Abstract
The immune modulating effects of selected herbs deserve careful studies to gain evidence-based support for their further development. We have been working hard on many items of medicinal herbs to gain insight into their immunomodulatory effects relevant to cancer treatment in particular, while infection control is not excluded. Nine of them have been selected to give the results of our exploration on their biological, particularly immunomodulatory activities. Since Hong Kong people especially favor one medicinal mushroom, viz. Coriolus versicolor, a number of clinical trials using Coriolus for cancer-related studies are included in this review. While immune modulation platforms are being built for relevant studies, a brief account on the research targets and related procedures are given.
1. Introduction
Special plants have enjoyed the genuine trust of people around the world as being good for health, sometimes with special emphases on longevity. Ancient China, in particular, has lists of choice plants to maintain health.
Since the Han Dynasty (206 B.C.–220 A.D.) remarkable documentations are available. One special area of intense interest and wisdom is related to infections and epidemics: from clinical presentations and treatment choices to public health measures. When bodily defense was mentioned in those days, although no assumption needs to be raised about immunological activities, it is also clear that advocates’ and narrators’ line of thought could be very near the modern concept of immunology at its most primitive level [1,2].
Herbal formulations have been listed for the treatment of various infections in epidemics, their indications of use, and ways of preparation, which are handed down to today to facilitate therapeutic and preventive measures. The rich collection of plant items used for infection control have been studied in the laboratory and clinically along the modern directions of immunological activities—first with reference to infection as described in the old days and later diversifying into other immunological areas, notably cancer.
This review will first briefly explore medicinal herbs in traditional Chinese medicine that have been studied in modern research platforms to identify their unique influences in some specific cellular and serological tests related to immunological activities. Some of these herbs have been selected in our home-ground to serve our aspirations on cancer treatment and epidemics.
2. The Hong Kong Experience: Immunological Properties of Medicinal Herbs Used Against Cancer
Numerous preclinical studies have demonstrated the antitumor activities of herbal medicines and their mechanisms of action. They may either directly inhibit the growth of tumors or indirectly exert an antitumor effect by enhancing the body’s immune function [1]. Over the past 15 years or more, our institute has carried out many preclinical investigations on the antitumor and/or immunomodulatory activities of various Chinese medicinal herbs and mushrooms (Table 1). For instance, in collaboration with the Memorial Sloan Kettering Cancer Center at New York, USA, the immunomodulatory activities of five medicinal herbs/mushrooms, namely Astragalus membranaceus, Coriolus versicolor, Curcuma longa, Echinacea purpurea, and Grifola frondosa, have been investigated using various types of preclinical models [3]. Promising results on Astragalus [4,5] and Curcuma longa [6,7] led to further investigations on these herbs in our institute. Furthermore, a panel of herbs, which are commonly prescribed to cancer patients according to traditional Chinese medicine (TCM) theory, have also been examined for their in vitro effects on cancer cells and/or on lymphocytes in our early screening program. Based on the results, seven medicinal herbs or mushrooms, namely Acanthopanax senticosus, Agaricus blazei, Curcuma longa, Ganoderma lucidum, Ganoderma sinense, Hedyotis diffusa, and Scutellaria baicalensis, with particular reference to their antiproliferative effects in cancer cells or immunomodulatory properties, have been further investigated for their mechanisms of action. In addition, since the establishment of our Partner State Key Laboratory in 2010, research collaboration with State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, has been focusing on several natural products, orchids as well as Yunnan wildly grown mushrooms. One of the edible mushrooms, Rubinoboletus ballouii, was shown for its immunomodulatory activities for the first time in our studies [8,9].

Table 1.
Medicinal plants and mushrooms with anticancer and immunomodulatory effects described in the text.
Hence, in this part of the review, the preclinical findings of the above-mentioned nine medicinal botanicals from our previous studies have been summarized. The clinical prospects of these individual botanicals, especially their potentials in cancer management with special focus on immunomodulation, have also been discussed.
2.1. Agaricus blazei
The Basidiomycete fungus Agaricus blazei (Agaricaceae family) is an edible mushroom, native to southern Brazil and was introduced to East Asia in the 1950s. It is commonly cultivated in Japan, China, and Brazil [10,11]. Our study firstly demonstrated the potent tumor-selective growth inhibitory activity of an ethanol–water extract of A. blazei against human leukemia NB-4 cells with the concentration that was required for 50 % inhibition (IC50 value) of 82.2 µg/mL. This extract at 500 mg/kg/day also inhibited the progression of NB-4 tumors in athymic nude mice by inducing apoptosis, which was indicated by DNA fragmentation in the tumors [12]. In view of the clinical studies on A. blazei, there were several other studies testing the efficacy of the water extract in patients with acute non-lymphoblastic leukemia [13], gynecological cancer [14], and prostate cancer [15]. Other clinical studies on the safety of A. blazei have also been conducted in cancer patients [16]; however, the dosages of the A. blazei extracts in these studies have not clearly reported. On the other hand, health supplements containing A. blazei were also shown to be effective in improving symptoms in inflammatory or allergic conditions [11].
2.2. Ganoderma lucidum and Ganoderma sinense
We have made attempts to study the antitumor and immunomodulatory effects of Ganoderma species, including different parts of the fruiting bodies, as demonstrated in human breast and liver cancer cells at effective concentrations (100–400 µg/mL) [17,18] and in S-180 sarcoma-bearing mice (at dosage 200 mg/kg) [19]. The highlights of these studies were the superior efficacies of the G. lucidum stipes, whose extract could exert higher cytotoxicity in cancer cells [18] and stronger immunomodulatory activities in terms of enhancing the proliferative responses and the cytokines interferon-γ (IFN-γ), interleukin-4 (IL-4) and interleukin-6 (IL-6) productions of the spleen lymphocytes of tumor-bearing mice [19], when compared with the efficacies of the G. lucidum pileus. On the other hand, the chemical and immunomodulatory activities of another Ganoderma species listed in Chinese Pharmacopoeia, G. sinense, were also investigated in our laboratory. The polysaccharide-enriched fraction of G. sinense hot water extract (50–400 µg/mL) was shown to have potent immunostimulating activities in human peripheral blood mononuclear cells (PBMCs) and monocyte-derived dendritic cells in terms of proliferative responses and cytokine productions [20]. Moreover, a series of polysaccharides were isolated from G. sinense and were characterized in detail [21,22,23]. Polysaccharide GSP-6B (molecular mass of 1.86 × 106 Da), with a backbone of (1→6)-linked-β-D-glucopyranosyl residues and branches at the O-3 position of every two sugar residues along the backbone, was shown to induce the release of interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) in PBMCs, with an effective range of 0.003 to 100 µg/mL [21]. While a protein-bound polysaccharide (GSP-4, at 200–800 µg/mL), with a molecular weight of 8.3 × 105 Da and the sugar residues t-; 1,3-; 1,4-; 1,6-; 1,3,4-; and 1,3,6-linked Glcp, t-linked Galp, and 1,6-linked Manp, was shown to modulate nitric oxide (NO), TNF-α, and IL-6 production in macrophage RAW264.7 cells. GSP-4 also enhanced the expression of inducible NO synthase mRNA in a dose-dependent manner [22]. Additionally, polysaccharide GSP-2 (at 30 µg/mL), with a molecular size of 32 kDa, a backbone of (1R4)– and (1R6)–Glcp, bearing terminal- and (1R3)–Glcp side-chains at the O-3 position of (1R6)–Glcp, was also isolated from G. sinense and was shown to induce the proliferation of mouse splenocytes targeting B cells only [23]. In addition, the potential antitumor small molecules in the stipe of G. sinense were identified using LC-QTOF-MS with a multivariate statistical tool [24].
