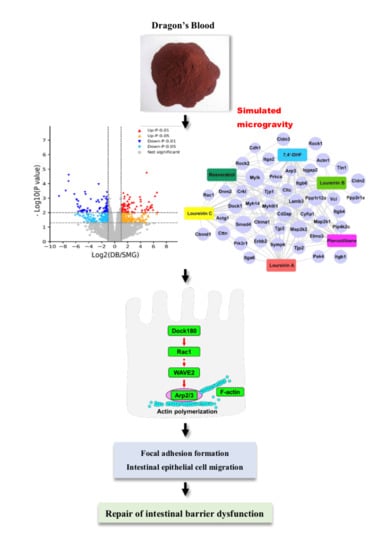
Dragon’s Blood Regulates Rac1-WAVE2-Arp2/3 Signaling Pathway to Protect Rat Intestinal Epithelial Barrier Dysfunction Induced by Simulated Microgravity
Abstract
:1. Introduction
2. Results
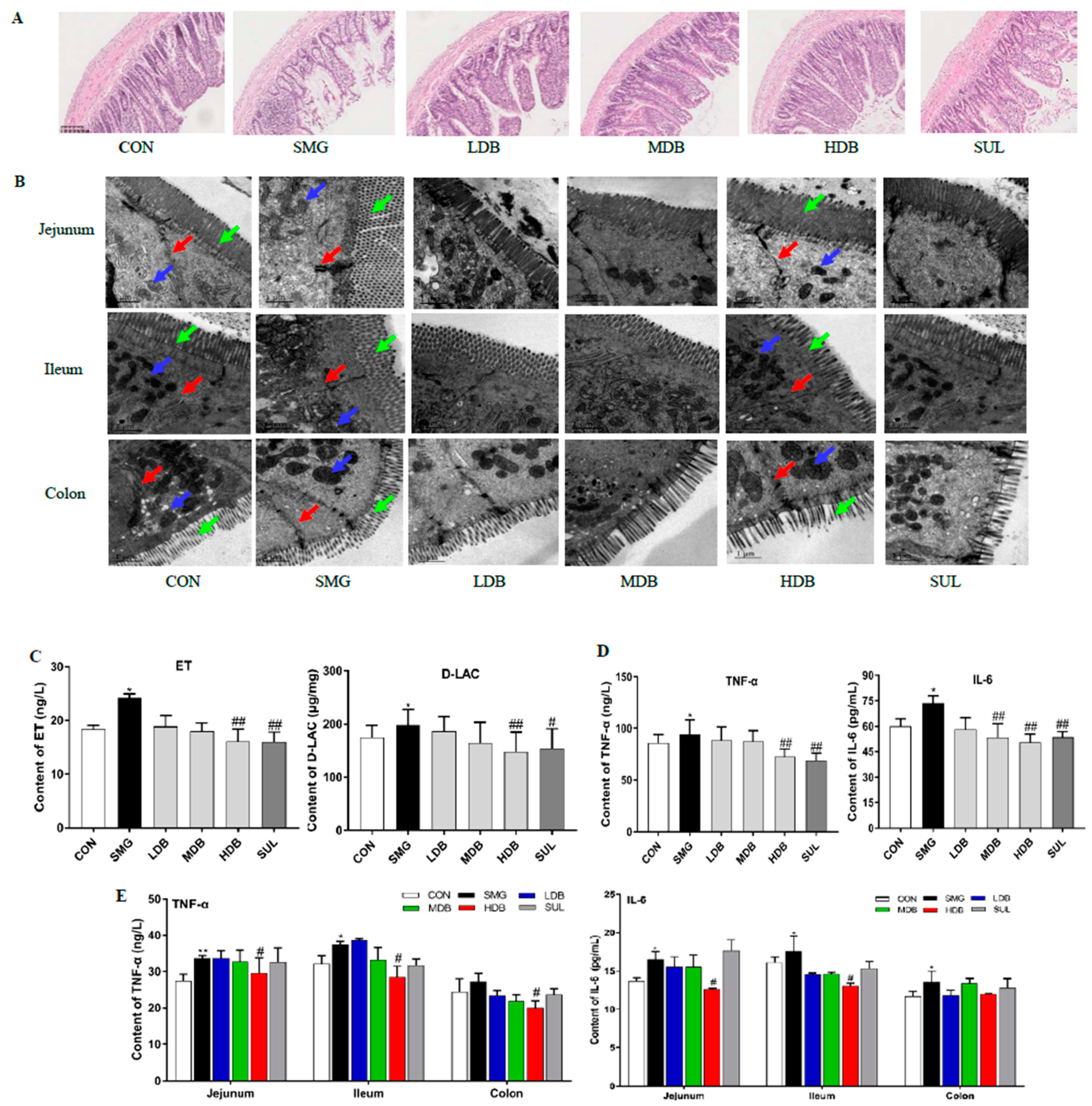
2.1. DB Attenuated IEB Injury in SMG Rats
2.2. DB Decreased Permeability and Inflammation in SMG Rat Intestine
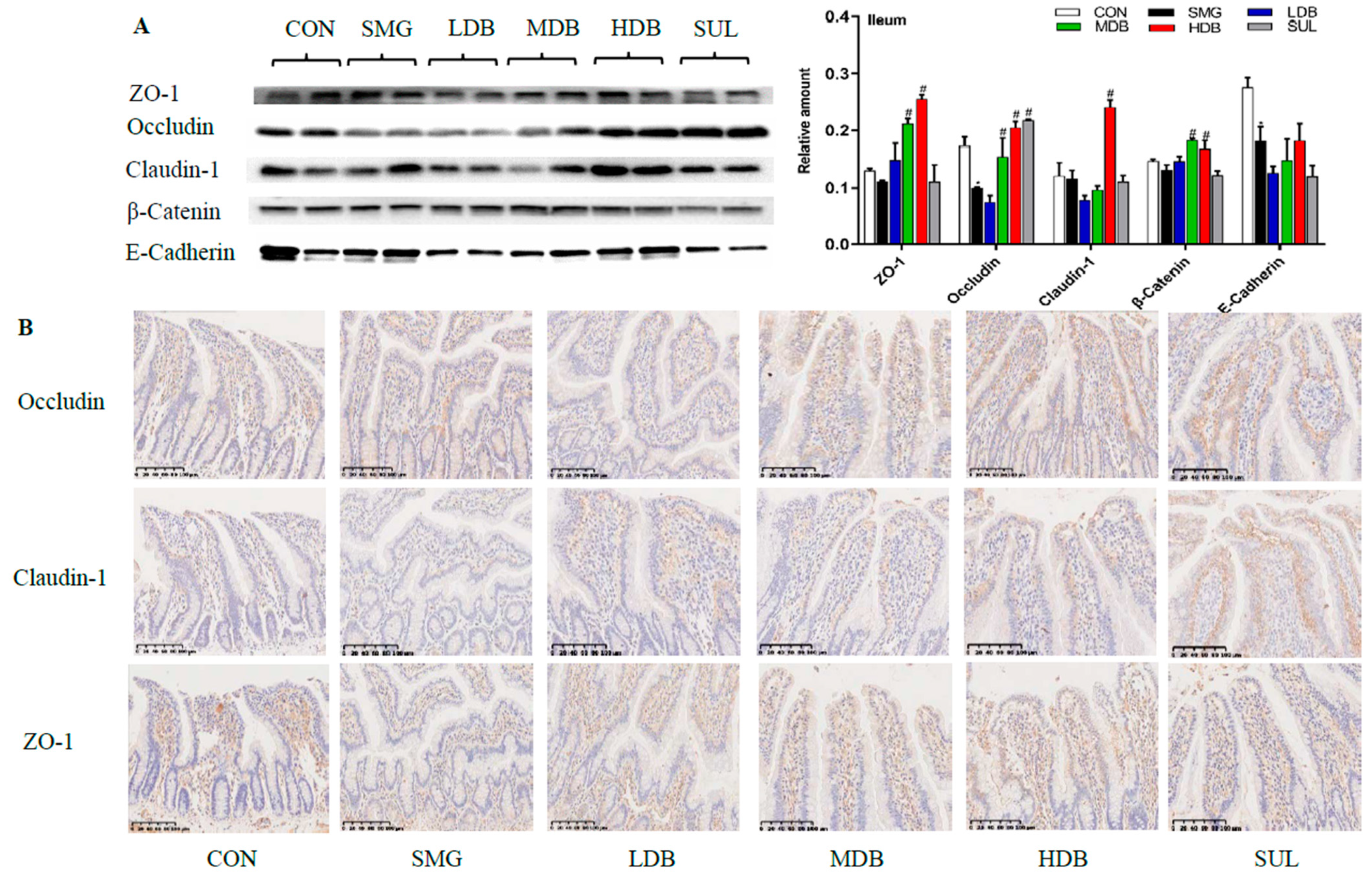
2.3. DB Protected Intestinal Barrier by Increasing the Expression of TJ and AJ Proteins in SMG Rats
2.4. DB Significantly Altered Intestinal Mucosa Proteins in SMG Rats Based on Proteomics
2.4.1. Identification of Differentially Expressed Protein (DEPs)
2.4.2. Gene-Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) Analysis
2.4.3. DB Regulated Proteins Related to IEB Damage in the Intestinal Mucosa of SMG Rats
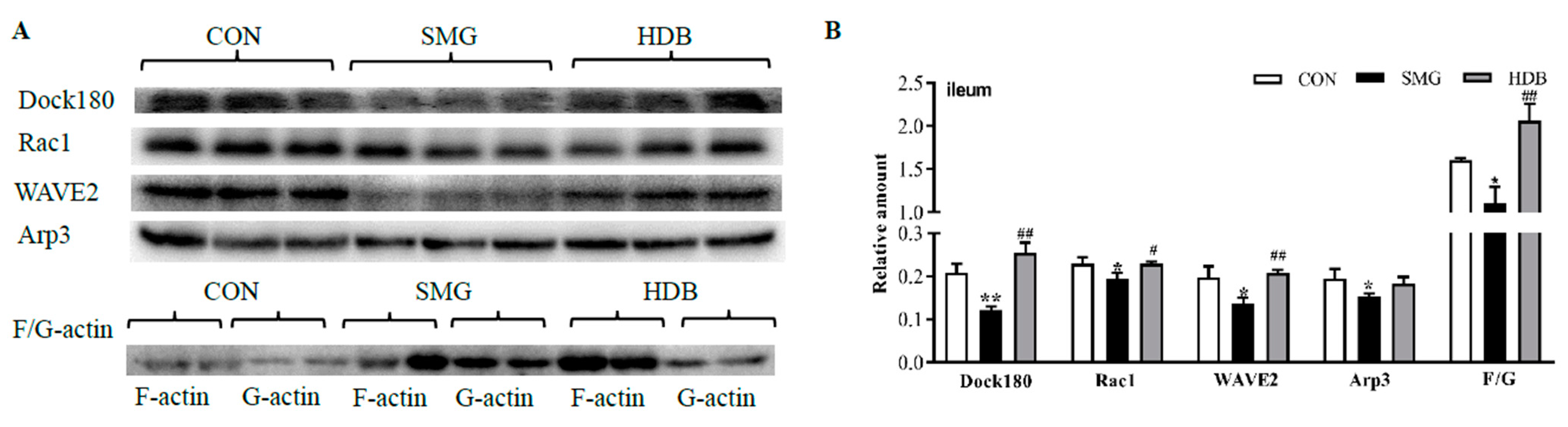
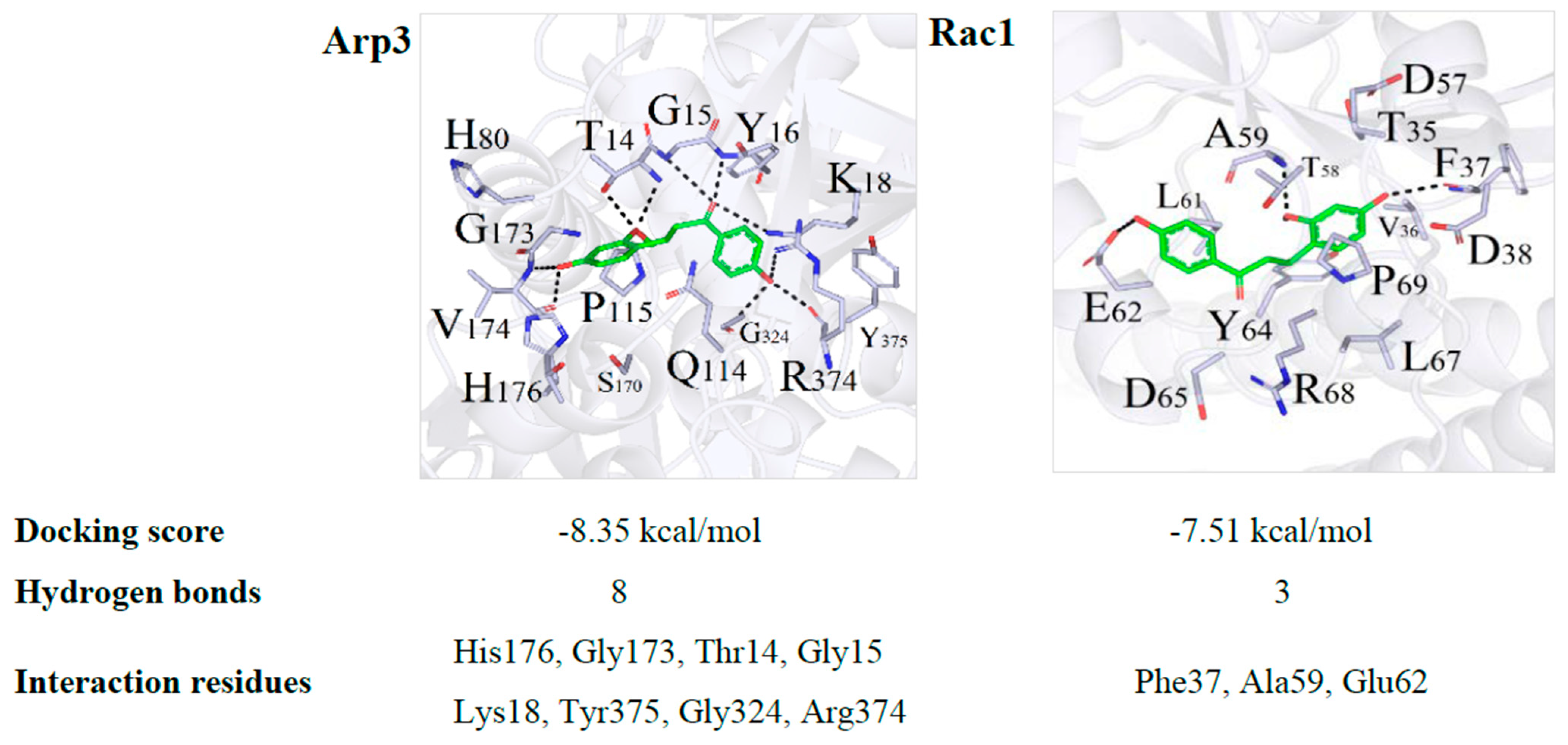
2.5. DB Protected IEB Dysfunction in SMG Rats via the Rac1-WAVE2-Arp2/3 Signaling Pathway
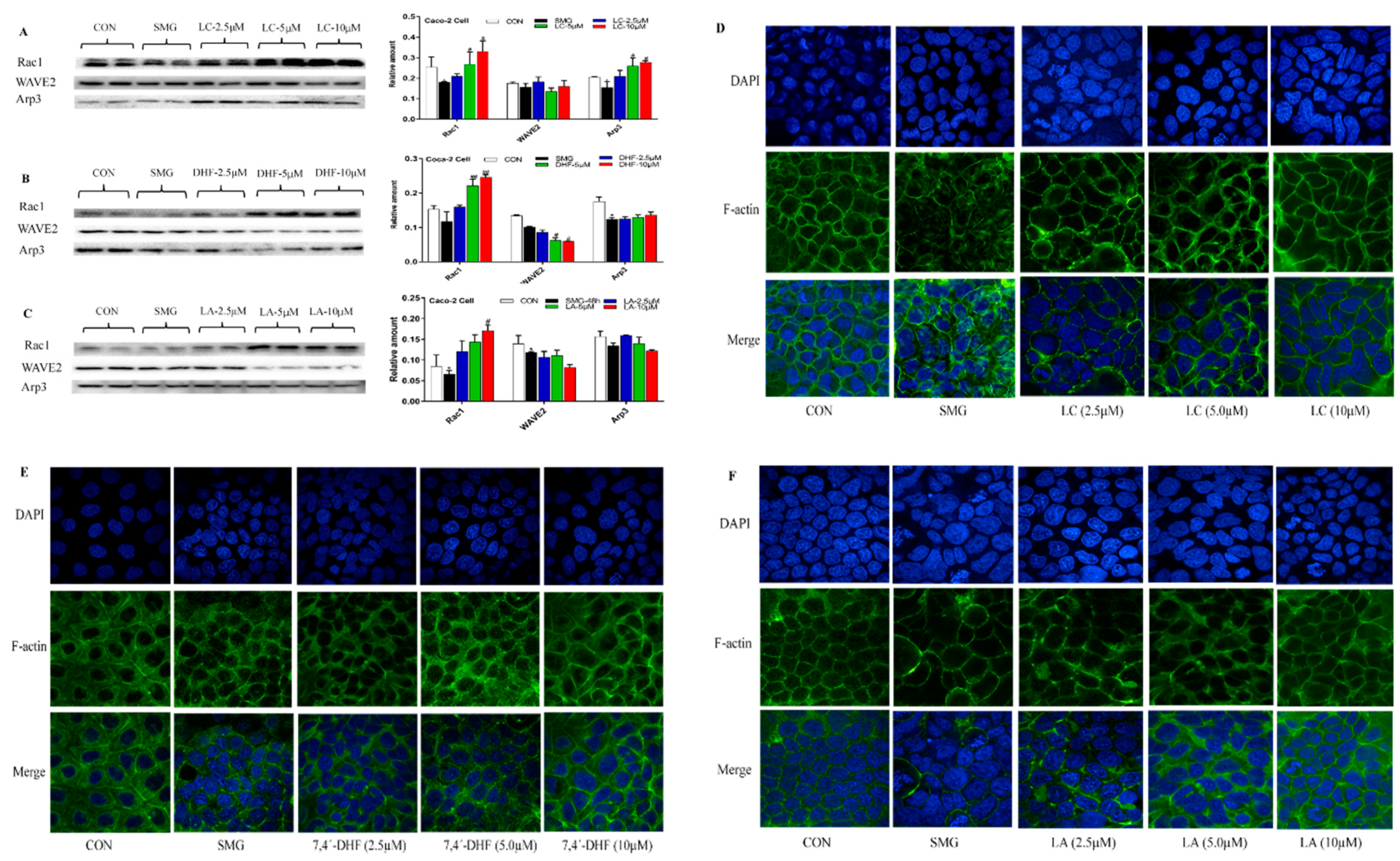
2.6. Effect of LC, LA, LB, and 7,4’-DHF on SMG-Induced IEB Injury in Caco-2 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animal Treatment and Sample Collection
4.3. Enzyme-Linked Immune Sorbent Assay (ELISA)
4.4. Histomorphology and Ultrastructure Observation
4.5. Western Blot Analysis
4.6. Immunohistochemistry Analysis
4.7. Proteomics
4.7.1. Sample Preparation
4.7.2. Protein Extraction and In-Gel Digestion
4.7.3. RP-HPLC-MS/MS Analysis
4.7.4. Protein Identification and Bioinformatic Analysis
4.8. Caco-2 Cell Experiment with Compounds in DB
4.8.1. Cell Culture and Microgravity Simulation
4.8.2. Effect of LA, LB, LC, and 7,4′-DHF on SMG-Treated Caco-2 Cells
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DB | Dagon’s Blood |
| IEB | intestinal epithelial barrier |
| LA | loureirin A |
| LC | loureirin C |
| TEM | transmission electron microscopy |
| D-LAC | D-lactic acid |
| IL-6 | interleukin-6 |
| E-cadherin | endothelial-cadherin |
| AJ | adherens junction |
| DEP | differentially expressed protein |
| TCM | traditional Chinese medicine |
| SMG | simulated microgravity |
| LB | loureirin B |
| 7,4′-DHF | 7,4′-dihydroxyflavone |
| ET | endotoxin |
| TNF-α | tumor necrosis factor-α |
| ZO-1 | zona occluden-1 |
| TJ | Tight junction |
| IHC | immunohistochemistry |
| FA | Focal adhesion |
References
- Thirsk, R.; Kuipers, A.; Mukai, C.; Williams, D. The space-flight environment: The international space station and beyond. CMAJ 2009, 180, 1216–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, B.; Grimm, D.; Strauch, S.M.; Erzinger, G.S.; Corydon, T.J.; Lebert, M.; Magnusson, N.E.; Infanger, M.; Richter, P.; Krüger, M. Influence of microgravity on apoptosis in cells, tissues, and other systems in vivo and in vitro. Int. J. Mol. Sci. 2020, 21, 9373. [Google Scholar] [CrossRef]
