The Benefits of Smart Nanoparticles in Dental Applications
Abstract
1. Introduction
- Preventive materials, including pit and fissure sealants, sealing agents, liner bases, and cements;
- Restorative materials, including primers, bonding agents, liners, amalgams, composite resin, compomers, hybrid ionomers, ceramics, and metal-ceramics;
- Auxiliary dental materials, including acid-etching solutions, impression materials, casting investment, gypsum, dental waxes, and finishing and polishing abrasives [11].
2. History of Dental Materials
- The introduction of porcelain (De Chennant, 1789);
- The discovery of the first dental amalgam in France (Taveau, 1816);
- The first ceramic tooth (Fonzi, 1808);
- The first imprint of a root canal (Maggiolo, 1809);
- The use of rubber and then celluloid for dentures;
- The first dental “tour” of the foot (Morrison, 1871) and the electric one (Green, 1874);
- The first substitution crown (Logan, 1884);
- Black—“the father of modern amalgams”—sets the proportions of alloys (1895);
- The polymerization of methyl methacrylate—starting point in the preparation of the first acrylic resin for dental use;
- The burning of ceramics on platinum foil (1936) and in vacuum (1940-1945);
- The appearance of polyelectrolyte cements as impression materials;
- The appearance of composite diacrylic resins;
- The extension of photopolymerization as a polymerization process for various dental materials;
- The removal of mercury from metal fillings and identification of new solutions;
- The introduction of titanium in dentistry;
- The development of smart dental materials as glass ionomer cements, resin-modified ionomer cements, compomers, ormocers, and cermets;
- The improvement of implantology [16].
- -
- The drug is released gradually, ensuring a constant concentration in the body at the value corresponding to the therapeutic field;
- -
- The approach helps their metabolism and excretion;
- -
- The side effects of drugs, caused by either overdoses or by their aggression, sometimes to both diseased and healthy cells, are reduced [20].
3. Smart Nanoparticles in Dentistry
- They can present antimicrobial, antiviral, and antifungal properties and as a consequence, can prevent biofilm formation when nanoparticles loaded with an antimicrobial agent are incorporated in resin composites [25];
- They enhance the mechanical properties of dental material, especially in restorative dentistry;
- They improve the bond strength between dentin and biomaterial;
- They prevent crack propagation and white spot lesions;
- They improve the fracture toughness of porcelain restorations [26].
- Enamel: The very hard, thin, and translucent layer that covers the surface of the dental crown;
- Dentin: A calcified tissue of the tooth situated inside the enamel and cementum;
- Cementum: Specialized calcified substance that is a part of the periodontium and covers the root of a tooth;
- Dental pulp: Unmineralized oral tissue situated in the center of the tooth that is composed of soft connective tissue, blood vessels, lymph vessels, and nervous elements.
3.1. Smart Nanoparticles as Drug Delivery Systems
- Bad breath (halitosis) is caused by gum diseases, dental caries, oral cancer, dry mouth, and bacteria on the tongue;
- Dental caries appear when acids, bacteria, and food form a plaque that covers the teeth [27];
- Gum diseases (gingivitis and periodontitis) represent an inflammation of the gums, as well as an infection of the tissues caused by the formation of a sticky, colorless plaque on teeth [28];
- Oral cancers, which include any malignant lesions on the gums, tongue, lips, cheek, floor of the mouth, and hard and soft palate [29];
- Mouth sores, which are inflammatory disorders characterized by small lesions that develop on the soft tissues in the mouth or at the base of the gums. The following disorders belong to this category: Canker sores (aphtous ulcers); fever blisters or cold sores caused by the Herpes simplex; oral thrush or candidiasis; angular cheilosis; fibrous inflammatory hyperplasia; and inflammatory papillary hyperplasia [30];
- Tooth erosion;
- Tooth sensitivity;
- Toothaches and dental emergencies.
3.1.1. Periodontal Diseases
- Depending on the type of treatment application, including personal (treatment of patients at home) and professional (at the dental office), (a) supra- and subgingival irrigation and (b) controlled release systems;
- According to the biodegradability of the system, biodegradable and non-biodegradable;
- Depending on the device type, devices with a sustained release of drugs that act in less than 24 h and therefore require multiple applications, and devices with a controlled release of drugs that follow the zero order kinetics and act for a longer period of time.
- Admirable ROS removal;
- Anti-inflammatory activity;
- Biodegradable behavior;
- A high biocompatibility and low systemic toxicity.
- Chitosan crosslinked nanoparticles obtained by the emulsion-dispersion technique loaded with sodium fluoride and cetylpyridinium chloride mixed with toothpaste lixivium in order to obtain a product that releases the active substance in a sustained manner [49];
- Chitosan crosslinked nanoparticles prepared by ionic gelation loaded with sodium fluoride used as dental delivery systems in protection against tooth decay [50];
- Chitosan-oligonucleotide complexes that are suitable for local therapeutic applications [51];
- Rose bengal-functionalized chitosan nanoparticles that can present several advantages, including an affinity to bacterial cells and their elimination, increased interaction and uptake caused by the smart nanoparticles, and singlet oxygen release after photoactivation of the photosensitizer (xanthenes with a negative charge) [52].
3.1.2. Dental Caries
- The anatomical site: Occlusal; proximal and cervical; linear enamel; and root caries;
- The type of lesion: Primary and secondary caries;
- The severity of disease: Acute; chronic; and arrested caries;
- The extent of caries: Incipient caries; occult caries; and cavitation;
- The tissue involved: Initial; superficial; moderate; deep; and deep complicate caries;
- The pathway of caries spread: Forward and backward caries;
- The number of tooth surfaces involved: Simple; compound; and complex caries;
- The chronology: Early childhood; adolescent; and adult caries.
3.1.3. Oral Cancer
- On the surface, edges, or back of the tongue;
- Inside the cheeks or on the “sky of the mouth”;
- Under the tongue or on the upper or lower lip;
- On the gums or inside the jaws.