On the other hand, five randomized controlled trials comparing the efficacy of G. lucidum medications to active or placebo control in cancer patients have been included in a systematic review and meta-analysis, in which the authors concluded that G. lucidum could be administered as an alternative adjuvant to conventional treatment in consideration of its potential of enhancing tumor response and stimulating host immunity [25]. In particular, in the clinical trial of breast cancer patients, G. lucidum spore powder treatment (1000 mg three times a day for 4 weeks) was shown to have significant improvements on physical well-being and fatigue. These patients also reported less anxiety and depression and better quality of life [26]. Nevertheless, there has been no clinical trial on G. sinense for cancer patients up till now.
2.3. Rubinoboletus ballouii
Rubinoboletus ballouii (RB) of the family Boletaceae, is an edible mushroom commonly found in the forest of Yunnan Province, China. Our study showed that the ethanol extract exerted anti-inflammatory effects using PBMCs. With bioassay-guided fractionation using high-speed counter current chromatography, two immunosuppressive compounds, 1-ribofuranosyl-s-triazin-2(1H)-one and pistillarin were isolated and characterized [8]. These compounds (at 20 and 100 µM) were shown to inhibit phytohemagglutinin-stimulated PBMC proliferation and inflammatory cytokine (TNF-α, IL-1β, IL-10, and IFN-γ) productions in a concentration-dependent manner. On the other hand, bioactive polysaccharides, which contain 80.6% (w/w) of neutral sugars including D-fucose, D-mannose, D-glucose, and D-galactose (1.7:1.4:1.0:1.8), and 12.5% (w/w) of proteins, were isolated from the water extract of RB and were evaluated for their immunomodulatory activities. Our results showed that the activation of monocyte-derived dendritic cells by RB polysaccharides (50 µg/mL) was mediated through the nuclear factor-κB (NF-κB) pathway via toll-like receptor-4 (TLR-4). Furthermore, RB polysaccharides at dosages of 40 and 200 mg/kg/day could restore the suppressed hematopoietic stem cell activity and upregulate the function of T helper cells in mice treated with cyclophosphamide [9]. These findings suggested that polysaccharides from Rubinoboletus ballouii exhibited immunostimulatory effects and have the potential to be developed as a health supplement for combined use with chemotherapeutic agents.
To date, there were only a few clinical reports on the poisoning activities of Boletus mushrooms [27,28]. While many natural compounds isolated from Boletus mushrooms were reported, such as those with apoptotic effect in cancer cells [29], antioxidant effect [30], and a liver-protective effect in mice [31]. Nevertheless, the immunomodulatory effect of RB is worth further investigating in the future.
2.4. Acanthopanax senticosus
The dried root and rhizome or stem of Acanthopanax senticosus (AS, also known as Siberian ginseng or Ciwujia in Chinese) is suggested to have immune-regulating function [46], such as the amelioration of inflammation and fatigue, as well as inhibition of tumor progression. In our laboratory, the potencies of AS aqueous extract (25–400 µg/mL) and ethanol extract (25–100 µg/mL) in regulating cytokine productions (IL-2, IFN-γ, and TNF-α) from PBMCs were determined. Our results suggested that AS aqueous extract, rather than ethanol extract or individual components (isofraxidin, syringin, or eleutheroside E), should be used in order to achieve its immunomodulatory properties [32]. In addition, the clinical efficacy of an AS-containing injection (Aidi injection) in advanced non-small-cell lung cancer patients was evaluated [47,48]. A clinical trial using AS alone has not yet been reported.
2.5. Astragalus membranaceus
Radix Astragali (Huangqi), the dried root of Astragalus membranaceus and Astragalus mongholicus, is a common Chinese herbal medicine as well as an ingredient of soup in Chinese cuisine. Being one of the immunomodulators studied in an National Institutes of Health (NIH)-supported research project, our previous study demonstrated that, when mice were immunized with immunogens (globo H-keyhole limpet hemocyanin (KLH) and GD3-KLH) present on the cell surface of cancer mixed with 95% ethanol extract of Astragalus (0.5–2 mg), significant immunological adjuvant activity could be observed [3]. Subjected to further fractionation, several flavonoids isolated from Radix Astragali, including astragalosides II (20–100 µg) and IV (20–100 µg), have been shown to have significant adjuvant activity in terms of antibody responses against cancer antigens and KLH [4]. On the other hand, the quality, purity, and uniformity of commercial Radix Astragali were assessed using chemical and genetic analyses, namely ion trap LC-MS and nuclear ribosomal DNA barcoding sequence analyses, in our collaborator laboratory in New York, USA [5]. In regard to the clinical trials on Radix Astragali alone, the majority of them focused on cardiovascular or kidney diseases [49,50]. In addition, the efficacies of Astragalus-containing Chinese herbal combinations have been evaluated in many clinical trials of advanced non-small-cell lung cancer patients [51]. Furthermore, the effects of Astragalus polysaccharides in combination with chemotherapeutics have also been examined in advanced non-small-cell lung cancer patients (250 mg/day, intravenous infusion) [52], and advanced pharyngeal or laryngeal squamous cell carcinoma patients (500 mg/day, intravenous infusion) [53].
2.6. Curcuma longa
Turmeric, the dried rhizome of the plant Curcuma longa (Zingiberaceae family), is a commonly used medicinal herb as well as a cooking herb in South or Southeast Asia and China for centuries. Thousands of preclinical studies reported the multifunctional bioactivities of curcumin [54], one of the major constituents of turmeric, including anticancer and anti-inflammatory effects in various cancer cells and animal models [55,56]. Apart from the collaborative study with Memorial Sloan Kettering Cancer Center in the U.S. on the immunological effects of turmeric, our institute has conducted a series of studies on the antitumor and anti-angiogenic activities of turmeric for a decade. We firstly confirmed the more potent antiproliferative activities of curcumin in turmeric ethanol extract than that of curcumin alone in colon cancer cells and endothelial cells [7]. We also reported the immunomodulatory activities (proliferation and TNF-α, IFN-γ productions) of turmeric polysaccharides (at 200 µg/mL) in PBMCs [6], and the anti-angiogenic activities of aromatic-turmerone in human endothelial cells, HMEC-1, (effective concentrations at 4.6–9.2 µM) and in vivo angiogenesis models (zebrafish and Matrigel plug in mice, effective concentration at 25 µg/mL) [33]. Meanwhile, we tested our hypothesis that the poor bioavailability of curcumin could be improved when it is used as a whole in turmeric extract. Results from both an in vitro Caco-2 cell monolayer study [34] and an in vivo pharmacokinetic study in mice [35] provided evidence that supported our hypothesis. Furthermore, the superior antitumor effects of turmeric extract (6.95 µg/mL, which contain 1.3 µg/mL curcumin) in colon-tumor-bearing mice than curcumin (1.3 µg/mL) alone was also demonstrated in our subsequent study [35]. On the other hand, we revealed that the antitumor activities of turmeric extract (400 mg/kg) plus bevacizumab (0.4 mg/kg) were comparable to those of the first-line chemotherapeutic, 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX), and without the hematological side effects caused by FOLFOX [36]. With higher levels of suppressed anti-angiogenesis and increased apoptosis found in tumors of turmeric-extract-treated mice than those of curcumin-treated mice, our findings from these studies provide strong evidence for the importance of turmeric ethanol extract (containing absorbable curcumin and other active components) in treating colon cancer. Last, but not least, the antimetastatic efficacies of turmeric extract were elucidated in our recent studies. Turmeric extract (200–400 mg/kg) was shown to decrease colon cancer cell migration and epithelial–mesenchymal transitions through multiple pathways, such as cofilin, focal adhesion kinase/phospho-Src (FAK/p-Src), protein kinase B (AKT), extracellular-signal-regulated kinase (Erk), and signal transducer and activator of transcription 3 (STAT3) pathways. It also decreased colon tumor burden and metastasis and enhanced immunity through T cell stimulation in a syngeneic orthotopic colon tumor mouse model [37]. In addition, turmeric extract (200 mg/kg) treatment in colon-patient-derived xenograft mice could reduce tumor progression, inhibit metastasis via modulating molecules involved in the Wnt and Src pathways, epithelial–mesenchymal transition (EMT)- and epidermal growth factor receptor (EGFR)-related pathways [38]. From these recent studies, the role of turmeric extract in regulating the colon tumor microenvironment has been revealed for the first time.
