Establishment of a Rapid Micropropagation System for Kaempferia parviflora Wall. Ex Baker: Phytochemical Analysis of Leaf Extracts and Evaluation of Biological Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. In Vitro Micropropagation
2.1.1. Surface Sterilization
2.1.2. Shoot Multiplication
2.1.3. Rooting and Acclimatization
2.2. Phytochemical Compositions
2.3. Antioxidant Ability
2.4. Enzyme Inhibitory Effects
3. Materials and Methods
3.1. In Vitro Micropropagation
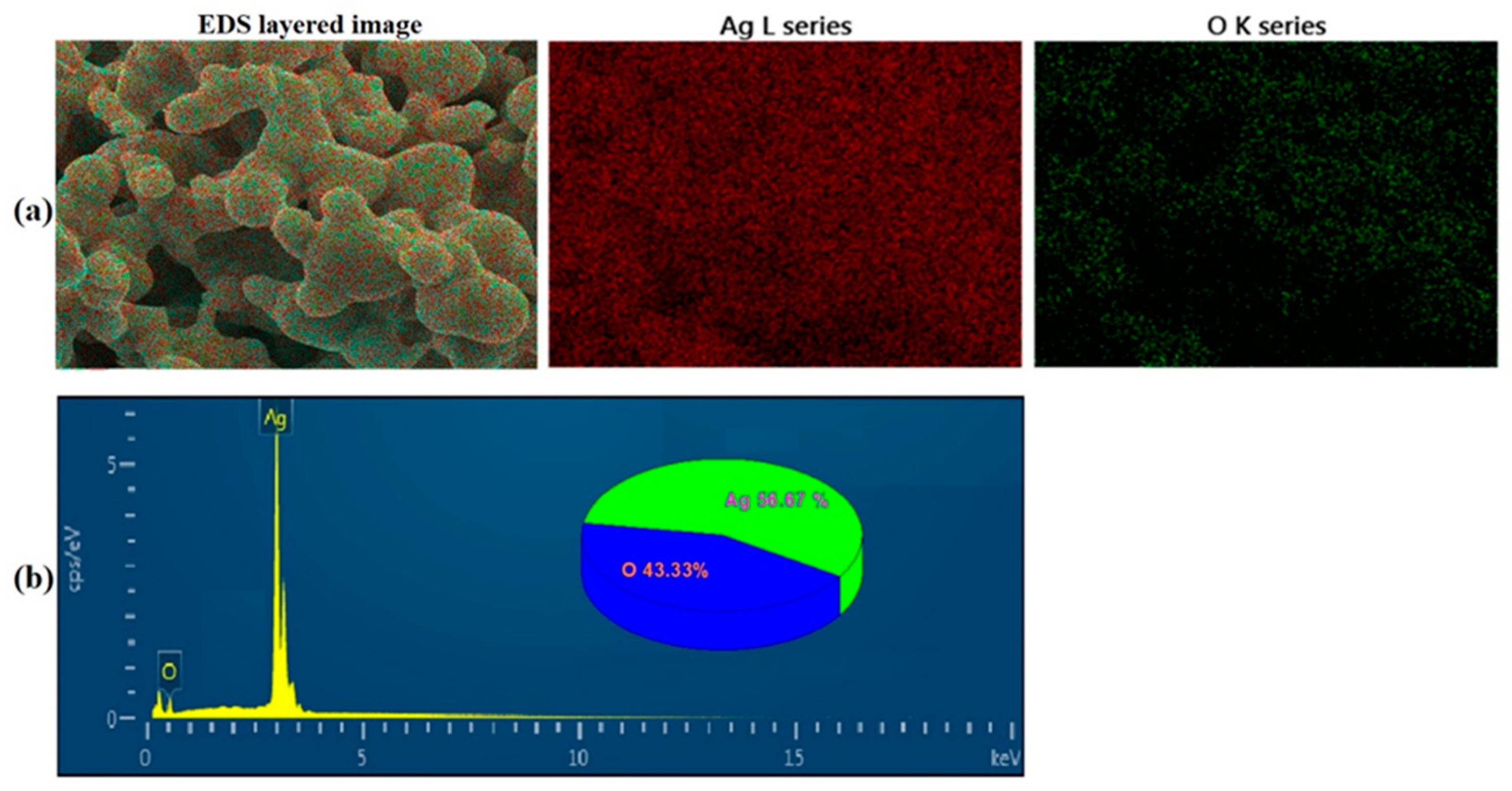
3.1.1. Synthesis and Characterization of Silver Oxide Nanoparticles (AgO NPs)
3.1.2. Plant Materials and Surface Decontamination
3.1.3. Shoot Multiplication
3.1.4. Rooting and Acclimatization
3.2. Phytochemical Analysis
3.2.1. Extract Preparation
3.2.2. Estimation of Total Phenolics Content (TPC) and Flavonoids Content (TFC)
3.2.3. Chemical Characterization
3.3. Biological Activities of K. parviflora Leaf Extracts
3.3.1. Antioxidant Assay
3.3.2. Enzyme Inhibition Assay
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yenjai, C.; Prasanphen, K.; Daodee, S.; Wongpanich, V.; Kittakoop, P. Bioactive flavonoids from Kaempferia parviflora. Fitoterapia 2004, 75, 89–92. [Google Scholar] [CrossRef]
- Rujjanawate, C.; Kanjanapothi, D.; Amornlerdpison, D.; Pojanagaroon, S. Anti-gastric ulcer effect of Kaempferia parviflora. J. Ethnopharmacol. 2005, 102, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Akase, T.; Shimada, T.; Terabayashi, S.; Ikeya, Y.; Sanada, H.; Aburada, M. Antiobesity effects of Kaempferia parviflora in spontaneously obese type II diabetic mice. J. Nat. Med. 2011, 65, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.P.; Luyen, B.T.T.; Lee, S.H.; Jang, H.D.; Kim, Y.H. Anti-osteoporotic and antioxidant activities by rhizomes of Kaempferia parviflora wall. Ex Baker. Nat. Prod. Sci. 2016, 22, 13–19. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kato, T.; Azuma, T.; Kikuzaki, H.; Abe, K. Anti-allergenic activity of polymethoxyflavones from Kaempferia parviflora. J. Funct. Foods 2015, 13, 100–107. [Google Scholar] [CrossRef]
- Paramee, S.; Sookkhee, S.; Sakonwasun, C.; Na Takuathung, M.; Mungkornasawakul, P.; Nimlamool, W.; Potikanond, S. Anti-cancer effects of Kaempferia parviflora on ovarian cancer SKOV3 cells. BMC Complement. Altern. Med. 2018, 18, 178. [Google Scholar]
- Sawasdee, P.; Sabphon, C.; Sitthiwongwanit, D.; Kokpol, U. Anticholinesterase activity of 7-methoxyflavones isolated from Kaempferia parviflora. Phytother. Res. 2009, 23, 1792–1794. [Google Scholar] [CrossRef]
- Tewtrakul, S.; Subhadhirasakul, S.; Karalai, C.; Ponglimanont, C.; Cheenpracha, S. Anti-inflammatory effects of compounds from Kaempferia parviflora and Boesenbergia pandurata. Food Chem. 2009, 115, 534–538. [Google Scholar] [CrossRef]
- Yoshino, S.; Awa, R.; Miyake, Y.; Fukuhara, I.; Sato, H.; Ashino, T.; Tomita, S.; Kuwahara, H. Daily intake of Kaempferia parviflora extract decreases abdominal fat in overweight and preobese subjects: A randomized, double-blind, placebo-controlled clinical study. Diabetes Metab. Syndr. Obes. 2018, 11, 447–458. [Google Scholar] [CrossRef]
- Azuma, T.; Kayano, S.-I.; Matsumura, Y.; Konishi, Y.; Tanaka, Y.; Kikuzaki, H. Antimutagenic and α-glucosidase inhibitory effects of constituents from Kaempferia parviflora. Food Chem. 2011, 125, 471–475. [Google Scholar] [CrossRef]
- Sutthanut, K.; Sripanidkulchai, B.; Yenjai, C.; Jay, M. Simultaneous identification and quantitation of 11 flavonoid constituents in Kaempferia parviflora by gas chromatography. J. Chromatogr. A 2007, 1143, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Nakao, K.; Murata, K.; Deguchi, T.; Itoh, K.; Fujita, T.; Higashino, M.; Yoshioka, Y.; Matsumura, S.-I.; Tanaka, R.; Shinada, T. Xanthine oxidase inhibitory activities and crystal structures of methoxyflavones from Kaempferia parviflora rhizome. Biol. Pharm. Bull. 2011, 34, 1143–1146. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Horigome, S.; Maeda, M.; Ho, H.-J.; Shirakawa, H.; Komai, M. Effect of Kaempferia parviflora extract and its polymethoxyflavonoid components on testosterone production in mouse testis-derived tumour cells. J. Funct. Foods 2016, 26, 529–538. [Google Scholar] [CrossRef]
