Vaccine Enthusiasm and Hesitancy in Cancer Patients and the Impact of a Webinar
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Statistical Methods
3. Results
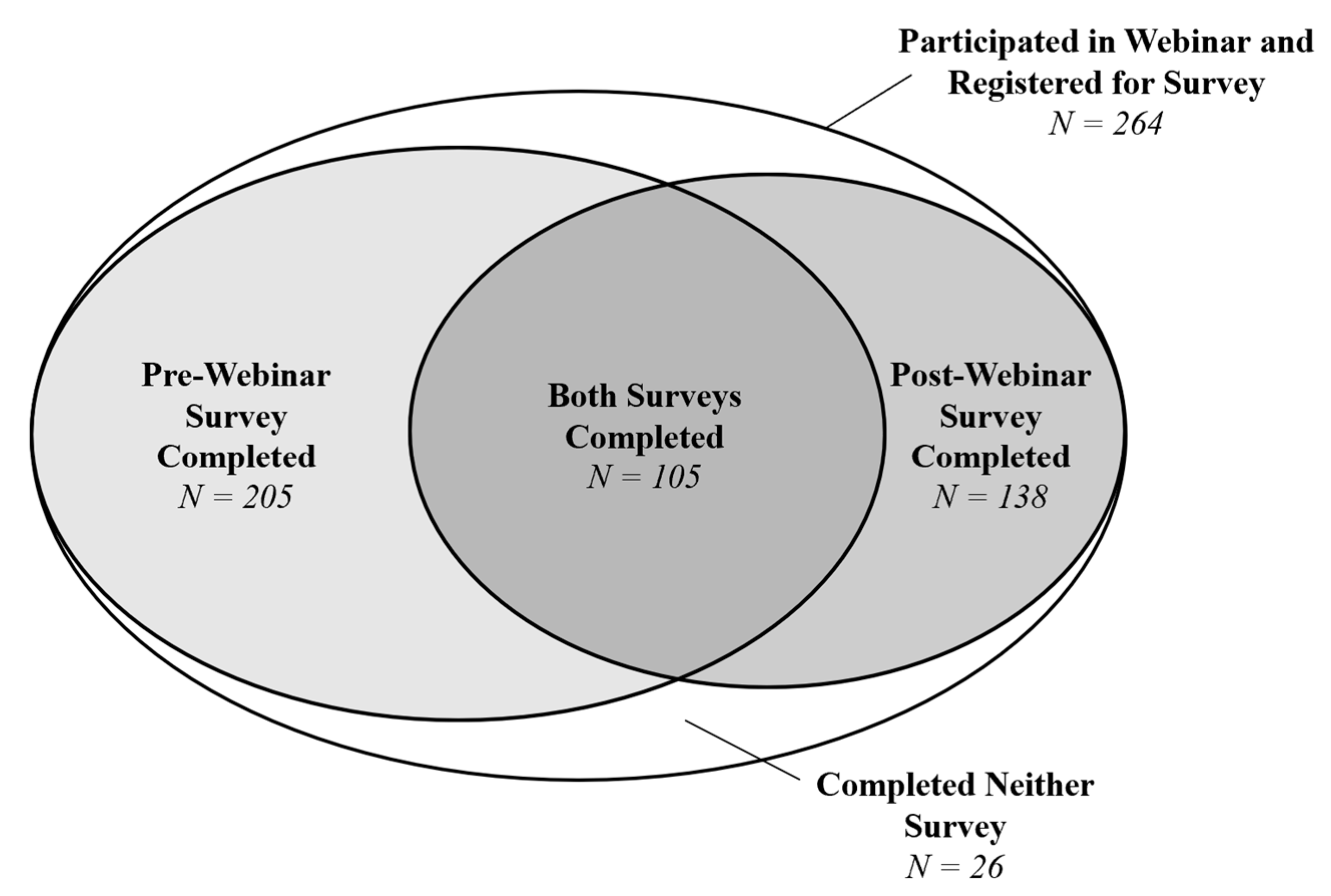
3.1. Participants and Characteristics
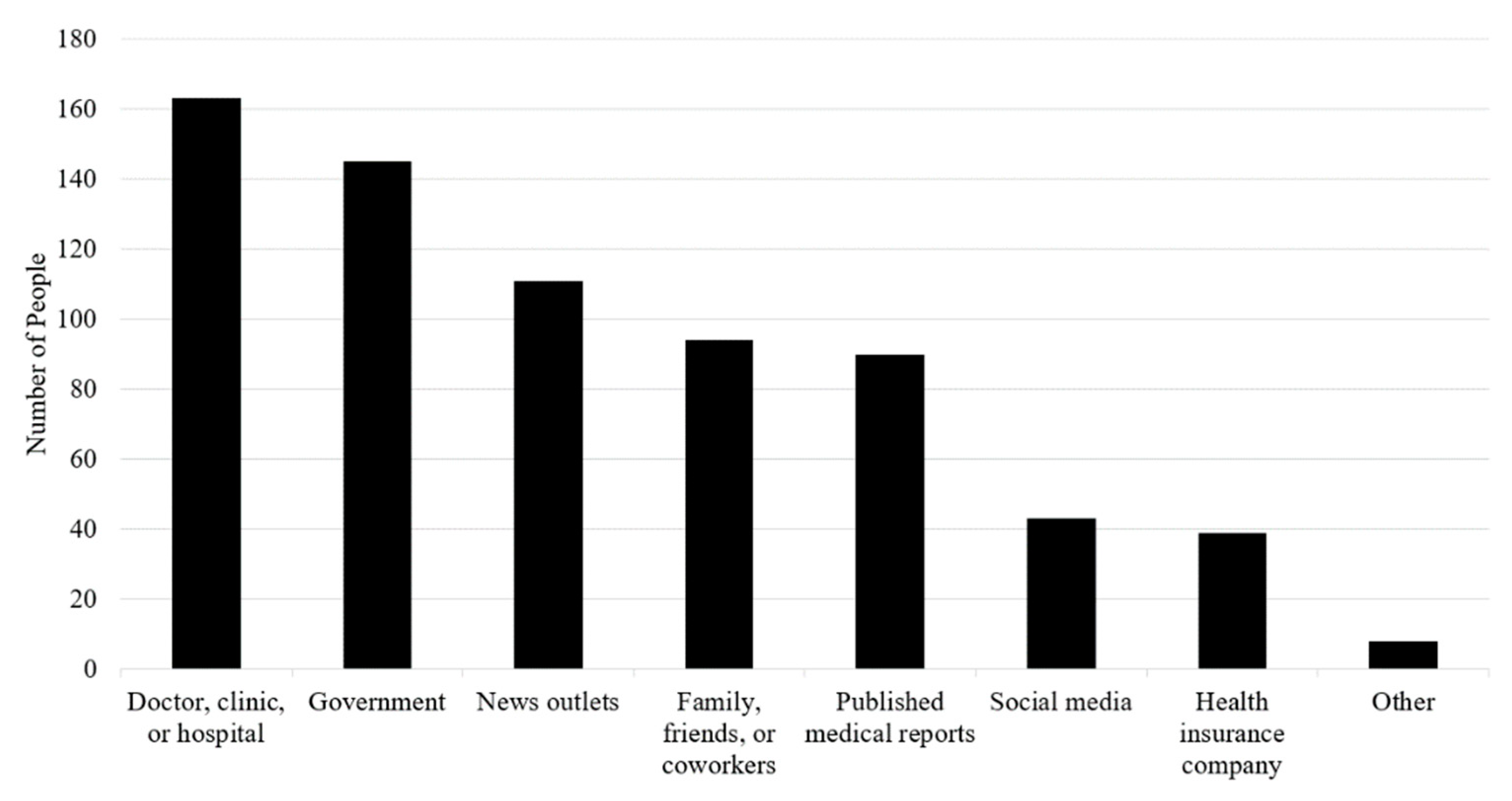
3.2. Where People Get Information about Vaccines against COVID-19
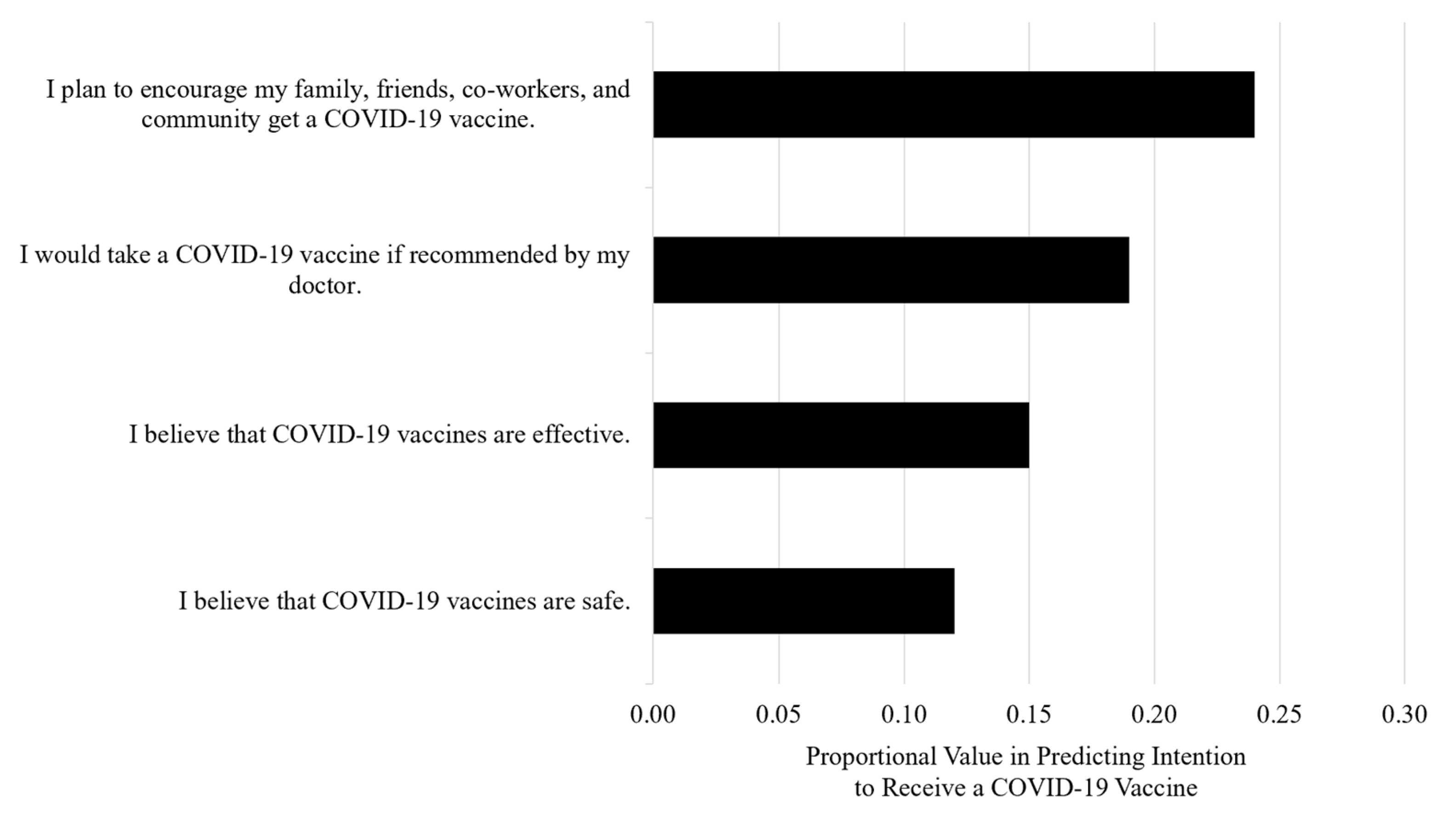
3.3. Characteristics Associated with the Intention to Receive a COVID-19 Vaccine
3.4. Webinar Impact on the Intention to Receive a COVID-19 Vaccine
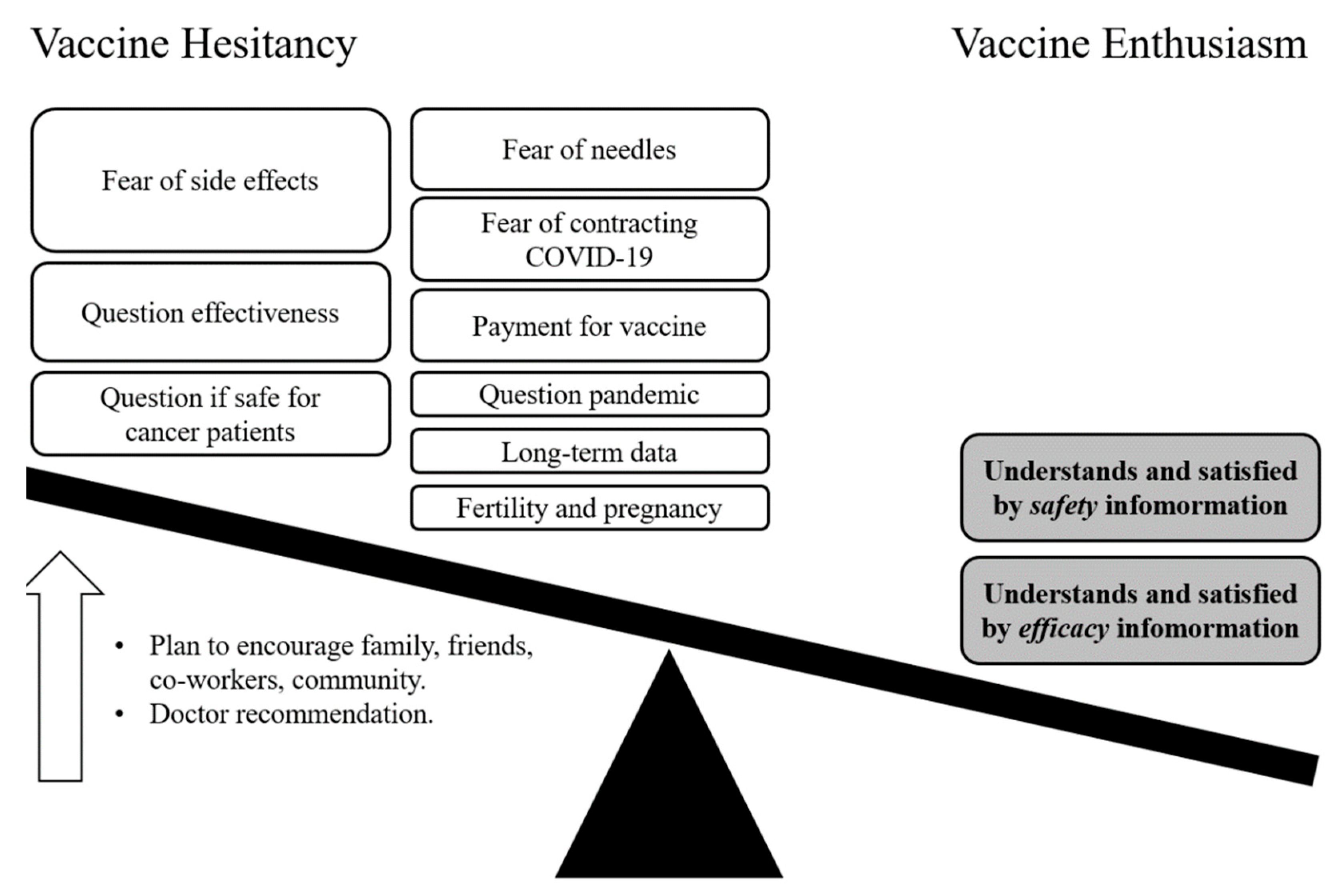
