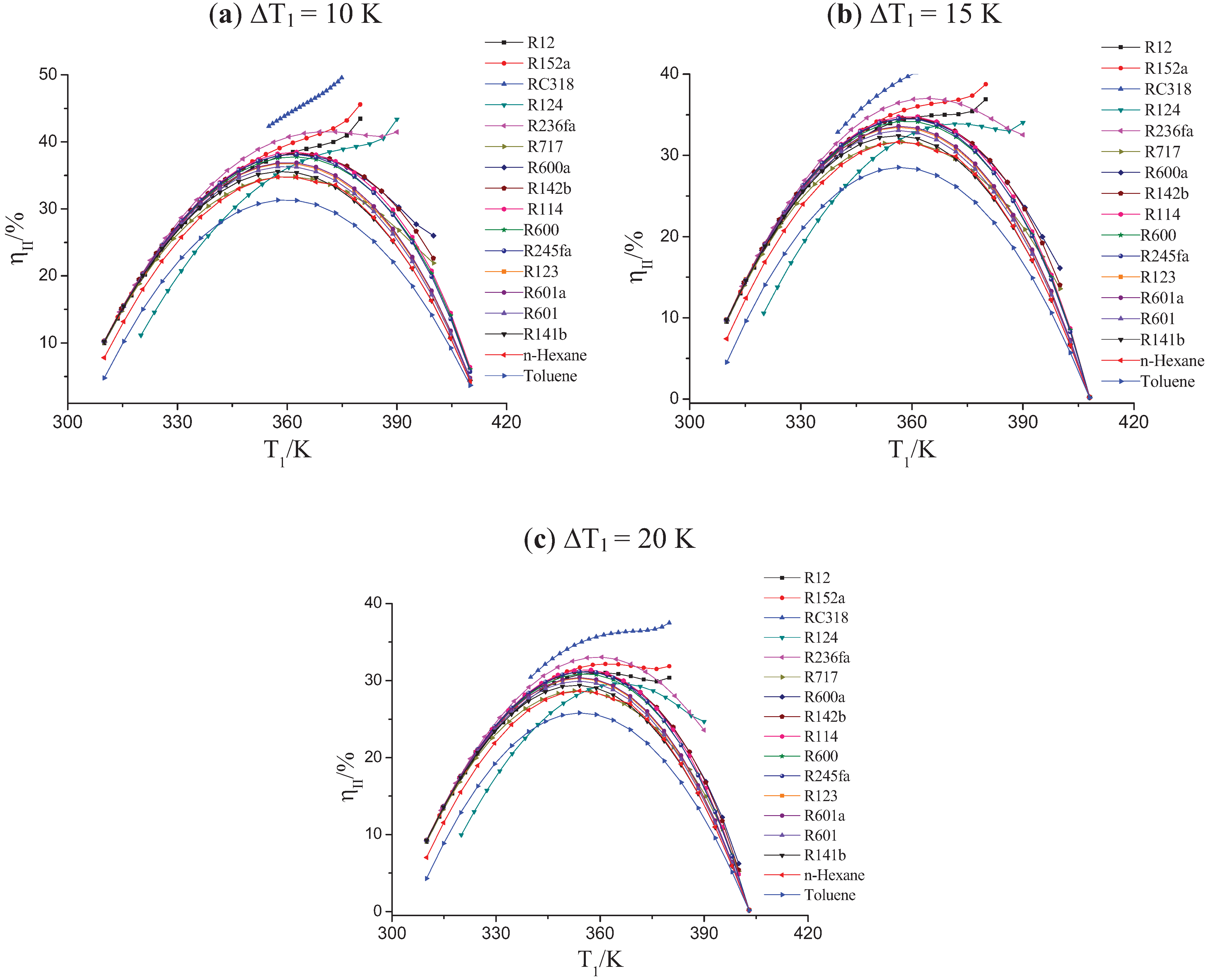
| Working Fluids | ∆Tc/K | ∆T1 = 10 K | ∆T1 = 15 K | ∆T1 = 20 K | |||
|---|---|---|---|---|---|---|---|
| Tb /K | ηII/% | Tb /K | ηII/% | Tb /K | ηII/% | ||
| R12 | −38.03 | --- | --- | --- | --- | 359.9 | 31.05 |
| R152a | −36.74 | --- | --- | --- | --- | 362.3 | 32.16 |
| RC318 | −34.77 | --- | --- | --- | --- | --- | --- |
| R124 | −27.7 | --- | --- | 372.2 | 33.88 | 365.7 | 29.60 |
| R236fa | −25.1 | 370.7 | 41.53 | 364.2 | 37.02 | 359.4 | 33.05 |
| R717 | −17.75 | 360.1 | 34.82 | 356.9 | 31.65 | 353.8 | 28.66 |
| R600a | −15.3 | 362.4 | 38.19 | 359 | 34.56 | 355.7 | 31.17 |
| R142b | −12.89 | 362.8 | 38.29 | 359.2 | 34.63 | 355.9 | 31.22 |
| R114 | −4.32 | 362.2 | 38.47 | 358.9 | 34.82 | 355.7 | 31.41 |
| R600 | 1.98 | 361.9 | 37.80 | 358.6 | 34.24 | 355.4 | 30.90 |
| R245fa | 4.05 | 361.7 | 38.14 | 358.5 | 34.56 | 355.4 | 31.20 |
| R123 | 33.68 | 359.7 | 36.80 | 356.8 | 33.47 | 354 | 30.30 |
| R601a | 37.25 | 359.9 | 36.95 | 357 | 33.58 | 354.2 | 30.40 |
| R601 | 46.55 | 359.5 | 36.35 | 356.6 | 33.06 | 353.8 | 29.95 |
| R141b | 56.81 | 358.6 | 35.60 | 355.9 | 32.43 | 353.2 | 29.41 |
| n-Hexane | 84.25 | 359.6 | 34.80 | 356.9 | 31.64 | 354.3 | 28.64 |
| Toluene | 168.6 | 359.6 | 31.36 | 357 | 28.51 | 354.5 | 25.80 |
4. Conclusions