- Bradbury, P.; Wu, H.; Choi, J.U.; Rowan, A.E.; Zhang, H.; Poole, K.; Lauko, J.; Chou, J. Modeling the impact of microgravity at the cellular level: Implications for human disease. Front. Cell Dev. Biol. 2020, 8, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demontis, G.C.; Germani, M.M.; Caiani, E.G.; Barravecchia, I.; Passino, C.; Angeloni, D. Human pathophysiological adaptations to the space environment. Front. Physiol. 2017, 8, 547. [Google Scholar] [CrossRef]
- Tarantino, U.; Cariati, I.; Marini, M.; D’Arcangelo, G.; Tancredi, V.; Primavera, M.; Iundusi, R.; Gasbarra, E.; Scimeca, M. Effects of simulated microgravity on muscle stem cells activity. Cell Physiol. Biochem. 2020, 54, 736–747. [Google Scholar] [PubMed]
- Smith, J.K. Osteoclasts and microgravity. Life 2020, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Putcha, L.; Berens, K.L.; Marshburn, T.H.; Ortega, H.J.; Billica, R.D. Pharmaceutical use by U.S. astronauts on space shuttle missions. Aviat. Space Environ. Med. 1999, 70, 705–708. [Google Scholar] [PubMed]
- Kast, J.; Yu, Y.; Seubert, C.N.; Wotring, V.E.; Derendorf, H. Drugs in space: Pharmacokinetics and pharmacodynamics in astronauts. Eur. J. Pharm. Sci. 2017, 109S, S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Eyal, S.; Derendorf, H. Medications in space: In search of a pharmacologist’s guide to the galaxy. Pharm. Res. 2019, 36, 148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yuan, M.; Cheng, C.; Xia, D.H.; Wu, S.W. Chinese herbal medicine effects on muscle atrophy induced by simulated microgravity. Aerosp. Med. Hum. Perform. 2018, 89, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.A.; Alexeeva, N.T.; Klochkova, S.V.; Nikityuk, D.B. Extracellular matrix collagen fiber structures of the gastrointestinal connective tissues in mice after a 30-day orbital flight. Vopr. Pitan. 2019, 88, 26–40. [Google Scholar]
- Alvarez, R.; Stork, C.A.; Sayoc-Becerra, A.; Marchelletta, R.R.; Prisk, G.K.; McCole, D.F. A Simulated microgravity environment causes a sustained defect in epithelial barrier function. Sci. Rep. 2019, 9, 17531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, M.; Wang, J.; Zhang, H.; Zhou, H.; Zhao, K. Simulated weightlessness perturbs the intestinal metabolomic profile of rats. Front. Physiol. 2019, 10, 1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.X.; Wang, Y.F.; He, J.; Li, P.P.; Jin, R.; Wang, K.; Xu, X.; Hao, J.; Zhang, Y.; Liu, H.J.; et al. Intestinal microbiota contributes to colonic epithelial changes in simulated microgravity mouse model. FASEB J. 2017, 31, 3695–3709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.B.; Wang, R.; Li, G.Q.; Cho, J.L.; Deng, Y.L.; Li, Y.J. Myosin light chain kinase mediates intestinal barrier dysfunction following simulated microgravity based on proteomic strategy. J. Proteom. 2021, 231, 104001. [Google Scholar] [CrossRef]