In the last two decades, over 75 clinical trials of curcumin/turmeric on chronic inflammatory diseases or cancers have been conducted [57,58,59,60]. The majority of the trials used curcumin alone as intervention, while some of them used mixtures of curcumin plus curcuminoids and/or turmeric oil. In general, these clinical trials have confirmed that curcumin/turmeric supplements can provide symptomatic relief, as well as improve tumor markers and other parameters of various cancer conditions [59].
2.7. Hedyotis Species
Hedyotis (Oldenlandia) diffusa is one of the herbs most commonly used in Chinese herbal medicines for treating cancer. Various studies demonstrated its anticancer effects in in vitro and in vivo models [61,62]. Our previous study developed a simple thin-layer chromatographic method to distinguish between H. diffusa and its allied species H. corymbosa [39]. In the subsequent studies, hedyotiscone A (25–50 µg/mL) isolated from H. corymbosa was found to reverse multidrug resistance in hepatoma cells by downregulating P-glycoprotein expression and MDR1 gene expression [40]. Additionally, 4-vinylphenol isolated from H. diffusa water extract was firstly proven for its anti-angiogenic activities in Matrigel and tumor-bearing mouse models (at 0.2–2 mg/kg) as well as in human endothelial cells (at 20–40 µg/mL) via the phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) pathway [41]. Despite many reports on the preclinical pharmacological activities of H. diffusa and the high usage of this herb in cancer patients clinically [63,64], proper clinical trials on this herb alone have not been reported. In contrast, the H. diffusa–containing formula was tested in chronic endometritis patients [65]. Further prospective clinical trials in cancer patients may be warranted in order to confirm the efficacies of this well known and commonly used “folk” anticancer herb.
2.8. Scutellaria barbata
Herba Scutellaria barbatae (SB), the whole plant of Scutellaria barbata, is commonly found as a functional component in numerous TCM prescriptions. SB is a popular herbal medicine, which has been demonstrated to have valuable therapeutic outcomes on anti-inflammation, detoxification, elimination of blood stasis, improvement of blood circulation, and anti-swelling, etc., under traditional Chinese medicine theory [66]. An active compound, designated pheophorbide a, has been isolated from SB in our earlier studies. Pheophorbide a was shown to possess photodynamic activity with apoptosis induction in human hepatocellular carcinoma (at 0.3 mg/kg) [42,43] and breast tumor (at 2.5 mg/kg) [44] mouse models. In addition, our recent study revealed the antitumor and antimetastatic effects of SB (615 mg/kg) and its chemical marker scutellarin (7 mg/kg) in a colon tumor mouse model [45]. In the same study, we also showed the regulation of cancer-metastasis-related proteins, such as e-cadherin, tetraspanin 8 (Tspan 8), and C-X-C motif chemokine receptor 4 (CXCR4), exerted by SB water extract.
In view of the clinical trials on SB, an SB aqueous extract (BZL101) has been tested in two phase I clinical trials of metastatic breast cancer [67,68]. Results showed promising clinical evidence of anticancer activity in women with metastatic breast cancer, with good safety profile, and it is well tolerated [68]. Nonetheless, similar to Hedyotis diffusa, Scutellaria barbata is always used in combination with other herbs; thus, no further clinical trials on this herb have been reported recently.
3. The Hong Kong Experience on Clinical Applications
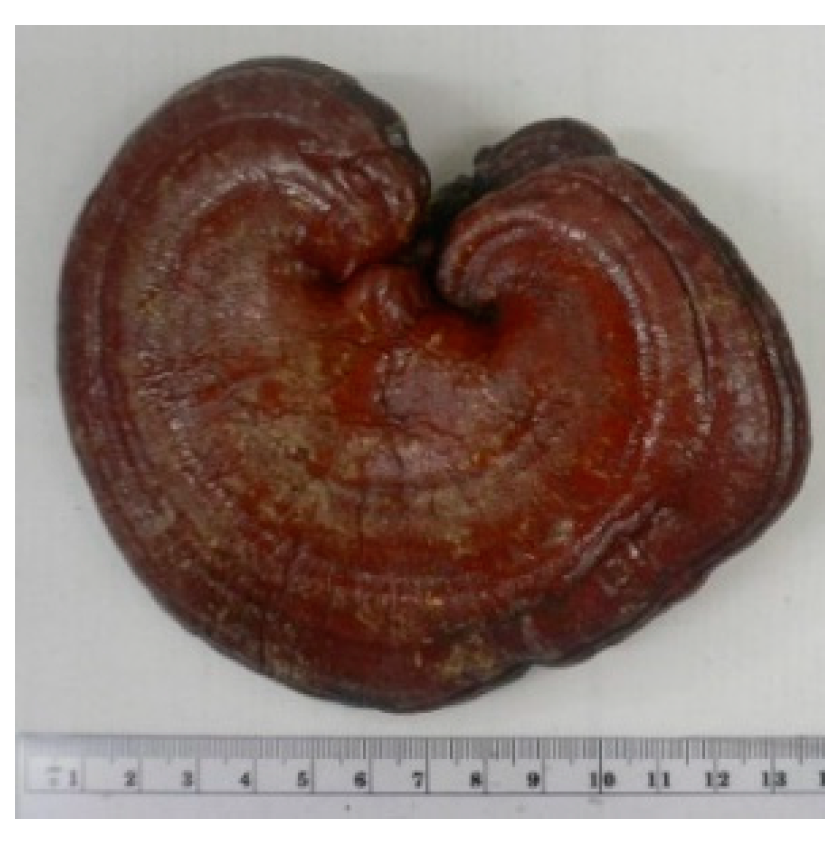
Research related to the immunological effects of medicinal herbs in Hong Kong has been influenced by the behavior of the general public in pursuit of better health. Inhabitants of the island city are fond of taking health supplements in the form of proprietary medicine originated from China and Hong Kong, which contains a rather limited number of herbs exemplified by those discussed in the last section. They give special favor to medicinal mushroom such as Ganoderma. Since the 1970s, a new fungal fruit body called Coriolus versicolor (Yun Zhi) has attracted extensive attention, both in the market and among health professionals. A strong belief that Coriolus and its extract polysaccharopeptide (PSP), could help cancer patients with regard to survival and alleviation of adverse effects during chemotherapy has developed.
The immunomodulating effects of PSP have been studied intensively from animal experiments to clinical trials, from in vivo studies to in vitro analysis, and from cell cultures to molecular and genomic explorations. The laboratory results greatly facilitated marketing, gaining substantial social and economic profits [69].
Apart from anticancer effects, PSP was also found to be antiviral (human immunodeficiency virus, HIV) and hepatoprotective. Other studies showed significant analgesic effects of PSP on acute and chronic inflammatory pain induced in rats. Since this analgesic effect could be antagonized by the cerebral intraventricular injection of anti-IL-2 serum, the central analgesic activity of PSP may be mediated by the IL-2 receptor. Since the immune system and nervous system provide two major forces in the maintenance of the internal equilibrium of the whole body, through cellular and molecular networks, PSP is assumed to be influential in this process [69] (Figure 1).

Figure 1.
Coriolus versicolor (Yunzhi).
Salvia miltiorrhiza (Danshen) has been one of the most commonly used medicinal herbs in traditional Chinese medicine, very much due to its vascular promotion activities, it has been used coupled with Coriolus in experimental studies and clinical trials. A number of studies have been completed in early 2000, and three examples are presented as follows.