3.2. Smart Materials in Restorative Dentistry
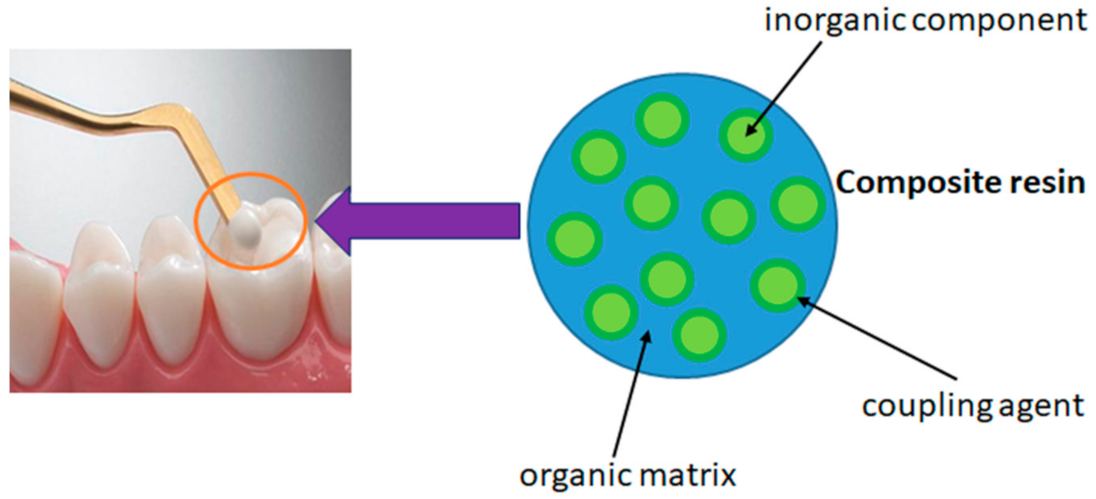
3.2.1. Nanocomposite Resin
3.2.2. Glass-Ionomer Cements
- Zinc polycarboxylate (ZPC) cements described by Smith, with zinc oxide being the cation source;
- The ionomer cements (GIC) described by Wilson and Kent, with fluoroaluminosilicate glass being the cation source;
- Alginate-based impression materials [106].
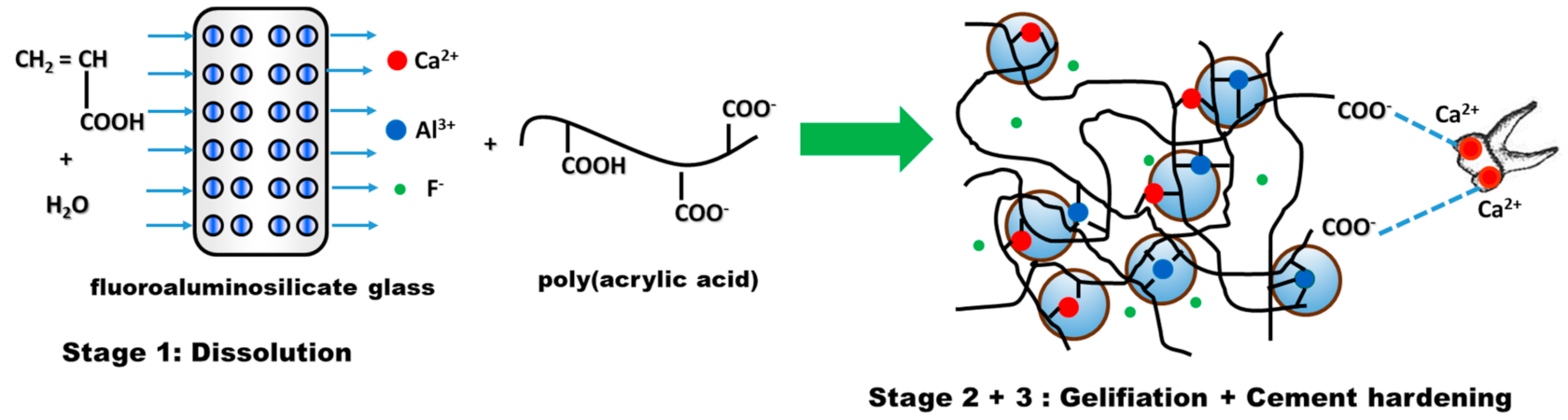
- Stage 1—Dissolution, which consists of mixing water-soluble polyacid with fine fluoroaluminosilicate glass powder [110]. Due to PAA ionization, hydrogen ions are released and attack the surface layer of glass powder, while the glass interior remains intact and will act as a filler in the cement matrix. The attack of the acid on the glass particles has the effect of first releasing the Ca2+ ions and then the Al3+ ones;
- Stage 2—Gelling. The released cations react with the carboxylate groups belonging to the polyacid chains, leading to the formation of ionic crosslinks, which result in the development of a hydrogel-type network around the glass particles. In this phase, water plays a very important role. If, in the first stage, the water is totally incorporated into the structure of the cement, during the gelling reaction, the cement paste must be protected from the addition of water, in order to prevent negative consequences for the formation of the cement [111];
- Stage 3—Cement hardening is a process that can take longer. In general, it is necessary for about 30 min for the reaction between Al ions and the reactive groups situated on the polyelectrolyte chain to take place [112]. The ionic crosslinking process is responsible for the hardening of the ionomer cement. Studies conducted by Wasson and Nicholson [113,114,115] suggest that dissolution of the glass fractions is achieved and the silicate network is responsible for the fragmentation of the cementation. This hypothesis is based on the fact that glass powders and acetic acid form cements after one week and their strength increases after a period of 6 months.
- Hybrid materials—GIC modified by the addition of resin (resin-modified ionomer cement);
- Composites modified by the addition of polyacid (compomers);
- Composites modified by the coupling of an inorganic network to the organic matrix (ormocers);
- GIC with the addition of modified resins by incorporating prepolymerized particles (giomers).
3.2.3. Resin-Modified Glass Ionomer Cements
- The synthesis of acrylic and methacrylic derivatives of amino acids, such as aspartic acid, glutamic acid, and α-alanine via the Schotten–Baumann reaction [131], and
- The copolymerization of monomers containing acryloyl and methacryloyl groups with acid acrylic.
