In Vitro Evaluation of No-Carrier-Added Radiolabeled Cisplatin ([189, 191Pt]cisplatin) Emitting Auger Electrons
Abstract
:1. Introduction
2. Results
2.1. In Vitro Property of n.c.a. Radio-Cisplatin
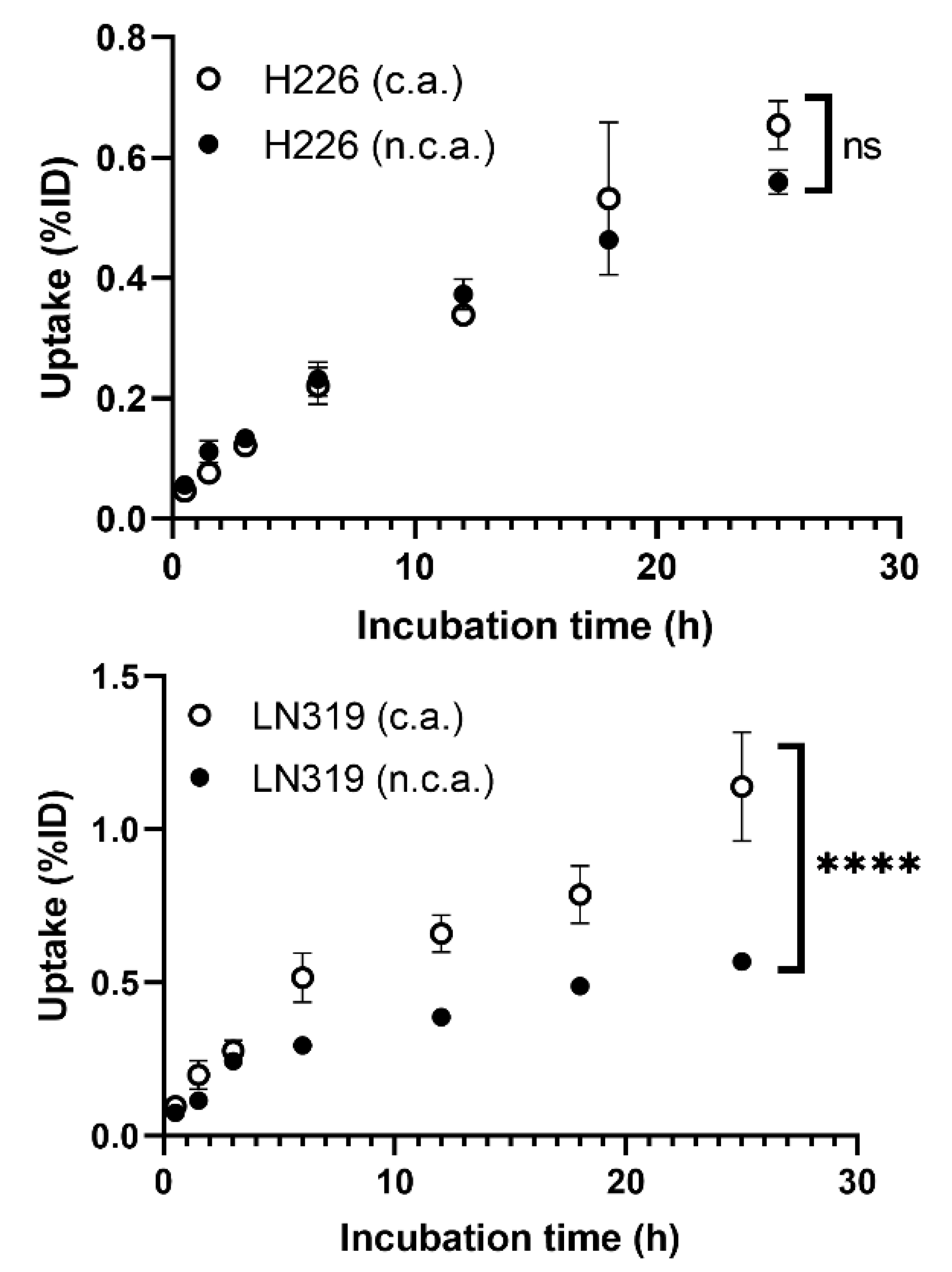
2.1.1. Cellular Uptake
2.1.2. Intracellular Distribution and DNA Binding
2.1.3. Cytotoxicity
2.2. DNA Damage Induced by n.c.a. Radio-Cisplatin
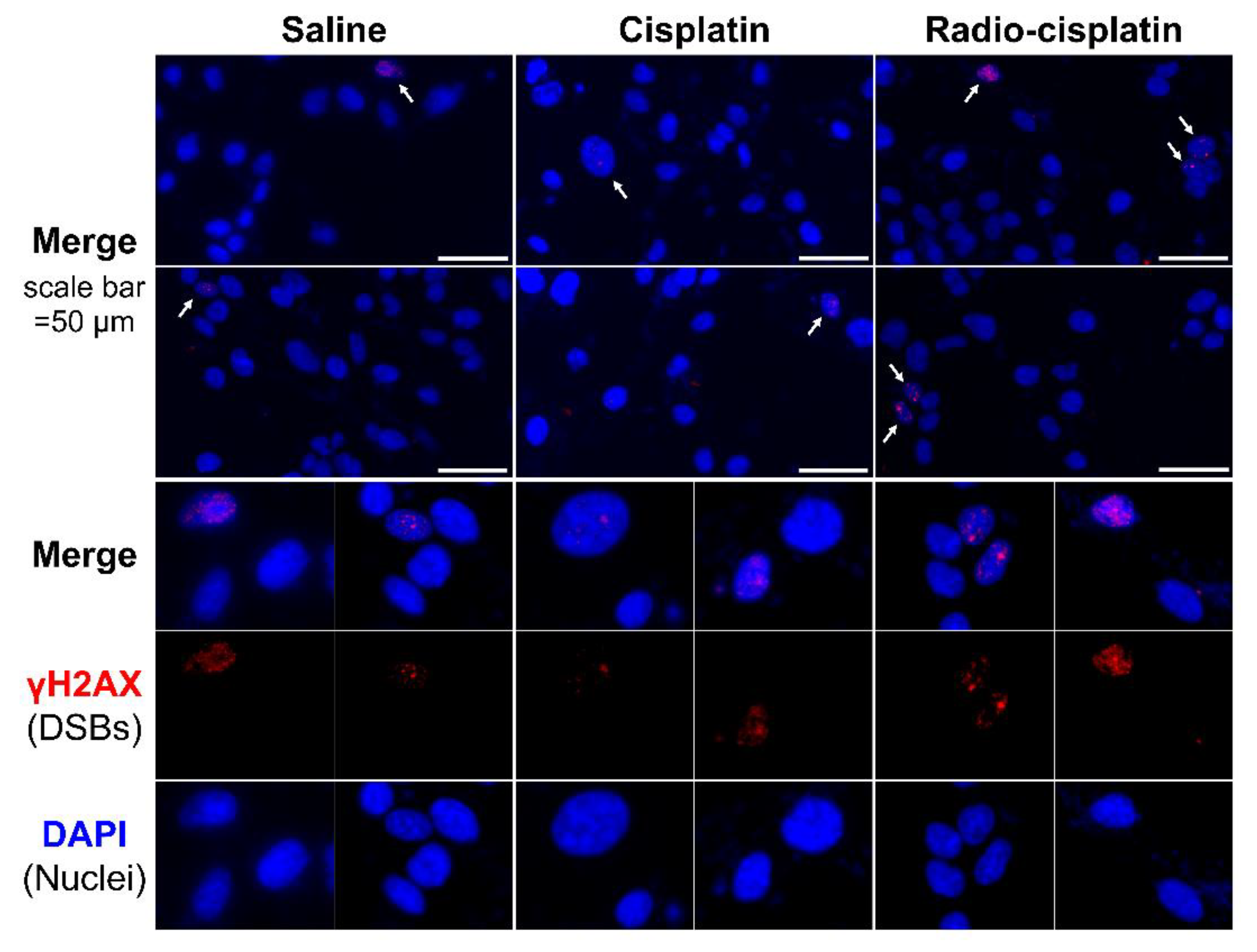
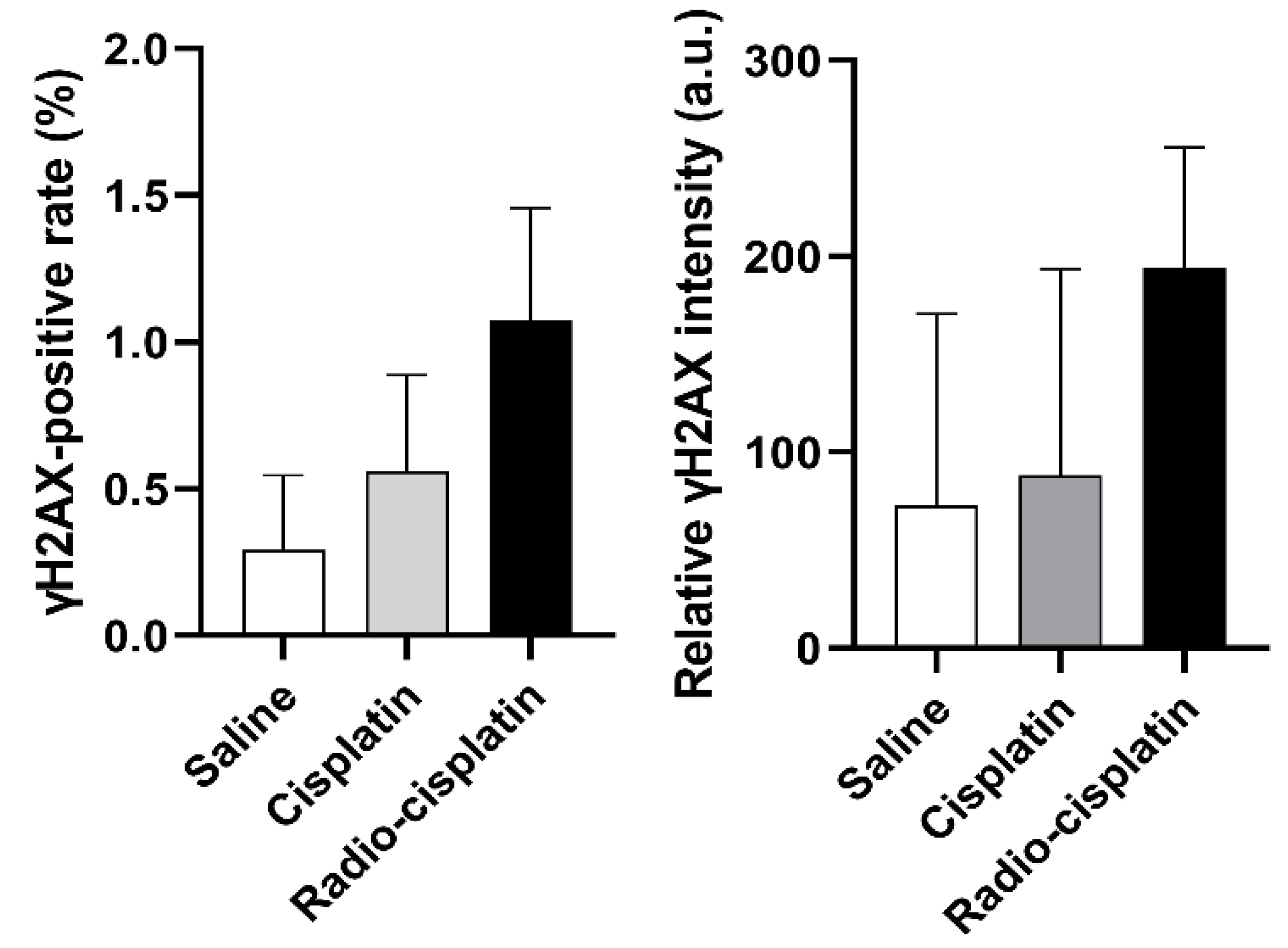
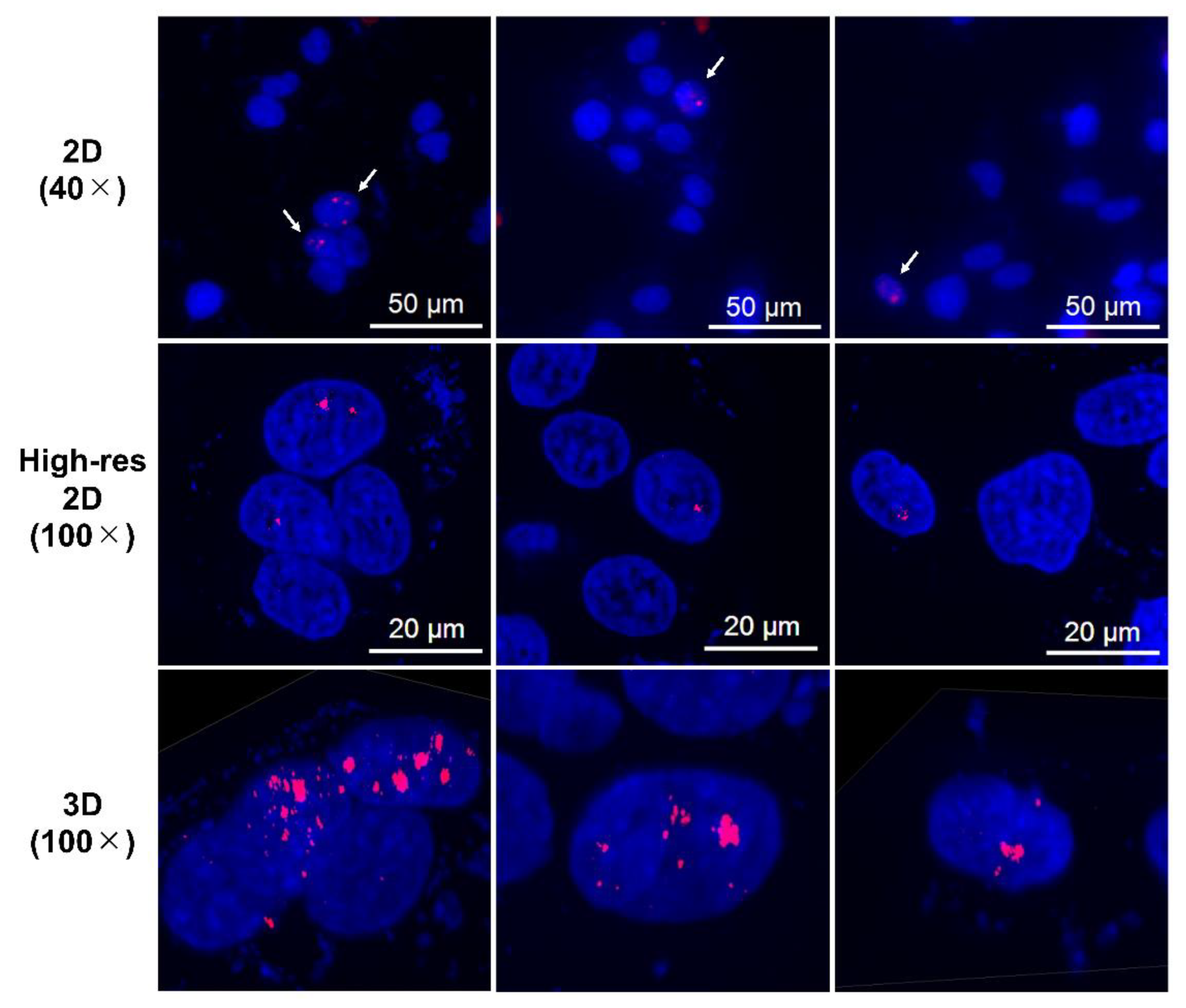
2.2.1. Cellular Uptake Immunofluorescence Assay for γH2AX
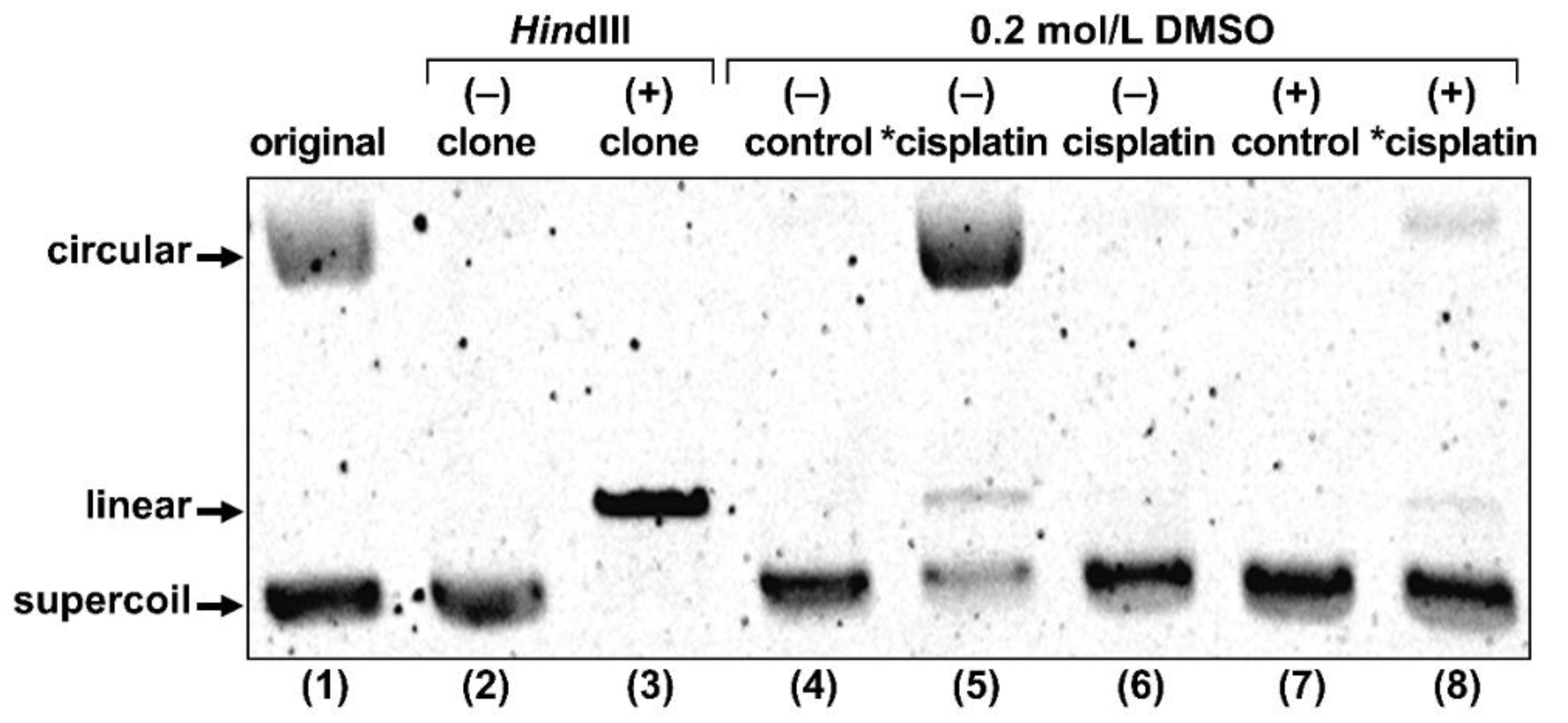
2.2.2. Gel Electrophoresis of Plasmid DNA
3. Discussion
3.1. In Vitro Property of n.c.a. Radio-Cisplatin
3.2. DNA Damage Induced by n.c.a. Radio-Cisplatin
4. Materials and Methods
4.1. General
4.2. Synthesis of Carrier-Free [189, 191Pt]cisplatin
4.3. Cell Culture
4.4. Cellular Uptake
4.5. Intracellular Distribution
4.6. DNA Binding
4.7. Sulforhodamine B (SRB) Assay
4.8. Live/Dead Viability/Cytotoxicity Assay
4.9. Immunofluorescence Staining of γH2AX
4.10. Gel Electrophoresis of Plasmid DNA
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Pt | platinum |
| Cisplatin | cis-diamminedichloroplatinum(II) |
| n.c.a. | no-carrier-added |
| c.a. | carrier-added |