- Ochiai, W.; Kobayashi, H.; Kitaoka, S.; Kashiwada, M.; Koyama, Y.; Nakaishi, S.; Nagai, T.; Aburada, M.; Sugiyama, K. Effect of the active ingredient of Kaempferia parviflora, 5, 7-dimethoxyflavone, on the pharmacokinetics of midazolam. J. Nat. Med. 2018, 72, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Tanaka, Y.; Kikuzaki, H. Phenolic glycosides from Kaempferia parviflora. Phytochemistry 2008, 69, 2743–2748. [Google Scholar] [CrossRef]
- Chaipech, S.; Morikawa, T.; Ninomiya, K.; Yoshikawa, M.; Pongpiriyadacha, Y.; Hayakawa, T.; Muraoka, O. Structures of two new phenolic glycosides, kaempferiaosides A and B, and hepatoprotective constituents from the rhizomes of Kaempferia parviflora. Chem. Pharm. Bull. 2012, 60, 62–69. [Google Scholar] [CrossRef]
- Pripdeevech, P.; Pitija, K.; Rujjanawate, C.; Pojanagaroon, S.; Kittakoop, P.; Wongpornchai, S. Adaptogenic-active components from Kaempferia parviflora rhizomes. Food Chem. 2012, 132, 1150–1155. [Google Scholar] [CrossRef]
- Sulaiman, M.R.; Zakaria, Z.A.; Duad, I.A.; Hidayat, M.T. Antinociceptive and anti-inflammatory activities of the aqueous extract of Kaempferia galanga leaves in animal models. J. Nat. Med. 2008, 62, 221–227. [Google Scholar] [CrossRef]
- Ali, M.S.; Dash, P.R.; Nasrin, M. Study of sedative activity of different extracts of Kaempferia galanga in Swiss albino mice. BMC Complementary Altern. Med. 2015, 15, 158. [Google Scholar] [CrossRef]
- Labrooy, C.D.; Abdullah, T.L.; Abdullah, N.A.P.; Stanslas, J. Optimum shade enhances growth and 5,7-dimethoxyflavone accumulation in Kaempferia parviflora Wall. ex Baker cultivars. Sci. Hortic. 2016, 213, 346–353. [Google Scholar] [CrossRef]
- Prathanturarug, S.; Apichartbutra, T.; Chuakul, W.; Saralamp, P. Mass propagation of Kaempferia parviflora Wall. ex Baker by in vitro regeneration. J. Hort. Sci. Biotechnol. 2007, 82, 179–183. [Google Scholar] [CrossRef]
- Labrooy, C.; Abdullah, T.L.; Stanslas, J. Influence of N6-benzyladenine and sucrose on in vitro direct regeneration and microrhizome induction of Kaempferia parviflora Wall. ex Baker, an important ethnomedicinal herb of Asia. Trop. Life Sci. Res. 2020, 31, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Chirangini, P.; Sinha, S.K.; Sharma, G.J. In vitro propagation and microrhizome induction in Kaempferia galanga Linn. and K. rotunda Linn. Indian J. Biotech. 2005, 4, 404–408. [Google Scholar]
- Mohanty, S.; Parada, R.; Singh, S.; Joshi, R.K.; Subudhi, E.; Nabak, S. Biochemical and molecular profiling of micropropagated and conventionally grown Kaempferia galanga. Plant Cell Tissue Organ Cult. 2011, 106, 39–46. [Google Scholar] [CrossRef]
- Zuraida, A.R.; Izzati, K.F.L.; Nazreena, O.A.; Omar, N. In vitro microrhizome formation in Kaempferia parviflora. Annu. Res. Rev. Biol. 2015, 5, 460–467. [Google Scholar] [CrossRef]
- Zuraida, A.R.; Nazreena, O.A.; Izzati, K.F.L.; Aziz, A. Establishment and optimization growth of shoot buds-derived callus and suspension cell cultures of Kaempferia parviflora. Am. J. Plant Sci. 2014, 5, 2693. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kim, D.H.; Gopal, J.; Sivanesan, I. Nanomaterials in plant tissue culture: The disclosed and undisclosed. RSC Adv. 2017, 7, 36492–36505. [Google Scholar] [CrossRef]
- Kim, D.H.; Enkhtaivan, G.; Saini, R.K.; Keum, Y.S.; Kang, K.W.; Sivanesan, I. Production of bioactive compounds in cladode culture of Turbinicarpus valdezianus (H.Moeller) Glass & R. C. Foster. Ind. Crop. Prod. 2019, 138, 111491. [Google Scholar]
- Kang, H.; Kang, K.W.; Kim, D.H.; Sivanesan, I. In vitro propagation of Gastrochilus matsuran (Makino) Schltr., an endangered epiphytic orchid. Plants 2020, 9, 524. [Google Scholar] [CrossRef]
- Song, K.; Sivanesan, I.; Ak, G.; Zengin, G.; Cziáky, Z.; Jekő, J.; Rengasamy, K.R.R.; Lee, O.N.; Kim, D.H. Screening of bioactive metabolites and biological activities of calli, shoots, and seedlings of Mertensia maritima (L.) Gray. Plants 2020, 9, 1551. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.M.; Ghosh, B. Micropropagation of Kaempferia angustifolia Roscoe: An aromatic, essential oil yielding, underutilized medicinal plant of Zingiberaceae family. J. Crop Sci. Biotechnol. 2018, 21, 147–153. [Google Scholar] [CrossRef]
- Leifert, C.; Cassells, A.C. Microbial hazards in plant tissue and cell cultures. In vitro Cell. Dev. Biol. Plant 2001, 37, 133–138. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomedicine 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Arab, M.; Yadollahi, M.; Hosseini-Mazinani, A.; Bagheri, S. Effects of antimicrobial activity of silver nanoparticles on in vitro establishment of G × N15 (hybrid of almond × peach) rootstock. J. Genet. Eng. Biotechnol. 2014, 12, 103–110. [Google Scholar] [CrossRef]