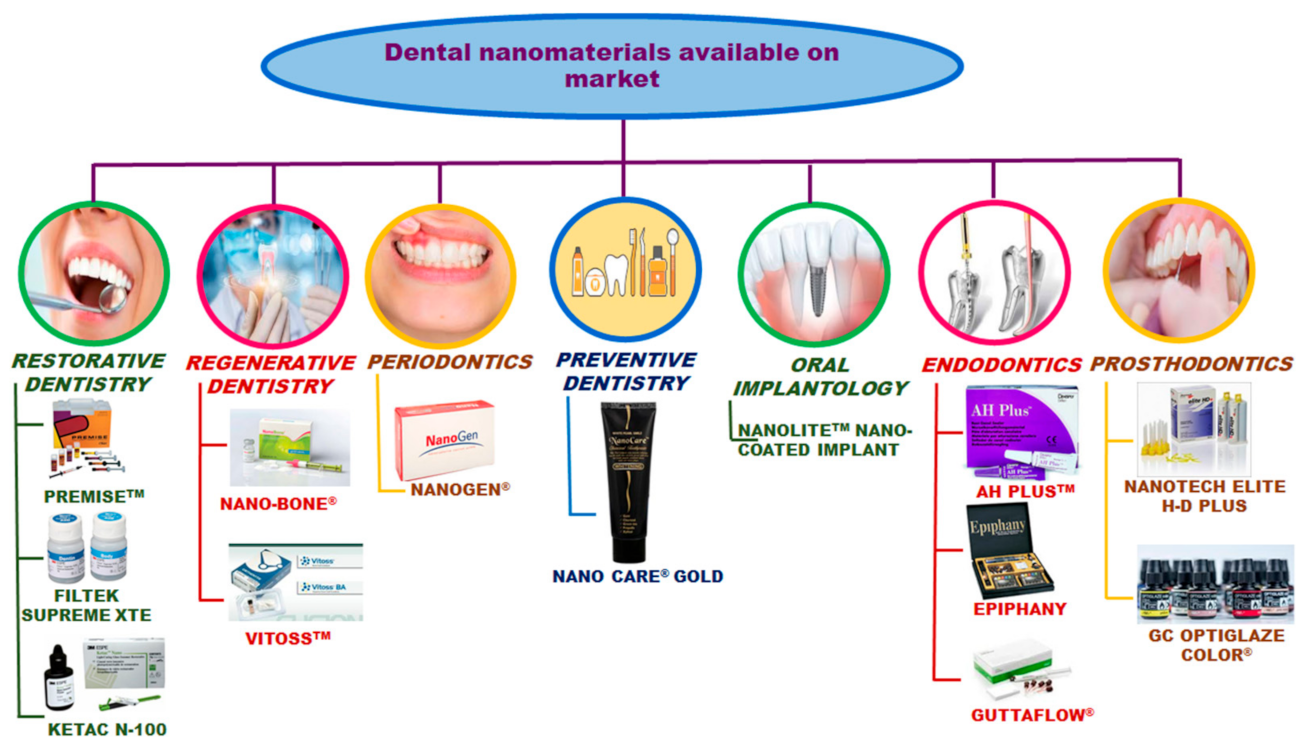
4. Commercial Dental Nanomaterials
- Excellent aesthetics;
- High initial polish;
- High fluoride release that is rechargeable;
- Ability to create a caries inhibition zone after acid exposure;
- Higher wear resistance;
- Fast and ease to handle.
5. Nanotoxicity
- They can produce irreversible damage to cells by oxidative stress;
- They can influence basic cellular processes;
- They can cause an inflammatory response.
6. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Johnston, W.M.; Kao, E.C. Assessment of appearance by visual observation and clinical colorimetry. J. Dent. Res. 1989, 68, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Peralta, S.L.; de Leles, S.B.; Dutra, A.L.; Guimares, V.B.S.; Piva, E.; Lund, R.G. Evaluation of physical-mechanical properties, antibacterial effect and cytotoxicity of temporary restorative materials. J. Appl. Oral Sci. 2018, 26, e20170562. [Google Scholar] [CrossRef] [PubMed]
- Peskeroy, C.; Culha, O. Comparative evolution of mechanical proprieties of dental nanomaterials. J. Nanomater. 2017, 6171578. [Google Scholar] [CrossRef]
- Pecho, O.E.; Ghinea, R.; Navarro do Amaral, E.A.; Cardona, J.C.; Della Bona, A.; Perez, M.M. Relevant optical properties for direct restorative materials. Dent. Mater. 2016, 32, e105–e112. [Google Scholar] [CrossRef]
- Pulgar, R.; Lucena, C.; Espinar, C.; Pecho, O.E.; Ruiz-Lopez, J.; Della Bona, A.; Perez, M.M. Optical and colorimetric evaluation of a multi-color polymer infiltrated ceramic-network material. Dent. Mater. 2019, 35, e131–e139. [Google Scholar] [CrossRef]
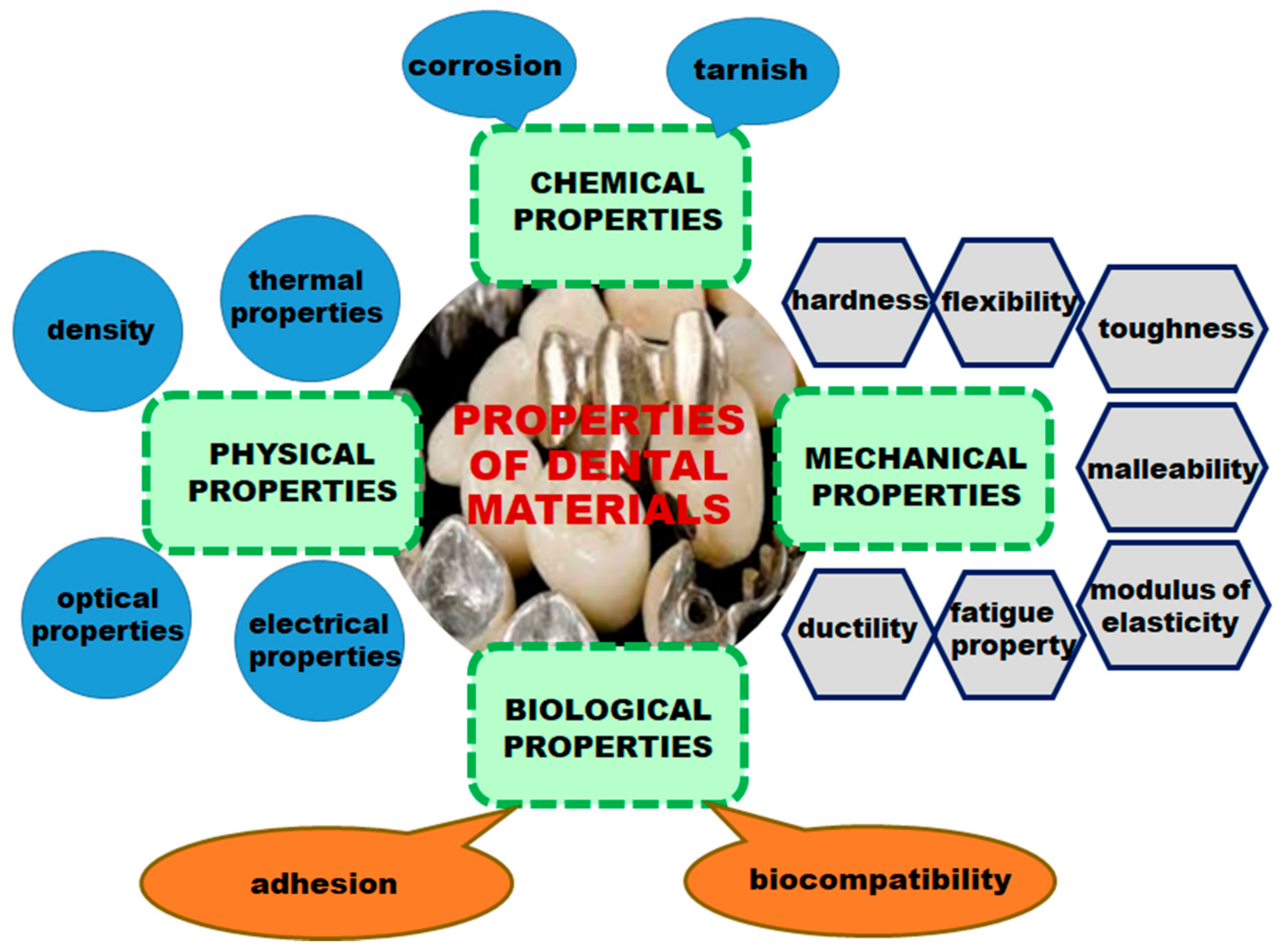
- Anusavice, K.J. Phillip’s Science of Dental Materials, 11th ed.; Saunders Co., Elsevier Science Ltd.: Bethesda, MD, USA, 2003. [Google Scholar]
- Robertson, T.M.; Heyman, H.O.; Swift, J.E.J., Jr. Studervant’s Art and Science of Operative Dentistry, 4th ed.; Mosby, Elsevier Science Ltd.: St. Louis, MO, USA, 2002; pp. 16–36. [Google Scholar]
- Arenholt-Bindslev, D. Environmental aspects of dental filling materials. Eur. J. Oral Sci. 1998, 106, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Anusavice, K.J. Biocompatibility. In Phillip’s Science of Dental Materials, 12th ed.; Anusavice, K.J., Shen, C., Rawls, H.R., Eds.; Saunders Co., Elsevier Science Ltd.: Bethesda, MD, USA, 2013; Chapter 7; pp. 111–147. [Google Scholar]
- Phillips, R.W.; Skinner, E.W. Skinner’s Science of Dental Materials, 9th ed.; Saunders Co., Elsevier Science Ltd.: Philadelphia, PA, USA, 1991; pp. 61–67. [Google Scholar]
- Manappallil, J.J. Basic Dental Materials, 4th ed.; Jaypee Brothers Medical Publishers (P) Ltd.: New Delhi, India, 2016; pp. 3–8. [Google Scholar]
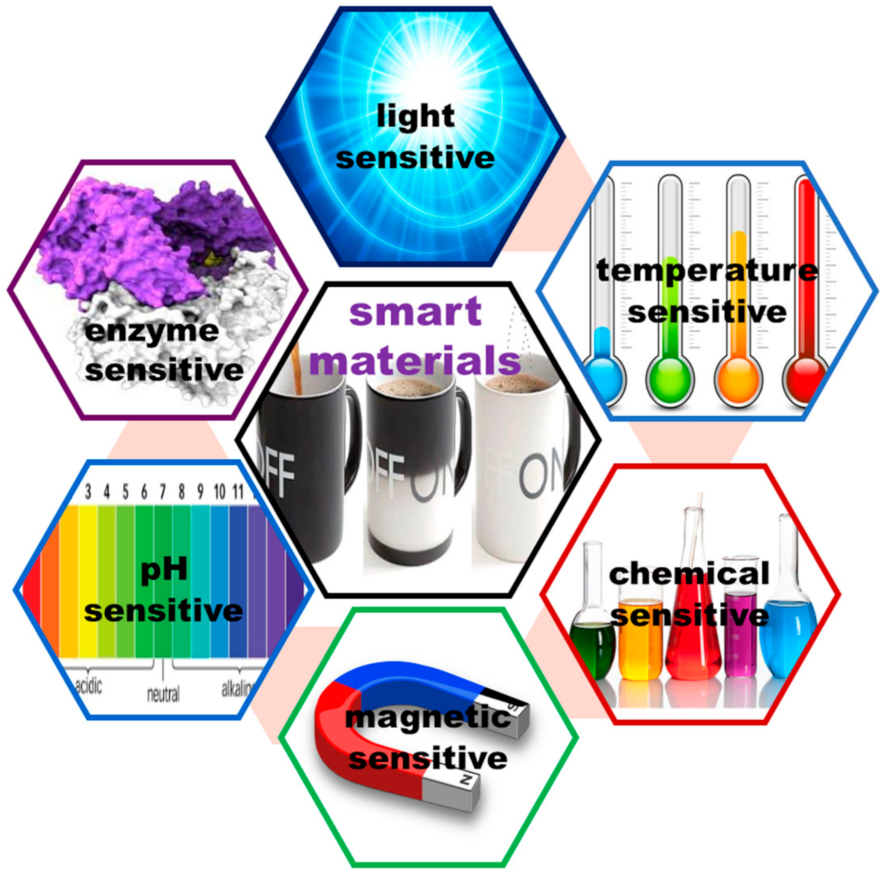
- Bahl, S.; Nagar, H.; Singh, I.; Sehgal, S. Smart materials types, properties and applications: A review. Mat. Today Proc. 2020, 28, 1302–1306. [Google Scholar] [CrossRef]
- Shanti, M.; Soma Sekhar, E.V.; Ankireddy, S. Smart materials in dentistry: Think smart! J. Pediatr. Dent. 2014, 2, 1–4. [Google Scholar] [CrossRef]
- Wynbrant, J. The Excruciating History of Dentistry: Toothsome Tales and Oral Oddities from Babylon to Braces; St. Martin’s Griffin Press: New York, NY, USA, 1998; pp. 3–26. [Google Scholar]
- Bremner, M.D.K. The Story of Dentistry from the Dawn of Civilization to the Present, 3rd ed.; Dental Items of Interest Pub. Co.: Brooklyn, UK, 1954. [Google Scholar]