| DSBs | DNA double strand breaks |
| LET | linear energy transfer |
| γH2AX | phosphorylated Histone H2AX |
| % ID | percent incubated dose |
| T1/2 | half-life |
| EC | electron capture |
| IT | internal conversion |
| ICP-MS | inductively coupled plasma mass spectrometry |
| a.u. | arbitrary unit |
| 2D | two-dimensional |
| 3D | three-dimensional |
References
- Lomax, M.E.; Folkes, L.K.; O’Neill, P. Biological consequences of radiation-induced DNA damage: Relevance to radiotherapy. Clin. Oncol. 2013, 25, 578–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassis, A.I.; Adelstein, S.J. Radiobiologic principles in radionuclide therapy. J. Nucl. Med. 2005, 46, 4S–12S. [Google Scholar]
- Kassis, A.I.; Fayad, F.; Kinsey, B.M.; Sastry, K.S.; Taube, R.A.; Adelstein, S.J. Radiotoxicity of 125I in mammalian cells. Radiat. Res. 1987, 111, 305–318. [Google Scholar] [CrossRef]
- Qaim, S.M. Nuclear data for production and medical application of radionuclides: Present status and future needs. Nucl. Med. Biol. 2017, 44, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Rebischung, C.; Hoffmann, D.; Stéfani, L.; Desruet, M.D.; Wang, K.; Adelstein, S.J.; Artignan, X.; Vincent, F.; Gauchez, A.S.; Zhang, H.; et al. First human treatment of resistant