- McShan, D.; Ray, P.C.; Yu, H. Molecular toxicity mechanism of nanosilver. J. Food Drug Anal. 2014, 22, 116–127. [Google Scholar] [CrossRef]
- Sarmast, M.; Salehi, H.; Khosh-Khui, M. Nano silver treatment is effective in reducing bacterial contaminations of Araucaria excelsa R. Br. var. glauca explants. Acta Biol. Hung. 2011, 62, 477–484. [Google Scholar] [CrossRef]
- Ahlawat, J.; Sehrawat, A.R.; Choudhary, R.; Yadav, S.H. Biologically synthesized silver nanoparticles eclipse fungal and bacterial contamination in micropropagation of Capparis decidua (FORSK.) Edgew: A substitute to toxic substances. Indian J. Exp. Biol. 2020, 58, 336–343. [Google Scholar]
- Abdi, G.; Salehi, H.; Khosh-Khui, M. Nano silver: A novel nanomaterial for removal of bacterial contaminants in valerian (Valeriana officinalis L.) tissue culture. Acta Physiol. Plant. 2008, 30, 709–714. [Google Scholar] [CrossRef]
- Song, K.; Kim, D.H.; Sivanesan, I. Effect of plant growth regulators on micropropagation of Hosta minor (Baker) Nakai through shoot tip culture. Propag. Ornam. Plants 2020, 20, 57–62. [Google Scholar]
- Kim, D.H.; Sivanesan, I. Somatic embryogenesis in Hosta minor (Baker) Nakai. Propag. Ornam. Plants 2019, 19, 24–29. [Google Scholar]
- Arigundam, U.; Variyath, A.M.; Siow, Y.L.; Marshall, D.; Debnath, S.C. Liquid culture for efficient in vitro propagation of adventitious shoots in wild Vaccinium vitis-idaea ssp. minus (lingonberry) using temporary immersion and stationary bioreactors. Sci. Hortic. 2020, 264, 109199. [Google Scholar] [CrossRef]
- Chithra, M.; Martin, K.P.; Sunandakumari, C.; Madhusoodanan, P.V. Protocol for rapid propagation and to overcome delayed rhizome formation in field established in vitro derived plantlets of Kaempferia galanga L. Sci. Hortic. 2005, 104, 113–120. [Google Scholar] [CrossRef]
- Mohanty, P.; Behera, S.; Swain, S.S.; Barik, D.P.; Naik, S.K. Micropropagation of Hedychium coronarium (J.) Koenig through rhizome bud. Physiol. Mol. Biol. Plants 2013, 19, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Kamila, P.K.; Rout, K.K.; Barik, D.P.; Panda, P.C.; Naik, S.K. An efficient plant regeneration protocol of an industrially important plant, Hedychium coronarium J. Koenig and establishment of genetic and biochemical fidelity of the regenerants. Ind. Crop. Prod. 2018, 126, 58–68. [Google Scholar] [CrossRef]
- Parida, R.; Mohanty, S.; Nayak, S. In vitro plant regeneration potential of genetically stable Globba marantina L., Zingiberaceous species and its conservation. Proc. Acad. Sci. India Sect. B 2018, 88, 321–327. [Google Scholar] [CrossRef]
- Jena, S.; Ray, A.; Sahoo, A.; Sahoo, S.; Kar, B.; Panda, P.C.; Nayak, S. High-frequency clonal propagation of Curcuma angustifolia ensuring genetic fidelity of micropropagated plants. Plant Cell Tissue Organ Cult. 2018, 135, 473–486. [Google Scholar] [CrossRef]
- Jing, H.; Strader, L.C. Interplay of auxin and cytokinin in lateral root development. Int. J. Mol. Sci. 2019, 20, 486. [Google Scholar] [CrossRef]
- Ionkova, I. Optimization of flavonoid production in cell cultures of Astragalus missouriensis Nutt. (Fabaceae). Pharmacogn. Mag. 2009, 5, 92–97. [Google Scholar]
- Jiao, J.; Gai, Q.Y.; Wang, X.; Qin, Q.P.; Wang, Z.Y.; Liu, J.; Fu, Y.J. Chitosan elicitation of Isatis tinctoria L. hairy root cultures for enhancing flavonoid productivity and gene expression and related antioxidant activity. Ind. Crop. Prod. 2018, 124, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Gharari, Z.; Bagheri, K.; Danafar, H.; Sharafi, A. Enhanced flavonoid production in hairy root cultures of Scutellaria bornmuelleri by elicitor induced over-expression of MYB7 and FNSП2 genes. Plant Physiol. Biochem. 2020, 148, 35–44. [Google Scholar] [CrossRef]
- Krongrawa, W.; Limmatvapirat, S.; Saibua, S.; Limmatvapirat, C. Effects of gamma irradiation under vacuum and air packaging atmospheres on the phytochemical contents, biological activities, and microbial loads of Kaempferia parviflora rhizomes. Radiat. Phys. Chem. 2020, 173, 108947. [Google Scholar] [CrossRef]