- Van Noort, R. Introduction to Dental Materials, 11th ed.; Mosby, Elsevier Science Ltd.: St. Louis, MO, USA, 2002; pp. 6–10. [Google Scholar]
- Ficai, D.; Sandulescu, M.; Ficai, A.; Andronescu, E.; Yetmez, M.; Agrali, O.B.; Elemek, E.; Gundruz, O.; Sahin, Y.M.; Oktar, F.N. Drug delivery systems for dental applications. Curr. Org. Chem. 2017, 21, 64–73. [Google Scholar] [CrossRef]
- Hanada, N. The dental drug delivery system for prevention of dental caries. Curr. Oral Health Rep. 2016, 3, 124–128. [Google Scholar] [CrossRef]
- Vasiliu, S.; Lungan, M.A.; Racovita, S.; Popa, M. Porous microparticles based on methacrylic copolymers and gellan as drug delivery systems. Polym. Int. 2020, 69, 1066–1080. [Google Scholar] [CrossRef]
- Cigu, T.A.; Vasiliu, S.; Racovita, S.; Lionte, C.; Sunel, V.; Popa, M.; Cheptea, C. Adsorption and release studies of new cephalosporin from chitosan-g-poly(glycidyl methacrylate) microparticles. Eur. Polym. J. 2016, 82, 132–152. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Corolani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From vhemical-physical applications to nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef]
- Foong, L.K.; Foroughi, M.M.; Mirhosseini, A.F.; Safaei, M.; Jahani, S.; Mostafavi, M.; Ebrahimpoor, N.; Sharifi, M.; Varma, S.R.; Khatami, M. Applications of nano-materials in diverse dentistry regimes. RSC Adv. 2020, 10, 15430–15460. [Google Scholar] [CrossRef]
- Pinon-Segundo, E.; Mendoza-Munoz, M.; Quintanar-Guerrero, D. Nanoparticles as dental drug-delivery systems. In Nanobiomaterials in Clinical Dentistry, 2nd ed.; Subramani, K., Ahmed, W., Hartsfield, J.K., Jr., Eds.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2019; Chapter 23; pp. 567–593. [Google Scholar]
- Peola, J.E. Nanodentistry: The benefits of nanotechnology in dentistry and its impact on oral health. J. Stud. Sci. Technol. 2017, 10, 45–50. [Google Scholar] [CrossRef]
- Kasraei, S.; Sami, L.; Hendi, S.; Arkani, M.Y.; Rezaci-Soufi, L.; Khamverdi, Z. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor. Dent. Endod. 2014, 39, 109–114. [Google Scholar] [CrossRef]
- Uno, M.; Kurachi, M.; Wakamatsu, N.; Doi, Y. Effects of adding silver nanoparticles on the toughening of dental porcelain. J. Prosthet. Dent. 2013, 109, 242–247. [Google Scholar] [CrossRef]
- Hanning, M.; Hanning, C. Nanotechnology and its role in caries therapy. Adv. Dent. Res. 2012, 24, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Sindhura Reddy, N.; Sowmya, S.; Bumgardner, J.D.; Chennazlei, K.P.; Biswas, R.; Jayakumar, R. Tetracycline nanoparticles loaded calcium sulfate composite beads for periodontal management. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 2080–2090. [Google Scholar] [CrossRef] [PubMed]
- Koopaie, M. Nanoparticulate systems for dental drug delivery. In Nanoengineered Biomaterials for Advanced Drug Delivery, 1st ed.; Mozafaei, M., Ed.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2020; pp. 525–559. [Google Scholar]
- Kupp, L.I.; Sheridan, P.J. Denture sore mouth. Dermatol. Clin. 2003, 21, 115–122. [Google Scholar] [CrossRef]
- Kalaydina, R.V.; Bajwa, K.; Qorri, B.; Decarlo, A.; Szewczuk, M.R. Recent advances in “smart” delivery systems for extended drug release in cancer therapy. Int. J. Nanomed. 2018, 13, 4727–4745. [Google Scholar] [CrossRef]