3.1. Testing the Immunomodulatory Effects of Coriolus versicolor and Salvia miltiorrhiza Capsules in Healthy Subjects—A Randomized, Double-Blind, Placebo-Controlled, Crossover Study
One hundred healthy subjects were recruited to take Yun Zhi (50 mg/kg body weight) plus Danshen (20 mg/kg body weight) or placebo capsules daily for four successive months and, after a 2 month wash-out period, crossover to take placebo or Yun Zhi plus Danshen capsules for another four successive months. Flow cytometry was used to assess the lymphocyte subtypes and concentration of T helper (Th) cell cytokines in culture supernatant. Gene expression of cytokines and cytokine receptors of peripheral blood mononuclear cells (PBMCs) was analyzed by a cDNA expression array. Results showed that regular oral consumption of Yun Zhi–Danshen capsules could significantly elevate PBMC gene expression of interleukin (IL)-2 receptor, increase the percentage and absolute counts of T helper cell and ratio of CD4+ (T helper)/CD8+ (T suppressor and cytotoxic T) cell, and significantly enhance the ex vivo production of typical Th1 cytokine interferon-gamma from PBMC activated by phytohemagglutinin and lipopolysaccharide (all p < 0.005). Such consumption had no adverse effects on the liver and renal functions and the biochemical profile [70].
3.2. Coriolus and Salvia for Patients Suffering from Nasopharyngeal Carcinoma
Twenty-seven patients with histologically proven nasopharyngeal carcinoma were recruited to take Yun Zhi (3.6 g daily) and Danshen (1.4 g daily) in the form of 12 combination capsules (TCM group) or placebo (12 capsules) daily for 16 weeks, respectively. Flow cytometry was used to assess the percentages and absolute counts of human lymphocyte subsets in whole blood. Plasma concentration of soluble interleukin-2 receptor and soluble tumor necrosis factor receptor 2 were measured by enzyme-linked immunosorbent assay (ELISA). Ex vivo production of tumor necrosis factor-alpha, interleukin (IL)-6, and IL-10 in the whole blood assay culture supernatant was measured by ELISA.
Results showed that the decreases in percentage and absolute count of T lymphocytes in the treatment group were less than those in the placebo group after they took the capsules for 16 weeks (both p < 0.05). Furthermore, the decreases in the absolute count of T suppressor cells, cytotoxic T lymphocytes, and T helper cells in the treatment group were significantly lower than those in the placebo group after they took the capsules for 16 weeks (both p < 0.05) [71].
3.3. Coriolus and Salvia for Breast Cancer Patients after Completion of Treatment
Bodily discomfort and fatigue are experienced by cancer patients undergoing treatment: from surgery and radiotherapy to chemotherapy, because of suppressed immunological functions. Eighty-two patients who completed treatment for breast cancer were recruited to take Yun Zhi (50 mg/kg body weight, 100% polysaccharopeptide (PSP)) and Danshen (20 mg/kg body weight) capsules every day for a total of 6 months. Ethylenediaminetetraacetic acid (EDTA) blood samples were collected every 2 months for the investigation of immunological functions. Flow cytometry was used to assess the percentages and absolute counts of human lymphocyte subsets in whole blood. Plasma level of soluble interleukin-2 receptor (sIL-2R) was measured by enzyme-linked immunosorbent assay (ELISA). Results showed that the absolute counts of T-helper lymphocytes (CD4+), the ratio of T-helper (CD4+)/T suppressor and cytotoxic lymphocytes (CD8+), and the percentage and the absolute counts of B-lymphocytes were significantly elevated in the treatment group. The plasma slL-2R concentration was also significantly decreased (all p < 0.05) among these patients [72].
Cancer patients were given the Coriolus and Salvia capsules after they completed standard treatment. They were expected to enjoy better quality of life, and more specifically, we critically looked at the immunological effects, which would offer quantitative data on clinical benefits. The follow-up periods were short, which would not allow longer-term studies on clinical benefits.
4. Common Target Areas That Investigators Used to Study the Bioactivities Related to Immunological Properties of Medicinal Plants:
4.1. Organs Related to the Provision of Relevant Cells and Cellular Activities: [73]
- bone marrow,
- thymus,
- lymph nodes and lymphatic system, and
- spleen.
Hypertrophy of these organs with increased cell production must be the most accepted indications of a medicinal herb’s immunological ability. Notable items in this group include Astragalus membranaceus, Angelica sinensis, and fungal varieties [2,73].
4.2. Activity of Macrophages: [74]
- engulfing ability and
- anticancer activities.
Polysaccharides in medicinal plants have been of major interest, and many examples are related to fungal species. Triterpenes, saponins, and nucleotides are other components of great interests, exemplified by Panax ginseng.
4.3. Immune Cells—T Cells, B Cells, Natural Killer (NK) Cells: [75,76]
- T cells’ responses give good pictures of anti-infection and anticancer influences,
- B cells could be studied on their responses to polysaccharide stimulation, and
- NK cell’s direct cytotoxic effects have been observed.
A large number of medicinal herbs, exemplified by Astragalus membranaceus, Hedyotis species, Acanthopanax senticosus, and Ledebouriella divaricata have been found influential.
4.4. Cytokines, Interleukin Production [77,78]
Herbal extracts mainly act on the macrophages and related cells. Cellular activities, however, produce cytokines and chemokines, which have far-reaching immunological effects. Studies, therefore, include their productions accepted as extended cellular effects. Selected herbs are those utilized in cellular studies [74]. Other studies include analysis of interferons, TNF-α, etc. [78,79,80].
5. Discussion
Medicinal plants have been used for centuries for the treatment of various diseases in many parts of the world. The utility of natural products as important resources for the discovery of new and safe drugs for human healthcare apparently is still important and is ongoing. It has been estimated that about 65% of the world’s population relies on plant-derived medicines for treating various diseases [81]. The demand for natural products has been growing during the past few decades. Herbal dietary supplements continued to experience strong sales growth in the United States in 2019, with an increase by 8.6% [82]. While a recent pilot survey in the UK showed that over 80% of participants used medicinal plants for multiple health benefits [83].
Cancer is one of the leading causes of death in the world, and it has caused the death of 9.9 million people in 2020 [84]. Cancers of the lung, breast, colorectum, liver, stomach, and prostate were listed as the top six cancers in causing mortality [84]. The use of complementary and alternative medicines (CAMs) by patients suffering from cancers is quite well documented [85]. Nowadays, many cancer patients use herbal medicines and natural products derived from plants or mushrooms to combat cancer, to strengthen the immune system, and to counter some possible side effects of the conventional treatment [86]. Approximately 60% of the anticancer agents that are currently available for clinical use or are in late stages of clinical trials are derived from natural products [87].
According to previous reviews, the most frequently used CAM modalities in Europe are herbal medicines [88]. In fact, herbal medicine is at the forefront of traditional medicine in Germany and Western Europe [89]. An Italian survey revealed that herbal medicine is frequently employed to improve quality of life [90]. Recent studies also showed that dissatisfaction with conventional medicine is the most important reason for the preferred use of herbal medicine in Germany [91]. In fact, a similar scenario has occurred with cancer patients worldwide. The use of Chinese herbal medicines (CHMs) as an adjuvant to cancer treatment is more frequent among cancer patients in Chinese communities, such as in China, Hong Kong, and Taiwan [59,92,93,94]. Plentiful clinical studies can be found on the efficacies of traditional Chinese medicines (TCMs), either combined with conventional cancer therapies or used alone, in cancer patients. The conclusions drawn from most of these studies suggest that, for cancer patients, TCM can be useful and effective, helping to control the disease, prolonging survival time, alleviating side effects of chemotherapy and radiotherapy, and improving the quality of life [95]. One systematic review summarized that traditional Chinese medicine preparations, combined with chemotherapy, may improve the objective response rates and disease control rates more than chemotherapy alone [96]. Throughout these years, pharmacognosists, pharmacologists, and biochemists are anticipating more and more scientific evidence on the efficacies of medicinal herbs, to be gained through modern pharmacological research approaches. This is also the mission of our institute. In this review, the preclinical studies of several medicinal herbs and mushrooms are chosen as examples. Their applications as adjuvant therapeutic agents in the management of cancer, particularly in the areas related to immunological support, are expected to gain increasing enthusiasm in future.