- Choi, M.H.; Kim, K.H.; Yook, H.S. Antioxidant activity and development of cosmetic materials of solvent extracts from Kaempferia parviflora. J. Korean Soc. Food Sci. Nutr. 2018, 47, 414–421. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The versatility of antioxidant assays in food science and safety—Chemistry, applications, strengths, and limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- Arumugam, R.; Sarikurkcu, C.; Mutlu, M.; Tepe, B. Sophora alopecuroides var. alopecuroides: Phytochemical composition, antioxidant and enzyme inhibitory activity of the methanolic extract of aerial parts, flowers, leaves, roots, and stems. S. Afr. J. Bot. 2020. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Yang, M.; Cao, J.; Khan, A.; Cheng, G. UHPLC-ESI-HRMS/MS analysis on phenolic compositions of different E Se tea extracts and their antioxidant and cytoprotective activities. Food Chem. 2020, 318, 126512. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Moldovan, C.; Zengin, G.; Bender, O.; Locatelli, M.; Simirgiotis, M.; Atalay, A.; Vodnar, D.C.; Rohn, S.; Crișan, G. UHPLC-QTOF-MS analysis of bioactive constituents from two Romanian Goji (Lycium barbarum L.) berries cultivars and their antioxidant, enzyme inhibitory, and real-time cytotoxicological evaluation. Food Chem. Toxicol. 2018, 115, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.B.; Zhang, N.; Van Der Pluijm, W. Global prevalence of type 2 diabetes over the next ten years (2018-2028). Diabetes 2018, 67 (Suppl. 1), 202. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Tipton, K.F. Assessment of enzyme inhibition: A review with examples from the development of monoamine oxidase and cholinesterase inhibitory drugs. Molecules 2017, 22, 1192. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2020, 338, 128119. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Miao, M. Inhibition of α-amylase by polyphenolic compounds: Substrate digestion, binding interactions and nutritional intervention. Trends Food Sci. Technol. 2020, 104, 190–207. [Google Scholar] [CrossRef]
- Mishra, P.; Kumar, A.; Panda, G. Anti-cholinesterase hybrids as multi-target-directed ligands against Alzheimer’s disease (1998–2018). Bioorg. Med. Chem. 2019, 27, 895–930. [Google Scholar] [CrossRef] [PubMed]
- Agunloye, O.M.; Oboh, G. Modulatory effect of caffeic acid on cholinesterases inhibitory properties of donepezil. J. Complement. Integr. Med. 2017, 15, 20170016. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.X.; Tian, J.H.; Yang, W.H.; Chen, S.G.; Liu, D.H.; Fang, H.T.; Zhang, H.L.; Ye, X.Q. Inhibition mechanism of ferulic acid against α-amylase and α-glucosidase. Food Chem. 2020, 317, 126346. [Google Scholar] [CrossRef] [PubMed]
- Shahwar, D.; Rehman, S.U.; Raza, M.A. Acetyl cholinesterase inhibition potential and antioxidant activities of ferulic acid isolated from Impatiens bicolor Linn. J. Med. Plants Res. 2010, 4, 260–266. [Google Scholar]
- Zhu, J.; Yang, H.; Chen, Y.; Lin, H.; Li, Q.; Mo, J.; Bian, Y.; Pei, Y.; Sun, H. Synthesis, pharmacology and molecular docking of multifunctional tacrine–ferulic acid hybrids as cholinesterase inhibitors against Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2018, 33, 496–506. [Google Scholar] [CrossRef]
- Ademosun, A.O.; Oboh, G.; Bello, F.; Ayeni, P.O. Antioxidative properties and effect of quercetin and its glycosylated form (Rutin) on acetylcholinesterase and butyrylcholinesterase activities. J. Evid. Based Complementary Altern. Med. 2016, 21, 11–15. [Google Scholar] [CrossRef]
- Dubey, S.; Ganeshpurkar, A.; Ganeshpurkar, A.; Bansal, D.; Dubey, N. Glycolytic enzyme inhibitory and antiglycation potential of rutin. Future J. Pharm. Sci. 2017, 3, 158–162. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; de La Rosa, L.A.; Bustos-Jaimes, I.; Alvarez-Parrilla, E. Inhibition of α-amylase by flavonoids: Structure activity relationship (SAR). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 206, 437–447. [Google Scholar] [CrossRef]
- Yong, N.L.; Ahmad, A.; Mohammad, A.W. Synthesis and characterization of silver oxide nanoparticles by a novel method. Int. J. Sci. Eng. Res. 2013, 4, 155–158. [Google Scholar]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Viticult. 1977, 28, 49–55. [Google Scholar]
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R. Sideritis galatica Bornm.: A source of multifunctional agents for the management of oxidative damage, Alzheimer’s’s and diabetes mellitus. J. Funct. Foods 2014, 11, 538–547. [Google Scholar] [CrossRef]