- Desai, P.; Thumma, N.J.; Wagh, P.R.; Zhan, S.; Ann, D.; Wang, J.; Prabhu, S. Cancer chemoprevention using nanotechnology-based approaches. Front. Pharmacol. 2020, 11, 323. [Google Scholar] [CrossRef]
- Galperina, S.; Kisich, K.; Iseman, M.D.; Heifets, L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am. J. Respir. Crit. Care Med. 2005, 172, 1487–1490. [Google Scholar] [CrossRef]
- Aleya, L.; Abdel-Daim, M.M. Nanoparticles and its biomedical applications in health and diseases: Special focus on drug delivery. Environ. Sci. Pollut. Res. 2020, 27, 19151–19168. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Bhardwaj, S.V. Local drug delivery in periodontology. Recent Adv. Pharm. Res. 2012, 1, 1–5. [Google Scholar]
- Liang, J.; Peng, X.; Zhou, X.; Cheng, L. Emerging applications of drug delivery systems in oral infectious diseases prevention and treatment. Molecules 2020, 25, 516. [Google Scholar] [CrossRef] [PubMed]
- Alsuraifi, A. Metallic nanoparticles in dental biomaterials: A review. AAJMS 2020, 3, 27–37. [Google Scholar]
- Ahuja, A.; Rahman, S.; Ali, J.; Khar, R.K. Site specific delivery system for treatment of periodontitits. Indian J. Pharm. Sci. 2003, 65, 106–112. [Google Scholar]
- Kaplish, V.; Walia, M.K.; Hari Kumar, S.L. Local drug delivery systems in the treatment of periodontitis: A review. Pharmacophore 2013, 4, 39–49. [Google Scholar]
- Dodwad, V.; Vaish, S.; Chhoka, M.; Mahajan, A. Magic bullet to treat periodontitits: A targeted approach. JPBMS 2012, 20, 1–5. [Google Scholar]
- Slots, J.; Rams, T.E. Local delivery of antimicrobial agents in the periodontal pocket. Periodontol 2000 1996, 10, 139–159. [Google Scholar] [CrossRef]
- Vyas, S.P.; Shihorkar, V.; Mishra, V. Controlled and targeted drug delivery strategies towards intraperiodontal pocket diseases. J. Clin. Pharm. Therapeut. 2000, 25, 21–42. [Google Scholar] [CrossRef] [PubMed]
- Tokajuk, G.; Niemirowicz, K.; Deptula, P.; Piktel, E.; Ciesluk, M.; Wileczewska, A.Z.; Dabrowski, J.R.; Bucki, R. Use of magnetic nanoparticles as a drug delivery system to improve chlorhexidine antimicrobial activity. Int. J. Nanomed. 2017, 12, 7833–7846. [Google Scholar] [CrossRef] [PubMed]
- Madhumathi, K.; Sampath Kumar, T.S. Regenerative potential and antibacterial activity of tetracycline loaded apatitic nanocarriers for the treatment of periodontitis. Biomed. Mater. 2014, 9, 035002. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Hiorth, M. Advanced drug delivery systems for local treatment of the oral cavity. Ther. Deliv. 2015, 6, 595–608. [Google Scholar] [CrossRef]
- Kovtun, A.; Kozlova, D.; Ganesan, K.; Biewald, C.; Seipold, N.; Gaengler, P.; Arnold, W.H.; Epple, M. Chlorhexidine-loaded calcium phosphate nanoparticles for dental maintenance treatment combination of mineralizing and antibacterial effect. RCS Adv. 2012, 2, 870–875. [Google Scholar] [CrossRef]
- Bao, X.; Zhao, J.; Sun, J.; Hu, M.; Yang, X. Polydopamine nanoparticles as efficient scavengers for reactive oxygen species in periodontal disease. ACS Nano 2018, 12, 8882–8892. [Google Scholar] [CrossRef]
- Vidal-Romero, G.; Zambrano-Zaragoza, M.L.; Martinez-Acevedo, L.; Leyva-Gomez, G.; Mendoza-Elvira, S.E.; Quintanar-Guerrero, D. Design and evaluation of pH-dependent nanosystems based on cellulose acetate phthalate, nanoparticles loaded with chlorhexidine for periodontal treatment. Pharmaceutics 2019, 11, 604. [Google Scholar] [CrossRef]
