Using Specified Risk Materials-Based Peptides for Oil Sands Fluid Fine Tailings Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. SRM Hydrolysis and Peptides Recovery
2.3. Characterization of SRM-Peptides, SKTs, and FFTs
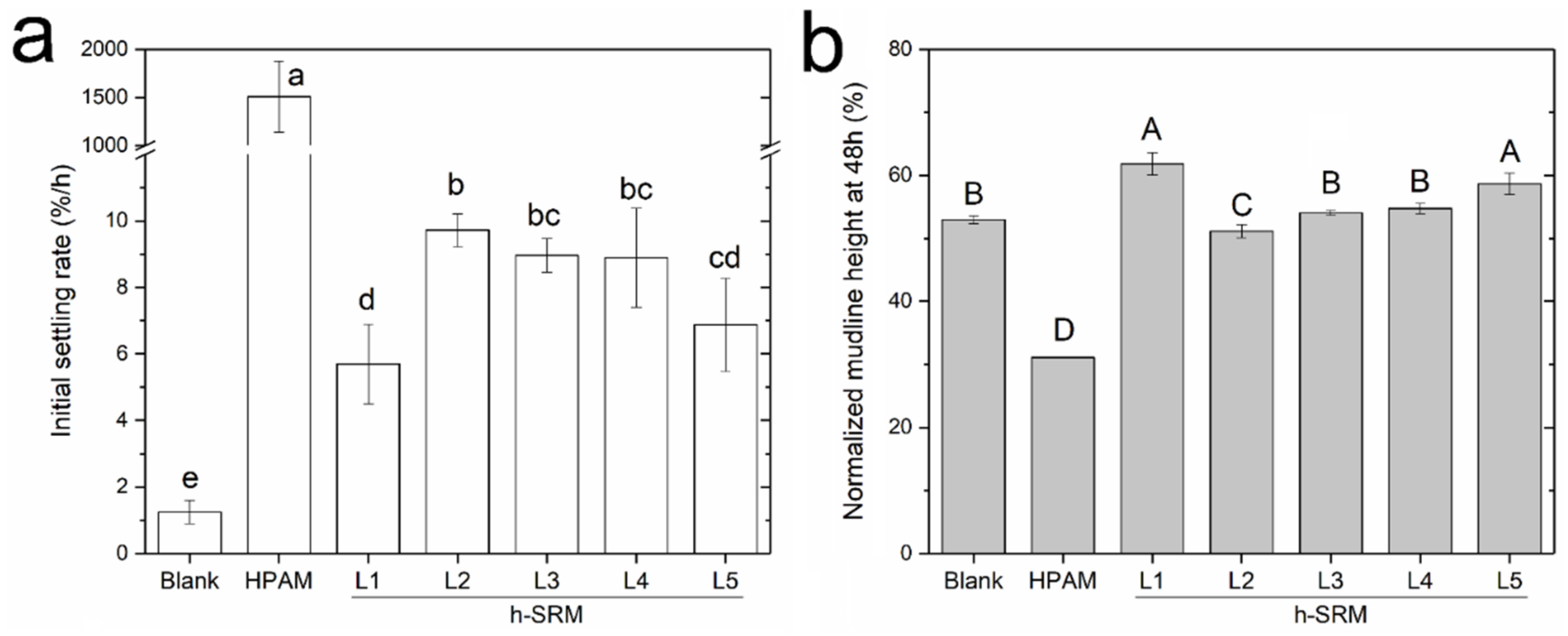
2.4. Cylinder Settling Test
