1. Introduction
Meat quality is a key evaluation criterion associated with the requirements that must be met to comply with consumers’ specifications, standards and expectations. Consumers’ ongoing demand for high quality standards in meat production, including safety, health promotion and personal beliefs, requires the development of new strategies to improve meat nutritional value [
1,
2]. There are several factors that underlie consumer preferences when purchasing a product, some of which are medical, the genetic predisposition to certain diseases, sensitivities or allergies, and others are related to cultural traditions and social evolution [
3]. According to the Food and Agriculture Organization (OECD/FAO) [
4], broiler meat is in the top of consumer preferences, being the most consumed type of meat due to its price and sustainable production [
5]. Current nutritional strategies are based on the use of natural ingredients in the formulation of poultry diets, to improve the nutritional value of the resulting animal products. Therefore, the tendency19 is to enrich the animal products in certain nutrients beneficial for human health, and thus available in an easier way.
There are several methods of broiler meat nutrients enrichment, including enrichment in polyunsaturated fatty acids (PUFA). A high-fatty acid diet is an important source of energy, to which is added a better adsorption of fat-soluble vitamins and all nutrients in the diet [
6,
7]. Polyunsaturated fatty acids omega-3 (
n − 3) and omega-6 (
n − 6) are essential fatty acids for the body’s proper functioning, but humans and animals cannot synthesize PUFA by their own metabolism. Inclusion of vegetable oils into broiler diets is a good strategy to improve the fatty acids profile in chicken meat [
8]. Flax is an important world crop primarily due to its high concentration of PUFA, especially its alpha-linolenic acid (ALA) content of more than 55%, beneficial to human health. The recovery of its final by-product, flaxseed meal, resulting from flaxseed oil extraction, is a solution for livestock feed and ingredients in many food products [
9], but also for the sustainable development of flaxseed production [
10]. Flaxseed meal is used in broiler diets as a valuable feed ingredient to enrich meat in PUFA
n − 3 [
11], but there is a certain limitation. The quality and shelf life of broiler meat are determined by lipid oxidation, and the result of this process is reflected in the decrease in nutritional value, flavor, texture, consistency and color. PUFA enrichment is associated with a high susceptibility to lipid peroxidation, so the inclusion of an antioxidant in diets becomes imperative.
Antioxidants are added to feed to maximize the oxidative stability of diets and meat and thus their quality remain unaffected [
12]. For a long time, the oxidative stability of meat was controlled using synthetic antioxidants. Consumers’ concerns about the possibility of consuming toxic products have led to the identification of natural antioxidants to replace synthetic ones [
13,
14]. Natural antioxidants have the advantage of important biochemical activity in reducing the effects of metabolic diseases. Their use, by delaying rancidity occurrence, also contributes to increasing the shelf life of meat, thus ensuring food safety and security.
Grapevine (
Vitis vinifera L.) is among the most popular crops in the world due to its nutritional properties, conferred mainly by their high content of polyphenols. Following the production of wine, the main use of grapes, approximately 20–30% of the resulting residue is grape pomace, a valuable source of nutrients with biotechnological potential, both in human and animal food. Grape pomace is a good alternative to synthetic antioxidants, a cheap source of bioactive substances, which also solves environmental problems regarding waste pollution [
15]. Grape seeds are part of grape pomace and contain an average of 6–20% oil [
16], which is usually extracted with solvents and refined before use [
17]. Recovery of grape seed oil (GSO) is an alternative for capitalizing on this by-product with high nutritional value [
18]. Due to its large worldwide production, grape seed oil is used in various applications, both for human use, in daily diet or in the cosmetics and pharmaceutical industry, but also in farm animal diets [
19]. Dabetic et al. [
17] reported the antioxidant, antibacterial and antifungal properties of grape seed oils. These properties allow the GSO to improve the oxidative stress and lipid profile of the products in which it is used, to decrease cholesterol level, have an inhibitory effect on the growth of some pathogens like
Staphylococcus aureus and
Escherichia coli and to show a cardioprotective effect due to the high content of unsaturated fatty acids (UFA), among many other health benefits [
20]. There are several studies [
8,
21,
22] that have shown the beneficial effect of using grape seed oil in broiler diets on health and blood plasma parameters, production performance, meat fatty acid content and oxidative stability.