neoplastic meningitis by intrathecal administration of MTX Plus 125IUdR. Int. J. Radiat. Biol. 2008, 84, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Vallis, K.A.; Reilly, R.M.; Scollard, D.; Merante, P.; Brade, A.; Velauthapillai, S.; Caldwell, C.; Chan, I.; Freeman, M.; Lockwood, G.; et al. Phase I trial to evaluate the tumor and normal tissue uptake, radiation dosimetry and safety of 111In-DTPA-human epidermal growth factor in patients with metastatic EGFR-positive breast cancer. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 181–192. [Google Scholar] [PubMed]
- Jong, M.; Valkema, R.; Jamar, F.; Kvols, L.K.; Kwekkeboom, D.J.; Breeman, W.A.P.; Bakker, W.H.; Smith, C.; Pauwels, S.; Krenning, E.P. Somatostatin receptor-targeted radionuclide therapy of tumors: Preclinical and clinical findings. Semin. Nucl. Med. 2002, 32, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Welt, S.; Scott, A.M.; Divgi, C.R.; Kemeny, N.E.; Finn, R.D.; Daghighian, F.; Germain, J.S.; Richards, E.C.; Larson, S.M.; Old, L.J. Phase I/II study of iodine 125-labeled monoclonal antibody A33 in patients with advanced colon cancer. J. Clin. Oncol. 1996, 14, 1787–1797. [Google Scholar] [CrossRef]