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of SRM-Peptides and Tailings Samples
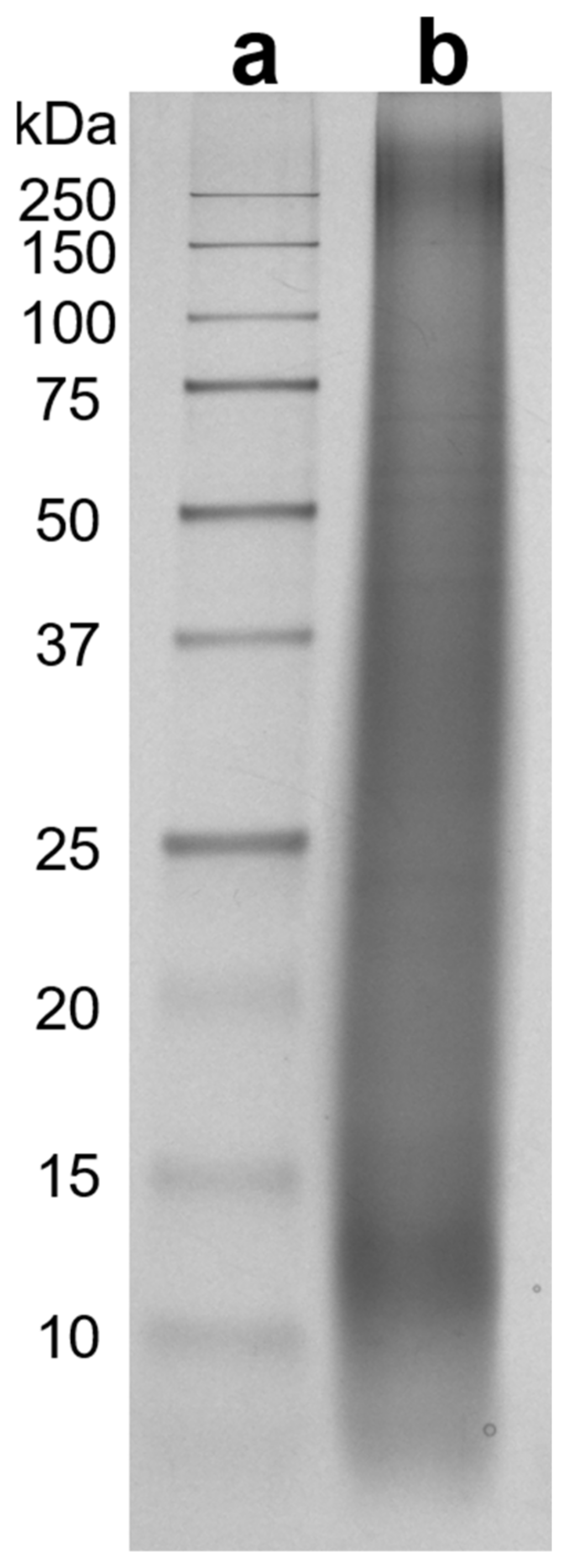
3.1.1. SRM-Peptides
3.1.2. Tailings Samples
3.2. Using SRM-Peptides in Treating Synthetic Kaolinite Tailings (SKTs)
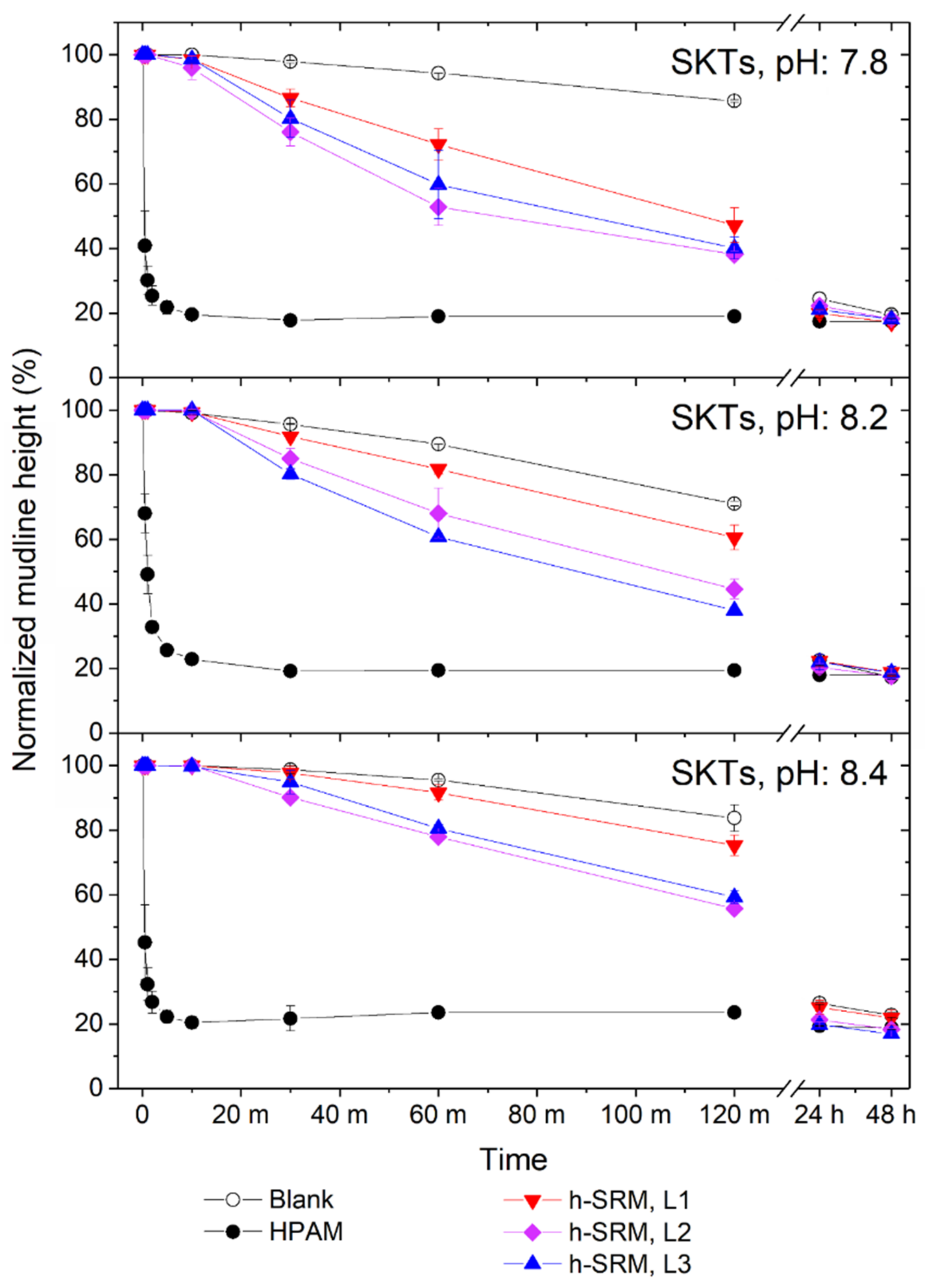
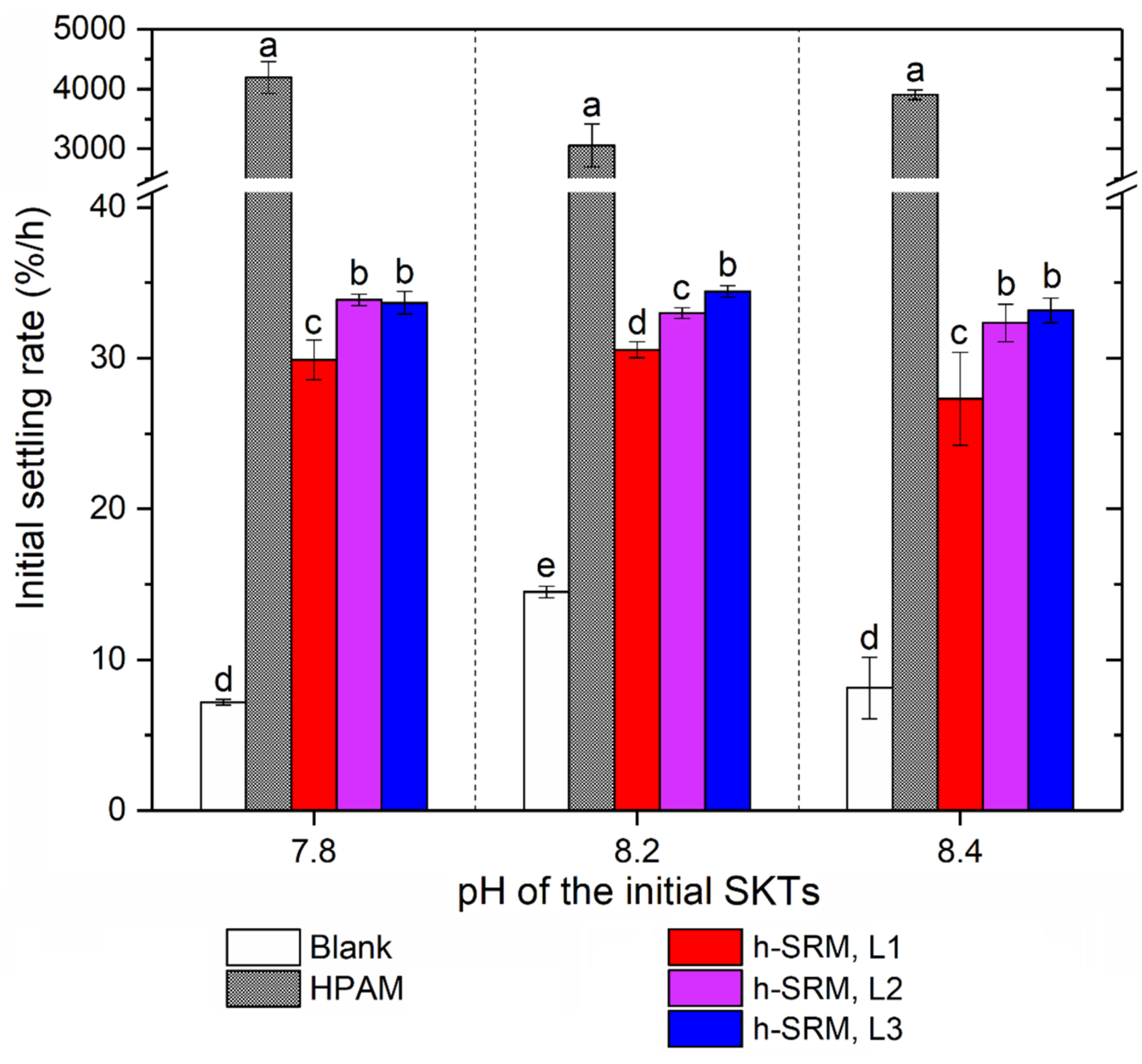