During the current COVID-19 pandemic, our research interests on the immunomodulatory effects of medicinal herbs have shifted to include their use as immuno-boosters for innate-immunological defense. Our efforts could be discussed in subsequent reports.
The academic editor of this journal, while reviewing this manuscript, brought forward the concern about the quality issues of the herbal materials and the methodological challenges associated with clinical studies. Indeed, the two issues deserve a separate review to reach a thorough recommendation. We tackle the quality issue through a stringent process: consisting of firstly acquiring the selected herbs from the most reliable seller, followed by comprehensive authentications, from gross examinations to chemical scrutiny; then storing the information in our regional herbal data authority to ensure that future repeated research material could be cross matched to prove identical criteria. With regard to clinical studies, we insist on taking reference from the clinical trial procedures so that safety comes first, followed by dosage identifications before the proper clinical trial could be planned.
Author Contributions
Medicinal herbal research: C.B.-S.L. and G.G.-L.Y.; clinical reports and whole review compilation: P.-C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This work was supported by the State Key Laboratory Fund provided by the Innovation and Technology Commission of Hong Kong.
Conflicts of Interest
The authors declare they have no conflict of interest.
References
- Wang, H.C. Ancent thoughts and practices related to immunology. China J. Immunol. 1993, 9, 237. (In Chinese) [Google Scholar]
- Kung, H.Y. Study on the immuno-pharmacology of 21 herbs. Pharmacol. Chin. Med. 1995, 30. [Google Scholar]
- Ragupathi, G.; Yeung, K.S.; Leung, P.C.; Lee, M.; Lau, C.B.S.; Vickers, A.; Hood, C.; Deng, G.; Cheung, N.K.; Cassileth, B.; et al. Evaluation of widely consumed botanicals as immunological adjuvants. Vaccine 2008, 26, 4860–4865. [Google Scholar] [CrossRef]
- Hong, F.; Xiao, W.; Ragupathi, G.; Lau, C.B.S.; Leung, P.C.; Yeung, K.S.; George, C.; Cassileth, B.; Kennelly, E.; Livingston, P.O. The known immunologically active components of Astragalus account for only a small proportion of the immunological adjuvant activity when combined with conjugate vaccines. Planta Med. 2011, 77, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.L.; Motley, T.J.; Unachukwu, U.J.; Lau, C.B.S.; Jiang, B.; Hong, F.; Leung, P.C.; Wang, Q.F.; Livingston, P.O.; Cassileth, B.R.; et al. Chemical and genetic assessment of variability in commercial Radix Astragali (Astragalus spp.) by ion trap LC-MS and nuclear ribosomal DNA barcoding sequence analyses. J. Agric. Food Chem 2011, 59, 1548–1556. [Google Scholar] [CrossRef]
- Yue, G.G.L.; Chan, B.C.; Hon, P.M.; Kennelly, E.J.; Yeung, S.K.; Cassileth, B.R.; Fung, K.P.; Leung, P.C.; Lau, C.B.S. Immunostimulatory activities of polysaccharide extract isolated from Curcuma longa. Int. J. Biol. Macromol. 2010, 47, 342–347. [Google Scholar] [CrossRef]
- Yue, G.G.L.; Chan, B.C.L.; Hon, P.M.; Lee, M.Y.H.; Fung, K.P.; Leung, P.C.; Lau, C.B.S. Evaluation of in vitro anti-proliferative and immunomodulatory activities of compounds isolated from Curcuma longa. Food Chem. Toxicol. 2010, 48, 2011–2020. [Google Scholar] [CrossRef]
- Li, L.F.; Chan, B.C.; Yue, G.G.L.; Lau, C.B.S.; Han, Q.B.; Leung, P.C.; Liu, J.K.; Fung, K.P. Two immunosuppressive compounds from the mushroom Rubinoboletus ballouii using human peripheral blood mononuclear cells by bioactivity-guided fractionation. Phytomedicine 2013, 20, 1196–1202. [Google Scholar] [CrossRef]
- Li, L.F.; Yue, G.G.L.; Chan, B.C.; Zeng, Q.; Han, Q.B.; Leung, P.C.; Fung, K.P.; Liu, J.K.; Lau, C.B.S. Rubinoboletus ballouii polysaccharides exhibited immunostimulatory activities through toll-like receptor-4 via NF-kappaB pathway. Phytother. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Kido, T.; Takaku, T.; Sumiyoshi, M.; Baba, K. Isolation of an anti-angiogenic substance from Agaricus blazei Murill: Its antitumor and antimetastatic actions. Cancer Sci. 2004, 95, 758–764. [Google Scholar] [CrossRef]
- Hetland, G.; Tangen, J.M.; Mahmood, F.; Mirlashari, M.R.; Nissen-Meyer, L.S.H.; Nentwich, I.; Therkelsen, S.P.; Tjonnfjord, G.E.; Johnson, E. Antitumor, anti-inflammatory and antiallergic effects of Agaricus blazei mushroom extract and the related medicinal Basidiomycetes mushrooms, Hericium erinaceus and Grifola frondosa: A review of preclinical and clinical studies. Nutrients 2020, 12, 1339. [Google Scholar] [CrossRef]
- Kim, C.F.; Jiang, J.J.; Leung, K.N.; Fung, K.P.; Lau, C.B.S. Inhibitory effects of Agaricus blazei extracts on human myeloid leukemia cells. J. Ethnopharmacol. 2009, 122, 320–366. [Google Scholar] [CrossRef] [PubMed]
- Hui, T.; Guo, L.; Wang, J.; Ito, H.; Simura, K. Clinical observation on treatment of acute nonlymphocytic leukemia with Agaricus blazei Murill. J. Lanzhou Med. Coll. 1994, 20, 169–171. [Google Scholar]
- Ahn, W.S.; Kim, D.J.; Chae, G.T.; Lee, J.M.; Bae, S.M.; Sin, J.I.; Kim, Y.W.; Namkoong, S.E.; Lee, I.P. Natural killer cell activity and quality of life were improved by consumption of a mushroom extract, Agaricus blazei Murill Kyowa, in gynecological cancer patients undergoing chemotherapy. Int, J. Gynecol. Cancer 2004, 14, 589–594. [Google Scholar] [CrossRef]
- Yoshimura, K.; Kamoto, T.; Ogawa, O.; Matsui, S.; Tsuchiya, N.; Tada, H.; Murata, K.; Yoshimura, K.; Habuchi, T.; Fukushima, M. Medical mushrooms used for biochemical failure after radical treatment for prostate cancer: An open-label study. Int. J. Urol. 2010, 17, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Mukai, H.; Watanabe, T.; Ando, M.; Katsumata, N. An alternative medicine, Agaricus blazei, may have induced severe hepatic dysfunction in cancer patients. Jpn, J. Clin. Oncol. 2006, 36, 808–810. [Google Scholar] [CrossRef]
- Yue, G.G.L.; Fung, K.P.; Tse, G.M.; Leung, P.C.; Lau, C.B.S. Comparative studies of various Ganoderma species and their different parts with regard to their antitumor and immunomodulating activities in vitro. J. Altern. Complement. Med. 2006, 12, 777–789. [Google Scholar] [CrossRef]
- Yue, G.G.L.; Lau, C.B.S.; Fung, K.P.; Leung, P.C. Studies on the anti-tumor activities of Ganoderma lucidum (Lingzhi): A comparison with two allied species and the different parts of their fruiting bodies. Environ. Mol. Mutagen. 2006, 47, 424. [Google Scholar]