- Kassis, A.I.; Tumeh, S.S.; Wen, P.Y.C.; Kortylewicz, J.B.; Abbeele, A.D.V.; Zimmerman, R.E.; Carvalho, P.A.; Garada, B.M.; DeSisto, W.C.; Bailey, N.O.; et al. Intratumoral administration of 5-[123I]iodo-2′ deoxyuridine in a patient with a brain tumor. J. Nucl. Med. 1966, 37, 19S–22S. [Google Scholar]
- Rosenkranz, A.A.; Slastnikova, T.A.; Karmakova, T.A.; Vorontsova, M.S.; Morozova, N.B.; Petriev, V.M.; Abrosimov, A.S.; Khramtsov, Y.V.; Lupanova, T.N.; Ulasov, A.V.; et al. Antitumor activity of auger electron emitter 111In delivered by modular nanotransporter for treatment of bladder cancer with EGFR overexpression. Front. Pharmacol. 2018, 9, 1331. [Google Scholar] [CrossRef] [Green Version]
- Cornelissen, B.; Vallis, K.A. Targeting the nucleus: An overview of auger-electron radionuclide therapy. Curr. Drug Discov. Technol. 2010, 7, 263–279. [Google Scholar] [CrossRef]
- Martina, R.F.; Feinendegend, L.E. The quest to exploit the Auger effect in cancer radiotherapy—A reflective review. Int. J. Radiat. Biol. 2016, 92, 617–632. [Google Scholar] [CrossRef]
- Buchegger, F.; Adamer, F.P.; Dupertuis, Y.M.; Delaloye, A.B. Auger radiation targeted into DNA: A therapy perspective. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1352–1363. [Google Scholar] [CrossRef]