The aim of the current study was to investigate the effects of grape seed oil supplementation as a natural antioxidant in PUFA-enriched broiler diets on growth performance, quality attributes for consumers, health lipid indices and oxidative stability of meat.
2. Materials and Methods
2.1. Ethical Procedure
The bird care and protocol (no. 2892/17 May 2019) used in this study was approved by the Ethics Committee of the National and Development Institute for Biology and Animal Nutrition (INCDBNA-IBNA), Balotesti. The experiment complied with the principles of Romanian Law 43/2014 for the handling and protection of animals used for experimental purposes, the EU Council Directive 98/58/EC concerning the protection of farmed animals and Directive 2010/63/EU on the protection of animals used for scientific purposes.
2.2. Experimental Design and Diets
This study was carried out on 120 14-day-old Cobb 500 unsexed broiler chickens, obtained from a local commercial hatchery. Different levels of grape seed oil (GSO) were tested as a source of natural antioxidants at 0% (GSO0, control), 1.5% (GSO1.5) and 3% (GSO3) levels by substituting the vegetable oil from the basal diet, in the presence of 4% flaxseed meal (FSM) (
Table 1). Flaxseed meal was included in all three diets to enrich them in PUFA. The plant materials (FSM and GSO) used in the new diet formulations were purchased from a local supplier in Romania. The broilers were weighed individually and randomly assigned to three treatments of 40 chicks each in a completely randomized design. According to the Cobb 500 Hybrid growth guide, the feeding program was divided into 2 feeding phases: grower (14–28 days) and finisher (29–42 days) using the HYBRIMIN Futter 5 Nutrition program. Feed and water were available ad libitum. The broilers were housed in semi-intensive system conditions, in a hall divided into 3 experimental spaces as growth boxes of 3 m
2 (each group was housed in a single box) and reared on the floor on permanent wood shaves litter (10–12 cm thick). The temperature and lighting programs were consistent with the recommendations of Hybrid. No mortality was recorded during the overall trial period.
2.3. Growth Performance and Broiler Slaughter
Throughout the experimental period (14–42 days) the following parameters were monitored: body weight (BW, g), average daily weight gain (ADWG, g/broiler/day), average daily feed intake (ADFI, g feed/broiler/day) and feed conversion ratio (FCR, g feed/g gain). BW was recorded weekly by weighing every broiler individually; ADWG was calculated by dividing total broiler weight by the number of experimental days; ADFI was calculated on a daily basis by subtracting the amount of rejected feed from the offered feed at the beginning and end of every 24 h (1 day); FCR values were calculated by dividing total feed consumed at total live weight.
At the end of the feeding-trial (42 days), 6 broilers per group were randomly selected and slaughtered by cervical dislocation according to the working protocol approved by the Ethics Commission of INCDBNA, Balotești. Six breast and six thigh meat samples per group were collected and were divided into several sections for each type of determination targeted. They were collected in plastic bags, labeled and distributed for the following determinations: instrumental color measurements; pH assessment; texture profile; fatty acid content; and lipid oxidation parameters.
2.4. Fatty Acids Determination
The fatty acid content of flaxseed meal, feeds and meat samples was determined using a gas chromatograph PerkinElmer Clarus 500 (Massachusetts, United States). The principle of the method consists of the transformation of fatty acids, from the sample under analysis, into methyl esters, followed by the separation of the components on the chromatographic column, their identification being made by reference to the standard chromatograms. The chromatograph has a flame ionization detector (FID) and capillary separation column with a high polar stationary phase TRACE TR-Fame, (Thermo Electron, Massachusetts, United States), with dimensions of 60 m × 0.25 mm × 0.25 μm film. The average amount of each fatty acid was used to calculate the sum of the total saturated (SFA), total monounsaturated (MUFA) and total polyunsaturated (PUFA) fatty acids. Indices of atherogenicity (AI) and thrombogenicity (TI), and the ratio of hypo and hypercholesterolemia (h/H), were calculated according to [
23,
24].