- La, B.G.; Capriotti, A.L.; Michelini, E.; Piovesana, S.; Calabretta, M.M.; Zenezini Chiozzi, R.; Roda, A.; Laganà, A. Proteomic analysis and bioluminescent reporter gene assays to investigate effects of simulated microgravity on Caco-2 cells. Proteomics 2017, 17, 15–16. [Google Scholar]
- Volk, N.; Lacy, B. Anatomy and physiology of the small bowel. Gastrointest. Endosc. Clin. North Am. 2017, 27, 1–13. [Google Scholar] [CrossRef]
- Sánchez de Medina, F.; Romero-Calvo, I.; Mascaraque, C.; Martínez-Augustin, O. Intestinal inflammation and mucosal barrier function. Inflamm. Bowel Dis. 2014, 20, 2394–2404. [Google Scholar] [CrossRef]
- Sun, J.; Liu, J.N.; Fan, B.; Chen, X.N.; Pang, D.R.; Zheng, J.; Zhang, Q.; Zhao, Y.F.; Xiao, W.; Tu, P.F.; et al. Phenolic constituents, pharmacological activities, quality control, and metabolism of Dracaena species: A review. J. Ethnopharmacol. 2019, 244, 112138. [Google Scholar] [CrossRef]
- Gao, L.G.; Liu, T.M.; Cheng, X.; Li, N. Wound healing activity of a traditional Chinese medicine (Longxuejie) in capsule dosage form. Pak. J. Pharm. Sci. 2020, 33, 445–448. [Google Scholar]
- Jiang, M.; Su, X.; Liu, J.L.; Zheng, C.L.; Li, X.G. Systems pharmacology-dissection of the molecular mechanisms of dragon’s blood in improving ischemic stroke prognosis. Evid. Based Complement. Altern. Med. 2020, 2020, 4858201. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.J.; Lv, J.C.; Yong, K.L.; Chen, X.; Liu, P.P.; Zhang, X.B. Antidiabetic effect of an active fraction extracted from dragon’s blood (Dracaena Cochinchinensis). J. Enzym. Inhib. Med. Chem. 2009, 24, 136–139. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Wang, Q.; Meng, H.; Zhang, Q.; Wu, Y.; Xiao, W.; Wang, Y.; Tu, P. Dragon’s Blood exerts cardio-protection against myocardial injury through PI3K-AKT-mTOR signaling pathway in acute myocardial infarction mice model. J. Ethnopharmacol. 2018, 227, 279–289. [Google Scholar] [CrossRef]
- Li, N.; Ma, Z.J.; Li, M.J.; Xing, Y.C.; Hou, Y. Natural potential therapeutic agents of neurodegenerative diseases from the traditional herbal medicine Chinese dragon’s blood. J. Ethnopharmacol. 2014, 152, 508–521. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Bleakley, B.; Gupta, R.K. Dragon’s blood: Botany, chemistry and therapeutic uses. J. Ethnopharmacol. 2008, 115, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.Y.; Yi, T.; Sze-To, C.M.; Zhu, L.; Peng, W.L.; Zhang, Y.Z.; Zhao, Z.Z.; Chen, H.B. A systematic review of the botanical, phytochemical and pharmacological profile of Dracaena cochinchinensis, a plant source of the ethnomedicine “dragon’s blood”. Molecules 2014, 19, 10650–10669. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Wang, Z.J.; Xu, Y.C. Longxuejie powder in combined with mesalazine tablets enema in treatment of ulcerative colitis. J. Chang. Univ. Chin. Med. 2016, 32, 115–117. [Google Scholar]