- Yue, G.G.L.; Fung, K.P.; Leung, P.C.; Lau, C.B.S. Comparative studies on the immunomodulatory and antitumor activities of the different parts of fruiting body of Ganoderma lucidum and Ganoderma spores. Phytother. Res. 2008, 22, 1282–1291. [Google Scholar] [CrossRef]
- Yue, G.G.L.; Chan, B.C.; Han, X.Q.; Cheng, L.; Wong, E.C.; Leung, P.C.; Fung, K.P.; Ng, M.C.; Fan, K.; Sze, D.M.; et al. Immunomodulatory activities of Ganoderma sinense polysaccharides in human immune cells. Nutr. Cancer 2013, 65, 765–774. [Google Scholar] [CrossRef]
- Han, X.Q.; Chan, B.C.L.; Dong, C.X.; Yang, Y.H.; Ko, C.H.; Yue, G.G.L.; Chen, D.; Wong, C.K.; Lau, C.B.S.; Tu, P.F.; et al. Isolation, structure characterization, and immunomodulating activity of a hyperbranched polysaccharide from the fruiting bodies of Ganoderma sinense. J. Agr. Food Chem. 2012, 60, 4276–4281. [Google Scholar] [CrossRef]
- Han, X.Q.; Chan, B.C.; Yu, H.; Yang, Y.H.; Hu, S.Q.; Ko, C.H.; Dong, C.X.; Wong, C.K.; Shaw, P.C.; Fung, K.P.; et al. Structural characterization and immuno-modulating activities of a polysaccharide from Ganoderma sinense. Int. J. Biol. Macromol. 2012, 51, 597–603. [Google Scholar] [CrossRef]
- Han, X.Q.; Yue, G.G.L.; Yue, R.Q.; Dong, C.X.; Chan, C.L.; Ko, C.H.; Cheung, W.S.; Luo, K.W.; Dai, H.; Wong, C.K.; et al. Structure elucidation and immunomodulatory activity of a beta glucan from the fruiting bodies of Ganoderma sinense. PLoS ONE 2014, 9, e100380. [Google Scholar] [CrossRef]
- Chan, K.M.; Yue, G.G.L.; Li, P.; Wong, E.C.; Lee, J.K.; Kennelly, E.J.; Lau, C.B.S. Screening and analysis of potential anti-tumor components from the stipe of Ganoderma sinense using high-performance liquid chromatography/time-of-flight mass spectrometry with multivariate statistical tool. J. Chromatogr. A 2017, 1487, 162–167. [Google Scholar] [CrossRef]
- Jin, X.; Ruiz Beguerie, J.; Sze, D.M.; Chan, G.C. Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database Syst. Rev. 2016, 4, CD007731. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Q.; Zhao, L.; Huang, X.; Wang, J.; Kang, X. Spore powder of Ganoderma lucidum improves cancer-related fatigue in breast cancer patients undergoing endocrine therapy: A pilot clinical trial. Evid Based Complement. Alternat. Med. 2012, 2012, 809614. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.A.; Klukowska-Rotzler, J.; Schenk-Jaeger, K.M.; Kupferschmidt, H.; Exadaktylos, A.K.; Lehmann, B.; Liakoni, E. Mushroom poisoning-a 17 year retrospective study at a level I university emergency department in Switzerland. Int. J. Environ. Res. Public Health 2018, 15, 2855. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Weinstein, S.A.; De Haro, L.; Bedry, R.; Schaper, A.; Rumack, B.H.; Zilker, T. Mushroom poisoning: A proposed new clinical classification. Toxicon 2019, 157, 53–65. [Google Scholar] [CrossRef]
- Lemieszek, M.K.; Ribeiro, M.; Guichard Alves, H.; Marques, G.; Nunes, F.M.; Rzeski, W. Boletus edulis ribonucleic acid—A potent apoptosis inducer in human colon adenocarcinoma cells. Food Funct. 2016, 7, 3163–3175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, Y.; Duan, X.; Tang, T.; Shen, Y.; Hu, B.; Liu, A.; Chen, H.; Li, C.; Liu, Y. Characterization and antioxidant activities of polysaccharides from thirteen Boletus mushrooms. Int. J. Biol. Macromol. 2018, 113, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Meng, B.; Li, L.; Wang, Y.; Zhang, Y.; Fang, X.; Wang, D. Boletus aereus protects against acute alcohol-induced liver damage in the C57BL/6 mouse via regulating the oxidative stress-mediated NF-kappaB pathway. Pharm. Biol. 2020, 58, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.M.; Yue, G.G.L.; Chan, Y.Y.; Kwok, H.F.; Gao, S.; Wong, C.W.; Lau, C.B.S. A review on the immunomodulatory activity of Acanthopanax senticosus and its active components. Chin. Med. 2019, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.G.L.; Kwok, H.F.; Lee, J.K.M.; Jiang, L.; Chan, K.M.; Cheng, L.; Wong, E.C.W.; Leung, P.C.; Fung, K.P.; Lau, C.B.S. Novel anti-angiogenic effects of aromatic-turmerone, essential oil isolated from spice turmeric. J. Funct. Foods 2015, 15, 243–253. [Google Scholar] [CrossRef]
- Yue, G.G.L.; Cheng, S.W.; Yu, H.; Xu, Z.S.; Lee, J.K.M.; Hon, P.M.; Lee, M.Y.H.; Kennelly, E.J.; Deng, G.; Yeung, S.K.; et al. The role of turmerones on curcumin transportation and p-glycoprotein activities in intestinal Caco-2 cells. J. Med. Food 2012, 15, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.G.L.; Jiang, L.; Kwok, H.F.; Lee, J.K.M.; Chan, K.M.; Fung, K.P.; Leung, P.C.; Lau, C.B.S. Turmeric ethanolic extract possesses stronger inhibitory activities on colon tumour growth than curcumin—The importance of turmerones. J. Funct. Foods 2016, 22, 565–577. [Google Scholar] [CrossRef]
- Yue, G.G.L.; Kwok, H.F.; Lee, J.K.M.; Jiang, L.; Wong, E.C.W.; Gao, S.; Wong, H.L.; Li, L.; Chan, K.M.; Leung, P.C.; et al. Combined therapy using bevacizumab and turmeric ethanolic extract (with absorbable curcumin) exhibited beneficial efficacy in colon cancer mice. Pharmacol. Res. 2016, 111, 43–57. [Google Scholar] [CrossRef]
- Li, M.; Yue, G.G.L.; Tsui, S.K.; Fung, K.P.; Lau, C.B.S. Turmeric extract, with absorbable curcumin, has potent anti-metastatic effect in vitro and in vivo. Phytomedicine 2018, 46, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yue, G.G.L.; Luo, L.; Tsui, S.K.W.; Fung, K.P.; Ng, S.S.M.; Lau, C.B.S. Turmeric is therapeutic in vivo on patient-derived colorectal cancer xenografts: Inhibition of growth, metastasis, and tumor recurrence. Front. Oncol. 2021, 10, 574827. [Google Scholar] [CrossRef]
- Lau, C.B.S.; Cheng, L.; Cheng, B.W.; Yue, G.G.L.; Wong, E.C.; Lau, C.P.; Leung, P.C.; Fung, K.P. Development of a simple chromatographic method for distinguishing between two easily confused species, Hedyotis diffusa and Hedyotis corymbosa. Nat. Prod. Res. 2012, 26, 1446–1450. [Google Scholar] [CrossRef]
- Yue, G.G.L.; Lee, J.K.M.; Cheng, L.; Chan, B.C.L.; Jiang, L.; Fung, K.P.; Leung, P.C.; Lau, C.B.S. Reversal of P-glycoprotein-mediated multidrug resistance in human hepatoma cells by hedyotiscone A, a compound isolated from Hedyotis corymbosa. Xenobiotica 2012, 42, 562–570. [Google Scholar] [CrossRef]
- Yue, G.G.L.; Lee, J.K.; Kwok, H.F.; Cheng, L.; Wong, E.C.; Jiang, L.; Yu, H.; Leung, H.W.; Wong, Y.L.; Leung, P.C.; et al. Novel PI3K/AKT targeting anti-angiogenic activities of 4-vinylphenol, a new therapeutic potential of a well-known styrene metabolite. Sci. Rep. 2015, 5, 11149. [Google Scholar] [CrossRef]