3.5. Changes in Beliefs and Perspectives on Vaccines against COVID-19
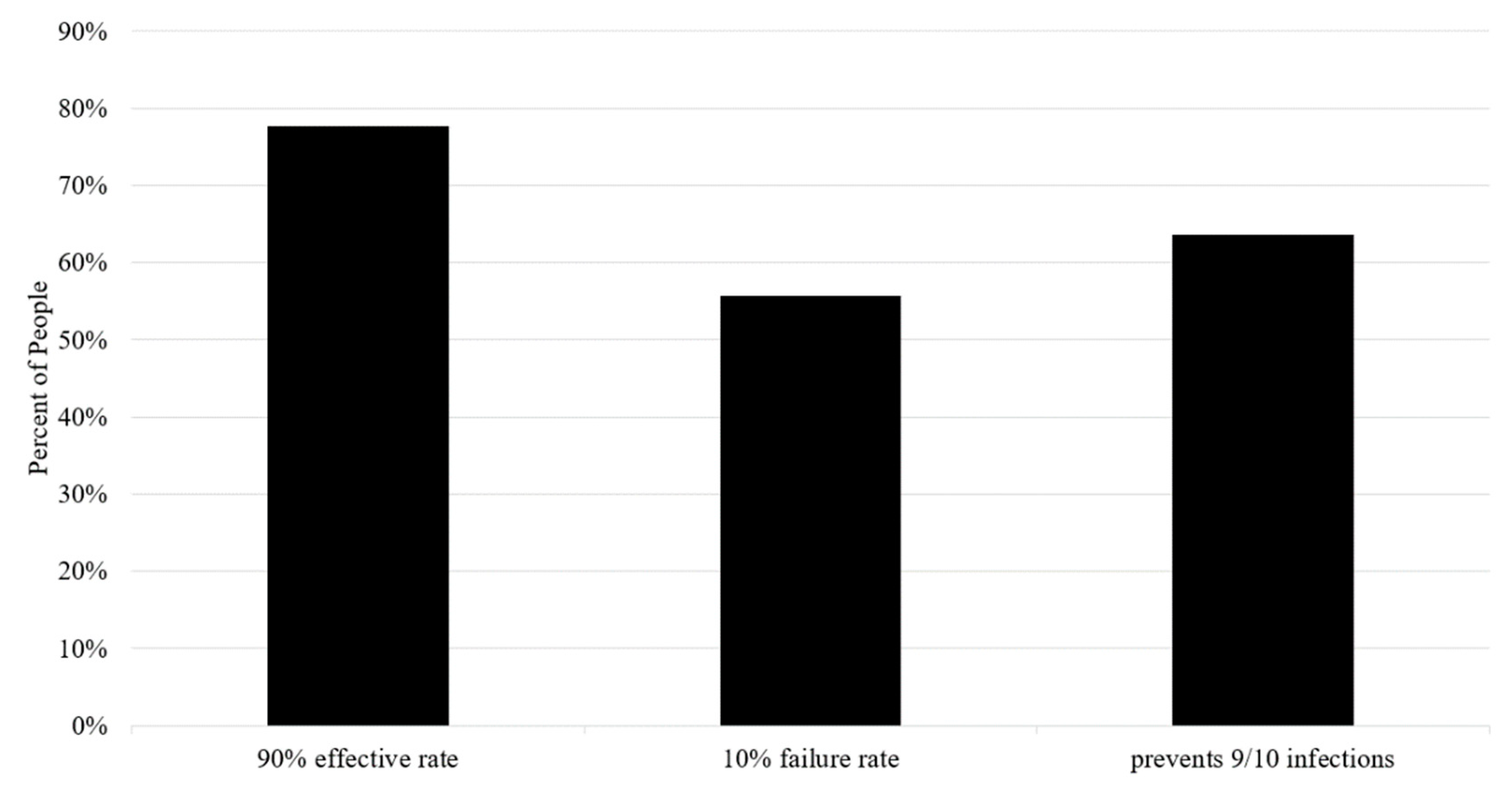
3.6. Participants’ Understanding of the Vaccine Depends on Communication Framing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- American Society of Hematology & American Society for Transplantation and Cellular Therapy. ASH-ASTCT COVID-19 and Vaccines: Frequently Asked Questions. Available online: https://www.hematology.org/covid-19/ash-astct-covid-19-and-vaccines (accessed on 18 March 2021).