2.5. Antioxidant Capacity
A method adapted from Ninfali et al. [
25] was used in order to determine the antioxidant capacity of grape seed oil. The method employed used hexane as solvent to solubilize both oil samples and 2,2-diphenyl-1-picrylhydrazyl (DPPH). The equipment used was a Jasco V 530 UV-VIS spectrometer, controlled with Spectra Manager software. The results were expressed as DPPH equivalents/g of sample, taking into consideration the density of the grape seed oil which was also determined.
2.6. Volatile Compounds
The analysis of volatile compounds from grape seed oil was performed by gas chromatography coupled with mass spectrometry (GC-MS), using Thermo Scientific equipment with an automatic Triplus autosampler. The GC-MS system consisted of a Focus GC gas chromatograph coupled with a Polaris Q ion mass spectrometer. The separation was performed on a DB-5MS type capillary column (non-polar stationary phase with 5% phenyl) with a length of 25 m, a diameter of 0.25 mm and 0.25 µm thickness film. The ion source and interface temperatures were 200 and 250 °C, and the detector operated in electron impact mode (70 eV). The detection was performed in the range m/z 35–300, the mass spectrometer operating in full-scan mode. A standard solution of alkanes for GC (even fraction C8–C20 in hexane) was analyzed according to the same temperature program and further used for Kovats retention indices calculation and compounds identification in oil samples. The results were expressed as a percentage.
2.7. Instrumental Color Measurements
Determination of meat color parameters in the CIELAB space by measuring the parameters L* (lightness), a* (saturation index in green/red), b* (saturation index in blue/yellow) and c* (metric chroma) and the total color difference (ΔE*) was performed according to the method described by Panaite et al. [
26] and Mancini et al. [
27], using a Konica Minolta CR-400 (Tokyo, Japan) colorimeter. The results were expressed as an average for three measurements/sample.
2.8. PH Assessment
The pH of the samples was measured using a Hach HQ30d pH-meter (Hach, Loveland, CO, USA), according to the SR ISO 2917: 2007 standard at 24 h post-mortem. To measure the pH, a meat–water mixture was prepared from 5 g of meat which was cut into pieces with a knife and mixed with 5 mL of distilled water, at neutral pH [
28]. At least three replicate measurements for each sample were carried out and the electrode was cleaned after each measurement. Buffer solutions of pH 7 and pH 4 were used for calibration of the pH meter.
2.9. Texture Profile Analysis
The chicken meat texture parameters were determined by a double cycle compression performed using a Perten TVT 6700 texturometer (Perten Instruments, Sweden), equipped with a Compression Platen cell. The analysis of the texture profile (TPA) resulting from the application of the compression test highlights as main texture parameters: hardness or firmness, springiness, resilience, cohesiveness and, as secondary parameter, gumminess. The thigh and breast meat samples were cut into cylindrical pieces of 10 mm thickness and 15 mm diameter, after which they were subjected to compression of 50% of the initial height. The principle of the method consisted of applying tension with a stainless-steel cylinder, with a 20 mm diameter on the meat samples, and the automatic recording of the resistance force that they opposed to deformation [
28]. The measurements on a sample were repeated at least three times, and the results were expressed as their average.
2.10. Lipid Oxidation Parameters Evaluation
Samples of breast and thigh meat were stored for 7 days in the refrigerator at a constant temperature of 4 °C before evaluating the lipid oxidation parameters. In order to carry out the assessment, the samples were minced and cryogenized with liquid nitrogen at a temperature of −180 °C, and therefore were grounded. The oxidative stability of the meat was determined by means of primary lipid degradation parameters, peroxide index, dienes and conjugated trienes, respectively, by secondary parameters, represented by the values of p-anisidine and those of thiobarbituric acid-reactive substances (TBARS), according to the method described by Untea et al. [
24]. The lipid peroxidation parameters were spectrophotometrically determined by using a V-530 Jasco (Japan Servo Co. Ltd., Tokyo, Japan) spectrophotometer, as follows:
The peroxide value was measured by the ferric thiocyanate method. To the lipid extract sample, a chloroform/methanol solution was added, and the mixture was vortexed. In the next step, xylenol orange solution and FeCl2 solution were added, the mixture was left for 5 min at room temperature and then measured.