- Rosenkranz, A.A.; Slastnikova, T.A.; Georgiev, G.P.; Zalutsky, M.R.; Sobolev, A.S. Delivery systems exploiting natural cell transport processes of macromolecules for intracellular targeting of Auger electron emitters. Nucl. Med. Biol. 2020, 80, 45–56. [Google Scholar] [CrossRef]
- Imstepf, S.; Pierroz, V.; Raposinho, P.; Bauwens, M.; Felber, M.; Fox, T.; Shapiro, A.B.; Freudenberg, R.; Fernandes, C.; Gama, S.; et al. Nuclear targeting with an auger electron emitter potentiates the action of a widely used antineoplastic drug. Bioconjugate Chem. 2015, 26, 2397–2407. [Google Scholar] [CrossRef] [PubMed]
- Balagurumoorthy, P.; Xu, X.; Wang, K.; Adelstein, S.J.; Kassis, A.I. Effect of distance between decaying 125I and DNA on Auger-electron induced double-strand break yield. Int. J. Radiat. Biol. 2012, 88, 998–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, E.; Quental, L.; Palma, E.; Oliveira, M.C.; Mendes, F.; Raposinho, P.; Correia, I.; Lavrado, J.; Maria, S.D.; Belchior, A.; et al. Evaluation of acridine orange derivatives as DNA-targeted radiopharmaceuticals for auger therapy: Influence of the radionuclide and distance to DNA. Sci. Rep. 2017, 7, 42544. [Google Scholar] [CrossRef]
