Antioxidant Vitamins and Prebiotic FOS and XOS Differentially Shift Microbiota Composition and Function and Improve Intestinal Epithelial Barrier In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Products
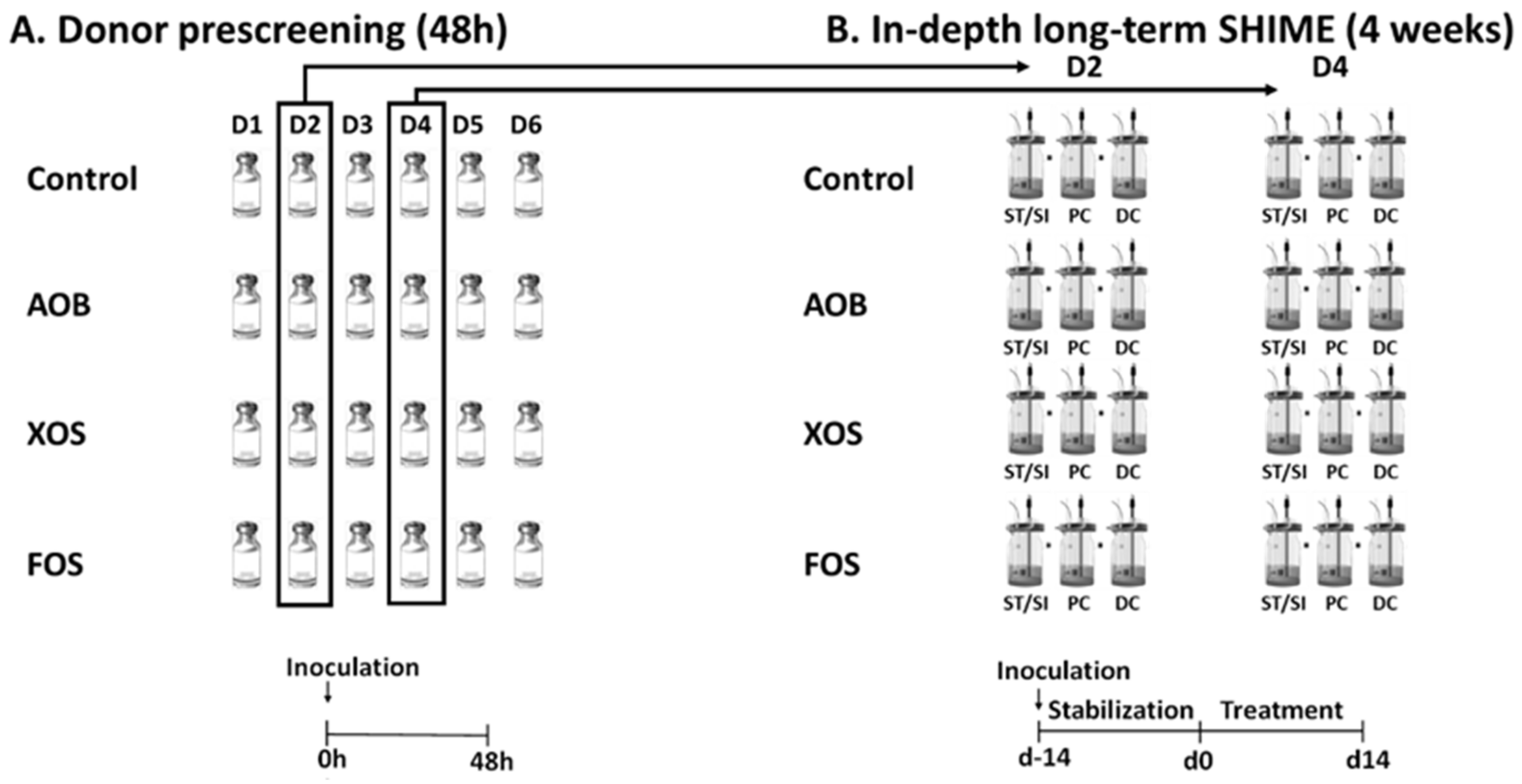
2.2. Experimental Design of Short-Term Colonic Incubations for Testing Interindividual Variability
2.3. Experimental Design of the Long-Term SHIME Study
2.4. Microbial Community Analysis by qPCR
2.5. Quantitative Microbial Community Analysis by 16S rRNA Gene Sequencing and Flow Cytometry
2.6. Metabolic Analysis
2.7. Caco-2 and HT29-MTX-E12 Cell Culture and Barrier Function
2.8. Redox Potential Measurements
2.9. Data and Statistical Analysis
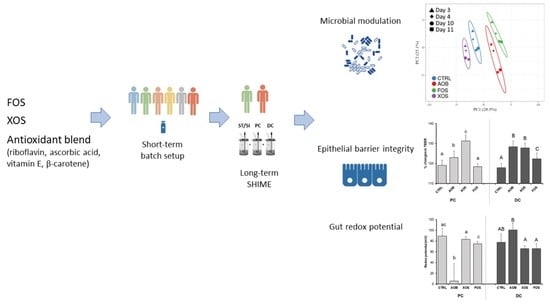
3. Results
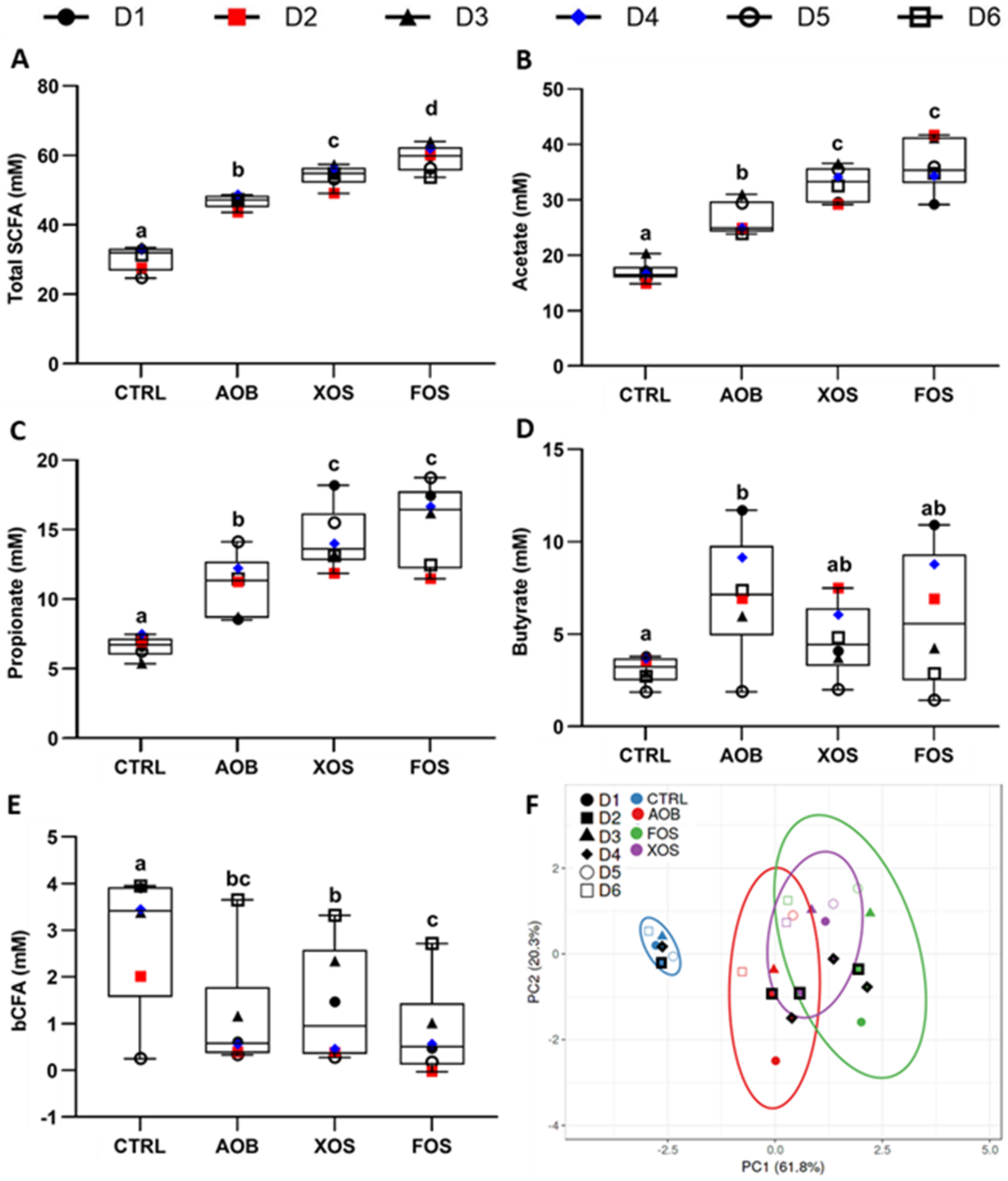
3.1. Part 1: Short-Term Fecal Batch Incubations
3.2. Part 2: Long-Term SHIME Study
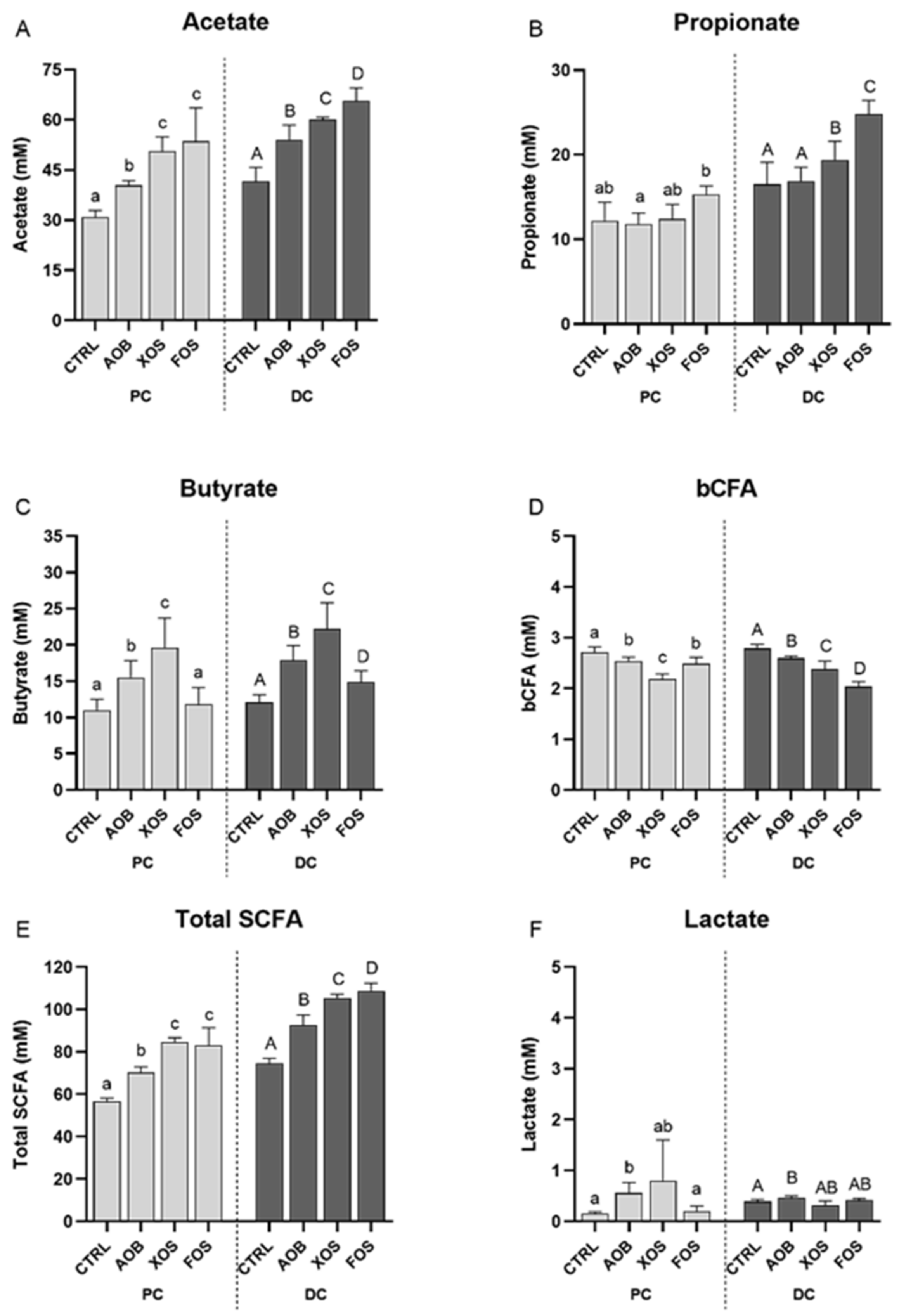
3.2.1. Effect of AOB, XOS and FOS on Metabolic Activity
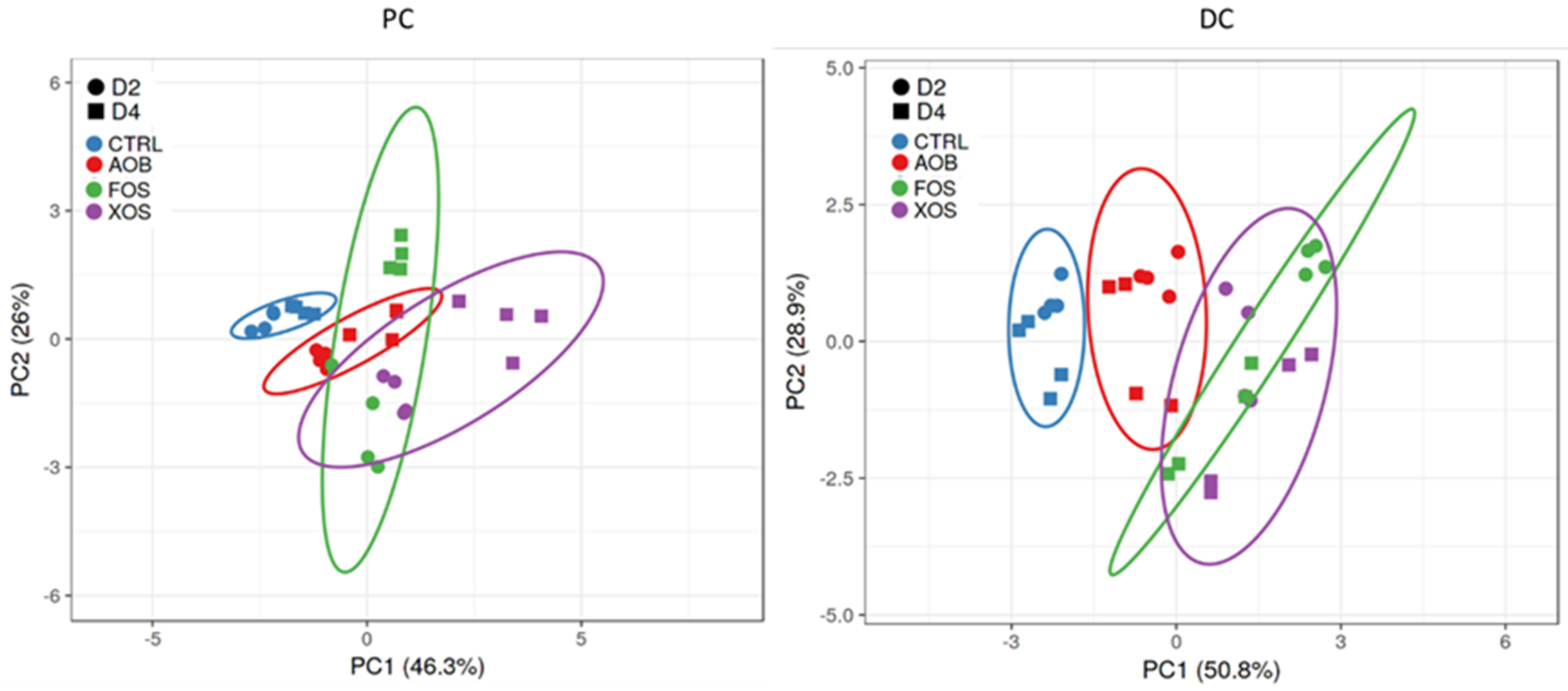