3.2.1. Tailings Settling and Sediment Condensation
3.2.2. Effect on Supernatant pH
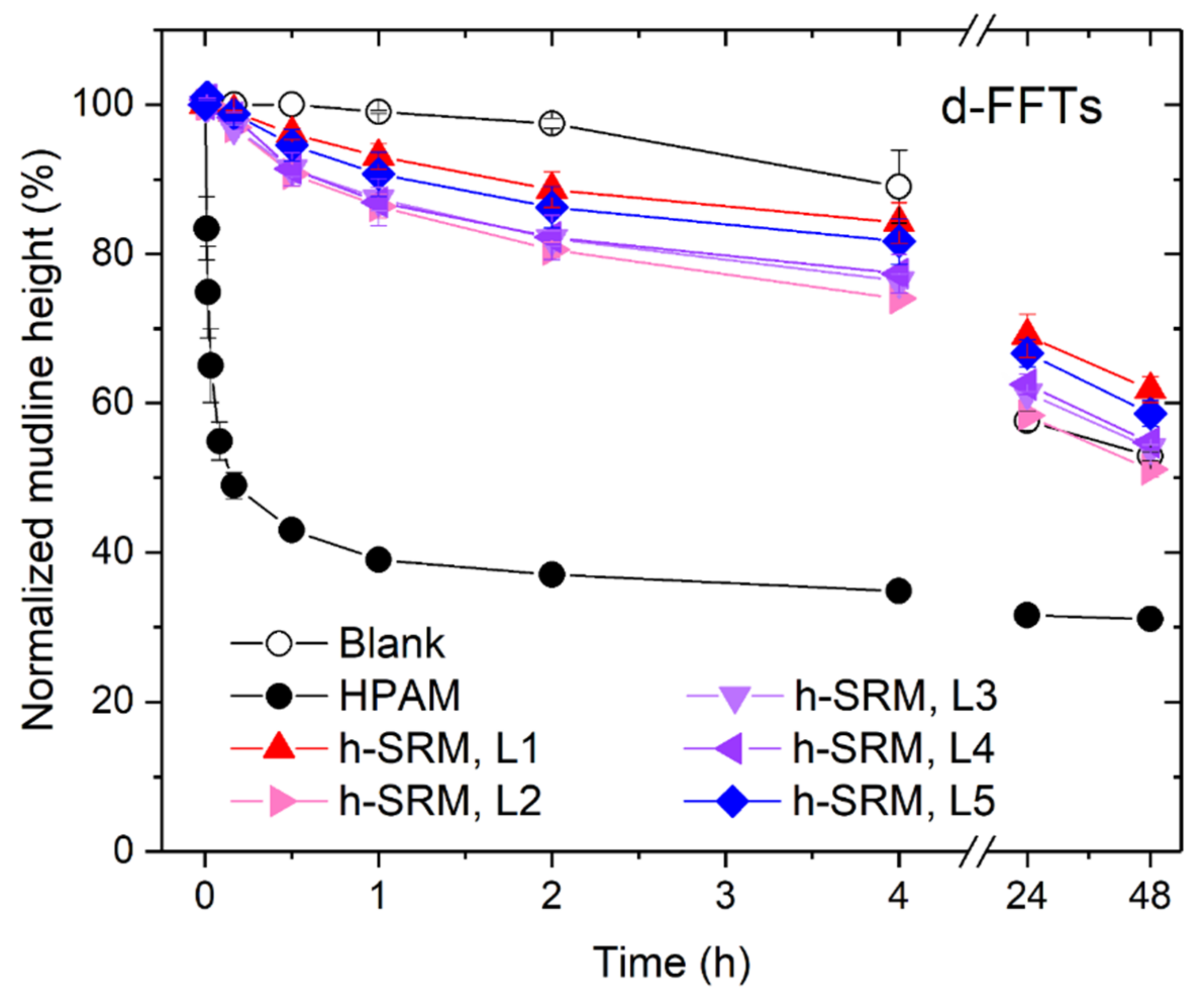
3.3. Using SRM-Peptides in Treating Diluted Industrial Fluid Fine Tailings (d-FFTs)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gosselin, P.; Hrudey, S.E.; Naeth, M.A.; Plourde, A.; Therrien, R.; Van Der Kraak, G.; Xu, Z. Environmental and Health Impacts of Canada’s Oil Sands Industry; Royal Society of Canada: Ottawa, ON, Canada, 2010. [Google Scholar]
- WISE. Chronology of Major Tailings Dam Failures; World Information Service on Energy: Doha, Qatar, 2021; Available online: https://www.wise-uranium.org/mdaf.html (accessed on 16 February 2021).
- AER. State of Fluid Tailings Management for Mineable Oil Sands; Alberta Energy Regulator: Calgary, AB, Canada, 2019. Available online: https://static.aer.ca/prd/documents/oilsands/2018-State-Fluid-Tailings-Management-Mineable-OilSands.pdf (accessed on 12 January 2021).
