Biochar with Alternate Wetting and Drying Irrigation: A Potential Technique for Paddy Soil Management
Abstract
1. Introduction
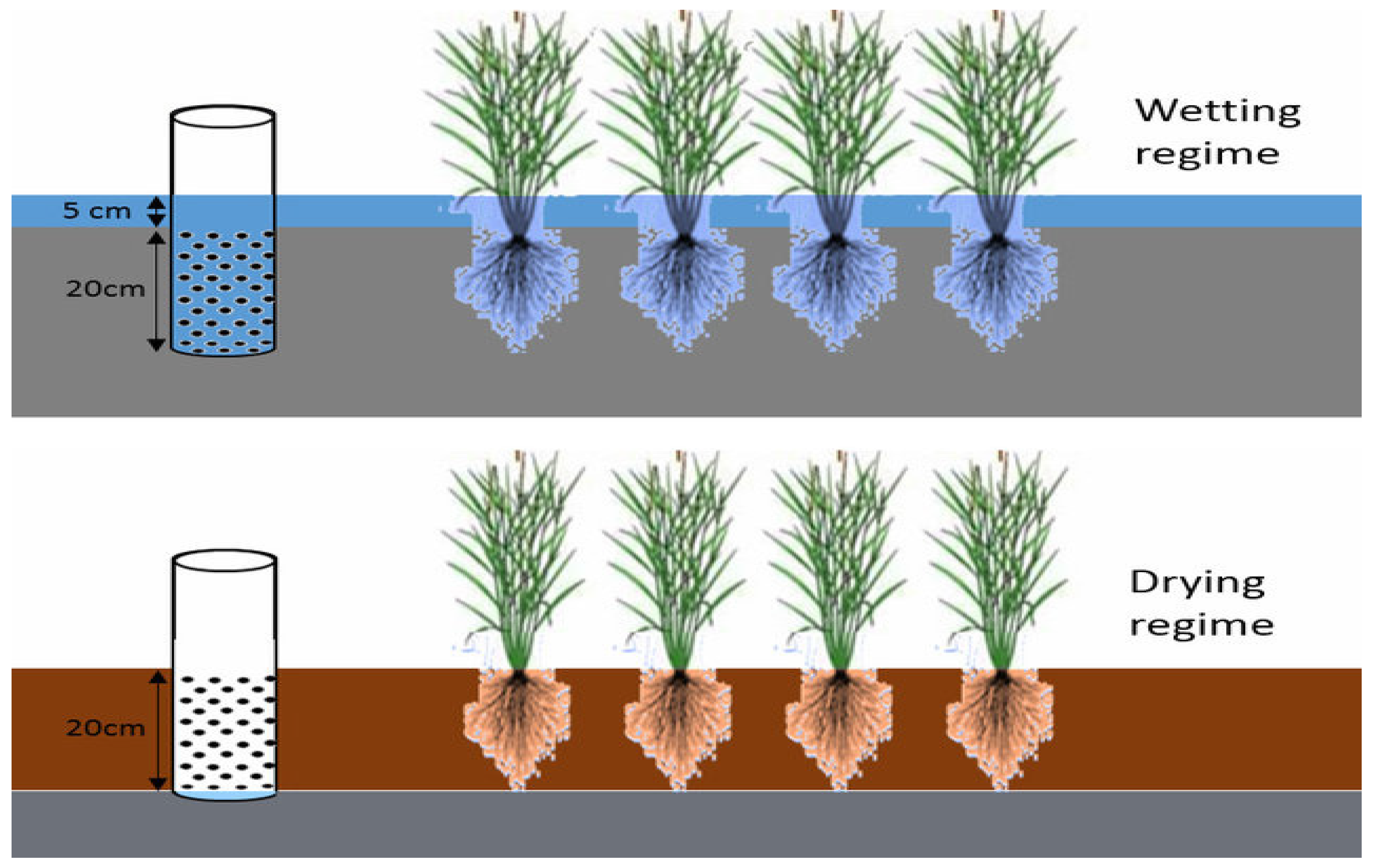
2. Adverse Effect of AWD on Paddy Soil Structure
3. Effect of AWD on Organic Carbon and Nitrogen Depletion
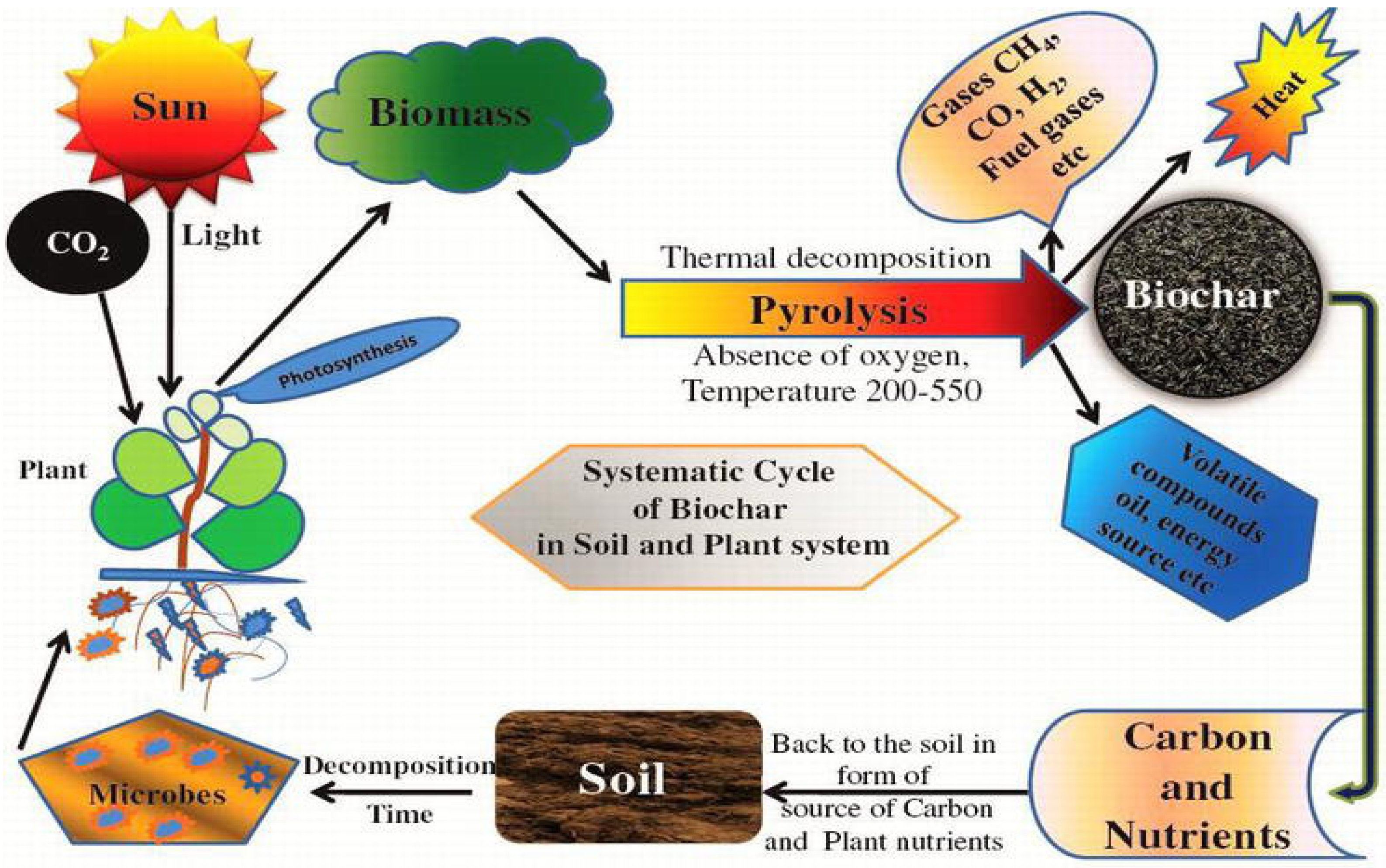
4. Characteristics and Types of Biochar
5. Potential of Biochar to Influence Different Chemical Properties of Soil
5.1. Role of Biochar on Soil Carbon Enhancement
5.2. Biochar Impact on Major Nutrient (N, P, K) Availability in Soil
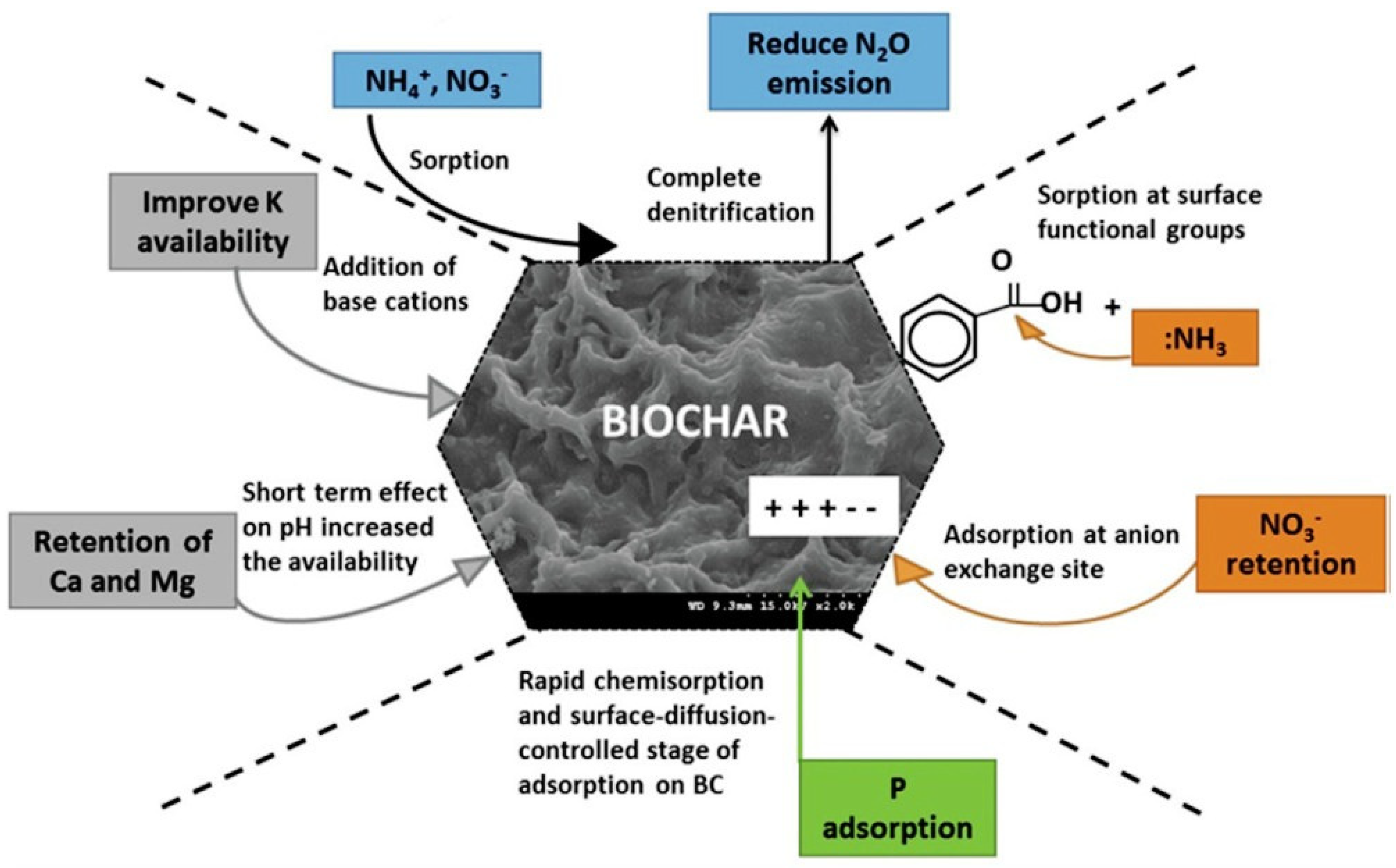
5.3. Capacity of Biochar to Retain Nutrients in Soil
6. Impact of Biochar on Physical and Hydrological Properties of Soil
6.1. Bulk Density
6.2. Soil Porosity
6.3. Soil Aggregate Stability
6.4. Soil Crack Formation
6.5. Soil Water Retention Properties
6.6. Hydraulic Conductivity of the Soil
7. Scope of Future Research
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amanullah, N.; Khan, S.-T.; Iqbal, A.; Fahad, S. Growth and Productivity Response of Hybrid Rice to Application of Animal Manures, Plant Residues and Phosphorus. Front. Plant Sci. 2016, 7, 1440. [Google Scholar] [CrossRef][Green Version]
- FAO. FAO Rice Market Monitor; FAO: Rome, Italy, 2018. [Google Scholar]
- IRRI. Rice Knowledge Bank; International Rice Research Institute: Los Baños, Philippines, 2018. [Google Scholar]
- Bouman, B.A.M.; Hengsdijk, H.; Hardy, B.; Bindraban, P.S.; Tuong, T.P. Water-wise rice production. In International Workshop on Water-Wise Rice Production; International Rice Research Institute (IRRI): Los Baños, Philippines, 2002; p. 356. [Google Scholar]
- Dong, N.M.; Brandt, K.K.; Sørensen, J.; Hung, N.N.; Hach, C.; Van Tan, P.S.; Dalsgaard, T. Effects of alternating wetting and drying versus continuous flooding on fertilizer nitrogen fate in rice fields in the Mekong Delta, Vietnam. Soil Biol. Biochem. 2012, 47, 166–174. [Google Scholar] [CrossRef]
- Wu, X.H.; Wang, W.; Yin, C.M.; Hou, H.J.; Xie, K.J.; Xie, X.L. Water consumption, grain yield, and water productivity in response to field water management in double rice systems in China. PLoS ONE 2017, 12, e0189280. [Google Scholar] [CrossRef] [PubMed]
- Lampayan, R.M.; Rejesus, R.M.; Singleton, G.R.; Bouman, B.A.M. Adoption and economics of alternate wetting and drying water management for irrigated lowland rice. Field Crop. Res. 2015, 170, 95–108. [Google Scholar] [CrossRef]
- Zhang, H.; Voroney, R.P.; Price, G.W. Effects of temperature and processing conditions on biochar chemical properties and their influence on soil C and N transformations. Soil Biol. Biochem. 2015, 83, 19–28. [Google Scholar] [CrossRef]
- Yao, F.; Huang, J.; Cui, K.; Nie, L.; Xiang, J.; Liu, X.; Wu, W.; Chen, M.; Peng, S. Agronomic performance of high-yielding rice variety grown under alternate wetting and drying irrigation. Field Crop. Res. 2012, 126, 16–22. [Google Scholar] [CrossRef]
- Khairi, M.N.; Mohd, M.S.J. Effects of Flooding and Alternate Wetting and Drying on the Yield Performance of Upland Rice. Pertanika Trop. Agric. Sci. 2016, 39, 299–309. [Google Scholar]
- Xu, Y.; Ge, J.; Tian, S.; Li, S.; Nguy-Robertson, A.L.; Zhan, M.; Cao, C. Effects of water-saving irrigation practices and drought resistant rice variety on greenhouse gas emissions from a no-till paddy in the central lowlands of China. Sci. Total Environ. 2015, 505, 1043–1052. [Google Scholar] [CrossRef]
- Riaz, A.; Khaliq, A.; Fiaz, S.; Noor, M.A.; Nawaz, M.M.; Mahboob, W.; Ullah, S. Weed Management in Direct Seeded Rice Grown under Varying Tillage Systems and Alternate Water Regimes. Planta Daninha 2018, 36, 59. [Google Scholar] [CrossRef]
- Carrijo, D.R.; Lundy, M.E.; Linquist, B.A. Rice yields and water use under alternate wetting and drying irrigation: A meta-analysis. Field Crop. Res. 2017, 203, 173–180. [Google Scholar] [CrossRef]
- Tan, Z.; Lin, C.S.K.; Ji, X.; Rainey, T.J. Returning biochar to fields: A review. Appl. Soil Ecol. 2017, 116, 1–11. [Google Scholar] [CrossRef]
- Garg, K.K.; Das, B.S.; Safeeq, M.; Bhadoria, P.B.S. Measurement and modeling of soil water regime in a lowland paddy field showing preferential transport. Agric. Water Manag. 2009, 96, 1705–1714. [Google Scholar] [CrossRef]
- Gordon, H.; Haygarth, P.M.; Bardgett, R.D. Drying and rewetting effects on soil microbial community composition and nutrient leaching. Soil Biol. Biochem. 2008, 40, 302–311. [Google Scholar] [CrossRef]
- Belder, P.; Spiertz, J.H.J.; Bouman, B.A.M.; Lu, G.; Tuong, T.P. Nitrogen economy and water productivity of lowland rice under water-saving irrigation. Field Crop. Res. 2005, 93, 169–185. [Google Scholar] [CrossRef]
- Abbasi, M.K.; Afsar, N.; Rahim, N. Effect of Wood Ash and Compost Application on Nitrogen Transformations and Availability in Soil-Plant Systems. Soil Sci. Soc. Am. J. 2013, 77, 558–567. [Google Scholar] [CrossRef]
- Johannes, L.S.J. Biochar for Environmental Management: Science, Technology and Implementation; Lehmann, S.J.J., Ed.; Taylor & Francis: London, UK, 2015; ISBN 9780203762264. [Google Scholar]
- Jien, S.-H.; Wang, C.-S. Effects of biochar on soil properties and erosion potential in a highly weathered soil. Catena 2013, 110, 225–233. [Google Scholar] [CrossRef]
- Obia, A.; Mulder, J.; Martinsen, V.; Cornelissen, G.; Børresen, T. In situ effects of biochar on aggregation, water retention and porosity in light-textured tropical soils. Soil Tillage Res. 2016, 155, 35–44. [Google Scholar] [CrossRef]
- Bakar, R.A.; Razak, Z.A.; Ahmad, S.H.; Seh-Bardan, B.J.; Tsong, L.C.; Meng, C.P. Influence of Oil Palm Empty Fruit Bunch Biochar on Floodwater pH and Yield Components of Rice Cultivated on Acid Sulphate Soil under Rice Intensification Practices. Plant Prod. Sci. 2015, 18, 491–500. [Google Scholar] [CrossRef]
- Laghari, M.; Mirjat, M.S.; Hu, Z.; Fazal, S.; Xiao, B.; Hu, M.; Chen, Z.; Guo, D. Effects of biochar application rate on sandy desert soil properties and sorghum growth. Catena 2015, 135, 313–320. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Shafi, M.I.; Beamont, E.; Anawar, H.M. Poultry Litter Biochar Increases Mycorrhizal Colonisation, Soil Fertility and Cucumber Yield in a Fertigation System on Sandy Soil. Agriculture 2020, 10, 480. [Google Scholar] [CrossRef]
- Mosharrof, M.; Uddin, M.K.; Jusop, S.; Sulaiman, M.F.; Shamsuzzaman, S.M.; Haque, A.N.A. Changes in Acidic Soil Chemical Properties and Carbon Dioxide Emission Due to Biochar and Lime Treatments. Agriculture 2021, 11, 219. [Google Scholar] [CrossRef]
- Major, J.; Rondon, M.; Molina, D.; Riha, S.J.; Lehmann, J. Nutrient Leaching in a Colombian Savanna Oxisol Amended with Biochar. J. Environ. Qual. 2012, 41, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Lu, T.; Wang, Y.; Chen, Y.; Lei, T. Sewage sludge biochar: Nutrient composition and its effect on the leaching of soil nutrients. Geoderma 2016, 267, 17–23. [Google Scholar] [CrossRef]
- Głąb, T.; Palmowska, J.; Zaleski, T.; Gondek, K. Effect of biochar application on soil hydrological properties and physical quality of sandy soil. Geoderma 2016, 281, 11–20. [Google Scholar] [CrossRef]
- Gamage, D.N.V.; Mapa, R.B.; Dharmakeerthi, R.S.; Biswas, A. Effect of rice-husk biochar on selected soil properties in tropical Alfisols. Soil Res. 2016, 54, 302. [Google Scholar] [CrossRef]