- Liu, H.; Chen, B.; Mao, Z.; Gao, C. Chitosan nanoparticles for loading of tooth paste actives and adhesion on tooth analogs. J. Appl. Polym. Sci. 2007, 106, 4248–4256. [Google Scholar] [CrossRef]
- Nguyen, S.; Escudero, C.; Seidiqi, N.; Smistad, G.; Hiorth, M. Fluoride loaded polymeric nanoparticles for dental delivery. Eur. J. Pharm. Sci. 2017, 104, 326–334. [Google Scholar] [CrossRef]
- Dung, T.H.; Lee, S.R.; Han, S.D.; Kim, S.J.; Ju, Y.M.; Kim, M.S.; Hoon, Y. Chitosan TPP nanoparticles as a release system of antisense oligonucleotide in the oral environment. J. Nanosci. Nanotechnol. 2007, 11, 3695–3699. [Google Scholar] [CrossRef]
- Shrestha, A.; Kirshen, A. Antibacterial efficacy of photosensitizer functionalized biopolymeric nanoparticles in the presence of tissue inhibitors in root canal. J. Endod. 2014, 40, 566–570. [Google Scholar] [CrossRef]
- Chang, P.C.; Tai, W.C.; Luo, H.T.; Lai, C.H.; Lin, H.H.; Lin, Z.J.; Chang, Y.C.; Lee, B.S. Core-shell poly(D,L-lactide-co-glycolide)-chitosan nanospheres with simvastatin-doxycycline for periodontal and osseous repair. Int. J. Biol. Macromol. 2020, 158, 627–635. [Google Scholar] [CrossRef]
- Hu, F.; Zhou, Z.; Xu, Q.; Fan, C.; Wang, L.; Ren, H.; Xu, S.; Ji, Q.; Chen, X. A novel pH-responsive quaternary ammonium chitosan-liposome nanoparticles for periodontal treatment. Int. J. Biol. Macromol. 2019, 129, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Feng, F.; Yu, M.C.; Wang, C.H.; Chang, P.C. Modulation of periodontitis progression using pH-responsive nanosphere encapsulating metronidazole or N-phenacylthiazolium bromide. J. Periodont. Res. 2018, 53, 22–28. [Google Scholar] [CrossRef]
- Ahmadian, E.; Shahi, S.; Yazdani, J.; Dizaj, S.M.; Sharifi, S. Local treatment of the dental carriers using nanomaterials. Biomed. Pharmacother. 2018, 108, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Sehgal, P. GV Black dental caries classification and preparation technique using optimal CNN-LSTM classifier. Multimed. Tools Appl. 2021, 80, 5255–5272. [Google Scholar] [CrossRef]
- Fisher, J.; Glick, M. A new model for caries classification and management. The FDI World Dental Federation caries matrix. JADA 2012, 143, 546–551. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, J.; Li, Y.; Lu, J.; Gao, L.; Xu, H.; Fan, M.; Yang, X. Enhanced nasal mucosal delivery and immunogenicity of anticaries DNA vaccine through incorporation of anionic liposomes in chitosan/DNA complexes. PLoS ONE 2013, 8, e71953. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Weir, M.D.; Melo, M.A.S.; Zhou, X.; Xu, H.H.K. Nanotechnology strategies for antibacterial and remineralizing composites and adhesives to tackle dental caries. Nanomedicine 2015, 10, 627–641. [Google Scholar] [CrossRef]
- Roveri, N.; Palazzo, B.; Iafisco, M. The role of biomimetism in developing nanostructured inorganic matrices for drug delivery. Expert Opin. Drug Deliv. 2008, 5, 861–887. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N. Tobacco use and oral cancer: A global perspective. J. Dent. Educ. 2001, 65, 328–339. [Google Scholar] [CrossRef]
- Scully, C. Oral squamous cell carcinoma; from a hypothesis about a virus, to concern about possible sexual transmission. Oral Oncol. 2002, 38, 227–234. [Google Scholar] [CrossRef]