- Zengin, G.; Uysal, A.; Diuzheva, A.; Gunes, E.; Jekő, J.; Cziáky, Z.; Picot-Allain, C.M.N.; Mahomoodally, M.F. Characterization of phytochemical components of Ferula halophila extracts using HPLC-MS/MS and their pharmacological potentials: A multi-functional insight. J. Pharmaceut. Biomed. Anal. 2018, 60, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef]
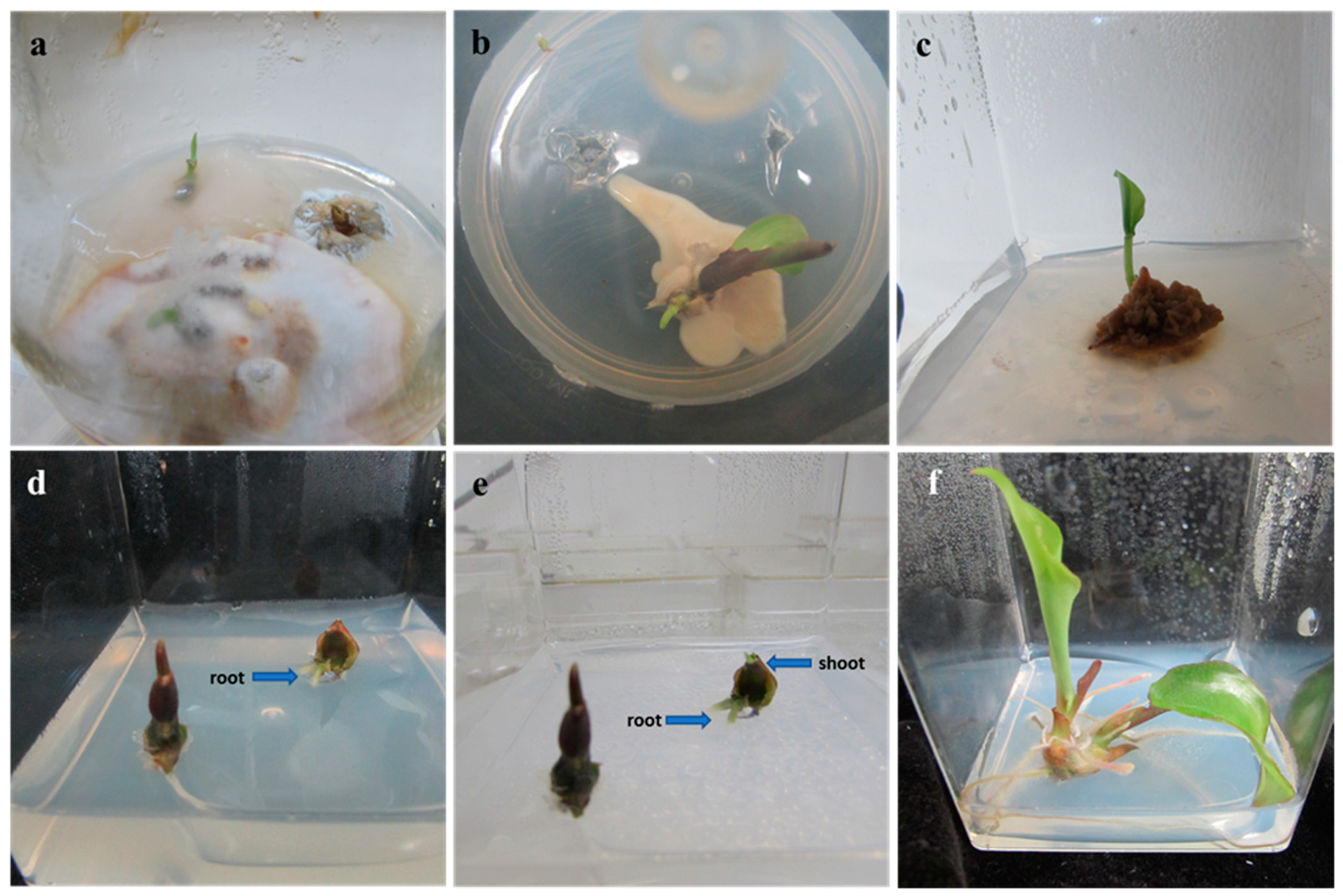


| Ag NPs (mg L−1) | Duration (min) | Decontamination (%) | Explant Survival (%) |
|---|---|---|---|
| 0 (Control) | 15.2 ± 3.3 j | 100 ± 0.0 a | |
| 25 | 30 | 23.7 ± 4.6 i | 100 ± 0.0 a |
| 50 | 44.2 ± 3.3 f | 100 ± 0.0 a | |
| 100 | 64.6 ± 4.9 c | 100 ± 0.0 a | |
| 200 | 94.9 ± 3.1 b | 73.7 ± 2.6 d | |
| 25 | 60 | 31.1 ± 4.6 h | 100 ± 0.0 a |
| 50 | 49.7 ± 3.9 e | 100 ± 0.0 a | |
| 100 | 100 ± 0.0 a | 100 ± 0.0 a | |
| 200 | 100 ± 0.0 a | 65.2 ± 3.6 f | |
| 25 | 90 | 38.1 ± 3.3 g | 91.7 ± 2.5 b |
| 50 | 56.7 ± 4.8 d | 85.2 ± 2.9 c | |
| 100 | 100 ± 0.0 a | 67.8 ± 3.1 e | |
| 200 | 100 ± 0.0 a | 44.3 ± 2.5 g | |
| Mean | 62.93 | 86.7 | |
| R-Square Coefficient of variation | 0.9896 | 0.9889 | |
| 5.34 | 2.28 | ||
| F-value | |||
| F-test | Conc | 27086.7 | 1775.4 |
| Duration | 251.4 | 1149.6 | |
| Conc * Duration | 53.4 | 89.0 | |
| p-value | |||
| Conc | 0.001 | 0.001 | |
| Duration | 0.001 | 0.001 | |
| Conc * Duration | 0.001 | 0.001 | |
| Factors | Decontamination (%) | Explant Survival (%) |
|---|---|---|
| 25 mg L−1 | 30.9 d | 97.2 a |
| 50 mg L−1 | 50.2 c | 95.1 b |
| 100 mg L−1 | 88.2 b | 89.3 c |
| 200 mg L−1 | 98.3 a | 61.1 d |
| LSD | 1.82 | 1.11 |
| 30 min | 56.8 c | 93.4 a |
| 60 min | 70.2 b | 91.3 b |
| 90 min | 73.7 a | 72.3 c |
| LSD | 1.58 | 0.96 |
| Cytokinin | Conc (µM) | Response (%) | No. of Shoots/Explant | No. of Roots/Shoot |
|---|---|---|---|---|
| Control (MS) | 0 | 22.6 ± 2.2 k | 1.2 ± 0.4 h | 2.3 ± 0.9 gh |
| 6-BA | 1 | 34.0 ± 3.0 i | 1.4 ± 0.5 gh | 4.3 ± 1.3 b–f |
| 2 | 53.9 ± 2.7 f | 2.6 ± 0.9 efg | 5.3 ± 1.0 b | |
| 4 | 64.1 ± 2.9 c | 4.2 ± 1.6 bc | 3.6 ± 1.0 c–f | |
| 8 | 78.8 ± 3.1 a | 6.3 ± 1.6 a | 4.6 ± 1.0 bcde | |
| 12 | 70.2 ± 4.7 b | 3.1 ± 1.1 cdef | 2.9 ± 0.9 fgh | |
| 6-KN | 1 | 23.0 ± 1.8 k | 1.3 ± 0.5 h | 3.2 ± 1.2 d–f |
| 2 | 29.4 ± 2.9 j | 2.8 ± 1.2 def | 6.6 ± 1.6 a | |
| 4 | 46.6 ± 3.1 g | 3.7 ± 1.3 bcde | 4.8 ± 1.5 bc | |
| 8 | 57.7 ± 2.9 e | 2.3 ± 1.0 fgh | 3.8 ± 1.3 c–f | |
| 12 | 61.1 ± 3.7 d | 3.4 ± 1.1 cdef | 1.9 ± 0.6 h | |
| TDZ | 1 | 39.7 ± 2.7 h | 2.9 ± 0.8 def | 3.1 ± 1.5 efgh |
| 2 | 67.8 ± 2.3 b | 4.7 ± 1.3 b | 5.0 ± 1.9 bc | |
| 4 | 49.2 ± 2.8 g | 3.8 ± 1.0 bcd | 4.7 ± 2.3 bcd | |
| 8 | 53.3 ± 2.7 f | 3.0 ± 1.5 def | 3.7 ± 1.5 c–f | |
| 12 | 32.7 ± 1.6 i | 2.6 ± 0.5 efg | 2.1 ± 1.3 h | |
| Mean | 49.0 | 3.08 | 3.87 | |
| R-Square | 0.9741 | 0.5997 | 0.4839 | |
| Coefficient of variation | 5.93 | 35.68 | 35.30 | |
| F-value | ||||
| F-test | Cyto | 378.49 | 6.72 | 1.20 |
| Conc | 405.83 | 14.33 | 20.83 | |
| Cyto * Conc | 192.41 | 10.61 | 2.26 | |
| p-value | ||||
| Cyto | 0.001 | 0.002 | 0.306 | |
| Conc | 0.001 | 0.001 | 0.001 | |
| Cyto * Conc | 0.001 | 0.001 | 0.028 | |
| Factors | Response (%) | No. of Shoots/Explant | No. of Roots/Shoot |
|---|---|---|---|
| 6-BA | 60.2 a | 3.5 a | 4.13 a |
| 6-KN | 43.6 b | 2.7 b | 4.07 a |
| TDZ | 48.5 c | 3.4 a | 3.71 a |
| LSD | 1.23 | 0.47 | 0.58 |
| 1 µM | 32.2 d | 1.9 c | 3.6 c |
| 2 µM | 50.4 c | 3.3 ab | 5.7 a |
| 4 µM | 53.3 b | 3.9 a | 4.3 b |
| 8 µM | 63.3 a | 3.9 a | 4.0 bc |
| 12 µM | 54.7 b | 3.0 b | 2.3 d |
| LSD | 1.59 | 0.61 | 0.75 |
| PGRs (µM) | Response (%) | No. of Shoots/Explant | No. of Roots/Shoot | |||
|---|---|---|---|---|---|---|
| 6-BA | 6-KN | TDZ | NAA | |||
| 0 | 0 | 0 | 0 | 22.6 ± 2.2 g | 1.2 ± 0.4 g | 2.3 ± 0.9 e |
| 8 | 2 | 0 | 0 | 89.0 ± 3.7 c | 7.8 ± 1.1 cde | 5.3 ± 1.4 cd |
| 8 | 4 | 0 | 0 | 86.1 ± 5.2 d | 9.2 ± 1.3 b | 4.2 ± 1.0 d |
| 8 | 6 | 0 | 0 | 76.2 ± 2.2 f | 6.2 ± 1.4 f | 2.8 ± 0.7 e |
| 8 | 0 | 0.5 | 0 | 97.2 ± 1.8 b | 12.2 ± 1.8 a | 4.3 ± 1.2 d |
| 8 | 0 | 1 | 0 | 90.9 ± 2.5 c | 8.1 ± 1.3 bcd | 2.7 ± 1.0 e |
| 8 | 0 | 2 | 0 | 83.6 ± 2.8 e | 6.4 ± 1.7 ef | 3.0 ± 1.1 e |
| 8 | 0 | 0.5 | 1 | 100 ± 0.0 a | 8.8 ± 1.6 bc | 6.3 ± 1.4 c |
| 8 | 0 | 0.5 | 2 | 100 ± 0.0 a | 6.8 ± 1.5 def | 8.1 ± 1.2 b |
| 8 | 0 | 0.5 | 4 | 100 ± 0.0 a | 5.9 ± 1.2 f | 9.7 ± 1.4 a |
| Mean | 84.6 | 7.3 | 4.9 | |||
| R-Square | 0.9878 | 0.8113 | 0.8255 | |||
| Coefficient of variation | 3.07 | 18.89 | 23.59 | |||
| F-value | 719.82 | 38.22 | 42.07 | |||
| IBA (µM) | Number of Roots/Shoot | Root Length (cm) | Shoot Length (cm) |
|---|---|---|---|
| 0 | 6.9 ± 1.5 f | 4.7 ± 1.3 d | 5.8 ± 0.5 c |