- Wong, J.T.F.; Chen, Z.; Wong, A.Y.Y.; Ng, C.W.W.; Wong, M.H. Effects of biochar on hydraulic conductivity of compacted kaolin clay. Environ. Pollut. 2018, 234, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, Z.; Singh, B.P.; Wang, H. The impact of crop residue biochars on silicon and nutrient cycles in croplands. Sci. Total Environ. 2019, 659, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Delvaux, B. Phytolith-rich biochar: A potential Si fertilizer in desilicated soils. GCB Bioenergy 2019, 11, 1264–1282. [Google Scholar] [CrossRef]
- Hamoud, Y.A.; Guo, X.; Wang, Z.; Chen, S.; Rasool, G. Effects of irrigation water regime, soil clay content and their combination on growth, yield, and water use efficiency of rice grown in South China. Int. J. Agric. Biol. Eng. 2018, 11, 126–136. [Google Scholar] [CrossRef]
- Staff, S.S. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys; USDA-NRCS: Washington, DC, USA, 1999. [Google Scholar]
- Dinka, T.M.; Lascano, R.J. Review Paper: Challenges and Limitations in Studying the Shrink-Swell and Crack Dynamics of Vertisol Soils. Open J. Soil Sci. 2012, 2, 82–90. [Google Scholar] [CrossRef]
- Bouman, B.A.; Tuong, T. Field water management to save water and increase its productivity in irrigated lowland rice. Agric. Water Manag. 2001, 49, 11–30. [Google Scholar] [CrossRef]
- Bottinelli, N.; Zhou, H.; Boivin, P.; Zhang, Z.B.; Jouquet, P.; Hartmann, C.; Peng, X. Macropores generated during shrinkage in two paddy soils using X-ray micro-computed tomography. Geoderma 2016, 265, 78–86. [Google Scholar] [CrossRef]
- Tan, X.; Shao, D.; Liu, H.; Yang, F.; Xiao, C.; Yang, H. Effects of alternate wetting and drying irrigation on percolation and nitrogen leaching in paddy fields. Paddy Water Environ. 2013, 11, 381–395. [Google Scholar] [CrossRef]
- Stewart, R.D.; Najm, M.R.A.; Rupp, D.E.; Lane, J.W.; Uribe, H.C.; Arumí, J.L.; Selker, J.S. Hillslope run-off thresholds with shrink-swell clay soils. Hydrol. Process. 2015, 29, 557–571. [Google Scholar] [CrossRef]
- Arnold, J.G.; Potter, K.N.; King, K.W.; Allen, P.M. Estimation of soil cracking and the effect on surface runoff in a Texas Blackland Prairie watershed. Hydrol. Process. 2005, 19, 589–603. [Google Scholar] [CrossRef]
- Ritchie, J.T.; Adams, J.E. Field Measurement of Evaporation from Soil Shrinkage Cracks. Soil Sci. Soc. Am. J. 1974, 38, 131–134. [Google Scholar] [CrossRef]
- Hamoud, Y.A.; Wang, Z.; Guo, X.; Shaghaleh, H.; Sheteiwy, M.; Chen, S.; Qiu, R.; Elbashier, M. Effect of Irrigation Regimes and Soil Texture on the Potassium Utilization Efficiency of Rice. Agronomy 2019, 9, 100. [Google Scholar] [CrossRef]
- Buresh, R.J.; Haefele, S.M. Changes in paddy soils under transition to water-saving and diversified cropping systems. In Proceedings of the 19th World Congress of Soil Science: Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010; pp. 9–12. [Google Scholar]
- Livsey, J.; Kätterer, T.; Vico, G.; Lyon, S.W.; Lindborg, R.; Scaini, A.; Da, C.T.; Manzoni, S. Do alternative irrigation strategies for rice cultivation decrease water footprints at the cost of long-term soil health? Environ. Res. Lett. 2019, 14, 074011. [Google Scholar] [CrossRef]
- Bouman, B.A.M.; Lampayan, R.M. Water Management in Irrigated Rice, Coping with Water Scarcity; Hardy, B., Ed.; International Rice Research Institute (IRRI): Los Baños, Philippines, 2007. [Google Scholar]
- Oliver, V.; Cochrane, N.; Magnusson, J.; Brachi, E.; Monaco, S.; Volante, A.; Courtois, B.; Vale, G.; Price, A.; Teh, Y.A. Effects of water management and cultivar on carbon dynamics, plant productivity and biomass allocation in European rice systems. Sci. Total Environ. 2019, 685, 1139–1151. [Google Scholar] [CrossRef]
- Alberto, M.C.R.; Wassmann, R.; Buresh, R.J.; Quilty, J.R.; Correa, T.Q.; Sandro, J.M.; Centeno, C.A.R. Measuring methane flux from irrigated rice fields by eddy covariance method using open-path gas analyzer. Field Crop. Res. 2014, 160, 12–21. [Google Scholar] [CrossRef]
- Moyano, F.E.; Manzoni, S.; Chenu, C. Responses of soil heterotrophic respiration to moisture availability: An exploration of processes and models. Soil Biol. Biochem. 2013, 59, 72–85. [Google Scholar] [CrossRef]
- Chang, R.; Jin, T.; Lü, Y.; Liu, G.; Fu, B. Soil Carbon and Nitrogen Changes following Afforestation of Marginal Cropland across a Precipitation Gradient in Loess Plateau of China. PLoS ONE 2014, 9, e85426. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Peng, S.; Xu, J.; He, Y.; Wang, Y. Effects of water saving irrigation and controlled release nitrogen fertilizer managements on nitrogen losses from paddy fields. Paddy Water Environ. 2015, 13, 71–80. [Google Scholar] [CrossRef]
- Pandey, A.; Mai, V.T.; Vu, D.Q.; Bui, T.P.L.; Mai, T.L.A.; Jensen, L.S.; de Neergaard, A. Organic matter and water management strategies to reduce methane and nitrous oxide emissions from rice paddies in Vietnam. Agric. Ecosyst. Environ. 2014, 196, 137–146. [Google Scholar] [CrossRef]
- Hoang, T.T.H.; Do, D.T.; Tran, T.T.G.; Ho, T.D.; Rehman, H.u. Incorporation of rice straw mitigates CH 4 and N 2 O emissions in water saving paddy fields of Central Vietnam. Arch. Agron. Soil Sci. 2019, 65, 113–124. [Google Scholar] [CrossRef]
- Barton, L.; Colmer, T.D. Irrigation and fertiliser strategies for minimising nitrogen leaching from turfgrass. Agric. Water Manag. 2006, 80, 160–175. [Google Scholar] [CrossRef]
- Sepaskhah, A.R.; Barzegar, M. Yield, water and nitrogen-use response of rice to zeolite and nitrogen fertilization in a semi-arid environment. Agric. Water Manag. 2010, 98, 38–44. [Google Scholar] [CrossRef]
- He, Y.; Lehndorff, E.; Amelung, W.; Wassmann, R.; Alberto, M.C.; von Unold, G.; Siemens, J. Drainage and leaching losses of nitrogen and dissolved organic carbon after introducing maize into a continuous paddy-rice crop rotation. Agric. Ecosyst. Environ. 2017, 249, 91–100. [Google Scholar] [CrossRef]
- Kaiser, K.; Kalbitz, K. Cycling downwards–dissolved organic matter in soils. Soil Biol. Biochem. 2012, 52, 29–32. [Google Scholar] [CrossRef]
- Bolan, N.S.; Adriano, D.C.; Kunhikrishnan, A.; James, T.; McDowell, R.; Senesi, N. Dissolved Organic Matter. Geoderma 2003, 113, 187–209. [Google Scholar]
- Siemens, J.; Kaupenjohann, M. Contribution of dissolved organic nitrogen to N leaching from four German agricultural soils. J. Plant Nutr. Soil Sci. 2002, 165, 675–681. [Google Scholar] [CrossRef]
- Jahangir, M.M.R.; Khalil, M.I.; Johnston, P.; Cardenas, L.M.; Hatch, D.J.; Butler, M.; Barrett, M.; O’flaherty, V.; Richards, K.G. Denitrification potential in subsoils: A mechanism to reduce nitrate leaching to groundwater. Agric. Ecosyst. Environ. 2012, 147, 13–23. [Google Scholar] [CrossRef]
- Nartey, O.D.; Zhao, B. Biochar Preparation, Characterization, and Adsorptive Capacity and Its Effect on Bioavailability of Contaminants: An Overview. Adv. Mater. Sci. Eng. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Noack, A.G. Black carbon in soils and sediments: Analysis, distribution, implications, and current challenges. Global Biogeochem. Cycles 2000, 14, 777–793. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Lehmann, J.; Hockaday, W.C.; Joseph, S.; Masiello, C.A. Temperature Sensitivity of Black Carbon Decomposition and Oxidation. Environ. Sci. Technol. 2010, 44, 3324–3331. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G.; Morgan, T.J. An overview of the organic and inorganic phase composition of biomass. Fuel 2012, 94, 1–33. [Google Scholar] [CrossRef]