Acknowledgments
References
- Wei, D.H.; Lu, X.S.; Lu, Z.; Gu, J.M. Performance analysis and optimization of organic Rankine cycle (ORC) for waste heat recovery. Energ. Convers. Manag. 2007, 48, 1113–1119. [Google Scholar] [CrossRef]
- Liu, B.T.; Chien, K.H.; Wang, C.C. Effect of working fluids on organic Rankine cycle for waste heat recovery. Energy 2004, 29, 1207–1217. [Google Scholar] [CrossRef]
- Heberle, F.; Brüggemann, D. Exergy based fluid selection for a geothermal Organic Rankine Cycle for combined heat and power generation. Appl. Therm. Eng. 2010, 30, 1326–1332. [Google Scholar] [CrossRef]
- Guo, T.; Wang, H.X.; Zhang, S.J. Comparative analysis of CO2-based transcritical Rankine cycle and HFC245fa-based subcritical organic Rankine cycle using low-temperature geothermal source. Sci. China 2010, 53, 1638–1646. [Google Scholar] [CrossRef]
- Kosmadakis, G.; Manolakos, D.; Papadakis, G. Parametric theoretical study of a two-stage solar organic Rankine cycle for RO desalination. Renew. Energ. 2010, 35, 989–996. [Google Scholar] [CrossRef]
- Facao, J.; Oliveira, A.C. Analysis of energetic, design and operational criteria when choosing an adequate working fluid for small ORC systems. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Lake Buena Vista, FL, USA, November 2009.