- Chan, J.Y.; Tang, P.M.; Hon, P.M.; Au, S.W.; Tsui, S.K.; Waye, M.M.; Kong, S.K.; Mak, T.C.; Fung, K.P. Pheophorbide a, a major antitumor component purified from Scutellaria barbata, induces apoptosis in human hepatocellular carcinoma cells. Planta Med. 2006, 72, 28–33. [Google Scholar] [CrossRef]
- Tang, P.M.; Chan, J.Y.; Au, S.W.; Kong, S.K.; Tsui, S.K.; Waye, M.M.; Mak, T.C.; Fong, W.P.; Fung, K.P. Pheophorbide a, an active compound isolated from Scutellaria barbata, possesses photodynamic activities by inducing apoptosis in human hepatocellular carcinoma. Cancer Biol. Ther. 2006, 5, 1111–1116. [Google Scholar] [CrossRef]
- Hoi, S.W.; Wong, H.M.; Chan, J.Y.; Yue, G.G.; Tse, G.M.; Law, B.K.; Fong, W.P.; Fung, K.P. Photodynamic therapy of Pheophorbide a inhibits the proliferation of human breast tumour via both caspase-dependent and -independent apoptotic pathways in in vitro and in vivo models. Phytother. Res. 2012, 26, 734–742. [Google Scholar] [CrossRef]
- Yue, G.G.L.; Chan, Y.Y.; Liu, W.; Gao, S.; Wong, C.W.; Lee, J.K.; Lau, K.M.; Lau, C.B.S. Effectiveness of Scutellaria barbata water extract on inhibiting colon tumor growth and metastasis in tumor-bearing mice. Phytother. Res. 2021, 35, 361–373. [Google Scholar] [CrossRef]
- Shen, M.L.; Zhai, S.K.; Chen, H.L.; Luo, Y.D.; Tu, G.R.; Ou, D.W. Immunomopharmacological effects of polysaccharides from Acanthopanax senticosus on experimental animals. Int. J. Immunopharmacol. 1991, 13, 549–554. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Yu, L.; Mo, T.; Wu, Q.; Zhou, Z. Aidi injection plus platinum-based chemotherapy for stage IIIB/IV non-small cell lung cancer: A meta-analysis of 42 RCTs following the PRISMA guidelines. J. Ethnopharmacol. 2018, 221, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Wang, C.; Zhou, M.; Hu, S.; Jiang, Y.; Huang, X.; Li, N.; Feng, J.; Tang, F.; Chen, X.; et al. Clinical efficacy and safety of Aidi injection plus paclitaxel-based chemotherapy for advanced non-small cell lung cancer: A meta-analysis of 31 randomized controlled trials following the PRISMA guidelines. J. Ethnopharmacol. 2019, 228, 110–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Guo, S.; Lin, W.; Zhang, B.; Chen, X.; Mo, H.; Zhan, T. The clinical efficacy and safety of the Chinese herbal medicine Astragalus (Huangqi) preparation for the treatment of acute myocardial infarction: A systematic review of randomized controlled trials. Medicine 2019, 98, e15256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shergis, J.L.; Yang, L.; Zhang, A.L.; Guo, X.; Zhang, L.; Zhou, S.; Zeng, L.; Mao, W.; Xue, C.C. Astragalus membranaceus (Huang Qi) as adjunctive therapy for diabetic kidney disease: An updated systematic review and meta-analysis. J. Ethnopharmacol. 2019, 239, 111921. [Google Scholar] [CrossRef]
- Dugoua, J.J.; Wu, P.; Seely, D.; Eyawo, O.; Mills, E. Astragalus-containing Chinese herbal combinations for advanced non-small-cell lung cancer: A meta-analysis of 65 clinical trials enrolling 4751 patients. Lung Cancer 2010, 1, 85–100. [Google Scholar]
- Guo, L.; Bai, S.P.; Zhao, L.; Wang, X.H. Astragalus polysaccharide injection integrated with vinorelbine and cisplatin for patients with advanced non-small cell lung cancer: Effects on quality of life and survival. Med. Oncol. 2012, 29, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Lin, C.Y.; Hsu, C.L.; Fan, K.H.; Huang, S.F.; Liao, C.T.; Lee, L.Y.; Ng, S.K.; Yen, T.C.; Chang, J.T.; et al. Incorporation of Astragalus polysaccharides injection during concurrent chemoradiotherapy in advanced pharyngeal or laryngeal squamous cell carcinoma: Preliminary experience of a phase II double-blind, randomized trial. J. Cancer Res. Clin. Oncol. 2020, 146, 33–41. [Google Scholar] [CrossRef]
- Gupta, N.; Verma, K.; Nalla, S.; Kulshreshtha, A.; Lall, R.; Prasad, S. Free radicals as a double-edged sword: The cancer preventive and therapeutic roles of curcumin. Molecules 2020, 25, 5390. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.S.; Hussain, M.B.; Sultan, M.T.; Arshad, M.S.; Waheed, M.; Shariati, M.A.; Plygun, S.; Hashempur, M.H. Biochemistry, safety, pharmacological activities, and clinical applications of turmeric: A mechanistic review. Evid Based Complement. Alternat. Med. 2020, 2020, 7656919. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocinska, K.; Zielinska, D.; et al. Turmeric and its major compound curcumin on health: Bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, M.; Schaffer, P.M.; Bar-Sela, G. An update on Curcuma as a functional food in the control of cancer and inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 605–611. [Google Scholar] [CrossRef] [PubMed]
- White, C.M.; Pasupuleti, V.; Roman, Y.M.; Li, Y.; Hernandez, A.V. Oral turmeric/curcumin effects on inflammatory markers in chronic inflammatory diseases: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019, 146, 104280. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Stojanovic-Radic, Z.; Matejic, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Rasoulpoor, S.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef]
- Niu, Y.; Meng, Q.X. Chemical and preclinical studies on Hedyotis diffusa with anticancer potential. J. Asian Nat. Prod. Res. 2013, 15, 550–565. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; He, J.; Tong, X.; Tang, L.; Liu, M. The Hedyotis diffusa Willd. (Rubiaceae): A review on phytochemistry, pharmacology, quality control and pharmacokinetics. Molecules 2016, 21, 710. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.T.; Chang, T.T.; Muo, C.H.; Wu, M.Y.; Sun, M.F.; Yeh, C.C.; Yen, H.R. Use of complementary traditional Chinese medicines by adult cancer patients in Taiwan: A nationwide population-based study. Integr. Cancer Ther. 2018, 17, 531–541. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Chen, H.Y.; Yang, S.H.; Lin, Y.H.; Chiu, J.H.; Lin, Y.H.; Chen, J.L. Hedyotis diffusa combined with Scutellaria barbata are the core treatment of Chinese herbal medicine used for breast cancer patients: A population-based study. Evid Based Complement. Alternat. Med. 2014, 2014, 202378. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.R.; Chen, M.Y.; Wang, M.D. Treatment of chronic endometritis by penning granule: A clinical randomized controlled trial. Zhongguo Zhong Xi Yi Jie He Za Zhi 2016, 36, 1055–1060. (In Chinese) [Google Scholar]