- Rawat, J.; Saxena, J.; Sanwal, P. Biochar: A Sustainable Approach for Improving Plant Growth and Soil Properties. In Biochar-an Imperative Amendment for Soil and the Environment; IntechOpen: London, UK, 2019. [Google Scholar]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biol. Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Jatav, H.S.; Singh, S.K.; Jatav, S.S.; Rajput, V.D.; Parihar, M.; Mahawer, S.K.; Singhal, R.K. Sukirtee Importance of Biochar in Agriculture and Its Consequence. In Applications of Biochar for Environmental Safety; Abbas, A.A.A., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Shakya, A.; Agarwal, T. Poultry Litter Biochar: An Approach towards Poultry Litter Management–A Review. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2657–2668. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.-M.; Dallmeyer, I.; Garcia-Perez, M. Influence of feedstock source and pyrolysis temperature on biochar bulk and surface properties. Biomass Bioenergy 2016, 84, 37–48. [Google Scholar] [CrossRef]
- Lv, D.; Xu, M.; Liu, X.; Zhan, Z.; Li, Z.; Yao, H. Effect of cellulose, lignin, alkali and alkaline earth metallic species on biomass pyrolysis and gasification. Fuel Process. Technol. 2010, 91, 903–909. [Google Scholar] [CrossRef]
- Nanda, S.; Mohanty, P.; Pant, K.K.; Naik, S.; Kozinski, J.A.; Dalai, A.K. Characterization of North American Lignocellulosic Biomass and Biochars in Terms of their Candidacy for Alternate Renewable Fuels. BioEnergy Res. 2013, 6, 663–677. [Google Scholar] [CrossRef]
- Li, M.; Liu, M.; Li, Z.; Jiang, C.; Wu, M. Soil N transformation and microbial community structure as affected by adding biochar to a paddy soil of subtropical China. J. Integr. Agric. 2016, 15, 209–219. [Google Scholar] [CrossRef]
- Wander, M.M.; Traina, S.J.; Stinner, B.R.; Peters, S.E. Organic and Conventional Management Effects on Biologically Active Soil Organic Matter Pools. Soil Sci. Soc. Am. J. 1994, 58, 1130–1139. [Google Scholar] [CrossRef]
- Yang, S.; Chen, X.; Jiang, Z.; Ding, J.; Sun, X.; Xu, J. Effects of Biochar Application on Soil Organic Carbon Composition and Enzyme Activity in Paddy Soil under Water-Saving Irrigation. Int. J. Environ. Res. Public Health 2020, 17, 333. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, L.B.; Johnson, B.G. Interacting effects of temperature, soil moisture and plant biomass production on ecosystem respiration in a northern temperate grassland. Agric. For. Meteorol. 2005, 130, 237–253. [Google Scholar] [CrossRef]
- Borken, W.; Matzner, E. Reappraisal of drying and wetting effects on C and N mineralization and fluxes in soils. Glob. Chang. Biol. 2009, 15, 808–824. [Google Scholar] [CrossRef]
- Butterly, C.R.; McNeill, A.M.; Baldock, J.A.; Marschner, P. Rapid changes in carbon and phosphorus after rewetting of dry soil. Biol. Fertil. Soils 2011, 47, 41–50. [Google Scholar] [CrossRef]
- El-Naggar, A.; El-Naggar, A.H.; Shaheen, S.M.; Sarkar, B.; Chang, S.X.; Tsang, D.C.W.; Rinklebe, J.; Ok, Y.S. Biochar composition-dependent impacts on soil nutrient release, carbon mineralization, and potential environmental risk: A review. J. Environ. Manag. 2019, 241, 458–467. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Anawar, H.M. Application of Biochars for Soil Constraints: Challenges and Solutions. Pedosphere 2015, 25, 631–638. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Awad, Y.M.; Yang, X.; Ryu, C.; Rizwan, M.; Rinklebe, J.; Tsang, D.C.W.; Ok, Y.S. Influence of soil properties and feedstocks on biochar potential for carbon mineralization and improvement of infertile soils. Geoderma 2018, 332, 100–108. [Google Scholar] [CrossRef]
- Laird, D.A.; Fleming, P.; Davis, D.D.; Horton, R.; Wang, B.; Karlen, D.L. Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 2010, 158, 443–449. [Google Scholar] [CrossRef]
- Durenkamp, M.; Luo, Y.; Brookes, P.C. Impact of black carbon addition to soil on the determination of soil microbial biomass by fumigation extraction. Soil Biol. Biochem. 2010, 42, 2026–2029. [Google Scholar] [CrossRef]
- El-Naggar, A.H.; Usman, A.R.A.; Al-Omran, A.; Ok, Y.S.; Ahmad, M.; Al-Wabel, M.I. Carbon mineralization and nutrient availability in calcareous sandy soils amended with woody waste biochar. Chemosphere 2015, 138, 67–73. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, M.; Nawaz, A.; Al-Sadi, A.M.; Solaiman, Z.M.; Alghamdi, S.S.; Ammara, U.; Ok, Y.S.; Siddique, K.H.M. Biochar for crop production: Potential benefits and risks. J. Soils Sediments 2017, 17, 685–716. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Lehmann, J.; Kinyangi, J.; Smernik, R.; Riha, S.J.; Engelhard, M.H. Long-term black carbon dynamics in cultivated soil. Biogeochemistry 2008, 89, 295–308. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Sohi, S.; Thies, J.E.; Skjemstad, J.O.; Luizão, F.J.; Engelhard, M.H.; Neves, E.G.; Wirick, S. Stability of biomass-derived black carbon in soils. Geochim. Cosmochim. Acta 2008, 72, 6069–6078. [Google Scholar] [CrossRef]
- Zhang, A.; Bian, R.; Pan, G.; Cui, L.; Hussain, Q.; Li, L.; Zheng, J.; Zheng, J.; Zhang, X.; Han, X.; et al. Effects of biochar amendment on soil quality, crop yield and greenhouse gas emission in a Chinese rice paddy: A field study of 2 consecutive rice growing cycles. Field Crop. Res. 2012, 127, 153–160. [Google Scholar] [CrossRef]
- Dong, D.; Feng, Q.; McGrouther, K.; Yang, M.; Wang, H.; Wu, W. Effects of biochar amendment on rice growth and nitrogen retention in a waterlogged paddy field. J. Soils Sediments 2015, 15, 153–162. [Google Scholar] [CrossRef]
- Bian, R.; Zhang, A.; Li, L.; Pan, G.; Zheng, J.; Zhang, X.; Zheng, J.; Joseph, S.; Chang, A. Effect of Municipal Biowaste Biochar on Greenhouse Gas Emissions and Metal Bioaccumulation in a Slightly Acidic Clay Rice Paddy. BioResources 2013, 9, 685–703. [Google Scholar] [CrossRef]
- Haefele, S.M.; Konboon, Y.; Wongboon, W.; Amarante, S.; Maarifat, A.A.; Pfeiffer, E.M.; Knoblauch, C. Effects and fate of biochar from rice residues in rice-based systems. Field Crop. Res. 2011, 121, 430–440. [Google Scholar] [CrossRef]
- Zhang, A.; Cui, L.; Pan, G.; Li, L.; Hussain, Q.; Zhang, X.; Zheng, J.; Crowley, D. Effect of biochar amendment on yield and methane and nitrous oxide emissions from a rice paddy from Tai Lake plain, China. Agric. Ecosyst. Environ. 2010, 139, 469–475. [Google Scholar] [CrossRef]
- Wei, C.; Gao, W.; Whalley, W.R.; Li, B. Shrinkage Characteristics of Lime Concretion Black Soil as Affected by Biochar Amendment. Pedosphere 2018, 28, 713–725. [Google Scholar] [CrossRef]
- Song, Y.; Zou, Y.; Wang, G.; Yu, X. Stimulation of nitrogen turnover due to nutrients release from aggregates affected by freeze-thaw in wetland soils. Phys. Chem. Earth Parts A 2017, 97, 3–11. [Google Scholar] [CrossRef]
- Liu, Y.; Lonappan, L.; Brar, S.K.; Yang, S. Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: A review. Sci. Total Environ. 2018, 645, 60–70. [Google Scholar] [CrossRef]
- Gao, S.; DeLuca, T.H. Influence of Biochar on Soil Nutrient Transformations, Nutrient Leaching, and Crop Yield. Adv. Plants Agric. Res. 2016, 4, 4. [Google Scholar] [CrossRef]