- Quoilin, S.; Declaye, S.; Tchanche, B.F.; Lemort, V. Thermo-economic optimization of waste heat recovery Organic Rankine Cycles. Appl. Therm. Eng. 2011, 31, 2885–2893. [Google Scholar] [CrossRef]
- Lakew, A.A.; Bolland, O. Working fluids for low-temperature heat source. Appl. Therm. Eng. 2010, 30, 1262–1268. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhao, L.; Wang, X.D. A comparative study of pure and zeotropic mixtures in low-temperature solar Rankine cycle. Appl. Energ. 2010, 87, 3366–3373. [Google Scholar] [CrossRef]
- Xu, R.J.; He, Y.L. A vapor injector-based novel regenerative organic Rankine cycle. Appl. Therm. Eng. 2011, 31, 1238–1243. [Google Scholar] [CrossRef]
- Zhang, S.J.; Wang, H.X.; Guo, T. Performance comparison and parametric optimization of subcritical Organic Rankine Cycle (ORC) and transcritical power cycle system for low-temperature geothermal power generation. Appl. Energ. 2011, 88, 2740–2754. [Google Scholar]
- Dai, Y.P.; Wang, J.F.; Lin, G. Parametric optimization and comparative study of organic Rankine cycle (ORC) for low grade waste heat recovery. Energ. Convers. Manag. 2009, 50, 576–582. [Google Scholar] [CrossRef]
- Saleh, B.; Koglbauer, G.; Wendland, M.; Fischer, J. Working fluids for low temperature organic Rankine cycles. Energy 2007, 32, 1210–1221. [Google Scholar] [CrossRef]
- Mago, P.J.; Chamra, L.M.; Somayaji, C. Performance analysis of different working fluids for use in organic Rankine cycles. Proc. IME J. Power Energ. 2007, 221, 255–264. [Google Scholar] [CrossRef]
- Young, J.B.; Minsung, K.; Chang, K.C.; Sung, J.K. Power-based performance comparison between carbon dioxide and R125 transcritical cycles for a low grade heat source. Appl. Energ. 2011, 88, 892–898. [Google Scholar]
- Roy, J.P.; Mishra, M.K.; Misra, A. Performance analysis of an Organic Rankine Cycle with superheating under different heat source temperature conditions. Appl. Energ. 2011, 88, 2995–3004. [Google Scholar] [CrossRef]
- Engineering Equation Solver, professional version, EES. F-Chart Software: Madision, WI, USA, 2006.
- Madhawa Hettiarachchi, H.D.; Golubovic, M.; Worek, W.M.; Ikegami, Y. Optimum design criteria for an Organic Rankine cycle using low-temperature geothermal heat sources. Energy 2007, 32, 1698–1706. [Google Scholar] [CrossRef]
- Papadopoulos, A.I.; Stijepovic, M.; Linke, P. On the systematic design and selection of optimal working fluids for Organic Rankine Cycles. Appl. Therm. Eng. 2010, 30, 760–769. [Google Scholar] [CrossRef]
- Tchanche, B.F.; Papadakis, G.; Lambrinos, G.; Frangoudakis, A. Fluid selection for a low-temperature solar organic Rankine cycle. Appl. Therm. Eng. 2009, 29, 2468–2476. [Google Scholar] [CrossRef]
- Chen, H.J.; Goswami, D.Y.; Stefanakos, E.K. A review of thermodynamic cycles and working fluids for the conversion of low-grade heat. Renew. Sustain. Energ. Rev. 2010, 14, 3059–3067. [Google Scholar] [CrossRef]
© 2012 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, C.; He, C.; Gao, H.; Xu, X.; Xu, J. The Optimal Evaporation Temperature of Subcritical ORC Based on Second Law Efficiency for Waste Heat Recovery. Entropy 2012, 14, 491-504. https://doi.org/10.3390/e14030491
Liu C, He C, Gao H, Xu X, Xu J. The Optimal Evaporation Temperature of Subcritical ORC Based on Second Law Efficiency for Waste Heat Recovery. Entropy. 2012; 14(3):491-504. https://doi.org/10.3390/e14030491
Chicago/Turabian StyleLiu, Chao, Chao He, Hong Gao, Xiaoxiao Xu, and Jinliang Xu. 2012. "The Optimal Evaporation Temperature of Subcritical ORC Based on Second Law Efficiency for Waste Heat Recovery" Entropy 14, no. 3: 491-504. https://doi.org/10.3390/e14030491
APA StyleLiu, C., He, C., Gao, H., Xu, X., & Xu, J. (2012). The Optimal Evaporation Temperature of Subcritical ORC Based on Second Law Efficiency for Waste Heat Recovery. Entropy, 14(3), 491-504. https://doi.org/10.3390/e14030491