The values of conjugated dienes and trienes were determined using a lipid extract sample dissolved in 2,2,4-trimethylpentane (iso-octane).
The p-anisidine value was determined by a method based on the reaction between p-anisidine and aldehydic compounds present in lipid extract samples in acidic conditions. The lipid extract sample was dissolved in iso-octane, the p-anisidine reagent was added to the cuvette, the sample was placed kept in the dark for 10 min and then the spectra was recorded.
The TBARS values were measured using third derivative spectrophotometry with some modifications [
29]. The sample was mixed with trichloroacetic acid and butyrate hydroxytoluene in ethanol, and centrifuged. The aliquots were mixed with aqueous thiobarbituric acid solution in a test tube and further incubated at 80 °C for 50 min. Following incubation, the sample was cooled under running water and measured. TBARS values were calculated against a standard curve obtained with 1,1,3,3-tetramethoxypropane.
2.11. Principal Component Analysis
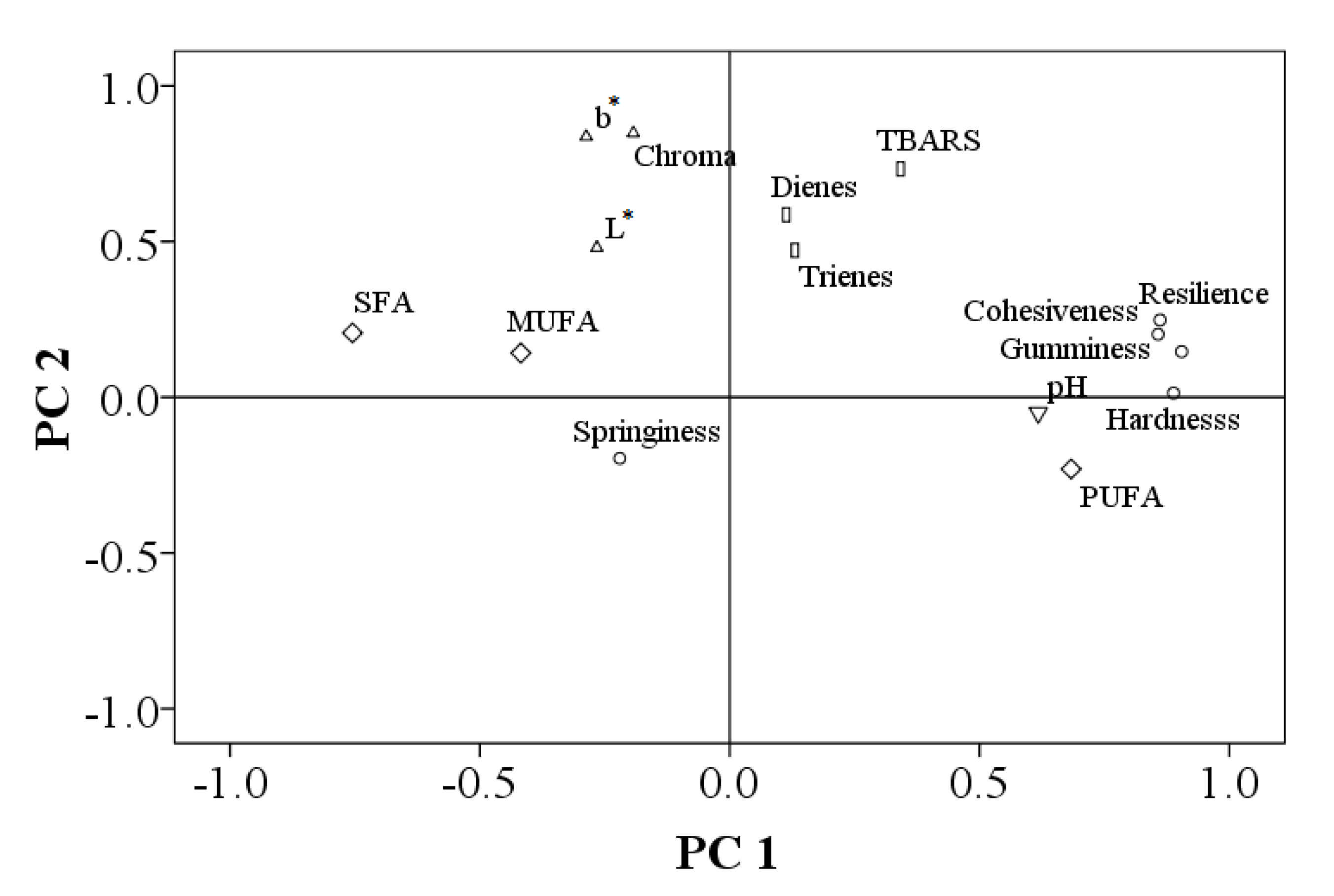
Principal Component Analysis (PCA) was performed to assess the relationships between the evaluated characteristics. The PCA technique converts the original variables into factors, namely principal components, in the conditions of minimal information loss. By applying this technique to the results, the differences and similarities between evaluated charactristics can be observed in the load graph.