- Streubel, J.D.; Collins, H.P.; Garcia-Perez, M.; Tarara, J.; Granatstein, D.; Kruger, C.E. Influence of Contrasting Biochar Types on Five Soils at Increasing Rates of Application. Soil Sci. Soc. Am. J. 2011, 75, 1402–1413. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.-Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Nelissen, V.; Rütting, T.; Huygens, D.; Staelens, J.; Ruysschaert, G.; Boeckx, P. Maize biochars accelerate short-term soil nitrogen dynamics in a loamy sand soil. Soil Biol. Biochem. 2012, 55, 20–27. [Google Scholar] [CrossRef]
- Case, S.D.C.; McNamara, N.P.; Reay, D.S.; Stott, A.W.; Grant, H.K.; Whitaker, J. Biochar suppresses N2O emissions while maintaining N availability in a sandy loam soil. Soil Biol. Biochem. 2015, 81, 178–185. [Google Scholar] [CrossRef]
- Basso, A.S.; Miguez, F.E.; Laird, D.A.; Horton, R.; Westgate, M. Assessing potential of biochar for increasing water-holding capacity of sandy soils. GCB Bioenergy 2013, 5, 132–143. [Google Scholar] [CrossRef]
- Cayuela, M.L.; van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- DeLuca, T.H.; MacKenzie, M.D.; Gundale, M.J.; Holben, W.E. Wildfire-Produced Charcoal Directly Influences Nitrogen Cycling in Ponderosa Pine Forests. Soil Sci. Soc. Am. J. 2006, 70, 448–453. [Google Scholar] [CrossRef]
- Ball, P.N.; MacKenzie, M.D.; DeLuca, T.H.; Montana, W.E.H. Wildfire and Charcoal Enhance Nitrification and Ammonium-Oxidizing Bacterial Abundance in Dry Montane Forest Soils. J. Environ. Qual. 2010, 39, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, K.; Miyamoto, T.; Shiono, T.; Shinogi, Y. Influence of Sugarcane Bagasse-derived Biochar Application on Nitrate Leaching in Calcaric Dark Red Soil. J. Environ. Qual. 2012, 41, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Xu, Y.; Liu, G.; Liu, Q.; Zhu, J.; Tu, C.; Amonette, J.E.; Cadisch, G.; Yong, J.W.H.; Hu, S. Impact of biochar application on nitrogen nutrition of rice, greenhouse-gas emissions and soil organic carbon dynamics in two paddy soils of China. Plant Soil 2013, 370, 527–540. [Google Scholar] [CrossRef]
- Knicker, H. “Black nitrogen”–an important fraction in determining the recalcitrance of charcoal. Org. Geochem. 2010, 41, 947–950. [Google Scholar] [CrossRef]
- Steiner, C.; Glaser, B.; Teixeira, W.G.; Lehmann, J.; Blum, W.E.H.; Zech, W. Nitrogen retention and plant uptake on a highly weathered central Amazonian Ferralsol amended with compost and charcoal. J. Plant Nutr. Soil Sci. 2008, 171, 893–899. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M. Biochar and the Nitrogen Cycle: Introduction. J. Environ. Qual. 2010, 39, 1218–1223. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Spokas, K.A.; Novak, J.M.; Lentz, R.D.; Cantrell, K.B. Biochar elemental composition and factors influencing nutrient retention. In Biochar for Envrionmental Management: Science, Technolody and Implementation; Lehmann, J., Joseph, S., Eds.; Routledge: London, UK, 2015; pp. 137–161. [Google Scholar]
- Soinne, H.; Hovi, J.; Tammeorg, P.; Turtola, E. Effect of biochar on phosphorus sorption and clay soil aggregate stability. Geoderma 2014, 219–220, 162–167. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, L.; Gao, B.; Xu, X.; Cao, X. The Interfacial Behavior between Biochar and Soil Minerals and Its Effect on Biochar Stability. Environ. Sci. Technol. 2016, 50, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Suksabye, P.; Pimthong, A.; Dhurakit, P.; Mekvichitsaeng, P.; Thiravetyan, P. Effect of biochars and microorganisms on cadmium accumulation in rice grains grown in Cd-contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar induced soil microbial community change: Implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Jośko, I.; Kuśmierz, M.; Futa, B.; Wielgosz, E.; Ligęza, S.; Pranagal, J. Microbiological, biochemical and ecotoxicological evaluation of soils in the area of biochar production in relation to polycyclic aromatic hydrocarbon content. Geoderma 2014, 213, 502–511. [Google Scholar] [CrossRef]
- Bhaduri, D.; Saha, A.; Desai, D.; Meena, H.N. Restoration of carbon and microbial activity in salt-induced soil by application of peanut shell biochar during short-term incubation study. Chemosphere 2016, 148, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, P.; Joseph, S.; Munroe, P.; Anawar, H.M.; Storer, P.; Gilkes, R.J.; Solaiman, Z.M. Influences of Biochar and Biochar-Mineral Complex on Mycorrhizal Colonisation and Nutrition of Wheat and Sorghum. Pedosphere 2015, 25, 686–695. [Google Scholar] [CrossRef]
- Karim, A.A.; Kumar, M.; Singh, S.K.; Panda, C.R.; Mishra, B.K. Potassium enriched biochar production by thermal plasma processing of banana peduncle for soil application. J. Anal. Appl. Pyrolysis 2017, 123, 165–172. [Google Scholar] [CrossRef]
- Martinsen, V.; Mulder, J.; Shitumbanuma, V.; Sparrevik, M.; Børresen, T.; Cornelissen, G. Farmer-led maize biochar trials: Effect on crop yield and soil nutrients under conservation farming. J. Plant Nutr. Soil Sci. 2014, 177, 681–695. [Google Scholar] [CrossRef]
- Wang, L.; Xue, C.; Nie, X.; Liu, Y.; Chen, F. Effects of biochar application on soil potassium dynamics and crop uptake. J. Plant Nutr. Soil Sci. 2018, 181, 635–643. [Google Scholar] [CrossRef]
- Liu, S.; Tang, W.; Yang, F.; Meng, J.; Chen, W.; Li, X. Influence of biochar application on potassium-solubilizing Bacillus mucilaginosus as potential biofertilizer. Prep. Biochem. Biotechnol. 2017, 47, 32–37. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, R.; Liu, R. Characterization of biochar from fast pyrolysis and its effect on chemical properties of the tea garden soil. J. Anal. Appl. Pyrolysis 2014, 110, 375–381. [Google Scholar] [CrossRef]
- Chen, L.; Liu, M.; Ali, A.; Zhou, Q.; Zhan, S.; Chen, Y.; Pan, X.; Zeng, Y. Effects of Biochar on Paddy Soil Fertility Under Different Water Management Modes. J. Soil Sci. Plant Nutr. 2020, 20, 1810–1818. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Khan, S.; Chao, C.; Waqas, M.; Arp, H.P.H.; Zhu, Y.-G. Sewage Sludge Biochar Influence upon Rice (Oryza sativa L) Yield, Metal Bioaccumulation and Greenhouse Gas Emissions from Acidic Paddy Soil. Environ. Sci. Technol. 2013, 47, 8624–8632. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Lu, S. Biochars improve aggregate stability, water retention, and pore-space properties of clayey soil. J. Plant Nutr. Soil Sci. 2014, 177, 26–33. [Google Scholar] [CrossRef]
- Madiba, O.F.; Solaiman, Z.M.; Carson, J.K.; Murphy, D.V. Biochar increases availability and uptake of phosphorus to wheat under leaching conditions. Biol. Fertil. Soils 2016, 52, 439–446. [Google Scholar] [CrossRef]
- Abrishamkesh, S.; Gorji, M.; Asadi, H.; Gh, B.-M.; Aa, P. Effects of rice husk biochar application on the properties of alkaline soil and lentil growth. Plant Soil Environ. 2016, 61, 475–482. [Google Scholar] [CrossRef]
- Gao, T.; Gao, M.; Peng, J.; Li, N. Effects of Different Amount of Biochar on Nitrogen, Phosphorus and Potassium Nutrients in Soil. Ser. Mater. Sci. Eng. 2018, 394, 022043. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.-K.; Yang, J.E.; Ok, Y.S. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef]
- Spokas, K.A.; Novak, J.M.; Venterea, R.T. Biochar’s role as an alternative N-fertilizer: Ammonia capture. Plant Soil 2012, 350, 35–42. [Google Scholar] [CrossRef]