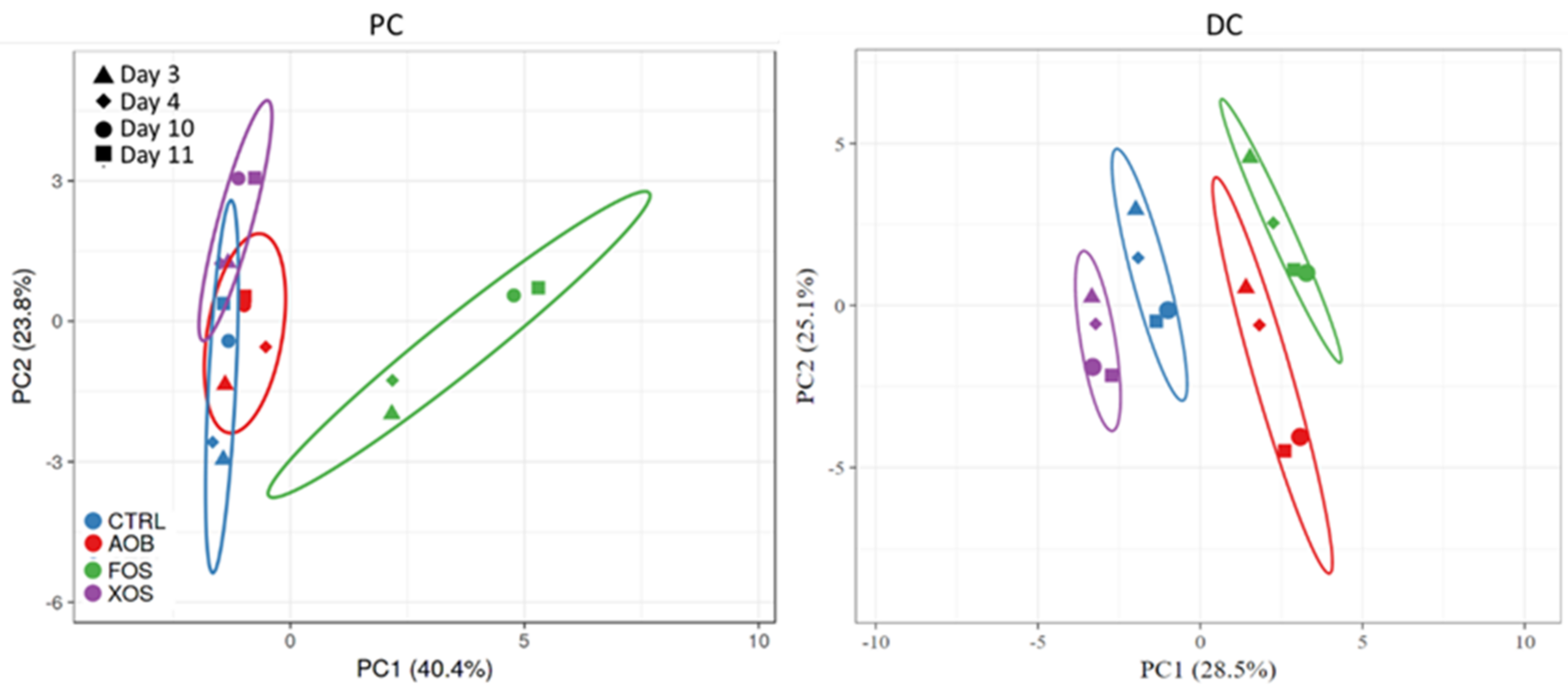
3.2.2. Effect of AOB, XOS and FOS on the Microbial Community Composition of the Proximal Colon
3.2.3. Effect of AOB, XOS and FOS on the Microbial Community Composition of the Distal Colon
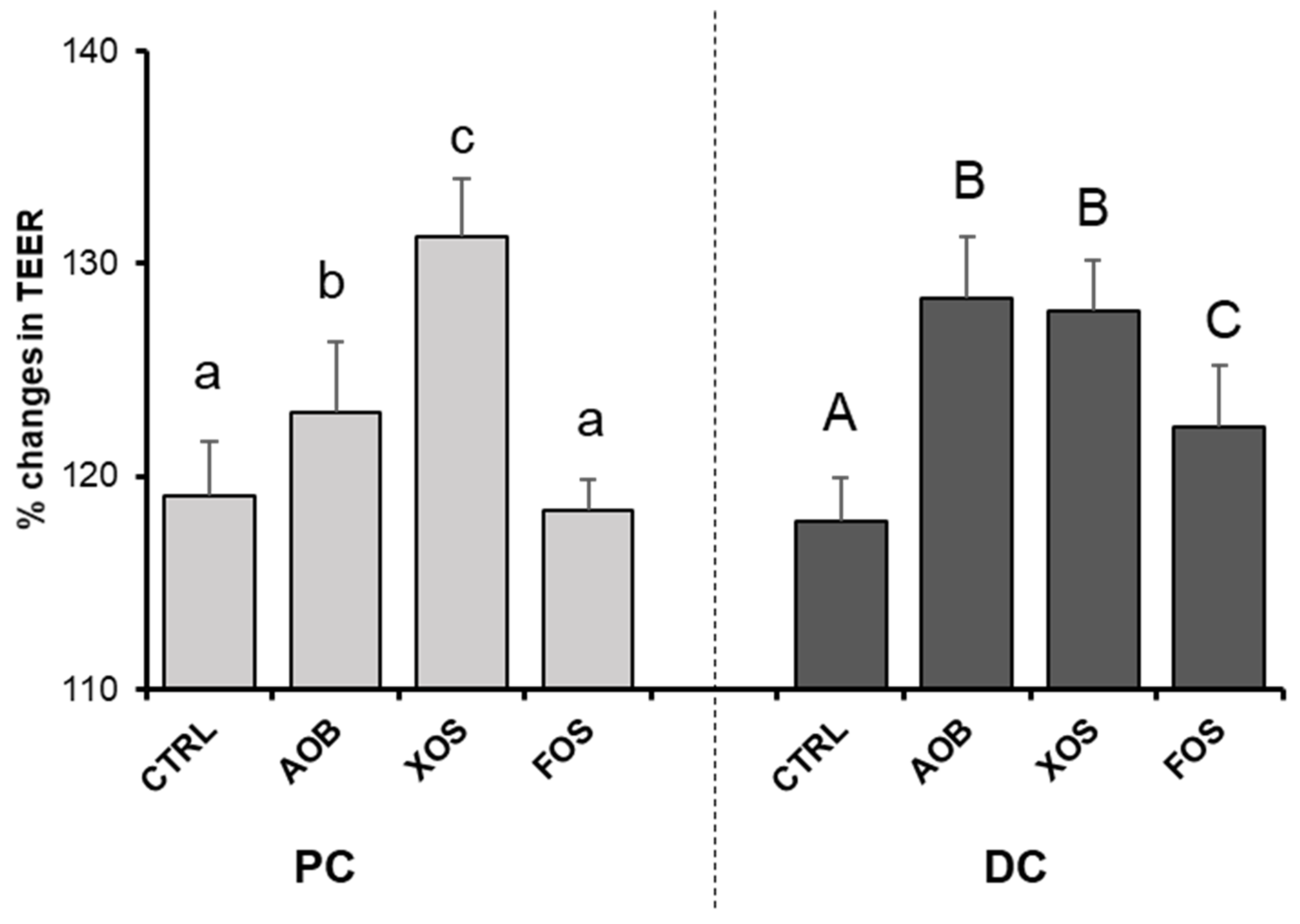
3.2.4. Effect of AOB, XOS and FOS on Gut Barrier Integrity
3.2.5. Effect of AOB, XOS and FOS on Gut Redox Potential
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vangay, P.; Ward, T.; Gerber, J.S.; Knights, D. Antibiotics, Pediatric Dysbiosis, and Disease. Cell Host Microbe 2015, 17, 553–564. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the Gut Microbiota in Disease. Microb. Ecology Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Petersen, C.; Round, J.L. Defining Dysbiosis and Its Influence on Host Immunity and Disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Antonio, S.; Gaetano, R.; Marco, L.V.; Gianluigi, F.; Annalisa, C.; Diego, F.; Michele, F.; Pasquale, G.; Pasquale, D.F. Polycystic