- Lin, Y.L.; Xiong, W.N.; Xiao, S.M.; Li, F.; Lu, Z.; Yan, J.Y.; Fang, X.W.; Cui, X.J.; Wen, Y.L.; Liang, J.Q.; et al. Pharmacoproteomics reveals the mechanism of Chinese dragon’s blood in regulating the RSK/TSC2/mTOR/ribosome pathway in alleviation of DSS-induced acute ulcerative colitis. J. Ethnopharmacol. 2020, 263, 113221. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Y.; Zhang, Y.Q.; Lei, Y.; Gao, X.M.; Zhai, H.Q.; Lin, N.; Tang, S.H.; Liang, R.X.; Ma, Y.; Li, D.F.; et al. A systems biology-based approach to uncovering the molecular mechanisms underlying the effects of dragon’s blood tablet in colitis, involving the integration of chemical analysis, ADME prediction, and network pharmacology. PLoS ONE 2014, 9, e101432. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.Y.; Wang, R.; Murtaza, H.; Jia, Q.T.; Tang, B.; Shan, S.Q.; Deng, Y.L.; Qing, H. Radioprotective effects of dragon’s blood and its extracts on radiation-induced myelosuppressive mice. J. Ethnopharmacol. 2014, 154, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Chen, B.; Gan, L.; Gao, T.; Qiao, J.Y.; Deng, Y.L. Effect of Drageny I on blood rheology and oxidative damage of myocardium in rats under simulated microgravity. Trans. Beijing Inst. Tech. 2013, 33, 1313–1316. [Google Scholar]
- Nday, C.M.; Frantzidis, C.; Jackson, G.; Bamidis, P.; Kourtidou-Papadeli, C. Neurophysiological changes in simulated microgravity: An animal model. Neurology 2019, 67, S221–S226. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.J.; Yue, Y.; Sun, H.P.; Jiang, S.Z.; Wu, X.Y. Compliance changes in femoral veins of rabbits after 21 days of simulated weightlessness. Aviakosm. Ekolog. Med. 2006, 40, 29–33. [Google Scholar]
- Taibbi, G.; Cromwell, R.L.; Zanello, S.B.; Yarbough, P.O.; Ploutz-Snyder, R.J.; Godley, B.F.; Vizzeri, G. Ocular outcomes comparison between 14- and 70-Day Head-Down-Tilt Bed rest. Invest. Ophthalmol. Vis. Sci. 2016, 57, 495–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeuninx, B.; Elhassan, Y.S.; Manolopoulos, K.N.; Sapey, E.; Rushton, A.B.; Edwards, S.J.; Morgan, P.T.; Philp, A.; Brook, M.S.; Gharahdaghi, N.; et al. The effect of short-term exercise prehabilitation on skeletal muscle protein synthesis and atrophy during bed rest in older men. J. Cachexia Sarcopenia Muscle 2021, 12, 52–69. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.P.; Vargis, E. Muscle atrophy marker expression differs between rotary cell culture system and animal studies. Biomed. Res. Int. 2019, 2019, 2042808. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Zhong, J.; Bian, Y.F.; Fan, Y.S.; Chen, Q.Y.; Liu, P.; Liu, Z.J. Rhein ameliorates lipopolysaccharide-induced intestinal barrier injury via modulation of Nrf2 and MAPKs. Life. Sci. 2019, 216, 168–175. [Google Scholar] [CrossRef] [PubMed]