- Hale, S.E.; Alling, V.; Martinsen, V.; Mulder, J.; Breedveld, G.D.; Cornelissen, G. The sorption and desorption of phosphate-P, ammonium-N and nitrate-N in cacao shell and corn cob biochars. Chemosphere 2013, 91, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Takaya, C.A.; Fletcher, L.A.; Singh, S.; Anyikude, K.U.; Ross, A.B. Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 2016, 145, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Rogovska, N.; Laird, D.A.; Karlen, D.L. Corn and soil response to biochar application and stover harvest. FieldCrop. Res. 2016, 187, 96–106. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Jaafar, N.M.; Clode, P.L.; Abbott, L.K. Biochar-Soil Interactions in Four Agricultural Soils. Pedosphere 2015, 25, 729–736. [Google Scholar] [CrossRef]
- Purakayastha, T.J.; Bera, T.; Bhaduri, D.; Sarkar, B.; Mandal, S.; Wade, P.; Kumari, S.; Biswas, S.; Menon, M.; Pathak, H.; et al. A review on biochar modulated soil condition improvements and nutrient dynamics concerning crop yields: Pathways to climate change mitigation and global food security. Chemosphere 2019, 227, 345–365. [Google Scholar] [CrossRef]
- Peng, X.; Ye, L.L.; Wang, C.H.; Zhou, H.; Sun, B. Temperature- and duration-dependent rice straw-derived biochar: Characteristics and its effects on soil properties of an Ultisol in southern China. Soil Tillage Res. 2011, 112, 159–166. [Google Scholar] [CrossRef]
- Pereira, E.I.P.; Suddick, E.C.; Mansour, I.; Mukome, F.N.D.; Parikh, S.J.; Scow, K.; Six, J. Biochar alters nitrogen transformations but has minimal effects on nitrous oxide emissions in an organically managed lettuce mesocosm. Biol. Fertil. Soils 2015, 51, 573–582. [Google Scholar] [CrossRef]
- McKenzie, B.M.; Tisdall, J.M.; Vance, W.H. Soil Physical Quality BT-Encyclopedia of Agrophysics. In Encyclopedia of Agrophysics; Gliński, J., Horabik, J., Lipiec, J., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 770–777. ISBN 978-90-481-3585-1. [Google Scholar]
- Verheijen, F.; Jeffery, S.; Bastos, A.C.; Van Der Velde, M.; Diafas, I. Biochar Application to Soils: A Critical Scientific Review of Effects on Soil Properties, Processes and Functions; European Comission: Luxembourg, 2010; Volume 8. [Google Scholar]
- Alghamdi, A.G. Biochar as a potential soil additive for improving soil physical properties—a review. Arab. J. Geosci. 2018, 11, 766. [Google Scholar] [CrossRef]
- Castellini, M.; Giglio, L.; Niedda, M.; Palumbo, A.D.; Ventrella, D. Impact of biochar addition on the physical and hydraulic properties of a clay soil. Soil Tillage Res. 2015, 154, 1–13. [Google Scholar] [CrossRef]
- Sun, Z.; Arthur, E.; de Jonge, L.W.; Elsgaard, L.; Moldrup, P. Pore Structure Characteristics After 2 Years of Biochar Application to a Sandy Loam Field. Soil Sci. 2015, 180, 41–46. [Google Scholar] [CrossRef]
- Ball BC, S.K. Gas movement. In Soil Analysis: Physical Methods; Smith, K.A., Ed.; Marcel Dekker: Madison, WI, USA, 1991; pp. 511–549. [Google Scholar]
- Schjønning, P.; Lamandé, M.; Berisso, F.E.; Simojoki, A.; Alakukku, L.; Andreasen, R.R. Gas Diffusion, Non-Darcy Air Permeability, and Computed Tomography Images of a Clay Subsoil Affected by Compaction. Soil Sci. Soc. Am. J. 2013, 77, 1977–1990. [Google Scholar] [CrossRef]
- Ramesh, T.; Bolan, N.S.; Kirkham, M.B.; Wijesekara, H.; Kanchikerimath, M.; Rao, C.S.; Sandeep, S.; Rinklebe, J.; Ok, Y.S.; Choudhury, B.U.; et al. Chapter One-Soil organic carbon dynamics: Impact of land use changes and management practices: A review. Adv. Agron. 2019, 156, 1–107. [Google Scholar]
- Hardie, M.; Clothier, B.; Bound, S.; Oliver, G.; Close, D. Does biochar influence soil physical properties and soil water availability? Plant Soil 2014, 376, 347–361. [Google Scholar] [CrossRef]
- Du, Z.; Chen, X.; Qi, X.; Li, Z.; Nan, J.; Deng, J. The effects of biochar and hoggery biogas slurry on fluvo-aquic soil physical and hydraulic properties: A field study of four consecutive wheat–maize rotations. J. Soils Sediments 2016, 16, 2050–2058. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, L.; Yang, W.; Hua, L.; Cai, C. Aggregate Stability under Long-Term Fertilization Practices: The Case of Eroded Ultisols of South-Central China. Sustainability 2019, 11, 1169. [Google Scholar] [CrossRef]
- Hortensius, D.; Welling, R. International standardization of soil quality measurements. Commun. Soil Sci. Plant Anal. 1996, 27, 387–402. [Google Scholar] [CrossRef]
- Cañasveras, J.C.; Barrón, V.; del Campillo, M.C.; Torrent, J.; Gómez, J.A. Estimation of aggregate stability indices in Mediterranean soils by diffuse reflectance spectroscopy. Geoderma 2010, 158, 78–84. [Google Scholar] [CrossRef]
- Yan, F.; Shi, Z.; Li, Z.; Cai, C. Estimating interrill soil erosion from aggregate stability of Ultisols in subtropical China. Soil Tillage Res. 2008, 100, 34–41. [Google Scholar] [CrossRef]
- Zhang, Q.; Du, Z.; Lou, Y.; He, X. A one-year short-term biochar application improved carbon accumulation in large macroaggregate fractions. Catena 2015, 127, 26–31. [Google Scholar] [CrossRef]
- Pal, D.K. Cracking Clay Soils (Vertisols): Pedology, Mineralogy and Taxonomy. In A Treatise of Indian and Tropical Soils; Springer International Publishing: Cham, Switzerland, 2017; pp. 9–42. [Google Scholar]
- Malongweni, S.O.; Kihara, Y.; Sato, K.; Tokunari, T.; Sobuda, T.; Mrubata, K.; Masunaga, T. Impact of agricultural waste on the shrink–swell behavior and cracking dynamics of expansive soils. Int. J. Recycl. Org. Waste Agric. 2019, 8, 339–349. [Google Scholar] [CrossRef]
- Wubie, A.A. Review on vertisol management for the improvement of crop productivity in Ethiopia. J. Biol. Agric. Healthc. 2015, 5, 92–102. [Google Scholar]
- Zong, Y.; Chen, D.; Lu, S. Impact of biochars on swell-shrinkage behavior, mechanical strength, and surface cracking of clayey soil. J. Plant Nutr. Soil Sci. 2014, 177, 920–926. [Google Scholar] [CrossRef]
- Herath, H.M.S.K.; Camps-Arbestain, M.; Hedley, M. Effect of biochar on soil physical properties in two contrasting soils: An Alfisol and an Andisol. Geoderma 2013, 209–210, 188–197. [Google Scholar] [CrossRef]
- Liu, X.H.; Han, Z.X. Effect of biochar on soil aggregates in the loess plateau: Results from incubation experiments. Int. J. Agric. Biol. 2012, 14, 975–979. [Google Scholar]
- Zhang, Y.; Gu, K.; Li, J.; Tang, C.; Shen, Z.; Shi, B. Effect of biochar on desiccation cracking characteristics of clayey soils. Geoderma 2020, 364, 114182. [Google Scholar] [CrossRef]
- Hansen, V.; Hauggaard-Nielsen, H.; Petersen, C.T.; Mikkelsen, T.N.; Müller-Stöver, D. Effects of gasification biochar on plant-available water capacity and plant growth in two contrasting soil types. Soil Tillage Res. 2016, 161, 1–9. [Google Scholar] [CrossRef]
- Pardo, G.S.; Sarmah, A.K.; Orense, R.P. Mechanism of improvement of biochar on shear strength and liquefaction resistance of sand. Géotechnique 2019, 69, 471–480. [Google Scholar] [CrossRef]
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phylogenetic Variation in the Silicon Composition of Plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef]
- Currie, H.A.; Perry, C.C. Silica in Plants: Biological, Biochemical and Chemical Studies. Ann. Bot. 2007, 100, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Pandis, C.; Spanoudaki, A.; Kyritsis, A.; Pissis, P.; Hernández, J.C.R.; Ribelles, J.L.G.; Pradas, M.M. Water sorption characteristics of poly(2-hydroxyethyl acrylate)/silica nanocomposite hydrogels. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 657–668. [Google Scholar] [CrossRef]