Ovary Syndrome and Probiotics: A Natural Approach to an Inflammatory Disease. Curr. Women Health Rev. 2021, 17, 14–20. [Google Scholar]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Liu, F.; Prabhakar, M.; Ju, J.; Long, H.; Zhou, H.-W. Effect of Inulin-Type Fructans on Blood Lipid Profile and Glucose Level: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. J. Clin. Nutr. 2017, 71, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Tandon, D.; Haque, M.M.; Gote, M.; Jain, M.; Bhaduri, A.; Dubey, A.K.; Mande, S.S. A Prospective Randomized, Double-Blind, Placebo-Controlled, Dose-Response Relationship Study to Investigate Efficacy of f Ructo- o Ligo s Accharides (FOS) on Human Gut Microflora. Sci. Rep. 2019, 9, 5473. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Lee, Y.-K.; Sybesma, W. Vitamins for the Gut Microbiome. Trends Mol. Med. 2019. [Google Scholar] [CrossRef]
- Gupta, U.C.; Gupta, S.C. Role of Vitamins in Human Health and Nutrition: Sources and Morbidity. Available online: https://www.eurekaselect.com/130852/article (accessed on 11 January 2021).
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Xu, Y.; Xiang, S.; Ye, K.; Zheng, Y.; Feng, X.; Zhu, X.; Chen, J.; Chen, Y. Cobalamin (Vitamin B12) Induced a Shift in Microbial Composition and Metabolic Activity in an in Vitro Colon Simulation. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.; Prietl, B.; Tauschmann, M.; Mautner, S.I.; Kump, P.K.; Treiber, G.; Wurm, P.; Gorkiewicz, G.; Högenauer, C.; Pieber, T.R. Effects of High Doses of Vitamin D3 on Mucosa-Associated Gut Microbiome Vary between Regions of the Human Gastrointestinal Tract. Eur. J. Nutr. 2016, 55, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Fangmann, D.; Theismann, E.-M.; Türk, K.; Schulte, D.M.; Relling, I.; Hartmann, K.; Keppler, J.K.; Knipp, J.-R.; Rehman, A.; Heinsen, F.-A.; et al. Targeted Microbiome Intervention by Microencapsulated Delayed-Release Niacin Beneficially Affects Insulin Sensitivity in Humans. Diabetes Care 2018, 41, 398–405. [Google Scholar] [CrossRef]
- Pham, V.T.; Fehlbaum, S.; Seifert, N.; Richard, N.; Sybesma, W.; Bruins, M.; Rehman, A.; Steinert, R.E. Effects of Colon-Targeted Vitamins on the Composition and Metabolic Activity of the Human Gut Microbiome—A Pilot Study. Gut Microbes 2021. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Taminiau, B.; Pinheiro, I.; Duysburgh, C.; Jacobs, H.; Pijls, L.; Marzorati, M. Arabinoxylo-Oligosaccharides and Inulin Impact Inter-Individual Variation on Microbial Metabolism and Composition, Which Immunomodulates Human Cells. J. Agric. Food Chem. 2018, 66, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Bioaugmentation as a Tool To Protect the Structure and Function of an Activated-Sludge Microbial Community Against a 3-Chloroaniline Shock Load | Applied and Environmental Microbiology. Available online: https://aem.asm.org/content/69/3/1511.short (accessed on 7 January 2021).