- Cawson, R.A. Leukoplakia and oral cancer. Proc. R. Soc. Med. 1969, 62, 610–614. [Google Scholar] [CrossRef]
- Lissowska, J.; Pilarska, A.; Pilarski, P.; Samolczyk-Wanyura, D.; Piekarczyk, J.; Bardin-Mikollajczak, A.; Zatonski, W.; Herrero, R.; Munoz, N.; Franceschi, S. Smoking, alcohol, diet, dentition and sexual practices in the epidemiology of oral cancer in Poland. Eur. J. Cancer Prev. 2003, 12, 25–33. [Google Scholar] [CrossRef]
- Koch, W.; Nance, M. Classification, clinical features, and molecular genetic models. In Epidemiology, Pathogenesis, and Prevention of Head and Neck Cancer; Olshan, A.F., Ed.; Springer: New York, NY, USA, 2010; pp. 1–21. [Google Scholar]
- Medina-Alarcon, K.P.; Voltan, A.R.; Fonseca-Santos, B.; Moro, I.J.; de Oliveira Souza, F.; Chorilli, M.; Soares, C.P.; dos Santos, A.G.; Mendes-Giannini, M.J.; Fusco-Almeida, A.M. Highlights in nanocarriers for the treatment against cervical cancer. Chimia 2017, 80, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Kumar Prajapati, V. Drug targeting to infectious diseases by nanoparticles surface functionalized with special biomolecules. Curr. Med. Chem. 2012, 19, 3196–3202. [Google Scholar] [CrossRef] [PubMed]
- Calixto, G.; Bernegossi, J.; Fonseca-Santos, B.; Chorilli, M. Nanotechnology-based drug delivery systems for treatment of oral cancer: A review. Int. J. Nanomed. 2014, 9, 3719–3735. [Google Scholar] [CrossRef]
- Tsang, R.Y.; Al-Fayea, T.; Au, H.J. Cisplatin overdose. Drug Saf. 2009, 32, 1109–1122. [Google Scholar] [CrossRef]
- Endo, K.; Ueno, T.; Kondo, S.; Wakisaka, N.; Murono, S.; Ito, M.; Katuoka, K.; Yoshizaki, T. Tumor-targeted chemotherapy with the nanopolymer-based drug NC-6004 for oral squamous cell carcinoma. Cancer Sci. 2013, 104, 369–374. [Google Scholar] [CrossRef]
- Gao, S.; Zheng, M.; Ren, X.; Tang, Y.; Liang, X. Local hyperthermia head and neck cancer: Mechanism, application and advance. Oncotarget 2016, 7, 57367–57378. [Google Scholar] [CrossRef]
- Cassano, D.; Pocoví-Martínez, S.; Voliani, V. Ultrasmall-in-Nano Approach: Enabling the Translation of Metal Nanomaterials to Clinics. Bioconj. Chem. 2018, 29, 4–16. [Google Scholar] [CrossRef]
- Vlamidis, Y.; Voliani, V. Bringing Again Noble Metal Nanoparticles to the Forefront of Cancer Therapy. Front. Bioeng. Biotechnol. 2018, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mapanao, A.K.; Santi, M.; Voliani, V. Combined chemo-photothermal treatment of three-dimensional head and neck squamous cell carcinomas by gold nano-architectures. J. Colloid Interface Sci. 2021, 582, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, L.; Huang, Y.; Liu, B.; Chi, H.R.; Shi, L.; Zhang, W.; Li, G.; Niu, Y.; Zhu, X. Synergistic therapy of chemotherapeutic drugs and MTH1 inhibitors using a pH-sensitive polymeric delivery system for oral squamous cell carcinoma. Biomater. Sci. 2017, 5, 2068–2078. [Google Scholar] [CrossRef] [PubMed]
- Marcazzan, S.; Varoni, E.M.; Blanco, E.; Lodi, G.; Ferrari, M. Nanomedicine, an emerging therapeutic strategy for oral cancer therapy. Oral Oncol. 2018, 76, 1–7. [Google Scholar] [CrossRef]
- Setten, R.L.; Rossi, J.J.; Han, S.P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef]
- Kim, D.H.; Rossi, J.J. Strategies for silencing human disease using RNA interference. Nat. Rev. Genet. 2007, 8, 173–184. [Google Scholar] [CrossRef]