- He, F.F.; Bao, D.; Su, H.; Wang, Y.M.; Lei, C.T.; Zhang, C.Y.; Ye, C.; Tang, H.; Wan, C.; You, C.Q.; et al. IL-6 increases podocyte motility via MLC-mediated focal adhesion impairment and cytoskeleton disassembly. J. Cell Physiol. 2018, 233, 7173–7181. [Google Scholar] [CrossRef] [PubMed]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta. 2008, 1778, 660–669. [Google Scholar] [CrossRef] [Green Version]
- Comajoan, P.; Gubern, C.; Huguet, G.; Serena, J.; Kádár, E.; Castellanos, M. Evaluation of common housekeeping proteins under ischemic conditions and/or rt-PA treatment in bEnd.3 cells. J. Proteom. 2018, 184, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.P.; Bai, J.; Zhang, Y.L.; Yang, X.L.; Xia, Y.L.; Lin, Q.Y.; Li, H.H. Blockade of intercellular adhesion molecule-1 prevents angiotensin II-induced hypertension and vascular dysfunction. Lab. Invest. 2020, 100, 378–386. [Google Scholar] [CrossRef]
- Rodgers, L.S.; Fanning, A.S. Regulation of epithelial permeability by the actin cytoskeleton. Cytoskeleton 2011, 68, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Burridge, K.; Guilluy, C. Focal adhesions, stress fibers and mechanical tension. Exp. Cell. Res. 2016, 343, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Suman, S.; Fornace, A.J., Jr.; Datta, K. Space radiation triggers persistent stress response, increases senescent signaling, and decreases cell migration in mouse intestine. Proc. Natl. Acad. Sci. USA 2018, 115, e9832–e9841. [Google Scholar] [CrossRef] [Green Version]
- Backert, S.; Schmidt, T.P.; Harrer, A.; Wessler, S. Exploiting the gastric epithelial barrier: Helicobacter pylori’s attack on tight and adherens junctions. Curr. Top. Microbiol. Immunol. 2017, 400, 195–226. [Google Scholar] [PubMed]
- Conway, J.; Jacquemet, G. Cell matrix adhesion in cell migration. Essays Biochem. 2019, 63, 535–551. [Google Scholar] [PubMed]
- Hight-Warburton, W.; Parsons, M. Regulation of cell migration by α4 and α9 integrins. Biochem. J. 2019, 476, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, K.W.; Wei, D.X.; Tian, Y.; Gao, Y.G.; Chen, Z.H.; Qian, A.R. The impact of spaceflight and simulated microgravity on cell adhesion. Int. J. Mol. Sci. 2020, 21, 3031. [Google Scholar] [CrossRef] [PubMed]
- Beltzner, C.C.; Pollard, T.D. Pathway of actin filament branch formation by Arp2/3 complex. J. Biol. Chem. 2008, 283, 7135–7144. [Google Scholar] [CrossRef] [Green Version]
- Beckham, Y.; Vasquez, R.J.; Stricker, J.; Sayegh, K.; Campillo, C.; Gardel, M.L. Arp2/3 inhibition induces amoeboid-like protrusions in MCF10A epithelial cells by reduced cytoskeletal-membrane coupling and focal adhesion assembly. PLoS ONE 2014, 9, e100943. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, N.; Tohyama, K.; Yamashiro, S. Mechanostress resistance involving formin homology proteins: G- and F-actin homeostasis-driven filament nucleation and helical polymerization-mediated actin polymer stabilization. Biochem. Biophys. Res. Commun. 2018, 506, 323–329. [Google Scholar] [CrossRef]