- Duysburgh, C.; Van den Abbeele, P.; Krishnan, K.; Bayne, T.F.; Marzorati, M. A Synbiotic Concept Containing Spore-Forming Bacillus Strains and a Prebiotic Fiber Blend Consistently Enhanced Metabolic Activity by Modulation of the Gut Microbiome in Vitro. Int. J. Pharm. X 2019, 1, 100021. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xia, X.; Tang, R.; Zhou, J.; Zhao, H.; Wang, K. Development of a Real-Time PCR Method for Firmicutes and Bacteroidetes in Faeces and Its Application to Quantify Intestinal Population of Obese and Lean Pigs. Lett. Appl. Microbiol. 2008, 47, 367–373. [Google Scholar] [CrossRef]
- De Paepe, K.; Kerckhof, F.-M.; Verspreet, J.; Courtin, C.M.; Van de Wiele, T. Inter-Individual Differences Determine the Outcome of Wheat Bran Colonization by the Human Gut Microbiome. Environ. Microbiol. 2017, 19, 3251–3267. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Ghyselinck, J.; Verstrepen, L.; Moens, F.; Van den Abbeele, P.; Said, J.; Smith, B.; Bjarnason, I.; Basit, A.W.; Gaisford, S. A 4-Strain Probiotic Supplement Influences Gut Microbiota Composition and Gut Wall Function in Patients with Ulcerative Colitis. Int. J. Pharm. 2020, 587, 119648. [Google Scholar] [CrossRef]
- Pham, V.T.; Seifert, N.; Richard, N.; Raederstorff, D.; Steinert, R.E.; Prudence, K.; Mohajeri, M.H. The Effects of Fermentation Products of Prebiotic Fibres on Gut Barrier and Immune Functions in Vitro. PeerJ 2018, 6, e5288. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A Web Tool for Visualizing Clustering of Multivariate Data Using Principal Component Analysis and Heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Wang, R.X.; Lee, J.S.; Campbell, E.L.; Colgan, S.P. Microbiota-Derived Butyrate Dynamically Regulates Intestinal Homeostasis through Regulation of Actin-Associated Protein Synaptopodin. Proc. Natl. Acad. Sci. USA 2020, 117, 11648–11657. [Google Scholar] [CrossRef] [PubMed]
- Bach Knudsen, K.E.; Lærke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Gundelund Nielsen, D.S.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; et al. Impact of Diet-Modulated Butyrate Production on Intestinal Barrier Function and Inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4. [Google Scholar] [CrossRef]
- González Hernández, M.A.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef]
- El Hage, R.; Hernandez Sanabria, E.; Calatayud Arroyo, M.; Van de Wiele, T. Supplementation of a Propionate-Producing Consortium Improves Markers of Insulin Resistance in an in Vitro Model of Gut-Liver Axis. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E742–E749. [Google Scholar] [CrossRef]
- Hoyles, L.; Snelling, T.; Umlai, U.-K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome–Host Systems Interactions: Protective Effects of Propionate upon the Blood–Brain Barrier. Microbiome 2018, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Diether, N.E.; Willing, B.P. Microbial Fermentation of Dietary Protein: An Important Factor in Diet–Microbe–Host Interaction. Microorganisms 2019, 7, 19. [Google Scholar] [CrossRef]
- Rios-Covian, D.; González, S.; Nogacka, A.M.; Arboleya, S.; Salazar, N.; Gueimonde, M.; de los Reyes-Gavilán, C.G. An Overview on Fecal Branched Short-Chain Fatty Acids Along Human Life and as Related With Body Mass Index: Associated Dietary and Anthropometric Factors. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Granado-Serrano, A.B.; Martín-Garí, M.; Sánchez, V.; Riart Solans, M.; Berdún, R.; Ludwig, I.A.; Rubió, L.; Vilaprinyó, E.; Portero-Otín, M.; Serrano, J.C.E. Faecal Bacterial and Short-Chain Fatty Acids Signature in Hypercholesterolemia. Sci. Rep. 2019, 9, 1772. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Wong, C.B.; Odamaki, T.; Xiao, J. Insights into the Reason of Human-Residential Bifidobacteria (HRB) Being the Natural Inhabitants of the Human Gut and Their Potential Health-Promoting Benefits. FEMS Microbiol. Rev. 2020, 44, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Duranti, S.; Ruiz, L.; Lugli, G.A.; Tames, H.; Milani, C.; Mancabelli, L.; Mancino, W.; Longhi, G.; Carnevali, L.; Sgoifo, A.; et al. Bifidobacterium Adolescentis as a Key Member of the Human Gut Microbiota in the Production of GABA. Sci. Rep. 2020, 10, 14112. [Google Scholar] [CrossRef]
- Allen, A.P.; Hutch, W.; Borre, Y.E.; Kennedy, P.J.; Temko, A.; Boylan, G.; Murphy, E.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Bifidobacterium Longum 1714 as a Translational Psychobiotic: Modulation of Stress, Electrophysiology and Neurocognition in Healthy Volunteers. Transl. Psychiatry 2016, 6, e939-e939. [Google Scholar] [CrossRef] [PubMed]