- Khan, M.; Shah, M.R. Sorption kinetics of water vapours in chromatographic silica gel. J. Chem. Soc. Pak. 2007, 29, 209–212. [Google Scholar]
- Bordoloi, S.; Gopal, P.; Boddu, R.; Wang, Q.; Cheng, Y.-F.; Garg, A. Soil-biochar-water interactions: Role of biochar from Eichhornia crassipes in influencing crack propagation and suction in unsaturated soils. J. Clean. Prod. 2019, 210, 847–859. [Google Scholar] [CrossRef]
- Kameyama, K.; Miyamoto, T.; Iwata, Y.; Shiono, T. Effects of Biochar Produced From Sugarcane Bagasse at Different Pyrolysis Temperatures on Water Retention of a Calcaric Dark Red Soil. Soil Sci. 2016, 181, 20–28. [Google Scholar] [CrossRef]
- Haque, A.N.A.; Uddin, M.K.; Sulaiman, M.F.; Amin, A.M.; Hossain, M.; Zaibon, S.; Mosharrof, M. Assessing the Increase in Soil Moisture Storage Capacity and Nutrient Enhancement of Different Organic Amendments in Paddy Soil. Agriculture 2021, 11, 44. [Google Scholar] [CrossRef]
- Amoozegar, A.; Warrick, A.W. Hydraulic Conductivity of Saturated Soils: Field Methods. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods; Klute, A., Ed.; ASA: Madison, WI, USA, 1986; pp. 735–770. [Google Scholar]
- Blanco-Canqui, H. Biochar and Soil Physical Properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef]
- Barnes, R.T.; Gallagher, M.E.; Masiello, C.A.; Liu, Z.; Dugan, B. Biochar-Induced Changes in Soil Hydraulic Conductivity and Dissolved Nutrient Fluxes Constrained by Laboratory Experiments. PLoS ONE 2014, 9, e108340. [Google Scholar] [CrossRef]
- Chen, C.; Wang, R.; Shang, J.; Liu, K.; Irshad, M.K.; Hu, K.; Arthur, E. Effect of Biochar Application on Hydraulic Properties of Sandy Soil under Dry and Wet Conditions. Vadose J. 2018, 17, 180101. [Google Scholar] [CrossRef]
- Liu, Z.; Dugan, B.; Masiello, C.A.; Barnes, R.T.; Gallagher, M.E.; Gonnermann, H. Impacts of biochar concentration and particle size on hydraulic conductivity and DOC leaching of biochar–sand mixtures. J. Hydrol. 2016, 533, 461–472. [Google Scholar] [CrossRef]
- Rogovska, N.; Laird, D.A.; Rathke, S.J.; Karlen, D.L. Biochar impact on Midwestern Mollisols and maize nutrient availability. Geoderma 2014, 230–231, 340–347. [Google Scholar] [CrossRef]
| Biochar Type | Chemical Properties | ||||||
|---|---|---|---|---|---|---|---|
| %C | %N | C/N | %Ash | pH | Total P (g kg−1) | Total K (g kg−1) | |
| Rice straw biochar | 55.7 | 1.1 | 50.2 | 28.1 | 9.88 | 3.05 | 53.08 |
| Peanut straw biochar | 54.7 | 1.8 | 31.3 | 30.3 | 10.25 | 2.78 | 38.35 |
| Corn straw biochar | 63.4 | 2.7 | 23.5 | 15.9 | 8.67 | 5.64 | 29.92 |
| Bamboo chips biochar | 89 | 0.2 | 498.9 | 2.7 | 9.5 | 0.81 | 10.81 |
| Pine chips biochar | 76.5 | 0.3 | 261.4 | 2.5 | 8.14 | 0.39 | 1.03 |
| Soil Type | Experiment Type and Duration | Crop | Biochar Material | Pyrolysis Temperature (°C) | Biochar Rate/Treatment | Effect | % Change | References |
|---|---|---|---|---|---|---|---|---|
| Sand | Incubation 6 months | - | Rice husk | ~600 | (% w/w) Control (0) 0.1 0.5 1.0 | Soil organic C (%) | [29] | |
| 0.86ef 0.93de 0.99d 1.23a | - 8 15 43 | |||||||
| Sandy loam | ||||||||
| 0.80f 0.93de 1.08c 1.32a | - 16 35 65 | |||||||
| Hydromorphic paddy soil | Pot 4 months | Rice | Rice straw | - | i. Biochar (0, 20, 40 t ha−1) with controlled irrigation ii. Biochar (40 t ha−1) with flooded irrigation | Greatly affected in water saving irrigation | Increased by 4 to 26.7% | [74] |
| Entic Halpudept | Field 2 consecutive cycles | Rice | Wheat straw | 350–550 | 0 t ha−1 10 t ha−1 20 t ha−1 40 t ha−1 | Soil organic C (g kg−1) | Increased | [88] |
| 1st cycle 23.2c 27.1b 29.5b 36.0a | 16.8% 27.2% 55.2% | |||||||
| 2nd cycle 23.5b 25.7ab 28.9ab 36.1a | 9.4% 23.0% 53.6% | |||||||
| Clay loam | Field 2 crop cycles | Rice | Bamboo chips and Rice straw | 600 | Soil organic C (g kg−1) | [89] | ||
| Control (No biochar and urea) Bamboo biochar (2.25 t ha−1) Rice straw biochar (2.25 t ha−1) Control + urea (435 kg ha−1) Bamboo biochar + urea Rice straw biochar + urea | 15.2b 24.73a 21.21a 14.18b 24.08a 20.89a | 62.7% 39.5% −6.7% 58.4% 37.4% | ||||||
| Entic Halpudept | Field 1 growth cycle | Rice and Wheat | Municipal biowaste | 450–550 | 0 t ha−1 40 t ha−1 | (SOC g kg−1) Rice 26.8b 32.2a | Increased 21% (rice) and 19% (wheat) | [90] |
| Wheat (SOC) 25.2b 29.9a | ||||||||
| Anthraquic Gleysols (Clay) | Field, 2 year | Rice | Rice husk (RH) | - | SOC (g kg−1) | [91] | ||
| Control Control + fertilizer RHB (4.13 kg m−2) RHB (4.13 kg m−2) +fertilizer Untreated Rice husk Untreated Ricehusk + fertilizer | 15.40b 14.90b 28.30a 28.70a 16.30b 16.40b | - −3.2% +83.7% +86.3% +5.8% +6.4% | ||||||
| China Entic Halpudept | Field 4 months | Rice | Wheat straw | 350–550 | i. Without N | (SOC g kg−1) | Increased | [92] |
| 0 t ha−1 10 t ha−1 40 t ha−1 | 23.5b 25.9b 36.9a | +2% +45% | ||||||
| i. With N | ||||||||
| 0 t ha−1 10 t ha−1 40 t ha−1 | 23.2b 27.1b 36.0a | +17% +55% |
| Total Nitrogen (TN) | ||||||||
| Soil Type | Experiment Type and Duration | Crop | Biochar Material | Pyrolysis Temperature (°C) | Biochar Rate/Treatment | Effects | % Change | References |
| Typic Sulfosaprists | Glasshouse 4 months | Rice | Oil palm empty fruit bunch | 300–400 | 0 t ha−1 10 t ha−1 20 t ha−1 40 t ha−1 | 0.28a 0.29a 0.28a 0.30a | - 3.6% 0.0% 7.1% | [22] |
| China Entic Halpudept | Field 2 consecutive cycles | Rice | Wheat straw | 350–550 | 0 t ha−1 10 t ha−1 20 t ha−1 40 t ha−1 | 1st cycle | [88] | |
| 2.07b 2.19b 2.11b 2.54a | +5.8% +1.9% +22.7% | |||||||
| 2nd cycle | ||||||||
| 1.98b 1.95b 2.16ab 2.27a | −1.5% +9.1% +14.6% | |||||||
| Clay loam | Field 2 crop cycles | Rice | Bamboo chips and Rice straw | 600 | Control (No biochar and urea) Bamboo biochar (2.25 t ha−1) Rice straw biochar (2.25 t ha−1) Control + urea (435 kg ha−1) Bamboo biochar + urea Rice straw biochar + urea | 2.13bc 2.32ab 2.38a 2.08c 2.17abc 2.39a | - +8.9% +11.7% −2.3% +1.9% +12.2% | [89] |
| Anthraquic Gleysols (Clay) | Field, 2 year | Rice | Rice husk | - | Control Control + fertilizer RHB (4.13 kg m−2) RHB (4.13 kg m−2) +fertilizer Untreated rice husk Untreated rice husk + fertilizer | 1.41b 1.39b 1.64a 1.63a 1.46b 1.48b | - −1.42% +16.31% +15.60% +3.55% +4.96% | [91] |
| EnticHalpudept | Field 4 months | Rice | Wheat straw | 350–550 | i. Without N | (TN g kg−1) | [92] | |
| 0 t ha−1 10 t ha−1 40 t ha−1 | 1.78d 2.12bcd 2.48ab | +19% +39% | ||||||
| i. With N | ||||||||
| 0 t ha−1 10 t ha−1 40 t ha−1 | 2.07cd 2.19abc 2.54a | +6% +23% | ||||||
| Acidic soil | Greenhouse 13 weeks | Rice | Sewage sludge | 550 | 0 g kg−1 5 g kg−1 10 g kg−1 | 0.04 0.18 0.26 | +350% +550% | [125] |
| Sandy loam | Incubation 60 days | - | Rice husk and Rice straw | 700 | Rice husk biochar (RHB) (0, 5, 10, 20, 50 g kg−1) Rice straw biochar (RSB) (0, 5, 10, 20, 50 g kg−1) | RHB and RSB increased up to 41% and 83% in sandy and, 23% and 66%in silty soil respectively | - | [123] |
| Silty loam | ||||||||
| Vertisol Clay | Incubation 180 days | - | Straw biochar (SB) Wood chip biochar (WCB) Wastewater biochar (WWB) | 500 | 0 g kg−1 20 g kg−1 40 g kg−1 60 g kg−1 | Increased significantly (p < 0.05) with increment rate | +16%, +11% and +14% by SB, WCB and WWB respectively | [126] |