- Vartiainen, M.K.; Machesky, L.M. The WASP-Arp2/3 pathway: Genetic insights. Curr. Opin. Cell. Biol. 2004, 16, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Kim, M.O.; Yun, S.P.; Ryu, J.M.; Park, J.H.; Seo, B.N.; Jeon, J.H.; Han, H.J. C(16)-Ceramide-induced F-actin regulation stimulates mouse embryonic stem cell migration: Involvement of N-WASP/Cdc42/Arp2/3 complex and cofilin-1/α-actinin. Biochim. Biophys. Acta. 2013, 1831, 350–360. [Google Scholar] [CrossRef]
- Li, H.; Yang, L.; Fu, H.; Yan, J.; Wang, Y.; Guo, H.; Hao, X.; Xu, X.; Jin, T.; Zhang, N. Association between Gαi2 and ELMO1/Dock180 connects chemokine signalling with Rac activation and metastasis. Nat. Commun. 2013, 4, 1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, D.; Paulino, M.; Polticelli, F.; Arredondo, F.; Williams, R.J.; Abin-Carriquiry, J.A. Structural evidence of quercetin multi-target bioactivity: A reverse virtual screening strategy. Eur. J. Pharm. Sci. 2017, 106, 393–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Wang, C.H.; Yan, H.; Zhang, R.; Zhao, J.B.; Qian, C.F.; Xiao, H.; Liu, H.Y. Inhibition of the Rac1-WAVE2-Arp2/3 signaling pathway promotes radiosensitivity via downregulation of cofilin-1 in U251 human glioma cells. Mol. Med. Rep. 2016, 13, 4414–4420. [Google Scholar] [CrossRef] [Green Version]
- Bauer, J. Microgravity and cell adherence. Int. J. Mol. Sci. 2020, 21, 2214. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Guo, J.; Wang, S.; Kang, L.; Deng, Y.; Li, Y. Simulated microgravity altered the metabolism of Loureirin B and the expression of major cytochrome P450 in liver of rats. Front. Pharmacol. 2018, 9, 1130. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Zhang, Y.S.; Wang, R.; Wei, L.Z.; Deng, Y.L.; Ren, W. Metabolic profiling of five flavonoids from Dragon’s Blood in human liver microsomes using high-performance liquid chromatography coupled with high resolution mass spectrometry. J. Chromatogr. 2017, 1052, 91–102. [Google Scholar] [CrossRef]
- Liu, H.Y.; Guo, J.J.; Li, Y.J.; Zhang, Y.S.; Wang, J.P.; Gao, J.Y.; Deng, Y.L.; Li, Y.Z. Investigation on intestinal proteins and drug metabolizing enzymes in simulated microgravity rats by a proteomics method. Molecules 2020, 25, 4391. [Google Scholar] [CrossRef]

| No. | Protein ID | Gene Name | Ratio of DB/SMG | KEGG Pathways |
|---|---|---|---|---|
| 1 | P63259 | Actin, gamma 1 (Actg1) | 0.1166 | CYTO SKEL, FA |
| 2 | F1LNG5 | Phosphoinositide-3-kinase regulatory subunit 1 (Pik3r1) | 2.8114 | CYTO SKEL, FA |
| 3 | A0A0G2JSK5 | Integrin subunit beta 1 (Itgb1) | 2.6357 | CYTO SKEL, FA |