- Zhu, Y.; Lu, Y.; Liu, Q.; Masliyah, J.; Xu, Z. Comprehensive Study on Cleaner Production of Heavy Oil from Athabasca Oil Sands Using Chemical Additives in Biodiesel-Assisted Ambient-Aqueous Bitumen Extraction Process. J. Clean. Prod. 2020, 277, 122940. [Google Scholar] [CrossRef]
- Wang, C.; Harbottle, D.; Liu, Q.; Xu, Z. Current state of fine mineral tailings treatment: A critical review on theory and practice. Miner. Eng. 2014, 58, 113–131. [Google Scholar] [CrossRef]
- Masliyah, J.; Zhou, Z.J.; Xu, Z.; Czarnecki, J.; Hamza, H. Understanding water-based bitumen extraction from Athabasca oil sands. Can. J. Chem. Eng. 2004, 82, 628–654. [Google Scholar] [CrossRef]
- Alamgir, A.; Harbottle, D.; Masliyah, J.; Xu, Z. Al-PAM assisted filtration system for abatement of mature fine tailings. Chem. Eng. Sci. 2012, 80, 91–99. [Google Scholar] [CrossRef]
- Piazza, G.; Garcia, R. Proteins and peptides as renewable flocculants. Bioresour. Technol. 2010, 101, 5759–5766. [Google Scholar] [CrossRef] [PubMed]
- Piazza, G.; McAloon, A.; Garcia, R. A renewable flocculant from a poultry slaughterhouse waste and preliminary estimate of production costs. Resour. Conserv. Recycl. 2011, 55, 842–848. [Google Scholar] [CrossRef]
- Wang, D.-C.; Yu, H.-Y.; Fan, X.; Gu, J.; Ye, S.; Yao, J.; Ni, Q.-Q. High aspect ratio carboxylated cellulose nanofibers cross-linked to robust aerogels for superabsorption-flocculants: Paving way from nanoscale to macroscale. Acs Appl. Mater. Interfaces 2018, 10, 20755–20766. [Google Scholar] [CrossRef]
- Conzatti, G.; Chamary, S.; De Geyter, N.; Cavalie, S.; Morent, R.; Tourrette, A. Surface functionalization of plasticized chitosan films through PNIPAM grafting via UV and plasma graft polymerization. Eur. Polym. J. 2018, 105, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Meier, M. Sustainable polymers: Reduced environmental impact, renewable raw materials and catalysis. Green Chem. 2014, 16, 1672. [Google Scholar] [CrossRef]
- Vajihinejad, V.; Gumfekar, S.P.; Bazoubandi, B.; Najafabadi, Z.R.; Soares, J.B.P. Water soluble polymer flocculants: Synthesis, characterization, and performance assessment. Macromol. Mater. Eng. 2019, 304, 1–43. [Google Scholar] [CrossRef] [Green Version]
- Gregory, J.; Barany, S. Adsorption and flocculation by polymers and polymer mixtures. Adv. Colloid Interface Sci. 2011, 169, 1–12. [Google Scholar] [CrossRef]
- Mekonnen, T.; Mussone, P.; Bressler, D. Valorization of rendering industry wastes and co-products for industrial chemicals, materials and energy. Crit. Rev. Biotech. 2016, 36, 120–131. [Google Scholar] [CrossRef]
- USDA-FSIS. Bovine Spongiform Encephalopathy (BSE) and Specified Risk Material (SRM) Guidance Materials and Resources; United States Department of Agriculture—Food Safety and Inspection Service: Omaha, NE, USA, 2015. Available online: https://www.fsis.usda.gov/guidelines/2015-0023 (accessed on 12 January 2021).
- Shui, T.; Khatri, V.; Chae, M.; Sokhansanj, S.; Choi, P.; Bressler, D.C. Development of a torrefied wood pellet binder from the cross-linking between specified risk materials-derived peptides and epoxidized poly (vinyl alcohol). Renew. Energy 2020, 162, 71–80. [Google Scholar] [CrossRef]
- Zhu, Y.; Chae, M.; Adhikari, B.; Khatri, V.; Kaminsky, H.; Mussone, P.; Bressler, D.C. Valorizing Biowaste for Wastewater Treatment: Dewatering Sludges Using Specified Risk Material-Based Flocculants for Industrial Sustainability. Acs Sustain. Chem. Eng. 2021, 9, 2037–2046. [Google Scholar] [CrossRef]
- Mekonnen, T.H.; Mussone, P.G.; Stashko, N.; Choi, P.Y.; Bressler, D.C. Recovery and characterization of proteinacious material recovered from thermal and alkaline hydrolyzed specified risk materials. Process Biochem. 2013, 48, 885–892. [Google Scholar] [CrossRef]