- Reissig, F.; Mamat, C.; Steinbach, J.; Pietzsch, H.J.; Freudenberg, R.; Retamal, C.N.; Caballero, J.; Kotzerke, J.; Wunderlich, G. Direct and auger electron-induced, single and double-strand breaks on plasmid DNA caused by 99mTc-labeled pyrene derivatives and the effect of bonding distance. PLoS ONE 2016, 11, e0161973. [Google Scholar] [CrossRef] [PubMed]
- Yasui, L.S.; Chen, K.; Wang, K.; Jones, T.P.; Caldwell, J.; Gusea, D.; Kassis, A.I. Using hoechst 33342 to target radioactivity to the cell nucleus. Radiat. Res. 2007, 167, 167–175. [Google Scholar] [CrossRef]
- Sato, N.; Kobayashi, H.; Saga, T.; Nakamoto, Y.; Ishimori, T.; Togashi, K.; Fujibayashi, Y.; Konishi, J.; Brechbiel, M.W. Tumor targeting and imaging of intraperitoneal tumors by use of antisense oligo-DNA complexed with dendrimers and/or avidin in mice. Clin. Cancer Res. 2001, 7, 3606–3612. [Google Scholar] [PubMed]
- Dahmen, V.; Pomplun, E.; Kriehuber, R. Iodine-125-labeled DNA-Triplex-forming oligonucleotides reveal increased cyto- and genotoxic effectiveness compared to Phosphorus-32. Int. J. Radiat. Biol. 2016, 92, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The next generation of platinum drugs: Targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [Green Version]
- NuDat 2.8. Available online: http://www.nndc.bnl.gov/nudat2/index.jsp (accessed on 1 March 2021).