| Phosphorus (P) | ||||||||
| Soil type | Experiment type and duration | Crop | Biochar material | Pyrolysis Temperature (°C) | Biochar rate/treatment | Effects | % Change | References |
| Typic Sulfosaprists | Glasshouse 4 months | Rice | Oil palm empty fruit bunch | 300–400 | 0 t ha−1 10 t ha−1 20 t ha−1 40 t ha−1 | 71.09ab 67.36b 72.71ab 100.01a | −5.2% +2.3% +40.7% | [22] |
| Dystroxerepts (Sand) | Field 160 days | Cucumber | Poultry litter biochar (PLB) | 450 | PLB combinedly applied with compound poultry manure and N, P | All treatment combination increased significantly (p < 0.05) over control | Up to +71% | [24] |
| Anthraquic Gleysols (Clay) | Field, 2 year | Rice | Rice husk | - | Available P | [91] | ||
| Control Control + fertilizer RHB (4.13 kg m−2) RHB (4.13 kg m−2) + fertilizer Untreated Rice husk Untreated Rice husk + fertilizer | 13.30bc 15.00a 14.70ab 15.70a 13.30bc 15.00a | - +12.78% +10.53% +18.05% +0.00% +12.78% | ||||||
| Sandy loam Silty loam | Incubation 60 days | - | Rice husk and Rice straw | 700 | Rice husk biochar (RHB) (0, 5, 10, 20, 50 g kg−1) Rice straw biochar (RSB) (0, 5, 10, 20, 50 g kg−1) | Increased with higher rate of both biochar in two types of soil. | Up to +171% | [123] |
| Vertisol Clay | Incubation 180 days | - | Straw biochar (SB) Wood chip biochar (WCB) Wastewater biochar (WWB) | 500 | 0 g kg−1 20 g kg−1 40 g kg−1 60 g kg−1 | Increased significantly (p < 0.05) with increment rate | +79%, +15% and +153% by SB, WCB and WWB respectively | [126] |
| Dystroxerepts (Sand) | Glasshouse 8 weeks | Wheat | Chicken manure and Wheat chaff | 450 | 0, 1, and 2% (w/w) | Increased microbial biomass P | Up to +48% | [127] |
| Potassium (K) | ||||||||
| Location and Soil type | Experiment type and duration | Crop | Biochar material | Pyrolysis Temperature (°C) | Biochar rate/treatment | Effects | % Change | References |
| Typic Sulfosaprists | Glasshouse 4 months | Rice | Oil palm empty fruit bunch | 300–400 | 0 t ha−1 10 t ha−1 20 t ha−1 40 t ha−1 | 0.09c 0.13bc 0.15ab 0.19a | +44.4% +66.7% +111.1% | [22] |
| Dystroxerepts (Sand) | Field 160 days | Cucumber | Poultry litter biochar (PLB) | 450 | PLB combinedly applied with compound poultry manure and N, P | All treatment combination increased significantly (p < 0.05) over control | Up to +82% | [24] |
| EnticHalpudept | Field 1 season | Rice and Wheat | Municipal biowaste | 450–550 | 0 t ha−1 40 t ha−1 | (mg kg−1) Rice 116b 148a Wheat 106b 129a | Increased 26% (rice) and 22% (wheat) | [90] |
| Anthraquic Gleysols (Clay) | Field, 2 year | Rice | Rice husk | - | (cmol kg−1) | [91] | ||
| Control Control + fertilizer RHB (4.13 kg m−2) RHB (4.13 kg m−2) + fertilizer Untreated rice husk Untreated ricehusk + fertilizer | 1.59b 1.65ab 1.70a 1.68ab 1.71a 1.70a | - +3.77% +6.92% +5.66% +7.55% +6.92% | ||||||
| Sandy loam, Sand | Field 1 year | Maize and Groundnut rotation | Maize cob | 350 | 0%, 2.5%, 5% and 10% | Significantly (p < 0.05) increased | 8 to 18 folds | [119] |
| Tea garden soil | Incubation 60 days | Rice husk | 550 | (% w/w) (0, 0.5, 1, 2, 4) | Maximum increased by 4% rate | 6.7 folds | [122] | |
| China Acidic soil | Greenhouse 13 weeks | Rice | Sewage sludge | 550 | (mg kg−1) | Increased +3% +23% | [125] | |
| 0 g kg−1 (Control) 5 g kg−1 10 g kg−1 | 305 315 374 | |||||||
| Sandy loam | Incubation 60 days | - | Rice husk and Rice straw | 700 | Rice husk biochar (RHB) (0, 5, 10, 20, 50 g kg−1) | Biochar doses increased in sandy and siltysoil and RSB performed better over RHB | up to 14 times | [123] |
| Silty loam | Rice straw biochar (RSB) (0, 5, 10, 20, 50 g kg−1) | |||||||
| Vertisol Clay | Incubation 180 days | - | Straw biochar (SB) Wood chip biochar (WCB) Wastewater biochar (WWB) | 500 | 0 g kg−1 20 g kg−1 40 g kg−1 60 g kg−1 | Increased significantly (p < 0.05) with increment rate | 97%, 36% and 10% by SB, WCB and WWB respectively | [126] |
| Clay loam | Pot, 70 days | Lentil | Rice husk | ~300 to ~500 | Rate: (% w/w) | (mg kg−1) | [128] | |
| Control (0) 0.4 0.8 1.6 2.4 3.3 | 108.00f 121.33e 140.00d 176.67c 218.67b 256.00a | - +12% +30% +64% +102% 137 | ||||||
| CEC (cmolc kg−1) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Soil Type | Experiment Type and Duration | Crop | Biochar Material | Pyrolysis Temperature (°C) | Biochar Rate/Treatment | Effects | % Change | References |
| Typic Sulfosaprists | Glasshouse 4 months | Rice | Oil palm empty fruit bunch | 300–400 | 0 t ha−1 10 t ha−1 20 t ha−1 40 t ha−1 | 24.26b 24.70ab 25.13ab 26.10a | +1.8% +3.6% +7.6% | [22] |
| Sand | Incubation 6 months | - | Rice husk | ~600 | (% w/w) 0 0.1 0.5 1.0 | Increased significantly (p < 0.05) | +17% +30% +31% | [29] |
| Sandy loam | ||||||||
| +2% +4% +17% | ||||||||
| Sandy loam | Incubation 60 days | - | Rice husk and Rice straw | 700 | Rice husk biochar (RHB) (0, 5, 10, 20, 50 g kg−1) | Higher dose of biochar increased CEC value in both soils | Increased up to 40% | [123] |
| Rice straw biochar (RSB) (0, 5, 10, 20, 50 g kg−1) | ||||||||
| Silty loam | ||||||||
| China Ultisol | Incubation 11 days | - | Rice straw | 250 300 350 400 450 | (% w/w) 0% (Control) 1% | Significantly (p < 0.05) increased | +4–17% | [138] |
| Loam | Greenhouse 42 days | Lettuce | Walnut shell | 900 | 10 metric t ha−1 | Significantly (p < 0.05) increased | +64% | [139] |
| Soil Type | Experiment Type and Duration | Crop | Biochar Material | Pyrolysis Temperature (°C) | Biochar Rate | Effect | % Change | References |
|---|---|---|---|---|---|---|---|---|
| Loamy sand | Incubation 4 months | - | Winter wheat and Miscanthus | 300 | Rate: (% m/m) | [28] | ||
| 0 0.5 1 2 4 | 1.8 1.59a 1.54b 1.46c 1.33d | - −11% −14% −19% −26% | ||||||
| Sand | Incubation 6 months | - | Rice husk | ~600 | (% w/w) | [29] | ||
| Control (0) 0.1 0.5 1.0 | 1.48a 1.39b 1.32c 1.27e | - −6 −11 −14 | ||||||
| Control (0) 0.1 0.5 1.0 | 1.41b 1.31cd 1.28de 1.24e | - −7 −9 −12 | ||||||
| Sandy loam | ||||||||
| Mesic typic Happludolls | Incubation 500 days | - | Wood | - | i. Biochar (0, 5, 10, 20 g kg−1) without manure ii. Biochar (0, 5, 10, 20 g kg−1) manure | Non significant | - | [82] |
| Entic Halpudept | Field 2 consecutive cycles | Rice | Wheat straw | 350–550 | 1st cycle | [88] | ||
| 0 t ha−1 10 t ha−1 20 t ha−1 40 t ha−1 | 0.99 0.96 0.91 0.89 | −3.0% −8.1% −10.1% | ||||||
| 2nd cycle | ||||||||
| 0 t ha−1 10 t ha−1 20 t ha−1 40 t ha−1 | 0.94 0.91 0.86 0.88 | −3.2% −8.5% −6.4% | ||||||
| Entic Halpudept | Field 4 months | Rice | Wheat straw | 350–550 | i. Without N | [92] | ||
| 0 t ha−1 10 t ha−1 40 t ha−1 | 1.01a 0.98ab 0.89c | −3% −12% | ||||||
| ii. With N | ||||||||
| 0 t ha−1 10 t ha−1 40 t ha−1 | 0.99ab 0.96ab 0.89c | −3% −10% | ||||||
| Sandy | Field 2 year | Maize | Birch wood | 500 | 0 t ha−1 20 t ha−1 40 t ha−1 100 t ha−1 | Decreased | Up to −16% | [144] |
| Loam | Field 4 year | Peanut shell | 350–500 | 0 Mg ha−1 28 Mg ha−1 | 1.36a 1.31b | −4% | [149] |
| Soil Type | Experiment Type and Duration | Crop | Biochar Material | Pyrolysis Temperature (°C) | Biochar Rate | Effect | % Change | References |
|---|---|---|---|---|---|---|---|---|
| Soil texture (Loamy sand) | Incubation 4 months | - | Winter wheat and Miscanthus | 300 | Rate: (w/w) | Total porosity (cm3 cm−3) | [28] | |
| 0% 0.5% 1% 2% 4% | 0.322 0.395d 0.414c 0.442b 0.489a | - +23 +29 +37 +52 | ||||||