- King, C.H.; Desai, H.; Sylvetsky, A.C.; LoTempio, J.; Ayanyan, S.; Carrie, J.; Crandall, K.A.; Fochtman, B.C.; Gasparyan, L.; Gulzar, N.; et al. Baseline Human Gut Microbiota Profile in Healthy People and Standard Reporting Template. PLoS ONE 2019, 14, e0206484. [Google Scholar] [CrossRef] [PubMed]
- Coyne, M.J.; Comstock, L.E. Niche-Specific Features of the Intestinal Bacteroidales. J. Bacteriol. 2008, 190, 736–742. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Salazar, N.; Gueimonde, M.; de los Reyes-Gavilan, C.G. Shaping the Metabolism of Intestinal Bacteroides Population through Diet to Improve Human Health. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Páez, A.; del Pugar, E.M.G.; López-Almela, I.; Moya-Pérez, Á.; Codoñer-Franch, P.; Sanz, Y. Depletion of Blautia Species in the Microbiota of Obese Children Relates to Intestinal Inflammation and Metabolic Phenotype Worsening. mSystems 2020, 5. [Google Scholar] [CrossRef]
- Zilm, P.S.; Rogers, A.H. Co-Adhesion and Biofilm Formation by Fusobacterium Nucleatum in Response to Growth PH. Anaerobe 2007, 13, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Zilm, P.S.; Mira, A.; Bagley, C.J.; Rogers, A.H. Effect of Alkaline Growth PH on the Expression of Cell Envelope Proteins in Fusobacterium Nucleatum. Microbiology 2010, 156, 1783–1794. [Google Scholar] [CrossRef] [PubMed]
- Magnúsdóttir, S.; Ravcheev, D.; de Crécy-Lagard, V.; Thiele, I. Systematic Genome Assessment of B-Vitamin Biosynthesis Suggests Co-Operation among Gut Microbes. Front. Genet. 2015, 6, 148. [Google Scholar] [CrossRef]
- Sharma, V.; Rodionov, D.A.; Leyn, S.A.; Tran, D.; Iablokov, S.N.; Ding, H.; Peterson, D.A.; Osterman, A.L.; Peterson, S.N. B-Vitamin Sharing Promotes Stability of Gut Microbial Communities. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Soto-Martin, E.C.; Warnke, I.; Farquharson, F.M.; Christodoulou, M.; Horgan, G.; Derrien, M.; Faurie, J.-M.; Flint, H.J.; Duncan, S.H.; Louis, P. Vitamin Biosynthesis by Human Gut Butyrate-Producing Bacteria and Cross-Feeding in Synthetic Microbial Communities. mBio 2020, 11. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.; Kim, S.; Lee, J.; Ha, J.; Oh, H.; Lee, Y.; Kim, Y.; Yoon, Y. Vitamin E (α-Tocopherol) Consumption Influences Gut Microbiota Composition. Int. J. Food Sci. Nutr. 2019, 1–5. [Google Scholar] [CrossRef]
- Million, M.; Tidjani Alou, M.; Khelaifia, S.; Bachar, D.; Lagier, J.-C.; Dione, N.; Brah, S.; Hugon, P.; Lombard, V.; Armougom, F.; et al. Increased Gut Redox and Depletion of Anaerobic and Methanogenic Prokaryotes in Severe Acute Malnutrition. Sci. Rep. 2016, 6, 26051. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Armstrong, N.; Khelaifia, S.; Guilhot, E.; Richez, M.; Lagier, J.-C.; Dubourg, G.; Chabriere, E.; Raoult, D. The Antioxidants Glutathione, Ascorbic Acid and Uric Acid Maintain Butyrate Production by Human Gut Clostridia in The Presence of Oxygen In Vitro. Sci. Rep. 2020, 10, 7705. [Google Scholar] [CrossRef]
- Million, M.; Raoult, D. Linking Gut Redox to Human Microbiome. Human Microbiome J. 2018, 10, 27–32. [Google Scholar] [CrossRef]
- Xu, J.; Xu, C.; Chen, X.; Cai, X.; Yang, S.; Sheng, Y.; Wang, T. Regulation of an Antioxidant Blend on Intestinal Redox Status and Major Microbiota in Early Weaned Piglets. Nutrition 2014, 30, 584–589. [Google Scholar] [CrossRef]


| Phylum | Family | PC | DC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CTRL | AOB | XOS | FOS | CTRL | AOB | XOS | FOS | |||
| Actinobacteria | Bifidobacteriaceae | 8.60 | 8.75 | 8.86 | 8.40 | 8.28 | 8.57 | 8.80 | 8.29 | |
| Bacteroidetes | Bacteroidaceae | 8.84 | 8.62 | 8.63 | 8.84 | 8.94 | 8.80 | 8.85 | 9.18 | |
| Prevotellaceae | 7.04 | 7.52 | 7.26 | 7.32 | 6.48 | 7.02 | 7.16 | 7.15 | ||
| Rikenellaceae | <LOQ | <LOQ | <LOQ | <LOQ | 6.84 | 6.99 | 7.33 | 7.91 | ||
| Tannerellaceae | 7.03 | 6.85 | 6.83 | <LOQ | 7.86 | 7.91 | 8.05 | 7.91 | ||
| Firmicutes | Erysipelotrichaceae | <LOQ | <LOQ | <LOQ | <LOQ | 7.37 | 7.54 | 7.13 | 6.77 | |
| Lachnospiraceae | 8.54 | 8.40 | 8.34 | 8.83 | 8.92 | 8.84 | 8.78 | 9.01 | ||
| Lactobacillaceae | 7.62 | 7.77 | 7.70 | 6.79 | 7.62 | 7.37 | 7.94 | 7.24 | ||
| Ruminococcaceae | 5.87 | 5.84 | <LOQ | 5.54 | 7.39 | 7.93 | 7.26 | 8.02 | ||
| Veillonellaceae | 7.91 | 7.93 | 7.52 | 7.94 | 7.55 | 7.72 | 8.14 | 8.11 | ||
| Fusobacteria | Fusobacteriaceae | <LOQ | <LOQ | <LOQ | <LOQ | 7.27 | <LOQ | 7.21 | 7.11 | |