- American Society of Clinical Oncology. COVID-19 Vaccine & Patients with Cancer. Available online: https://www.asco.org/asco-coronavirus-resources/covid-19-vaccines-patients-cancer (accessed on 18 March 2021).
- National Comprehensive Cancer Network. Cancer and COVID-19 Vaccination (Version 1.0 1/22/2021). Available online: https://www.nccn.org/covid-19/pdf/COVID-19_Vaccination_Guidance_V1.0.pdf (accessed on 18 March 2021).
- Lazarus, J.V.; Ratzan, S.C.; Palayew, A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Kimball, S.; El-Mohandes, A. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2020, 27, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.A.; Bloomstone, S.J.; Walder, J.; Crawford, S.; Fouayzi, H.; Mazor, K.M. Attitudes Toward a Potential SARS-CoV-2 Vaccine. Ann. Intern. Med. 2020, 173, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, N.E. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef]
- Lin, C.; Tu, P.; Beitsch, L.M. Confidence and Receptivity for COVID-19 Vaccines: A Rapid Systematic Review. Vaccines 2020, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Attwell, K.; Betsch, C.; Dubé, E.; Sivelä, J.; Gagneur, A.; Suggs, L.S.; Picot, V.; Thomson, A. Increasing vaccine acceptance using evidence-based approaches and policies: Insights from research on behavioural and social determinants presented at the 7th Annual Vaccine Acceptance Meeting. Int. J. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Press Ganey Associates LLC. Vaccine Hesitancy and Acceptance: Data Segmentation Helps Address Barriers 4 February 2021. Available online: https://www.pressganey.com/resources/white-papers/vaccine-hesitancy-and-acceptance (accessed on 18 March 2021).