| 1 | 11.0 ± 1.7 d | 5.6 ± 0.4 c | 6.1 ± 0.4 c |
| 2 | 24.0 ± 2.5 a | 7.8 ± 0.5 b | 9.8 ± 0.5 a |
| 4 | 15.8 ± 2.4 b | 8.9 ± 0.5 a | 6.6 ± 0.4 b |
| 8 | 13.2 ± 1.5 c | 5.8 ± 0.5 c | 4.4 ± 0.3 e |
| 12 | 8.9 ± 1.1 e | 3.2 ± 0.7 e | 5.3 ± 0.4 d |
| Mean | 13.29 | 5.99 | 6.3 |
| R-Square | 0.9096 | 0.8911 | 0.9499 |
| Coefficient of variation | 14.02 | 11.69 | 6.45 |
| F-Value | 96.55 | 78.53 | 182.02 |
| Sources of Leaves | Total Phenolic Content (mg GAE/g) | Total Flavonoid Content (mg RE/g) |
|---|---|---|
| In vitro cultures | 14.07 ± 0.09 | 1.55 ± 0.07 |
| The greenhouse | 18.28 ± 0.20 | 0.96 ± 0.06 |
| No. | Name | Formula | Rt | [M + H]+ | [M − H]− | Fragment 1 | Fragment 2 | Fragment 3 | Fragment 4 | Fragment 5 | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Caffeic acid | C9H8O4 | 15.22 | 179.03444 | 135.0438 | 107.0492 | |||||
| 2 | Vicenin-2 (Apigenin-6,8-di-C-glucoside) | C27H30O15 | 19.39 | 595.16630 | 577.1575 | 559.1442 | 457.1131 | 325.0707 | 295.0601 | ||
| 3 1 | Ferulic acid | C10H10O4 | 19.94 | 193.05009 | 178.0261 | 149.0600 | 137.0226 | 134.0360 | 121.0278 | ||
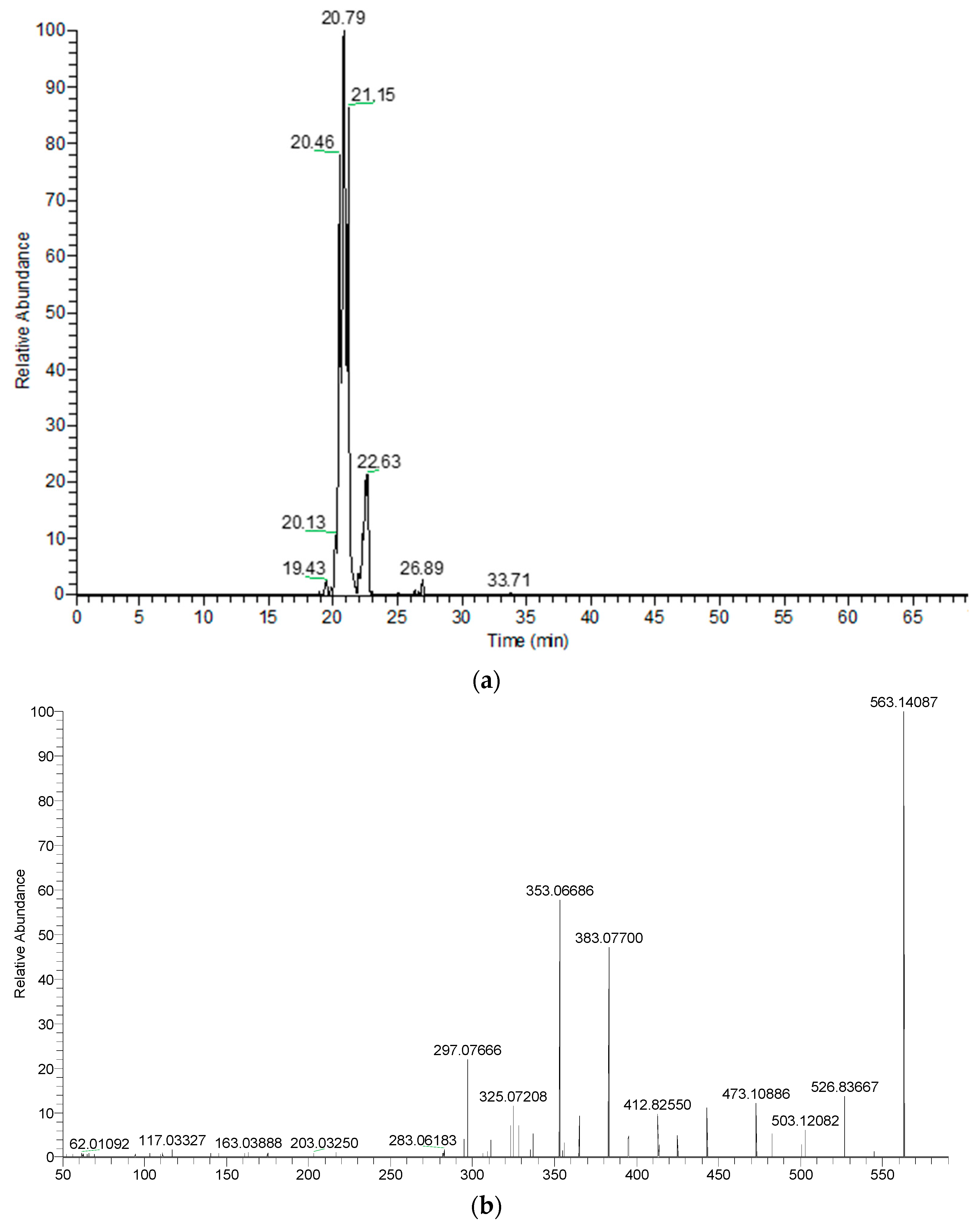
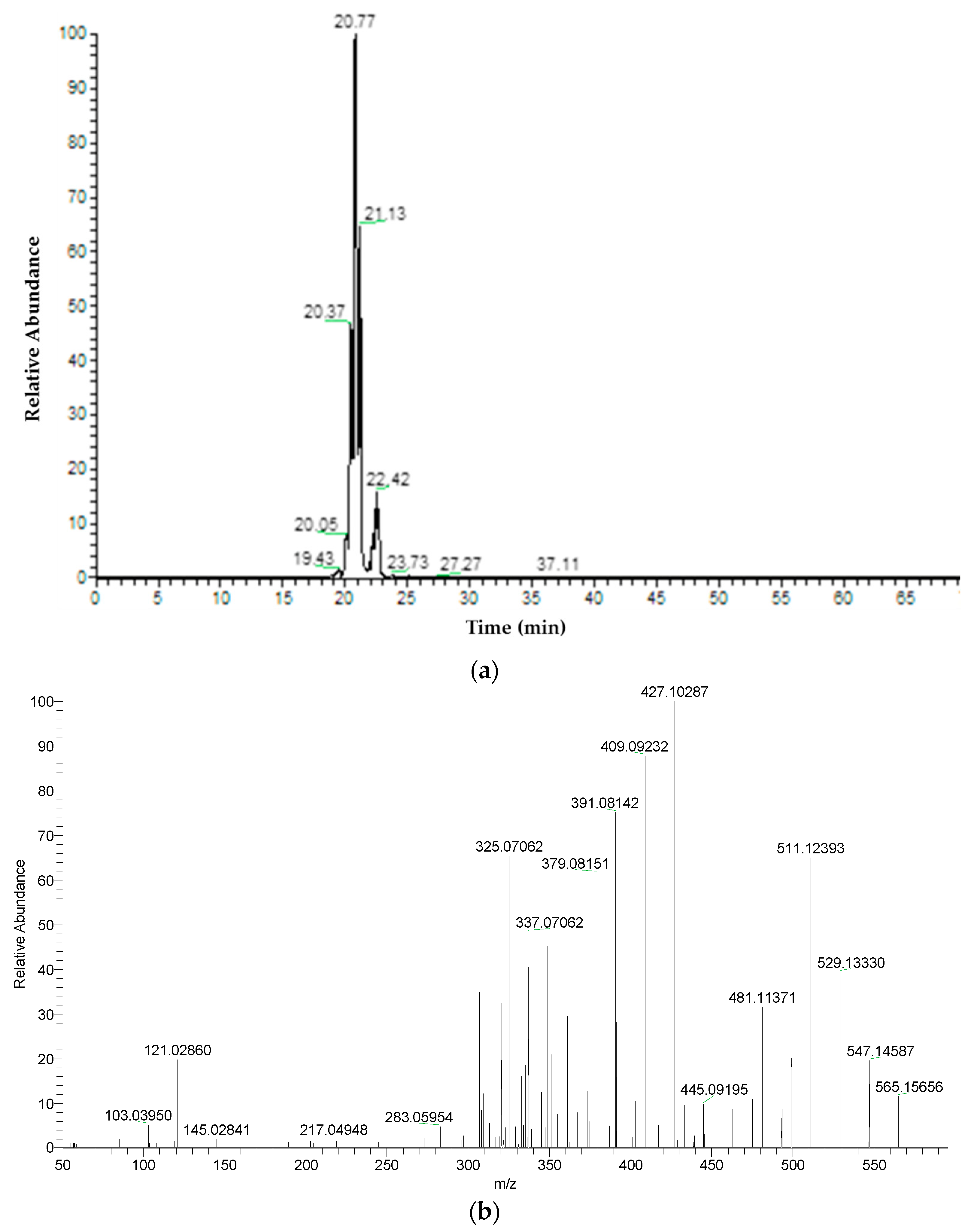
| 4 | Apigenin-C-hexoside-C-pentoside isomer 1 | C26H28O14 | 20.37 | 565.15574 | 547.1459 | 511.1239 | 427.1029 | 409.0923 | 295.0602 | ||
| 5 | Apigenin-C-hexoside-C-pentoside isomer 2 | C26H28O14 | 20.77 | 565.15574 | 547.1455 | 511.1242 | 427.1030 | 379.0814 | 295.0602 | ||
| 6 | Apigenin-C-hexoside-C-pentoside isomer 3 | C26H28O14 | 21.13 | 565.15574 | 547.1458 | 511.1236 | 469.1133 | 379.0813 | 295.0602 | ||
| 7 1 | Rutin (Quercetin-3-O-rutinoside) | C27H30O16 | 23.54 | 611.16122 | 465.1043 | 303.0499 | 129.0549 | 85.0289 | 71.0497 | [15] | |
| 8 | Lumichrome | C12H10N4O2 | 24.45 | 243.08821 | 216.0769 | 200.0820 | 198.0665 | 172.0870 | 145.0761 | ||
| 9 1 | Cosmosiin (Apigenin-7-O-glucoside) | C21H20O10 | 24.50 | 433.11347 | 271.0601 | 153.0185 | 119.0493 | ||||
| 10 | Isorhamnetin-3-O-rutinoside (Narcissin) | C28H32O16 | 25.55 | 623.16122 | 315.0513 | 314.0432 | 299.0202 | 271.0243 | 243.0300 | [15] | |
| 11 | Tamarixetin-3-O-rutinoside | C28H32O16 | 25.74 | 623.16122 | 315.0513 | 314.0435 | 299.0198 | 271.0250 | 243.0294 | [16] | |
| 12 | Syringetin-3-O-rutinoside | C29H34O17 | 25.96 | 653.17178 | 345.0616 | 344.0539 | 329.0303 | 315.0151 | 301.0363 | [16] | |
| 13 | Acacetin-7-O-glucoside (Tilianin) | C22H22O10 | 29.06 | 447.12913 | 285.0757 | 270.0523 | 269.0444 | 242.0579 | [16] | ||
| 14 | Methoxy-trihydroxy(iso)flavanone | C16H14O6 | 30.20 | 303.08687 | 193.0499 | 167.0340 | 163.0390 | 145.0285 | |||
| 15 1 | Apigenin (4’,5,7-Trihydroxyflavone) | C15H10O5 | 30.29 | 269.04500 | 225.0547 | 201.0548 | 151.0026 | 149.0233 | 117.0331 | ||
| 16 | Isokaempferide (3-Methoxy-4’,5,7-trihydroxyflavone) | C16H12O6 | 30.97 | 299.05556 | 284.0329 | 256.0371 | 255.0297 | 227.0342 | |||
| 17 | Undecanedioic acid | C11H20O4 | 31.36 | 215.12834 | 197.1176 | 153.1272 | 125.0955 | 57.0331 | |||
| 18 | 3,4’,5,7-Tetramethoxyflavone or 3’,4’,5,7-Tetramethoxyflavone | C19H18O6 | 32.44 | 343.11817 | 328.0942 | 327.0862 | 314.0793 | 313.0707 | 285.0765 | [11] | |
| 19 | Pinocembrin (5,7-Dihydroxyflavanone) | C15H12O4 | 32.77 | 255.06573 | 213.0547 | 151.0023 | 145.0645 | 107.0124 | 83.0122 | ||
| 20 | 3,3’,4’,5,7-Pentamethoxyflavone | C20H20O7 | 32.92 | 373.12873 | 358.1046 | 357.0968 | 343.0810 | 327.0863 | 312.0990 | [11] | |
| 21 | Kaempferide (4’-Methoxy-3,5,7-trihydroxyflavone) | C16H12O6 | 33.09 | 299.05556 | 284.0328 | 256.0378 | 227.0340 | 151.0030 | |||
| 22 | Dihydroxy-methoxy(iso)flavone-O-acetylhexoside | C24H24O11 | 33.21 | 489.13969 | 285.0758 | 270.0522 | 269.0442 | 242.0564 | |||
| 23 | Dimethoxy-trihydroxy(iso)flavone | C17H14O7 | 33.35 | 329.06613 | 314.0434 | 299.0198 | 271.0249 | 227.0338 | |||
| 24 | Dodecanedioic acid | C12H22O4 | 33.81 | 229.14399 | 211.1334 | 167.1433 | |||||
| 25 | 5,7-Dimethoxyflavanone | C17H16O4 | 33.82 | 285.11268 | 181.0497 | 166.0261 | 138.0317 | 131.0494 | 103.0548 | [15] | |
| 26 1 | Chrysin (5,7-Dihydroxyflavone) | C15H10O4 | 33.85 | 255.06573 | 209.0595 | 153.0184 | 129.0340 | 103.0547 | 67.0185 | ||
| 27 | 5,7-Dimethoxyflavone | C17H14O4 | 34.13 | 283.09704 | 268.0729 | 267.0652 | 239.0703 | 238.0622 | 225.0538 | [11] | |
| 28 1 | Galangin (3,5,7-Trihydroxyflavone) | C15H10O5 | 34.75 | 271.06065 | 242.0572 | 215.0701 | 165.0181 | 153.0184 | 105.0336 | ||