| 4 | Q6T487 | Actinin, alpha 1 (Actn1) | 3.4880 | CYTO SKEL, FA |
| 5 | Q99MI8 | Actinin alpha 4 (Actn4) | 1.8886 | CYTO SKEL, FA |
| 6 | R9PXU6 | Vinculin (Vcl) | 2.4851 | CYTO SKEL, FA |
| 7 | F1LQT3 | Rho-associated coiled-coil containing protein kinase 2 (Rock2) | 2.3299 | CYTO SKEL, FA |
| 8 | A0A0G2JWJ0 | Protein phosphatase 1, regulatory subunit 12A (Ppp1r12a) | 4.0816 | CYTO SKEL, FA |
| 9 | A0A0G2K470 | Integrin alpha 2 (Itga2) | 2.2878 | CYTO SKEL, FA |
| 10 | D3ZN37 | Rho-associated coiled-coil containing protein kinase 1 (Rock1) | 2.4038 | CYTO SKEL, FA |
| 11 | D3ZZW1 | Dedicator of cyto-kinesis 1 (Dock1) | 3.5549 | CYTO SKEL, FA |
| 12 | Q01986 | Mitogen-activated protein kinase kinase 1 (Map2k1) | 1.6345 | CYTO SKEL, FA |
| 13 | Q5U2U2 | V-crk avian sarcoma virus CT10 oncogene homolog-like (Crkl) | 1.9489 | CYTO SKEL, FA |
| 14 | B5DF62 | p21- (RAC1)-activated kinase 4 (Pak4) | 1.9673 | CYTO SKEL, FA |
| 15 | F1LSD3 | Integrin subunit beta 4 (Itgb4) | 1.9011 | CYTO SKEL, FA |
| 16 | F1M775 | Diaphanous-related formin 1(Dia1) | 2.2056 | CYTO SKEL, FA |
| 17 | G3V667 | Integrin subunit alpha 6 (Itga6) | 1.7947 | CYTO SKEL, FA |
| 18 | P35465 | p21- (RAC1)-activated kinase (Pak1) | 2.1580 | CYTO SKEL, FA |
| 19 | Q6AYF4 | Integrin subunit beta 6(Itgb6) | 1.5949 | CYTO SKEL, FA |
| 20 | P36506 | Mitogen-activated protein kinase kinase 2 (Map2k2) | 5.2687 | CYTO SKEL |
| 21 | A0A0G2K472 | Cytoplasmic FMR1 interacting protein 1(Cyfip1) | 1.8093 | CYTO SKEL |
| 22 | F1LW74 | IQ motif containing GTPase activating protein 2 (Iqgap2) | 2.0964 | CYTO SKEL |
| 23 | O88370 | Phosphatidylinositol-5-phosphate 4-kinase type 2 gamma (Pip4k2c) | 1.5063 | CYTO SKEL |
| 24 | P26431 | Solute carrier family 9 member A1 (Slc9a1) | 6.2933 | CYTO SKEL |
| 25 | P31977 | Ezrin (Ezr) | 2.0838 | CYTO SKEL |
| 26 | P55161 | NCK-associated protein 1 (Nckap1) | 1.6742 | CYTO SKEL |
| 27 | Q5WQV5 | Radixin (Rdx) | 6.2933 | CYTO SKEL |
| 28 | G3V7Q7 | IQ motif containing GTPase activating protein 1 (Iqgap1) | 1.5901 | CYTO SKEL, AJ |
| 29 | F1M2P8 | Protein kinase C, alpha (Prkca) | 1.9988 | FA |
| 30 | D4A8D5 | Filamin B (Flnb) | 2.0076 | FA |
| 31 | F1LPI5 | Laminin subunit beta 3 (Lamb3) | 3.0845 | FA |
| 32 | D4A7U1 | Zyxin (Zyx) | 6.4350 | FA |
| 33 | G3V852 | Talin 1 (Tln1) | 2.5176 | FA |
| 34 | A0A0G2JT61 | Rrb-b2 receptor tyrosine kinase 2 (Erbb2) | 2.3015 | FA, AJ |
| 35 | A0A0G2JYB2 | Claudin 2 (Cldn2) | 3.5026 | TJ |
| 36 | A0A0G2K8M3 | Tight junction protein 3 (Tjp3) | 1.6807 | TJ |
| 37 | D4A4X4 | Cingulin (Cgn) | 1.6955 | TJ |
| 38 | F1LNF0 | Myosin heavy chain 14 (Myh14) | 1.9354 | TJ |
| 39 | F1LSH0 | Symplekin (Sympk) | 2.5214 | TJ |
| 40 | F1M7H7 | Membrane associated guanylate kinase (Magi1) | 9.2851 | TJ |
| 41 | G3V6P7 | Myosin, heavy chain 9, non-muscle-like 1 (Myh9l1) | 1.7921 | TJ |