- McNeil, D. How Much Herd Immunity Is Enough? Available online: https://www.nytimes.com/2020/12/24/health/herd-immunity-covid-coronavirus.html (accessed on 18 March 2021).
- Clark, S.J.; Cowan, A.E.; Wortley, P.M. Influenza vaccination attitudes and practices among US registered nurses. Am. J. Infect. Control 2009, 37, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Hollmeyer, H.G.; Hayden, F.; Poland, G.; Buchholz, U. Influenza vaccination of health care workers in hospitals—A review of studies on attitudes and predictors. Vaccine 2009, 27, 3935–3944. [Google Scholar] [CrossRef] [PubMed]
- Posfay-Barbe, K.M. How Do Physicians Immunize Their Own Children? Differences Among Pediatricians and Nonpediatricians. Pediatrics 2005, 116, e623–e633. [Google Scholar] [CrossRef]
- Dubé, E.; Laberge, C.; Guay, M.; Bramadat, P.; Roy, R.; Bettinger, J.A. Vaccine hesitancy. Hum. Vaccines Immunother. 2013, 9, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Larson, H.J.; Jarrett, C.; Schulz, W.S.; Chaudhuri, M.; Zhou, Y.; Dube, E.; Schuster, M.; MacDonald, N.E.; Wilson, R.; Hesitancy, S.W.G.o.V. Measuring vaccine hesitancy: The development of a survey tool. Vaccine 2015, 33, 4165–4175. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Wong, E.L.Y.; Huang, J.; Cheung, A.W.L.; Law, K.; Chong, M.K.C.; Ng, R.W.Y.; Lai, C.K.C.; Boon, S.S.; Lau, J.T.F.; et al. Acceptance of the COVID-19 vaccine based on the health belief model: A population-based survey in Hong Kong. Vaccine 2021, 39, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Kreps, S.; Prasad, S.; Brownstein, J.S.; Hswen, Y.; Garibaldi, B.T.; Zhang, B.; Kriner, D.L. Factors Associated With US Adults’ Likelihood of Accepting COVID-19 Vaccination. JAMA Netw. Open 2020, 3, e2025594. [Google Scholar] [CrossRef]
- Jarrett, C.; Wilson, R.; O’Leary, M.; Eckersberger, E.; Larson, H.J. Strategies for addressing vaccine hesitancy—A systematic review. Vaccine 2015, 33, 4180–4190. [Google Scholar] [CrossRef]
- Wilson, S.L.; Wiysonge, C. Social media and vaccine hesitancy. BMJ Glob. Health 2020, 5, e004206. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, J. COVID-19 Conspiracies and Beyond: How Physicians Can Deal with Patients’ Misinformation. JAMA 2021, 325, 208. [Google Scholar] [CrossRef] [PubMed]
- Dror, A.A.; Eisenbach, N.; Taiber, S.; Morozov, N.G.; Mizrachi, M.; Zigron, A.; Srouji, S.; Sela, E. Vaccine hesitancy: The next challenge in the fight against COVID-19. Eur. J. Epidemiol. 2020, 35, 775–779. [Google Scholar] [CrossRef] [PubMed]
- van de Water, L.F.; van Kleef, J.J.; Dijksterhuis, W.P.M.; Henselmans, I.; van den Boorn, H.G.; Vaarzon Morel, N.M.; Schut, K.F.; Daams, J.G.; Smets, E.M.A.; van Laarhoven, H.W.M. Communicating treatment risks and benefits to cancer patients: A systematic review of communication methods. Qual. Life Res. 2020, 29, 1747–1766. [Google Scholar] [CrossRef] [PubMed]

| Pre-Webinar Survey Completed (N = 205) | Both Surveys Completed (N = 105) | Post-Webinar Survey Completed (N = 138) | ||||