- Schägger, H. Tricine-SDS-PAGE. Nat. Protoc. 2006, 1, 16. [Google Scholar] [CrossRef]
- Kaminsky, H. Demystifying the methylene blue index. In 4th International Oil Sands Tailings Conference; The University of Alberta Geotechnical Centre and the Oil Sands Tailings: Edmonton, AB, Canada, 2014. [Google Scholar]
- COSIA. Unified Fines Method for Minus 44 Micron Material and for Particle Size Distribution; Canada’s Oil Sands Innovation Alliance: Calgary, AB, Canada, 2016. [Google Scholar]
- Mekonnen, T.H.; Mussone, P.G.; El-Thaher, N.; Choi, P.; Bressler, D.C. Subcritical hydrolysis and characterization of waste proteinaceous biomass for value added applications. J. Chem. Technol. Biotechnol. 2015, 90, 476–483. [Google Scholar] [CrossRef]
- Napper, D. Polymeric Stabilization of Colloidal Dispersions; Academic Press: London, UK, 1983; Volume 3. [Google Scholar]
- Lee, B.J.; Fettweis, M.; Toorman, E.; Molz, F.J. Multimodality of a particle size distribution of cohesive suspended particulate matters in a coastal zone. J. Geophys. Res. Oceans 2012, 117. [Google Scholar] [CrossRef]
- Zhang, G.; Yin, H.; Lei, Z.; Reed, A.H.; Furukawa, Y. Effects of exopolymers on particle size distributions of suspended cohesive sediments. J. Geophys. Res. Ocean. 2013, 118, 3473–3489. [Google Scholar] [CrossRef]
- Masliyah, J.; Czarnecki, J.; Xu, Z. Handbook on Theory and Practice of Bitumen Recovery from Athabasca Oil Sands. Vol. I. Theoretical Basis; Kingsley Knowledge Publishing: Edmonton, AB, Canada, 2010; ISBN 978-1-926832-03-6. [Google Scholar]
- FTFC. Advances in Oil Sands Tailings Research; Fine Tailings Fundamentals Consortium: Edmonton, AB, Canada, 1995. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; John Wiley & Sons, Inc.: Toronto, ON, Canada, 2012. [Google Scholar]
- CAPP. Oil Sands Tailings; Canadian Association of Petroleum Producers: Calgary, AB, Canada, 2015; Available online: https://www.capp.ca/explore/tailings-ponds/ (accessed on 12 January 2021).
- Zhu, Y.; Yan, C.; Liu, Q.; Masliyah, J.H.; Xu, Z. Biodiesel-Assisted Ambient Aqueous Bitumen Extraction (BA3BE) from Athabasca Oil Sands. Energy Fuels 2018, 32, 6565–6576. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, B. Biodegradation of silk biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, W. A viscosity correlation for mixtures of heavy oil, bitumen, and petroleum fractions. Soc. Pet. Eng. J. 1984, 24, 277–282. [Google Scholar] [CrossRef]
- Wang, X.; Feng, X.; Xu, Z.; Masliyah, J.H. Polymer aids for settling and filtration of oil sands tailings. Can. J. Chem. Eng. 2010, 88, 403–410. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Gong, Y.; Kaminsky, H.; Chae, M.; Mussone, P.; Bressler, D.C. Using Specified Risk Materials-Based Peptides for Oil Sands Fluid Fine Tailings Management. Materials 2021, 14, 1582. https://doi.org/10.3390/ma14071582
Zhu Y, Gong Y, Kaminsky H, Chae M, Mussone P, Bressler DC. Using Specified Risk Materials-Based Peptides for Oil Sands Fluid Fine Tailings Management. Materials. 2021; 14(7):1582. https://doi.org/10.3390/ma14071582
Chicago/Turabian StyleZhu, Yeling, Yuki Gong, Heather Kaminsky, Michael Chae, Paolo Mussone, and David C. Bressler. 2021. "Using Specified Risk Materials-Based Peptides for Oil Sands Fluid Fine Tailings Management" Materials 14, no. 7: 1582. https://doi.org/10.3390/ma14071582
APA StyleZhu, Y., Gong, Y., Kaminsky, H., Chae, M., Mussone, P., & Bressler, D. C. (2021). Using Specified Risk Materials-Based Peptides for Oil Sands Fluid Fine Tailings Management. Materials, 14(7), 1582. https://doi.org/10.3390/ma14071582