| Sand | Incubation 6 months | - | Rice husk | ~600 | (% w/w) Control (0) 0.1 0.5 1.0 | % Porosity | [29] | |
| 44.02e 47.42d 50.06c 52.2a | - +8% +14% +19% | |||||||
| Sandy loam | 46.79d 50.56bc 51.82ab 53.08a | - +8% +11% +13% | ||||||
| Vertisol Clay | Incubation 180 days | - | Straw biochar (SB) Wood chip biochar (WCB) Wastewater biochar (WWB) | 500 | 0 g kg−1 20 g kg−1 40 g kg−1 60 g kg−1 | Increased significantly (p < 0.05) with increment rate | 100%, 68% and 36% by SB, WCB and WWB respectively | [126] |
| Vertisol (Clay) | Column study 2.5 years | - | Fruit tree | 500 | 0% 1% 3% | Increased significantly (p < 0.05) | - +13% +37% | [143] |
| Sandy | Field 2 year | Maize | Birch wood | 500 | 0 t ha−1 20 t ha−1 40 t ha−1 100 t ha−1 | Increased | Upto+14% | [144] |
| Soil Type | Experiment Type and Duration | Crop | Biochar Material | Pyrolysis Temperature (°C) | Biochar Rate | Effect | % Change | References |
|---|---|---|---|---|---|---|---|---|
| Sand, Sandy loam, loamy sand | Field 1 year | Maize and Soybean | Corn cob and rice husk | 300–350 | 0–4% | Significantly increased (p > 0.05) | +7 to 20% | [21] |
| Vertisol Clay | Incubation 180 days | - | Straw biochar (SB) Wood chip biochar (WCB) Wastewater biochar (WWB) | 500 | 0 g kg−1 20 g kg−1 40 g kg−1 60 g kg−1 | Increased significantly (p < 0.05) with increment rate | 21%, 84% and 140% by SB, WCB and WWB respectively | [126] |
| China Ultisol | Incubation 11 days | - | Rice straw | 250 300 350 400 450 | (% w/w) 0% (Control) 1% | Non significant | - | [138] |
| Sandy loam | Field 1 year | Maize | Corn cob | 360 | 0, 4.5, 9 t ha−1 | Non significant | - | [154] |
| Alfisol (Silt loam) | Incubation 295 days | - | Corn stover | Control 350 550 | Control 7.18 t C ha−1 | Increased | >+17% | [159] |
| Andisol (Silt loam) | Control 350 550 | Control 7.18 t C ha−1 | +7–15% | |||||
| Sandy loam | Incubation 11 months | - | Pine sawdust | 0, 4, 8, 16 (g kg−1) | NS | - | [160] | |
| Increased Significantly | +20 to +37% | |||||||
| Silt loam |
| Soil Type | Experiment Type and Duration | Crop | Biochar Material | Pyrolysis Temperature (°C) | Biochar Rate | Effect | % Change | References |
|---|---|---|---|---|---|---|---|---|
| Vertisol (Silty clay) | Not available | - | Mixed Corn straw and Peanut shell | 450 | 0 g kg−1 50 g kg−1 100 g kg−1 150 g kg−1 | Decreased cracking area density with increasing biochar rates | 33.6%, 52.1% 56.9% for 50, 100 and 150 g kg−1 respectively | [93] |
| Inceptisol Clay | Incubation 280 days | 450 | (w/w) | Crack area density (%) | [156] | |||
| Rice husk | 0% 2% 5% 10% | 12.68a 8.87b 6.42bc 4.84c | −30% −49% −62% | |||||
| Sugarcane bagasse | 0% 2% 5% 10% | 12.68a 9.00b 4.79c 3.82c | −29% −62% −70% | |||||
| Vertisol Clay | Incubation 180 days | - | Straw biochar (SB) Wood chip biochar (WCB) Wastewater biochar (WWB) | 500 | 0 g kg−1 20 g kg−1 40 g kg−1 60 g kg−1 | All biochars reduced surface crack formation | 60 g kg−1 of SB, WCB and WWB decreased 14, 17, 19% surface area cracking density respectively | [158] |
| Pukou (Clay) Xiashu (Clay) | Not available | - | Wood | 500 | 0, 0.5, 2, 4 and 6% (w/w) | Reduced cracking ratio and number | 16.85 and 32.26% respectively | [161] |
| Soil Type | Experiment Type and Duration | Crop | Biochar Material | Pyrolysis Temperature (°C) | Biochar Rate/Treatment | Effect | % Change | References |
|---|---|---|---|---|---|---|---|---|
| Clay loam | Field 2 crop cycles | Rice | Bamboo chips and Rice straw | 600 | Soil moisture (g g−1) | [89] | ||
| Control (No biochar and urea) Bamboo biochar (2.25 t ha−1) Rice straw biochar (2.25 t ha−1) Control + urea (435 kg ha−1) Bamboo biochar + urea Rice straw biochar + urea | 0.33 0.34 0.38 0.35 0.36 0.38 | +3.0% +15.2% +6.1% +9.1% +15.2% | ||||||
| Vertisol (Silty clay) | Not available | Mixed Corn straw and Peanut shell | 450 | (0, 50, 100, 150) g kg−1 | Increased gravimetric water content | - | [93] | |
| Vertisol Clay | Incubation 180 days | - | Straw biochar (SB) Wood chip biochar (WCB) Wastewater biochar (WWB) | 500 | 0 g kg−1 20 g kg−1 40 g kg−1 60 g kg−1 | Increased significantly (p < 0.05) with increment rate for straw biochar | 1.4%, 6.1% and 18.4% respectively | [126] |
| Vertisol (Clay) | Column study 2.5 years | - | Fruit trees | 500 | 0% 1% 3% | Increased significantly (p < 0.05) at maximum biochar dose | - | [143] |
| Alfisol (Silt loam) | Incubation 295 days | - | Corn stover | Control 350 550 | Control 7.18 t C ha−1 | Increased plant available water | - | [159] |
| Andisol (Silt loam) | Control 350 550 | Control 7.18 t C ha−1 | ||||||
| Sandy loam | Pot 4 months | Barley | Pine wood Wheat straw | 1200 750 | 0 and 1% | Increased AWC | 17 to 42% | [162] |
| Loamy sand | Column study 3 months | - | Water hyacinth | 350–400 | 0, 2, 5 and 10% (w/w) | Increased soil moisture with increasing soil biochar content | - | [168] |
| Clay | Laboratory 180 days | - | Sugarcane | 400–800 | 0, 1, 3, 5, 10% (w/w) | Increased AWC with increment biochar rate greater than 3% | ~60% | [169] |
| Soil Type | Experiment Type and Duration | Crop | Biochar Material | Pyrolysis Temperature (°C) | Biochar Rate | Effect | % Change | References |
|---|---|---|---|---|---|---|---|---|
| Sand | Incubation 6 months | - | Rice husk | ~600 | (% w/w) Control (0) 0.1 0.5 1.0 | Decreased significantly (p < 0.05) | - −54 −78 | [29] |
| Sandy loam | ||||||||
| - −82 −148 | ||||||||
| Kaolin clay | Column study | - | Peanut shell | 500 | 0% 5% 20% | 1.2 × 10−9 m s−1 2.1 × 10−9 m s−1 1.3 × 10−9 m s−1 | +75% +8% | [30] |
| Alfisol (Silt loam) | Incubation 295 days | - | Corn stover | Control 350 550 | Control 7.18 t C ha−1 | Increased | 139% | [159] |
| Andisol (Silt loam) | Control 350 550 | Control 7.18 t C ha−1 | ||||||
| Sand | Column study | - | Wood | 400 | 0% 10% | Decreased | −92% | [173] |
| Organic soil | Decreased | −67% | ||||||
| Clay loam | Increased | +328% | ||||||
| Sand | Column study | - | Wood | 400 | 0–10% (w/w) | Decreased | ~72 ± 3% | [175] |
| Loam, Silt loam, Silty clay loam | Field 4 year | Maize | Hardwood | 400 | 0, 9.9, 18.4 Mg ha−1 | Non significant | - | [176] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, A.N.A.; Uddin, M.K.; Sulaiman, M.F.; Amin, A.M.; Hossain, M.; Solaiman, Z.M.; Mosharrof, M. Biochar with Alternate Wetting and Drying Irrigation: A Potential Technique for Paddy Soil Management. Agriculture 2021, 11, 367. https://doi.org/10.3390/agriculture11040367
Haque ANA, Uddin MK, Sulaiman MF, Amin AM, Hossain M, Solaiman ZM, Mosharrof M. Biochar with Alternate Wetting and Drying Irrigation: A Potential Technique for Paddy Soil Management. Agriculture. 2021; 11(4):367. https://doi.org/10.3390/agriculture11040367
Chicago/Turabian StyleHaque, Ahmad Numery Ashfaqul, Md. Kamal Uddin, Muhammad Firdaus Sulaiman, Adibah Mohd Amin, Mahmud Hossain, Zakaria M. Solaiman, and Mehnaz Mosharrof. 2021. "Biochar with Alternate Wetting and Drying Irrigation: A Potential Technique for Paddy Soil Management" Agriculture 11, no. 4: 367. https://doi.org/10.3390/agriculture11040367
APA StyleHaque, A. N. A., Uddin, M. K., Sulaiman, M. F., Amin, A. M., Hossain, M., Solaiman, Z. M., & Mosharrof, M. (2021). Biochar with Alternate Wetting and Drying Irrigation: A Potential Technique for Paddy Soil Management. Agriculture, 11(4), 367. https://doi.org/10.3390/agriculture11040367