2.12. Statistical Analysis
All results were statistically processed for each quality parameter of the chicken samples. The analytical data were compared by analysis of variance (ANOVA and t test), using the StatView program for Windows. The results of this experiment are presented as mean values, the differences being considered statistically significant at p < 0.05.
4. Discussion
4.1. Chemical Composition of Plant Materials
Flaxseed meal is recognized worldwide due to its rich composition of PUFA
n − 3 fatty acid and alpha-linolenic acid, and also its high-quality protein. Available data in the literature show for flaxseed meal a content higher than 33% for ALA [
30]; 42.93% PUFA
n − 3; 70.23% total PUFA; and a value of 0.64 for
n − 6/
n − 3 ratio [
31].
Grape seed oil composition is closely related to the grape variety from which it is obtained, environmental and climatic conditions, soil type, harvesting time and seed maturation degree [
32,
33]. The oil composition includes bioactive compounds, such as free fatty acids, vitamin E (tocopherols and tocotrienols), polyphenols and steroids (campesterol, beta-sitosterol and stigmasterol) [
34]. In some countries, grape seed oil is used as a natural food preservative [
35]. However, there are poor references in the literature regarding the antioxidant capacity of grape seed oil; available data indicated a concentration of 42.18 mmol of Trolox equivalent/g measured by oxygen radical absorbance capacity assay [
20]. GC-MS analysis of grape seed oil allowed in the present study to establish its composition by identifying the main classes of compounds, and based on them the evaluation of the antioxidant potential, necessary to complete the diets with a rich lipid profile. The principal components of the profile established were linoleic acid ethyl ester, benzyl benzoate and 10,13-eicosadienoic acid methyl ester.
Other components with antioxidant, anti-tumor, anti-inflammatory, antiatherogenic, diuretic, antifungal and antiviral properties such as sesquiterpenes (β guaiene), triterpene saponins (lysergic acid) or monoterpene phenols (thymol) [
36,
37] are present in the grape seed oil composition with considerable concentrations.
4.2. Effect of Grape Seed Oil on Growth Performance of Broiler
In our study, although there were no significant effects of grape seed oil inclusion in broiler diets, there was an improvement of final weight in the GSO3 group, compared to the GSO group. The results reveal that between the two experimental groups that included grape seed oil (GSO1.5 and GSO3), a higher level of inclusion (GSO3) led to significantly higher average daily weight gain and body weight gain (
p < 0.05). Beneficial effects of grape seed oil use were reported by Tekeli et al. [
21] on feed conversion rate, which was improved (
p < 0.05) when it included a level of 1.5% GSO in broiler diets. Similar results regarding the feed conversion rate improvement and a significant increase (
p < 0.05) in the average of broiler body weight were obtained using 1% grape seed oil [
38]. At a supplementation level of 2.5% GSO in diets, no significant effects (
p < 0.05) were recorded during the entire experimental period on the broiler production performance [
39].