|---|---|---|---|---|---|---|
| Characteristic | Number | Percent | Number | Percent | Number | Percent |
| Age (years) | ||||||
| 18–26 | 10 | 5% | 4 | 4% | 4 | 3% |
| 27–29 | 4 | 2% | 0 | 0% | 1 | 1% |
| 30–39 | 20 | 10% | 9 | 9% | 9 | 7% |
| 40–49 | 32 | 6% | 14 | 13% | 15 | 11% |
| 50–59 | 35 | 17% | 15 | 14% | 15 | 11% |
| 60–64 | 33 | 16% | 20 | 19% | 25 | 18% |
| 65–69 | 19 | 9% | 11 | 11% | 1 | 1% |
| 70–79 | 39 | 19% | 23 | 22% | 25 | 18% |
| 80 years or older | 11 | 5% | 9 | 9% | 11 | 8% |
| Prefer not to answer | 2 | 1% | 0 | 0% | 32 | 23% |
| Gender Identity | ||||||
| Man | 40 | 20% | 25 | 24% | 27 | 20% |
| Woman | 161 | 79% | 78 | 74% | 88 | 57% |
| Nonbinary, genderqueer, or genderfluid | 1 | 0.5% | 1 | 1% | 0 | 0% |
| Prefer not to answer | 3 | 2% | 1 | 1% | 23 | 17% |
| Sexual Orientation | ||||||
| Heterosexual or “straight” | 191 | 93% | 97 | 92% | 108 | 78% |
| Homosexual, gay, or lesbian | 3 | 1.5% | 2 | 2% | 3 | 2% |
| Bisexual | 2 | 1% | 0 | 0% | 1 | 1% |
| Other | 1 | 0.5% | 1 | 1% | 1 | 1% |
| Prefer not to answer | 8 | 4% | 4 | 4% | 25 | 18% |
| Race | ||||||
| American Indian or Alaska Native | 2 | 1% | 0 | 0% | 1 | 1% |
| Asian or Asian American | 10 | 5% | 3 | 3% | 3 | 2% |
| Black or African American | 12 | 6% | 7 | 7% | 8 | 6% |
| Native Hawaiian or Other Pacific Islander | 1 | 0.5% | 1 | 1% | 1 | 1% |
| White | 169 | 82% | 90 | 86% | 99 | 72% |
| Other | 7 | 3% | 2 | 2% | 2 | 1 |
| Prefer not to answer | 6 | 3% | 2 | 2% | 24 | 17% |
| Ethnicity | ||||||
| Hispanic or Latinx | 18 | 9% | 5 | 5% | 5 | 4% |
| Not Hispanic or Latinx | 175 | 85% | 96 | 91% | 107 | 78% |
| Prefer not to answer | 12 | 6% | 4 | 4% | 26 | 19% |
| Highest Level of Education | ||||||
| High school or equivalent | 7 | 3% | 3 | 3% | 4 | 3% |
| Some college credits | 15 | 7% | 7 | 7% | 9 | 7% |
| Associate’s degree | 15 | 7% | 7 | 7% | 9 | 7% |
| Bachelor’s degree | 60 | 29% | 29 | 28% | 32 | 23% |
| Graduate or professional degree | 103 | 50% | 56 | 53% | 60 | 43% |
| Prefer not to answer | 5 | 2% | 3 | 3% | 24 | 17% |
| Economics | ||||||
| Number of people in household | ||||||
| 1 | 37 | 18% | 24 | 23% | 28 | 20% |
| 2 | 106 | 52% | 58 | 55% | 65 | 47% |
| 3 | 31 | 15% | 10 | 10% | 11 | 8% |
| 4 | 17 | 8% | 7 | 7% | 8 | 6% |
| 5 | 8 | 4% | 2 | 2% | 2 | 1% |
| 6 | 3 | 1.5% | 2 | 2% | 2 | 1% |
| 7 | 2 | 1% | 2 | 2% | 2 | 1% |
| 8 | 0 | 0% | 0 | 0% | 0 | 0% |
| 9 or more | 1 | 0.5% | 0 | 0% | 0 | 0% |
| Prefer not to answer | 0 | 0% | 0 | 0% | 20 | 14% |
| Total household income for 2020 | ||||||
| Less than $15,000 | 2 | 1% | 1 | 1% | 1 | 1% |
| $15,000 to $19,999 | 5 | 2% | 3 | 3% | 3 | 2% |
| $20,000 to $24,999 | 3 | 1.5% | 1 | 1% | 1 | 1% |
| $25,000 to $34,999 | 6 | 3% | 4 | 4% | 4 | 3% |
| $35,000 to $49,999 | 9 | 4% | 8 | 8% | 10 | 7% |
| $50,000 to $74,999 | 25 | 12% | 12 | 11% | 15 | 11% |
| $75,000 to $99,999 | 29 | 14% | 15 | 14% | 15 | 11% |
| $100,000 and above | 71 | 35% | 29 | 28% | 32 | 23% |