- Chen, Q.; Rahman, K.; Wang, S.J.; Zhou, S.; Zhang, H. Scutellaria barbata: A review on chemical constituents, pharmacological activities and clinical applications. Curr. Pharm. Des. 2020, 26, 160–175. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.; Shtivelman, E.; Perez, A.; Vogel, C.; Franco, S.; Tan Chiu, E.; Melisko, M.; Tagliaferri, M.; Cohen, I.; Shoemaker, M.; et al. Phase I trial and antitumor effects of BZL101 for patients with advanced breast cancer. Breast Cancer Res. Treat. 2007, 105, 17–28. [Google Scholar] [CrossRef]
- Perez, A.T.; Arun, B.; Tripathy, D.; Tagliaferri, M.A.; Shaw, H.S.; Kimmick, G.G.; Cohen, I.; Shtivelman, E.; Caygill, K.A.; Grady, D.; et al. A phase 1B dose escalation trial of Scutellaria barbata (BZL101) for patients with metastatic breast cancer. Breast Cancer Res. Treat. 2010, 120, 111–118. [Google Scholar] [CrossRef]
- Li, X.Y. Advances in Immunomodulatin studies of PSP. In Advanced Research in PSP 1999; Yang, Q.Y., Ed.; Winsor Health Products Ltd.: Hong Kong, China, 1999. [Google Scholar]
- Wong, C.K.; Tse, P.S.; Wong, E.L.Y.; Leung, P.C.; Fung, K.P.; Lam, C.W.K. Immunomodulatory effects of Yunzhi and Danshen capsules in health subjects—A randomized, double-blind, placebo-controlled, crossover study. Int. Immunopharmacol. 2004, 4, 201–211. [Google Scholar] [CrossRef]
- Bao, Y.X.; Wong, C.K.; Leung, S.F.; Chan, A.T.C.; Li, P.W.; Wong, E.L.Y.; Leung, P.C.; Fung, K.P.; Yin, Y.B.; Lam, C.W.K. Clinical studies of immunomodulatory activities of Yunzhi-Danshen in patients with nasopharyngeal carcinoma. J. Altern. Complement. Med. 2006, 12, 771–776. [Google Scholar] [CrossRef]
- Wong, C.K.; Bao, Y.X.; Wong, E.L.Y.; Leung, P.C.; Fung, K.P.; Lam, C.W.K. Immunomodulatory activities of Yunzhi and Danshen in post-treatment breast cancer patients. Am. J. Chin. Med. 2005, 33, 381–395. [Google Scholar] [CrossRef]
- Wai, S.Y. Influence of Astragalus on the thymes of mice of University of Pharmacology. Am. J. Chin. Med. 1991, 22, 359. [Google Scholar]
- Wang, S.L. Effects of 8 herbal extracts on the intraperitoneal macrophages. J. Bai J.Y. Univ. 1990, 16, 325. [Google Scholar]
- Ko, S.T. Five anti-aging herbal extracts acting on T cells multiplication and IL2 production. J. China Univ. Pharmacol. 1990, 21, 43. [Google Scholar]
- Yang, G.Z.; Bao, T.; Geng, P.L. Immunoregulatory effects of ginsenoside in vivo and in vitro. J. Tradit. Chin. Med. 1986, 6, 191–194. [Google Scholar]
- Wang, B.K. Effect of Lycuim barbarum polysaccharides on the immune responses of T, CTL and NK cells in normal and cyclophosphamide treated mice. China J. Pharmacol. Toxicol. 1990, 4, 39. [Google Scholar]
- Lei, L.S. Effects of Ganoderma polysaccharides on T cell subpopulation and production of IL2 in mixed lymphocyte responses. Aceta Pharm Sin. 1992, 27, 331. [Google Scholar]
- Chou, K.Y. Human immune suppression is inducable by trichosanthin via CD8 cell mediated pathway. J. Cell Res. 1994, 4, 17. [Google Scholar] [CrossRef][Green Version]
- Sasaki, H.; Nishimura, H.; Morota, T.; Chin, M.; Mitsuhashi, H.; Komatsu, Y.; Maruyama, H.; Guo-Rui, T.; Wei, H.; Yu-Lang, X. Immunosuppressive principles of Rehmannia glutinosa var. hueichingensis. Planta Med. 1989, 55, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Allkin, B. Medicines: At least 28,187 plant species are currently recorded as being of medicinal use. In State of the World’s Plants 2017; Willis, K.J., Ed.; Royal Botanic Kew Gardens: London, UK, 2017. [Google Scholar]
- Smith, T.; May, G.; Eckl, V.; Reynolds, C.M. US Sales of Herbal Supplements Increase by 8.6% in 2019. HerbalGram 2020, 127, 54–69. [Google Scholar]
- Zahn, R.; Perry, N.; Perry, E.; Mukaetova-Ladinska, E.B. Use of herbal medicines: Pilot survey of UK users’ views. Complement. Ther. Med. 2019, 44, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.fr/today (accessed on 26 January 2021).
- Deng, G.; Cassileth, B. Complementary or alternative medicine in cancer care-myths and realities. Nat. Rev. Clin. Oncol 2013, 10, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Werneke, U.; Earl, J.; Seydel, C.; Horn, O.; Crichton, P.; Fannon, D. Potential health risks of complementary alternative medicines in cancer patients. Br. J. Cancer 2004, 90, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Eardley, S.; Bishop, F.L.; Prescott, P.; Cardini, F.; Brinkhaus, B.; Santos-Rey, K.; Vas, J.; von Ammon, K.; Hegyi, G.; Dragan, S.; et al. A systematic literature review of complementary and alternative medicine prevalence in EU. Forsch Komplementmed 2012, 19, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Enioutina, E.Y.; Salis, E.R.; Job, K.M.; Gubarev, M.I.; Krepkova, L.V.; Sherwin, C.M. Herbal Medicines: Challenges in the modern world. Part 5. status and current directions of complementary and alternative herbal medicine worldwide. Expert Rev. Clin. Pharmacol. 2017, 10, 327–338. [Google Scholar] [CrossRef]
- Menniti-Ippolito, F.; Gargiulo, L.; Bologna, E.; Forcella, E.; Raschetti, R. Use of unconventional medicine in Italy: A nation-wide survey. Eur, J. Clin. Pharmacol. 2002, 58, 61–64. [Google Scholar] [CrossRef]
- Welz, A.N.; Emberger-Klein, A.; Menrad, K. Why people use herbal medicine: Insights from a focus-group study in Germany. BMC Complement. Altern Med. 2018, 18, 92. [Google Scholar] [CrossRef]
- Lam, Y.C.; Cheng, C.W.; Peng, H.; Law, C.K.; Huang, X.; Bian, Z. Cancer patients’ attitudes towards Chinese medicine: A Hong Kong survey. Chin. Med. 2009, 4, 25. [Google Scholar] [CrossRef]
- Lu, C.L.; Li, X.; Zhou, H.M.; Zhang, C.; Yang, Y.Y.; Feng, R.L.; Long, C.J.; Deng, F.Y.; Li, J.C.; Cao, Z.M.; et al. Randomised controlled trials of traditional Chinese medicine in cancer care published in Chinese: An overview. Lancet 2019, 394, S26. [Google Scholar] [CrossRef]
- So, T.H.; Chan, S.K.; Lee, V.H.; Chen, B.Z.; Kong, F.M.; Lao, L.X. Chinese medicine in cancer treatment—how is it practised in the east and the west? Clin. Oncol. 2019, 31, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Zhao, C.; Deng, L.I.; Chen, J.; Yu, B.; Wu, X.; Pang, P.; Chen, X. Efficacy of traditional Chinese medicine in treating cancer. Biomed. Rep. 2016, 4, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, Y.; Fang, C.; Zhao, L.; Lin, L.; Lu, L. Traditional Chinese medicine preparation combined therapy may improve chemotherapy efficacy: A systematic review and meta-analysis. Evid. Based Complement. Alternat. Med. 2019, 5015824. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).