4.3. Effect of Grape Seed Oil on Color Parameters and the pH of the Chicken Meat
Meat color is one of the most important buying factors, because depending on it, consumers make a first impression of the product. According to Marcinkowska-Lesiak et al. [
40], meat color is considered a freshness and quality indicator of the product. There are several factors that influence the chicken meat color, namely genetic factors, applied diet, muscle fiber characteristics or heme pigments [
41]. A high level of PUFA also influences the color and aroma of the meat [
42]. In the present study, the new tested diets influenced the chicken meat. Supplementing the broiler diet with a double amount of grape seed oil led to the
L* parameter being increased in the GSO3 group compared to the GSO1.5 group, suggesting an increase in thigh meat lightness with an increasing level of oil addition, from 1.5 to 3%. Corroborated with the recorded results for the
L* parameter, the values of parameter
a* indicated a decrease (
p < 0.05) in the red color of thigh meat, compared to the GSO0 and GSO1.5 groups. According to Mirshekar et al. [
43], an increased degree of meat lightness is due to a lower pH value, resulting also in higher water losses. It was reported [
44] that meat lightness is influenced by collagen deposits on muscles, which are white and accentuate as broilers become older. Another factor that influences the meat color is the myoglobin content increase in the meat depending also on the broiler age. According to Savadkoohi et al. [
45], instrumentally measured color changes can be considered visible if
∆E > 2. In this study, the supplementation of broiler diets with GSO led to a visible change in color in the case of thigh meat at the maximum level of inclusion evaluated of 3% (GSO3). The trend was different in the case of breast meat, which had a visibly different color at a lower inclusion level of GSO of 1.5% (GSO1.5), compared to the GSO0 group.
Determining the meat pH is important because there is a correlation between its values and physico-chemical characteristics, such as color and hardness [
46]. In this study, the pH measurement indicated lower values in the groups that included grape seed oil (GSO1.5 and GSO3) compared to the GSO0 group, the differences being significant (
p < 0.05) for thigh meat. The pH values indicated a high degree of broiler meat freshness and the beneficial effect of GSO inclusion in broiler diets. According to the limits established by different authors regarding the quality of chicken meat reflected by the measured pH value, the results obtained in this study are within the normal limits, between 5.9 and 6.2 [
47].
4.4. Effect of Grape Seed Oil on Texture Parameters
Along with color, texture is considered an important decision factor for meat quality [
48]. The proteolysis of key myofibrillar proteins is responsible for structural integrity in muscle fiber preservation. The weakening of muscle fibers is caused postmortem by proteolytic degradation, and thus, the meat becomes harder [
40]. The texture profile analysis of broiler meat, thigh and breast revealed no significant treatment effects, which indicates that no negative effect of grape seed oil feeding was observed in the present study.
4.5. Effect of Grape Seed Oil on Meat Fatty Acid Content
This study assessed the quality attributes of chicken meat for consumers, changes in its fatty acid content and the consequent effects on meat oxidative stability under the influence of grape seed oil in the presence of flaxseed meal. The observed variation in the fatty acid composition of broiler meat was due to the differentiated inclusion of GSO in diets, because the level of flaxseed meal was the same in all three treatments. The present study clearly shows that the inclusion of grape seed oil in diets had significant effects, especially on the fatty acids of thigh meat. These effects were visible in the content of increased SFA, lower PUFA and PUFA n − 3, and the increase in n − 6/n − 3 ratio value. Regarding the breast meat, the SFA content increased, but there was an improvement in the ratio of PUFA n − 6/n − 3 between experimental groups (GSO1.5 vs. GSO3).
According to Barroeta [
49], the inclusion of PUFA-rich oils in broiler diets results in a lower deposition of body fat, even if PUFA have a higher content of metabolizable energy than SFA. There are several factors that influence the effectiveness of fat utilization in broiler diets, including their chemical composition, inclusion rate or chick age. They are joined by anti-nutritional factors (e.g., tannins, phytic acid and trypsin inhibitor) contained by grape seeds or the raw residues from the GSO extraction industries [
50,
51]. These compounds, present in grape-derived products, interact with fats by decreasing lipase secretion and consequently lipolysis process, leading to lipid malabsorption [
52], and finally affecting the fatty acids profile.