| 29 | 4’,5,7-Trimethoxyflavone | C18H16O5 | 34.81 | 313.10760 | 298.0837 | 297.0761 | 269.0809 | 255.0649 | 227.0711 | [11] | |
| 30 | 3,5,7-Trimethoxyflavone | C18H16O5 | 34.98 | 313.10760 | 298.0836 | 297.0758 | 280.0729 | 279.0652 | 252.0778 | [11] | |
| 31 | Dihydroxy-methoxy(iso)flavone | C16H12O5 | 35.05 | 283.06065 | 268.0375 | 267.0294 | 239.0344 | 211.0393 | |||
| 32 | Ayanin (3’,5-Dihydroxy-3,4’,7-trimethoxyflavone) | C18H16O7 | 35.20 | 345.09743 | 330.0733 | 329.0661 | 315.0499 | 287.0551 | 259.0602 | [15] | |
| 33 | 3,4’,5,7-Tetramethoxyflavone or 3’,4’,5,7-Tetramethoxyflavone | C19H18O6 | 35.44 | 343.11817 | 328.0940 | 327.0862 | 310.0837 | 285.0760 | 282.0886 | [11] | |
| 34 | Dihydroxy-dimethoxy(iso)flavone | C17H14O6 | 35.60 | 315.08686 | 300.0628 | 299.0548 | 272.0680 | 271.0602 | 257.0445 | ||
| 35 | Retusin (5-Hydroxy-3,3’,4’,7-tetramethoxyflavone) | C19H18O7 | 37.10 | 359.11308 | 344.0890 | 343.0812 | 329.0655 | 301.0706 | [11] | ||
| 36 | Pinostrobin (5-Hydroxy-7-methoxyflavanone) | C16H14O4 | 37.14 | 271.09704 | 229.0864 | 173.0599 | 167.0339 | 131.0494 | 103.0547 | [15] | |
| 37 | Tectochrysin (5-Hydroxy-7-methoxyflavone) | C16H12O4 | 38.08 | 269.08138 | 254.0573 | 226.0624 | 167.0338 | [11] | |||
| 38 | 4’,7-Dimethoxy-5-hydroxyflavone | C17H14O5 | 38.75 | 299.09195 | 284.0678 | 256.0728 | [11] | ||||
| 39 | 5-Hydroxy-3,7-dimethoxyflavone | C17H14O5 | 38.94 | 299.09195 | 284.0678 | 283.0601 | 256.0728 | 255.0649 | 241.0496 | [11] | |
| 40 | 5-Hydroxy-3,4’,7-trimethoxyflavone | C18H16O6 | 39.39 | 329.10252 | 314.0784 | 313.0707 | 299.0552 | 285.0756 | 271.0598 | [11] |
| No. | Name | Formula | Rt | [M + H]+ | [M − H]− | Fragment 1 | Fragment 2 | Fragment 3 | Fragment 4 | Fragment 5 | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Caffeic acid | C9H8O4 | 15.19 | 179.03444 | 135.0438 | 107.0488 | |||||
| 2 | Vicenin-2 (Apigenin-6,8-di-C-glucoside) | C27H30O15 | 19.38 | 595.16630 | 577.1556 | 559.1453 | 457.1132 | 325.0707 | 295.0602 | ||
| 3 1 | Ferulic acid | C10H10O4 | 19.95 | 193.05009 | 178.0261 | 149.0596 | 137.0231 | 134.0360 | 121.0278 | ||
| 4 | Apigenin-C-hexoside-C-pentoside isomer 1 | C26H28O14 | 20.43 | 565.15574 | 547.1460 | 511.1242 | 427.1027 | 409.0920 | 295.0602 | ||
| 5 | Apigenin-C-hexoside-C-pentoside isomer 2 | C26H28O14 | 20.78 | 565.15574 | 547.1451 | 511.1239 | 427.1030 | 379.0813 | 295.0601 | ||
| 6 | Apigenin-C-hexoside-C-pentoside isomer 3 | C26H28O14 | 21.14 | 565.15574 | 547.1455 | 511.1245 | 469.1141 | 379.0813 | 295.0602 | ||
| 7 1 | Rutin (Quercetin-3-O-rutinoside) | C27H30O16 | 23.53 | 611.16122 | 465.1033 | 303.0499 | 129.0549 | 85.0290 | 71.0498 | [15] | |
| 8 | Lumichrome | C12H10N4O2 | 24.46 | 243.08821 | 216.0767 | 200.0819 | 198.0662 | 172.0869 | 145.0760 | ||
| 10 | Isorhamnetin-3-O-rutinoside (Narcissin) | C28H32O16 | 25.55 | 623.16122 | 315.0512 | 314.0433 | 299.0203 | 271.0254 | 243.0298 | [15] | |
| 11 | Tamarixetin-3-O-rutinoside | C28H32O16 | 25.74 | 623.16122 | 315.0512 | 314.0440 | 299.0192 | 271.0250 | 243.0288 | [16] | |
| 12 | Syringetin-3-O-rutinoside | C29H34O17 | 25.97 | 653.17178 | 345.0618 | 344.0536 | 329.0306 | 315.0142 | 301.0355 | [16] | |
| 13 | Acacetin-7-O-glucoside (Tilianin) | C22H22O10 | 29.06 | 447.12913 | 285.0757 | 270.0522 | 269.0443 | 242.0567 | [16] | ||
| 15 1 | Apigenin (4’,5,7-Trihydroxyflavone) | C15H10O5 | 30.29 | 269.04500 | 225.0555 | 201.0546 | 151.0019 | 149.0230 | 117.0330 | ||
| 41 | Dihydroxy-methoxy(iso)flavone isomer 1 | C16H12O5 | 30.85 | 283.06065 | 268.0377 | 240.0423 | 239.0346 | 211.0394 | |||
| 16 | Isokaempferide (3-Methoxy-4’,5,7-trihydroxyflavone) | C16H12O6 | 30.97 | 299.05556 | 284.0328 | 256.0379 | 255.0297 | 227.0341 | |||
| 17 | Undecanedioic acid | C11H20O4 | 31.37 | 215.12834 | 197.1175 | 153.1272 | 125.0959 | 57.0331 | |||
| 18 | 3,4’,5,7-Tetramethoxyflavone or 3’,4’,5,7-Tetramethoxyflavone | C19H18O6 | 32.45 | 343.11817 | 328.0943 | 327.0863 | 314.0783 | 313.0707 | 285.0760 | [11] | |
| 19 | Pinocembrin (5,7-Dihydroxyflavanone) | C15H12O4 | 32.78 | 255.06573 | 213.0556 | 151.0024 | 145.0649 | 107.0121 | 83.0123 | ||
| 20 | 3,3’,4’,5,7-Pentamethoxyflavone | C20H20O7 | 32.93 | 373.12873 | 358.1045 | 357.0966 | 343.0811 | 327.0863 | 312.0991 | [11] | |
| 22 | Dihydroxy-methoxy(iso)flavone-O-acetylhexoside | C24H24O11 | 33.20 | 489.13969 | 285.0757 | 270.0523 | 269.0440 | 242.0566 | |||
| 24 | Dodecanedioic acid | C12H22O4 | 33.81 | 229.14399 | 211.1332 | 167.1425 | |||||