| Proteobacteria | Burkholderiaceae | 7.31 | 7.24 | 7.05 | 7.29 | 7.20 | 7.29 | 7.63 | 7.38 | |
| Desulfovibrionaceae | 6.82 | 6.30 | 5.80 | <LOQ | 7.33 | 7.19 | 7.16 | 7.37 | ||
| Enterobacteriaceae | 6.86 | 6.67 | 6.10 | 6.24 | 6.19 | 6.18 | 5.62 | 5.99 | ||
| Pseudomonadaceae | 6.27 | 5.77 | <LOQ | <LOQ | 7.48 | 7.12 | 7.24 | 7.46 | ||
| Verrucomicrobia | Akkermansiaceae | <LOQ | <LOQ | <LOQ | <LOQ | 8.29 | 8.15 | 8.13 | 8.16 | |
| Phylum | Family | OTU | Related to | PC | DC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CTRL | AOB | XOS | FOS | CTRL | AOB | XOS | FOS | ||||
| Actinobacteria | Bifidobacteriaceae | OTU 01 | Bifidobacterium adolescentis | 8.39 | 8.37 | 8.74 | 7.79 | 8.05 | 8.16 | 8.67 | 7.75 |
| OTU 07 | Bifidobacterium longum | 8.16 | 8.44 | 8.21 | 8.27 | 7.87 | 8.34 | 8.18 | 8.14 | ||
| Bacteroidetes | Bacteroidaceae | OTU 04 | Bacteroides dorei | 8.04 | 8.11 | 8.46 | 8.61 | 8.19 | 8.54 | 8.41 | 8.31 |
| OTU 05 | Bacteroides fragilis | 8.45 | 7.90 | 7.65 | 5.62 | 8.66 | 7.89 | 7.93 | 7.53 | ||
| OTU 11 | Bacteroides ovatus | 6.08 | 5.89 | 6.93 | 7.31 | 7.44 | 6.90 | 7.83 | 8.93 | ||
| OTU 13 | Bacteroides uniformis | <LOQ | <LOQ | <LOQ | <LOQ | 6.37 | 6.22 | 7.16 | 6.17 | ||
| OTU 08 | Bacteroides xylanisolvens | 8.34 | 8.02 | 7.73 | 7.45 | 8.05 | 7.59 | 8.22 | 8.36 | ||
| OTU 20 | Prevotella salivae | 7.04 | 7.52 | 7.26 | 7.31 | 6.48 | 7.02 | 7.16 | 7.15 | ||
| Firmicutes | Erysipelotrichaceae | OTU 17 | Clostridium innocuum | <LOQ | <LOQ | <LOQ | <LOQ | 5.46 | 7.07 | <LOQ | 5.50 |
| Lachnospiraceae | OTU 03 | Clostridium clostridioforme | 8.38 | 8.01 | 8.20 | 8.40 | 8.62 | 8.28 | 8.36 | 8.51 | |
| OTU 06 | Lachnoclostridium sp. | 7.80 | 7.68 | 6.74 | 8.08 | 6.27 | 6.56 | 6.07 | 6.85 | ||
| OTU 16 | Blautia wexlerae | 5.84 | 7.68 | 5.52 | 8.34 | 7.16 | 7.78 | 6.71 | 8.39 | ||
| OTU 14 | Eubacterium rectale | <LOQ | <LOQ | <LOQ | <LOQ | 7.11 | 6.35 | 7.65 | 7.88 | ||
| OTU 30 | Clostridium sp. | <LOQ | <LOQ | <LOQ | <LOQ | 6.59 | 7.45 | 6.83 | 6.68 | ||
| OTU 34 | Lachnospiraceae spp. | <LOQ | <LOQ | <LOQ | <LOQ | 6.66 | 6.59 | 7.63 | 7.60 | ||
| OTU 26 | Blautia coccoides | <LOQ | <LOQ | <LOQ | <LOQ | 7.83 | 7.32 | 7.72 | 7.20 | ||
| OTU 28 | Lachnospiraceae spp. | 6.77 | 6.89 | 7.43 | 6.43 | 6.91 | 7.03 | 7.54 | 6.95 | ||
| Lactobacillaceae | OTU 12 | Pediococcus acidilactici | 7.62 | 7.76 | 7.70 | 6.79 | 7.62 | 7.37 | 7.93 | 7.23 | |
| Ruminococcaceae | OTU 18 | Subdoligranulum sp. | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 7.16 | <LOQ | 7.52 | |
| OTU 24 | Faecalibacterium prausnitzii | <LOQ | <LOQ | <LOQ | <LOQ | 6.42 | 7.52 | 6.37 | 7.72 | ||
| Veillonellaceae | OTU 09 | Anaeroglobus geminatus | 7.76 | 7.83 | 7.19 | 7.12 | 7.31 | 7.62 | 8.10 | 7.91 | |
| OTU 22 | Dialister invisus | 7.26 | 7.22 | 7.17 | 7.76 | 7.10 | 6.91 | 7.00 | 7.44 | ||
| Fusobacteria | Fusobacteriaceae | OTU 33 | Fusobacterium nucleatum | <LOQ | <LOQ | <LOQ | <LOQ | 7.27 | <LOQ | 7.21 | 7.11 |
| Verrucomicrobia | Akkermansiaceae | OTU 15 | Akkermansia muciniphila | <LOQ | <LOQ | <LOQ | <LOQ | 8.29 | 8.15 | 8.13 | 8.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, V.T.; Calatayud, M.; Rotsaert, C.; Seifert, N.; Richard, N.; Van den Abbeele, P.; Marzorati, M.; Steinert, R.E. Antioxidant Vitamins and Prebiotic FOS and XOS Differentially Shift Microbiota Composition and Function and Improve Intestinal Epithelial Barrier In Vitro. Nutrients 2021, 13, 1125. https://doi.org/10.3390/nu13041125
Pham VT, Calatayud M, Rotsaert C, Seifert N, Richard N, Van den Abbeele P, Marzorati M, Steinert RE. Antioxidant Vitamins and Prebiotic FOS and XOS Differentially Shift Microbiota Composition and Function and Improve Intestinal Epithelial Barrier In Vitro. Nutrients. 2021; 13(4):1125. https://doi.org/10.3390/nu13041125
Chicago/Turabian StylePham, Van T., Marta Calatayud, Chloë Rotsaert, Nicole Seifert, Nathalie Richard, Pieter Van den Abbeele, Massimo Marzorati, and Robert E. Steinert. 2021. "Antioxidant Vitamins and Prebiotic FOS and XOS Differentially Shift Microbiota Composition and Function and Improve Intestinal Epithelial Barrier In Vitro" Nutrients 13, no. 4: 1125. https://doi.org/10.3390/nu13041125
APA StylePham, V. T., Calatayud, M., Rotsaert, C., Seifert, N., Richard, N., Van den Abbeele, P., Marzorati, M., & Steinert, R. E. (2021). Antioxidant Vitamins and Prebiotic FOS and XOS Differentially Shift Microbiota Composition and Function and Improve Intestinal Epithelial Barrier In Vitro. Nutrients, 13(4), 1125. https://doi.org/10.3390/nu13041125