Indices of atherogenicity (AI) and thrombogenicity (TI) and the ratio of hypo- and hyper-cholesterolemia (h/H) are health and nutritional lipid indices [
53]. The results obtained in this study provide the overview for a nutritional assessment of fatty acids and their health-related lipid indices. Because the AI index is a significant indicator of cardiovascular disease risk [
54], its decrease in thigh meat suggests a reduced risk for broilers to develop this type of disease, corroborated with the level of GSO inclusion in the diet. According to Garaffo et al. [
55], the TI index indicates the possibility of clots forming in the blood vessels. The results obtained in the present study show an increased TI value in the GSO1.5 group, but as the level of inclusion in the diet (GSO3) was increased, its value was no longer influenced, being comparable to that obtained in the GSO0 group. In addition to AI and IT indices, the hypo- and hyper-cholesterolemia ratio is an additional index that contributes to the evaluation of the effect that fatty acids have on cholesterol metabolism [
53]. The decrease in h/H ratio value in the GSO1.5 group reflects the lower PUFA content in the thigh meat, compared to the GSO0 group.
4.6. Effect of Grape Seed Oil on Lipid Oxidation Parameters
It is well known that lipid oxidation is one of the main processes of quality deterioration in meat and meat products [
42]. Oxidative stress occurs as a result of an imbalance between the production of various oxidizing chemical species and the natural antioxidant capacity of cells to annihilate these species [
56]. The inclusion of grape seed oil at different levels in PUFA-enriched broiler diets has been carried out to manipulate the oxidative stability of meat and to prevent its quality deterioration. In the current study, the antioxidant effect of GSO on meat lipid oxidation could be observed most on thigh meat, compared to the breast meat samples. Apart from the peroxide index, all other lipid degradation parameters of thigh meat were significantly (
p < 0.05) improved by the new feed diets. Overall, the inclusion of a 1.5% GSO level indicated for thigh meat the best results regarding the main degradation parameters, conjugated dienes and trienes, but also on one of the secondary ones, p-anisidine. Although there could be observed an improvement for breast meat in terms of lipid oxidation, only the TBARS parameter from the GSO3 group was significantly (
p < 0.05) influenced. Unlike thigh meat, for breast the best results were obtained at a rate of 3% GSO inclusion in broiler diets. In a similar study [
22] it was shown that the inclusion of 1% GSO led to an improvement in the oxidative status of broiler meat, by significantly (
p < 0.05) decreasing the malonaldehyde and peroxide index values. A decrease in the malondialdehyde concentration in broiler meat has also been reported by Bander [
38], when using a level of 1% GSO.
4.7. The Relationship between Meat Characteristics
PCA results show a bi-plot of the evaluated characteristics of the two PCs and the positive or negative relationship between them. The main contributor characteristics on the first principal component (PC1) were the textural parameters, hardness, gumminess, cohesiveness and resilience, along with the pH and polyunsaturated fatty acids. These characteristics were negatively correlated with the textural parameter springiness and monounsaturated (MUFA) and saturated fatty acids (SFA). Along the PC1, an inverse relationship could be observed between cohesiveness and springiness, which was expected. The second principal component, PC2, showed a close relationship between luminosity (L*), b* parameter and chroma and between dienes, trienes and TBARS lipid degradation parameters. There was a direct significant (p < 0.05) relationship between the b* parameter and chroma (r = 0.965), between b* and L* (r = 0.674), between L* and chroma (r = 0.544) and between dienes and trienes (r = 0.825). Additionally, the secondary parameter of lipid degradation, TBARS, was directly correlated with chroma (r = 0.511), a relationship which can be explained by the fact that lipid oxidation changes meat chroma, affecting the meat quality. Meat hardness was negatively correlated with saturated fatty acids (r = −0.542), whereas a high positive correlation was found between hardness and gumminess (r = 0.954), resilience (r = 0.751) and cohesiveness (r = 0.758). A high negative relationship was found between saturated fatty acids and polyunsaturated fatty acids (r = −0.923).