| Prefer not to answer | 55 | 27% | 32 | 31% | 57 | 41% |
| Health Insurance | ||||||
| Employer offered | 133 | 65% | 59 | 56% | 65 | 47% |
| Private purchase | 37 | 18% | 21 | 20% | 26 | 19% |
| Medicare | 67 | 33% | 44 | 42% | 49 | 36% |
| Veterans Affairs, Tricare | 14 | 7% | 7 | 7% | 8 | 6% |
| Medicaid | 6 | 3% | 3 | 3% | 3 | 2% |
| No health insurance | 1 | 0.5% | 0 | 0% | 0 | 0% |
| Other (student health) | 7 | 3% | 3 | 3% | 8 | 6% |
| Prefer not to answer | 0 | 0% | 0 | 0% | 13 | 9% |
| Political Affiliation | ||||||
| Democrat | 69 | 34% | 38 | 36% | 47 | 34% |
| Republican | 39 | 19% | 19 | 18% | 19 | 14% |
| Independent | 29 | 14% | 12 | 11% | 14 | 10% |
| Other | 4 | 2% | 1 | 1% | 2 | 1% |
| Prefer not to answer | 64 | 31% | 35 | 33% | 56 | 41% |
| Connections to Cancer | ||||||
| Have cancer and actively receiving treatment | 55 | 27% | 35 | 33% | 40 | 29% |
| Cancer survivor and not receiving treatment | 59 | 29% | 24 | 24% | 29 | 17% |
| Caregiver to a cancer patient | 20 | 10% | 12 | 11% | 12 | 9% |
| Friend or family member to a cancer patient | 47 | 23% | 25 | 24% | 27 | 20% |
| Health care provider | 39 | 19% | 15 | 14% | 15 | 11% |
| Academic researcher or research staff member | 18 | 9% | 12 | 11% | 14 | 10% |
| Government employee | 8 | 4% | 3 | 3% | 3 | 2% |
| Community-based organization that serves people with cancer | 20 | 10% | 10 | 10% | 10 | 7% |
| Health insurance company employee | 1 | 0.5% | 0 | 0% | 0 | 0% |
| No connection to cancer | 2 | 1% | 0 | 0% | 0 | 0% |
| Other (caregiver to person with other disease, for-profit business, family of health care worker) | 12 | 6% | 6 | 6% | 8 | 6% |
| Characteristic | Coefficient | Significance | Model Importance |
|---|---|---|---|
| I plan to encourage my family, friends, co-workers, and community to get a COVID-19 vaccine. | 0.260 | p < 0.0001 | 0.548 |
| I would take a COVID-19 vaccine if recommended by my doctor. | 0.252 | p < 0.0001 | 0.351 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelkar, A.H.; Blake, J.A.; Cherabuddi, K.; Cornett, H.; McKee, B.L.; Cogle, C.R. Vaccine Enthusiasm and Hesitancy in Cancer Patients and the Impact of a Webinar. Healthcare 2021, 9, 351. https://doi.org/10.3390/healthcare9030351
Kelkar AH, Blake JA, Cherabuddi K, Cornett H, McKee BL, Cogle CR. Vaccine Enthusiasm and Hesitancy in Cancer Patients and the Impact of a Webinar. Healthcare. 2021; 9(3):351. https://doi.org/10.3390/healthcare9030351
Chicago/Turabian StyleKelkar, Amar H., Jodian A. Blake, Kartikeya Cherabuddi, Hailee Cornett, Bobbie L. McKee, and Christopher R. Cogle. 2021. "Vaccine Enthusiasm and Hesitancy in Cancer Patients and the Impact of a Webinar" Healthcare 9, no. 3: 351. https://doi.org/10.3390/healthcare9030351
APA StyleKelkar, A. H., Blake, J. A., Cherabuddi, K., Cornett, H., McKee, B. L., & Cogle, C. R. (2021). Vaccine Enthusiasm and Hesitancy in Cancer Patients and the Impact of a Webinar. Healthcare, 9(3), 351. https://doi.org/10.3390/healthcare9030351