| 42 | Q3ZB99 | Tight junction protein 2 (Tjp2) | 1.8268 | TJ |
| 43 | Q4QQT4 | Protein phosphatase 2 scaffold subunit A beta (Ppp2r1b) | 1.6361 | TJ |
| 44 | Q5XI34 | Protein phosphatase 2 scaffold subunit A alpha (Ppp2r1a) | 1.5793 | TJ |
| 45 | Q63400 | Claudin 3 (Cldn3) | 2.4558 | TJ |
| 46 | A0A0G2K2P5 | Tight junction protein 1 (Tjp1) | 1.9539 | TJ, AJ |
| 47 | Q66HL2 | Cortactin (Cttn) | 3.6887 | TJ, BACT INVAS |
| 48 | A0A0A0MY48 | Dynamin 2 (Dnm2) | 1.8772 | BACT INVAS |
| 49 | A0A0G2K4S6 | Engulfment and cell motility 1 (Elmo1) | 5.6117 | BACT INVAS |
| 50 | F1LRS8 | CD2-associated protein (Cd2ap) | 3.8926 | BACT INVAS |
| 51 | F1M779 | Clathrin heavy chain (Cltc) | 2.0538 | BACT INVAS |
| 52 | Q499U2 | Engulfment and cell motility 3 (Elmo3) | 1.8629 | BACT INVAS |
| 53 | Q5U302 | Catenin alpha 1 (Ctnna1) | 2.0367 | BACT INVAS, AJ |
| 54 | A0A0G2JXW2 | SMAD family member 4 | 2.4516 | AJ |
| 55 | D3ZZZ9 | Catenin delta 1 (Ctnnd1) | 2.1570 | AJ |
| 56 | D4A5C0 | Nectin cell adhesion molecule 3 (Nectin3) | 1.5708 | AJ |
| 57 | G3V8P4 | Protein tyrosine phosphatase, receptor type, F (Ptprf) | 2.5484 | AJ |
| TJ, AJ and Rac1/WAVE2/Arp2/3 pathway proteins | ||||
| 58 | Q6RUV5 | Ras-related C3 botulinum toxin substrate 1 (Rac1) | 1.1689 | - |
| 59 | A0A0G2K5T9 | Wiskott-Aldrich syndrome protein family member 2 (WAVE2) | 1.5264 | - |
| 60 | Q4V7C7 | Actin-related protein 3 (Arp3) | 1.1846 | - |
| 61 | Q6P6T5 | Occludin (Ocln) | 2.4612 | - |
| 62 | P56745 | Claudin-1 (Cldn1) | 1.4483 | - |
| 63 | Q9R0T4 | E-Cadherin (E-Cdhn) | 2.3361 | - |
| 64 | Q9WU82 | β-catenin (Ctnnb) | 2.2410 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, S.; Liu, H.; Cui, Y.; Deng, Y. Dragon’s Blood Regulates Rac1-WAVE2-Arp2/3 Signaling Pathway to Protect Rat Intestinal Epithelial Barrier Dysfunction Induced by Simulated Microgravity. Int. J. Mol. Sci. 2021, 22, 2722. https://doi.org/10.3390/ijms22052722
Li Y, Liu S, Liu H, Cui Y, Deng Y. Dragon’s Blood Regulates Rac1-WAVE2-Arp2/3 Signaling Pathway to Protect Rat Intestinal Epithelial Barrier Dysfunction Induced by Simulated Microgravity. International Journal of Molecular Sciences. 2021; 22(5):2722. https://doi.org/10.3390/ijms22052722
Chicago/Turabian StyleLi, Yujuan, Shan Liu, Huayan Liu, Yaoyuan Cui, and Yulin Deng. 2021. "Dragon’s Blood Regulates Rac1-WAVE2-Arp2/3 Signaling Pathway to Protect Rat Intestinal Epithelial Barrier Dysfunction Induced by Simulated Microgravity" International Journal of Molecular Sciences 22, no. 5: 2722. https://doi.org/10.3390/ijms22052722
APA StyleLi, Y., Liu, S., Liu, H., Cui, Y., & Deng, Y. (2021). Dragon’s Blood Regulates Rac1-WAVE2-Arp2/3 Signaling Pathway to Protect Rat Intestinal Epithelial Barrier Dysfunction Induced by Simulated Microgravity. International Journal of Molecular Sciences, 22(5), 2722. https://doi.org/10.3390/ijms22052722