- Radionuclide Decay Data. Available online: https://hps.org/publicinformation/radardecaydata.cfm (accessed on 1 March 2021).
- Howell, R.W.; Kassis, A.I.; Adelstein, S.J.; Rao, D.V.; Wright, H.A.; Hamm, R.N.; Turner, J.E.; Sastry, K.S.R. Radiotoxicity of 195mPt labeled trans-platinum(II) in mammalian cells. Radiat. Res. 1994, 140, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Azure, M.T.; Sastry, K.S.R.; Archer, R.D.; Howell, R.W.; Rao, D.V. Microscale synthesis of carboplatin labeled with the Auger emitter Pt-193m: Radiotoxicity versus chemotoxicity of the antitumor drug in mammalian cells. In Biophysical Aspects of Auger Processes; American Association of Physicists in Medicine: Alexandria, VA, USA, 1992; pp. 336–351. [Google Scholar]
- Areberg, J.; Johnsson, A.; Wennerberg, J. In Vitro toxicity of 191Pt-labeled cisplatin to a human cervical carcinoma cell line (ME-180). Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 1275–1280. [Google Scholar] [CrossRef]
- Areberg, J.; Wennerberg, J.; Johnsson, A.; Norrgren, K.; Mattsson, S. Antitumor effect of radioactive cisplatin (191Pt) on nude mice. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 827–832. [Google Scholar] [CrossRef]
- Norrgren, K.; Sjölin, M.; Björkman, S.; Areberg, J.; Johnsson, A.; Johansson, L.; Mattsson, S. Comparative renal, hepatic, and bone marrow toxicity of cisplatin and radioactive cisplatin (191Pt) in wistar rats. Cancer Biother. Radiopharm. 2006, 21, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Obata, H.; Minegishi, K.; Nagatsu, K.; Ogawa, M.; Zhang, M.R. Synthesis of no-carrier-added [188, 189, 191Pt]cisplatin from a cyclotron produced 188, 189, 191PtCl42- complex. Sci. Rep. 2021, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Obata, H.; Minegishi, K.; Nagatsu, K.; Zhang, M.R.; Shinohara, A. Production of 191Pt from an iridium target by vertical beam irradiation and simultaneous alkali fusion. Appl. Radiat. Isot. 2019, 149, 31–37. [Google Scholar] [CrossRef]
- Gately, D.P.; Howell, S.B. Cellular accumulation of the anticancer agent cisplatin: A review. Br. J. Cancer 1993, 67, 1171–1176. [Google Scholar] [CrossRef] [Green Version]
- Eljack, N.D.; Ma, H.M.; Drucker, J.; Shen, C.; Hambley, T.W.; New, E.J.; Friedrich, T.; Clarke, R.J. Mechanisms of cell uptake and toxicity of the anticancer drug cisplatin. Metallomics 2014, 6, 2126–2133. [Google Scholar] [CrossRef] [Green Version]
- Gale, G.R.; Morris, C.R.; Atkins, L.M.; Smith, A.B. Binding of an antitumor platinum compound to cells as influenced by physical factors and pharmacologically active agents. Cancer Res. 1973, 33, 813–818. [Google Scholar]
- Ogawa, M.; Gale, G.R.; Keirn, S.S. Effects of cis-diamminedichloroplatinum (NSC 119875) on murine and human hemopoietic precursor cells. Cancer Res. 1975, 35, 1398–1401. [Google Scholar] [PubMed]
- Binks, S.P.; Dobrota, M. Kinetics and mechanism of uptake of platinum-based pharmaceuticals by the rat small intestine. Biochem. Pharmacol. 1990, 40, 1329–1336. [Google Scholar] [CrossRef]
- EI-Kareh, A.W.; Secomb, T.W. A mathematical model for cisplatin cellular pharmacodynamics. Neoplasia 2003, 5, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Ishida, S.; Lee, J.; Thiele, D.J.; Herskowitz, I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc. Natl. Acad. Sci. USA 2002, 99, 14298–14302. [Google Scholar] [CrossRef] [Green Version]
- Howell, S.B.; Safaei, R.; Larson, C.A.; Sailor, M.J. Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol. Pharmacol. 2010, 77, 887–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yonezawa, A.; Masuda, S.; Yokoo, S.; Katsura, T.; Inui, K. Cisplatin and oxaliplatin, but not carboplatin and nedaplatin, are substrates for human organic cation transporters (SLC22A1–3 and multidrug and toxin extrusion family). J. Pharmacol. Exp. Ther. 2006, 319, 879–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groessl, M.; Zava, O.; Dyson, P.J. Cellular uptake and subcellular distribution of ruthenium-based metallodrugs under clinical investigation versus cisplatin. Metallomics 2011, 3, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.A.; Howell, S.B. Cellular pharmacology of cisplatin: Perspectives on mechanisms of acquired resistance. Cancer Cells 1990, 2, 35–43. [Google Scholar] [PubMed]
- Rodríguez, M.C.; García, R.Á.F.; Blanco, E.; Bettmer, J.; Bayón, M.M. Quantitative evaluation of cisplatin uptake in sensitive and resistant individual cells by single-cell ICP-MS (SC-ICP-MS). Anal. Chem. 2017, 89, 11491–11497. [Google Scholar] [CrossRef] [PubMed]
- Amable, L.; Smith, S.; Stephan, C. New Research Evaluating Cisplatin Uptake in Ovarian Cancer Cells by Single Cell ICP-MS; Application Note; ICP-MASS Spectrometry; PerkinElmer, Inc.: Waltham, MA, USA, 2017; Available online: https://www.perkinelmer.com/lab-solutions/resources/docs/APP-NexION-2000-ICP-MS-Single-Cell-Cancer-Research-013176_01.pdf (accessed on 1 March 2021).