| 25 | 5,7-Dimethoxyflavanone | C17H16O4 | 33.82 | 285.11268 | 181.0496 | 166.0258 | 138.0316 | 131.0493 | 103.0545 | [15] | |
| 26 1 | Chrysin (5,7-Dihydroxyflavone) | C15H10O4 | 33.85 | 255.06573 | 209.0596 | 153.0182 | 129.0341 | 103.0546 | 67.0185 | ||
| 27 | 5,7-Dimethoxyflavone | C17H14O4 | 34.14 | 283.09704 | 268.0730 | 267.0650 | 239.0702 | 238.0626 | 225.0549 | [11] | |
| 28 1 | Galangin (3,5,7-Trihydroxyflavone) | C15H10O5 | 34.76 | 271.06065 | 242.0576 | 215.0700 | 165.0183 | 153.0182 | 105.0339 | ||
| 29 | 4’,5,7-Trimethoxyflavone | C18H16O5 | 34.82 | 313.10760 | 298.0836 | 297.0761 | 269.0808 | 255.0662 | 227.0694 | [11] | |
| 30 | 3,5,7-Trimethoxyflavone | C18H16O5 | 35.00 | 313.10760 | 298.0839 | 297.0757 | 280.0730 | 279.0654 | 252.0782 | [11] | |
| 31 | Dihydroxy-methoxy(iso)flavone isomer 2 | C16H12O5 | 35.03 | 283.06065 | 268.0377 | 267.0294 | 239.0345 | 211.0394 | |||
| 32 | Ayanin (3’,5-Dihydroxy-3,4’,7-trimethoxyflavone) | C18H16O7 | 35.21 | 345.09743 | 330.0733 | 329.0653 | 315.0499 | 287.0549 | 259.0601 | [15] | |
| 33 | 3,4’,5,7-Tetramethoxyflavone or 3’,4’,5,7-Tetramethoxyflavone | C19H18O6 | 35.45 | 343.11817 | 328.0944 | 327.0863 | 310.0835 | 285.0754 | 282.0886 | [11] | |
| 34 | Dihydroxy-dimethoxy(iso)flavone | C17H14O6 | 35.60 | 315.08686 | 300.0629 | 299.0552 | 272.0679 | 271.0602 | 257.0436 | ||
| 35 | Retusin (5-Hydroxy-3,3’,4’,7-tetramethoxyflavone) | C19H18O7 | 37.10 | 359.11308 | 344.0890 | 343.0815 | 329.0656 | 301.0706 | [11] | ||
| 36 | Pinostrobin (5-Hydroxy-7-methoxyflavanone) | C16H14O4 | 37.14 | 271.09704 | 229.0859 | 173.0598 | 167.0339 | 131.0494 | 103.0546 | [15] | |
| 37 | Tectochrysin (5-Hydroxy-7-methoxyflavone) | C16H12O4 | 38.09 | 269.08138 | 254.0571 | 226.0626 | 167.0342 | [11] | |||
| 39 | 5-Hydroxy-3,7-dimethoxyflavone | C17H14O5 | 38.94 | 299.09195 | 284.0680 | 283.0602 | 256.0729 | 255.0650 | 241.0494 | [11] | |
| 40 | 5-Hydroxy-3,4’,7-trimethoxyflavone | C18H16O6 | 39.38 | 329.10252 | 314.0784 | 313.0707 | 299.0550 | 285.0757 | 271.0602 | [11] |
| Sources of Leaves and Standards | DPPH | ABTS | CUPRAC | FRAP | PBD | Chelating |
|---|---|---|---|---|---|---|
| In vitro cultures | >3 b | 2.99 ± 0.20 c | 2.82 ± 0.03 c | 1.94 ± 0.01 c | >3 b | 0.59 ± 0.01 b |
| The greenhouse | >3 b | 2.20 ± 0.05 b | 2.07 ± 0.01 b | 1.52 ± 0.02 b | >3 b | 0.70 ± 0.09 c |
| Trolox | 0.06 ± 0.01 a | 0.09 ± 0.01 a | 0.11 ± 0.01 a | 0.04 ± 0.01 a | 0.52 ± 0.02 a | nt |
| EDTA | nt | nt | nt | nt | nt | 0.02 ± 0.001 a |
| Sources of Leaves and Standards | AChE | BChE | Tyrosinase | Amylase |
|---|---|---|---|---|
| In vitro cultures | 1.15 ± 0.04 b | 0.95 ± 0.10 c | 0.71 ± 0.01 b | 1.41 ± 0.01 b |
| The greenhouse | 1.07 ± 0.10 b | 1.67 ± 0.11 b | 0.71 ± 0.01 b | 1.37 ± 0.05 b |
| Galantamine | 0.003 ± 0.001 a | 0.007 ± 0.002 a | nt | nt |
| Kojic acid | nt | nt | 0.08 ± 0.001 a | nt |
| Acarbose | nt | nt | nt | 0.68 ± 0.01 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-Y.; Kim, K.-S.; Ak, G.; Zengin, G.; Cziáky, Z.; Jekő, J.; Adaikalam, K.; Song, K.; Kim, D.-H.; Sivanesan, I. Establishment of a Rapid Micropropagation System for Kaempferia parviflora Wall. Ex Baker: Phytochemical Analysis of Leaf Extracts and Evaluation of Biological Activities. Plants 2021, 10, 698. https://doi.org/10.3390/plants10040698
Park H-Y, Kim K-S, Ak G, Zengin G, Cziáky Z, Jekő J, Adaikalam K, Song K, Kim D-H, Sivanesan I. Establishment of a Rapid Micropropagation System for Kaempferia parviflora Wall. Ex Baker: Phytochemical Analysis of Leaf Extracts and Evaluation of Biological Activities. Plants. 2021; 10(4):698. https://doi.org/10.3390/plants10040698
Chicago/Turabian StylePark, Han-Yong, Kyung-Su Kim, Gunes Ak, Gokhan Zengin, Zoltán Cziáky, József Jekő, Kathalingam Adaikalam, Kihwan Song, Doo-Hwan Kim, and Iyyakkannu Sivanesan. 2021. "Establishment of a Rapid Micropropagation System for Kaempferia parviflora Wall. Ex Baker: Phytochemical Analysis of Leaf Extracts and Evaluation of Biological Activities" Plants 10, no. 4: 698. https://doi.org/10.3390/plants10040698
APA StylePark, H.-Y., Kim, K.-S., Ak, G., Zengin, G., Cziáky, Z., Jekő, J., Adaikalam, K., Song, K., Kim, D.-H., & Sivanesan, I. (2021). Establishment of a Rapid Micropropagation System for Kaempferia parviflora Wall. Ex Baker: Phytochemical Analysis of Leaf Extracts and Evaluation of Biological Activities. Plants, 10(4), 698. https://doi.org/10.3390/plants10040698