- Drewinko, B.; Brown, B.; Gottlieb, J.A. The effect of cis-diamminedichloroplatinum(II) on cultured human lymphoma cells and its therapeutic implications. Cancer Res. 1973, 33, 3091–3095. [Google Scholar] [PubMed]
- Olive, P.L.; Banáth, J.P. Kinetics of H2AX phosphorylation after exposure to cisplatin. Cytom. Part B Clin. Cytom. J. Int. Soc. Anal. Cytol. 2009, 76, 79–90. [Google Scholar] [CrossRef] [PubMed]

| Fraction of 191Pt (% ID) | [191Pt]cisplatin | ||
|---|---|---|---|
| Number Per Cell | Radioactivity (Bq) Per Cell | ||
| Cell | 0.1 * | 175 | 5 × 10−4 |
| Nucleus | 0.02 | 35 | 1 × 10−4 |
| DNA | 0.0004 | 1 | 2 × 10−6 |
| Treatment | Survival (%) * | |
|---|---|---|
| H226 | LN319 | |
| Saline | 100 ± 7 | 100 ± 11 |
| Cisplatin | ||
| 1 ng (17 nM) | 100 ± 9 | 95 ± 14 |
| 1 μg (17 µM) | 63 ± 7 | 48 ± 8 |
| [189, 191Pt]cisplatin | ||
| 189Pt, 58 kBq; 191Pt, 34 kBq | 113 ± 18 | 92 ± 4 |
| 189Pt, 120 kBq; 191Pt, 70 kBq | 91 ± 15 | 87 ± 13 |
| Treatment | Live * | Dead * | D/L Ratio |
|---|---|---|---|
| Saline | 900 | 6.77 | 1.00 |
| Cisplatin | 862 | 6.94 | 1.07 |
| 2.5 ng (11 nM) | |||
| [189, 191Pt]cisplatin | 829 | 7.27 | 1.17 |
| 189Pt, 75 kBq; 191Pt, 50 kBq |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obata, H.; Tsuji, A.B.; Sudo, H.; Sugyo, A.; Minegishi, K.; Nagatsu, K.; Ogawa, M.; Zhang, M.-R. In Vitro Evaluation of No-Carrier-Added Radiolabeled Cisplatin ([189, 191Pt]cisplatin) Emitting Auger Electrons. Int. J. Mol. Sci. 2021, 22, 4622. https://doi.org/10.3390/ijms22094622
Obata H, Tsuji AB, Sudo H, Sugyo A, Minegishi K, Nagatsu K, Ogawa M, Zhang M-R. In Vitro Evaluation of No-Carrier-Added Radiolabeled Cisplatin ([189, 191Pt]cisplatin) Emitting Auger Electrons. International Journal of Molecular Sciences. 2021; 22(9):4622. https://doi.org/10.3390/ijms22094622
Chicago/Turabian StyleObata, Honoka, Atsushi B. Tsuji, Hitomi Sudo, Aya Sugyo, Katsuyuki Minegishi, Kotaro Nagatsu, Mikako Ogawa, and Ming-Rong Zhang. 2021. "In Vitro Evaluation of No-Carrier-Added Radiolabeled Cisplatin ([189, 191Pt]cisplatin) Emitting Auger Electrons" International Journal of Molecular Sciences 22, no. 9: 4622. https://doi.org/10.3390/ijms22094622
APA StyleObata, H., Tsuji, A. B., Sudo, H., Sugyo, A., Minegishi, K., Nagatsu, K., Ogawa, M., & Zhang, M.-R. (2021). In Vitro Evaluation of No-Carrier-Added Radiolabeled Cisplatin ([189, 191Pt]cisplatin) Emitting Auger Electrons. International Journal of Molecular Sciences, 22(9), 4622. https://doi.org/10.3390/ijms22094622