- Jin, L.; Wang, Q.; Chen, J.; Wang, Z.; Xin, H.; Zhang, D. Efficient Delivery of Therapeutic siRNA by Fe3O4 Magnetic Nanoparticles into Oral Cancer Cells. Pharmaceutics 2019, 11, 615. [Google Scholar] [CrossRef]
- Chou, L.Y.T.; Ming, K.; Chan, W.C.W. Strategies for the intracellular delivery of nanoparticles. Chem. Soc. Rev. 2011, 40, 233–245. [Google Scholar] [CrossRef]
- Piao, L.; Zhang, M.; Datta, J.; Xie, X.; Su, T.; Li, H.; Teknos, T.N.; Pan, Q. Lipid-based nanoparticle delivery of Pre-miR-107 inhibits the tumorigenicity of head and neck squamous cell carcinoma. Mol. Ther. 2012, 20, 1261–1269. [Google Scholar] [CrossRef]
- Murdoch, C.; Reeves, K.J.; Hearnden, V.; Colley, H.; Massignani, M.; Canton, I.; Madsen, J.; Blanazs, A.; Armes, S.P.; Lewis, A.L.; et al. Internalization and biodistribution of polymersomes into oral squamous cell carcinoma cells in vitro and in vivo. Nanomedicine 2010, 5, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Hearnden, V.; Lomas, H.; Macneil, S.; Thornhill, M.; Murdoch, C.; Lewis, A.; Madsen, J.; Blanazs, A.; Armes, S.; Battaglia, G. Diffusion studies of nanometer polymersomes across tissue engineered human oral mucosa. Pharm. Res. 2009, 26, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Tanaka, M.; Tanaka, T. Oral carcinogenesis and oral cancer chemoprevention: A review. Pathol. Res. Int. 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- Holpuch, A.S.; Hummel, G.J.; Tong, M.; Seghi, G.A.; Pei, P.; Ma, P.; Mumper, R.J.; Mallery, S.R. Nanoparticles for local drug delivery to the oral mucosa. Proof of principle studies. Pharm. Res. 2010, 27, 1224–1236. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.E.; Cretan, M.S.; Purcar, V.; Popa, M.; Daraba, O.M.; Atanase, L.I.; Ochiuz, L. Drug delivery system based on pH-sensitive biocompatible poly(2-vinyl pyridine)-b-poly(ethylene oxide) nanomicelles loaded with curcumin and 5-Fluorouracil. Polymers 2020, 12, 1450. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, D.S.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly bioavailable forms of curcumin and promising avenues for curcumin-based research and application: A review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, C.; Pereira, P.; Schellenberg, P.; Coutinho, P.J.; Gama, F.M. Self-assembled dextrin nanogel as curcumin delivery system. J. Biomater. Nanobiotechnol. 2012, 3, 178–184. [Google Scholar] [CrossRef]
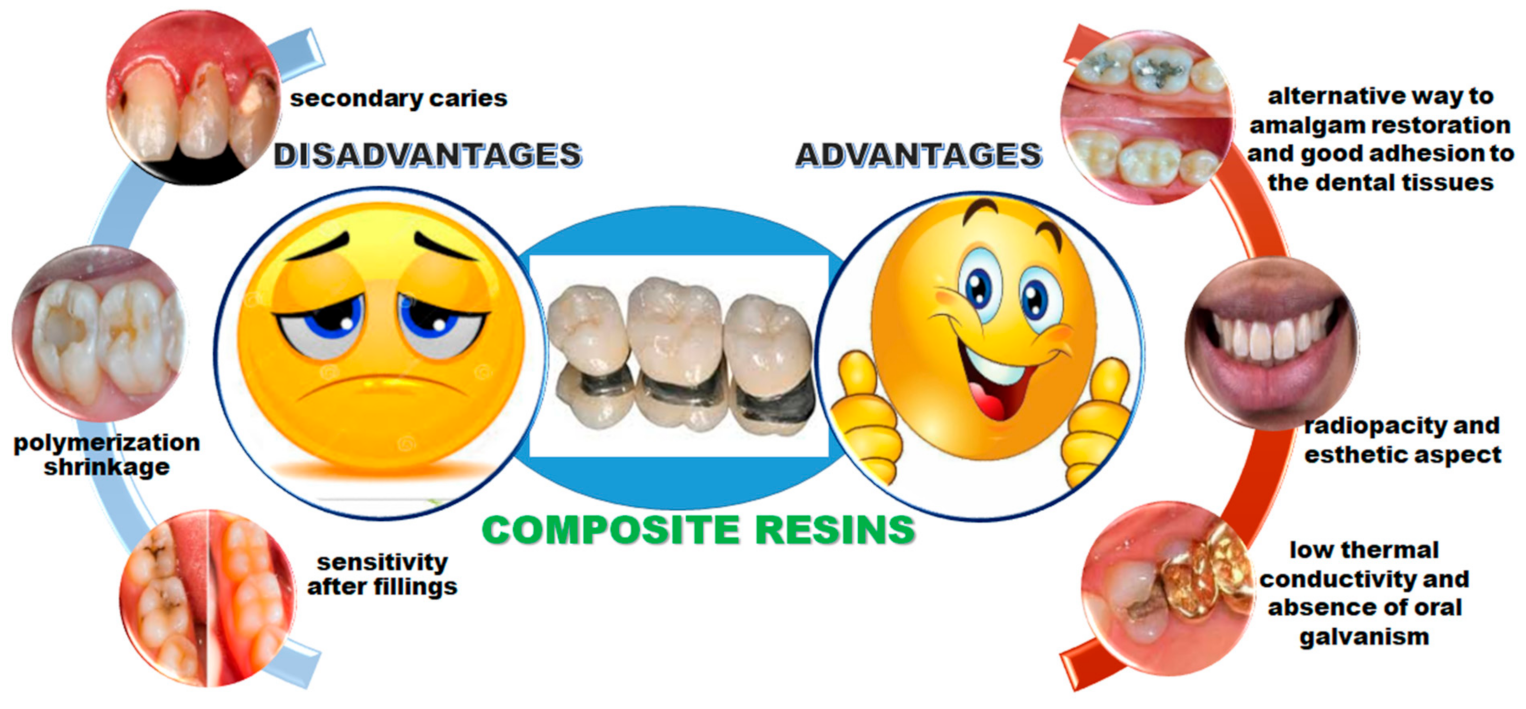
- Azeem, R.A.; Sureshbabu, N.M. Clinical performance of direct versus indirect composite restorations in posterior teeth: A systematic review. J. Conserv. Dent. 2018, 21, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Antonelli da Veiga, A.M.; Carneiro Cunha, A.; Ferreira, D.M.T.P.; da Silva Fidalgo, T.K.; Chianca, T.K.; Rodrigues Reis, K.; Cople Maia, L. Longetivity of direct and indirect resin composite restorations in permanent posterior teeth: A systematic review and meta-analysis. J. Dent. 2016, 54, 1–12. [Google Scholar] [CrossRef]
- Malek, M.; Farzaneh, F.; Samani, Y.; Pachenari, F.; Pachenari, H. The applications of nanotechnology in restorative dentistry: A review study. Nanomed. J. 2019, 6, 241–249. [Google Scholar] [CrossRef]
- Khurshid, Z.; Zafar, M.; Qasim, S.; Shahab, S.; Naseem, M.; AbuReqaiba, A. Advances in nanotechnologies for restorative dentistry. Materials 2015, 8, 717–731. [Google Scholar] [CrossRef]
- Tanaka, T.; Taira, H.; Shintani, H.; Wakasa, M.; Yamaki, M. Residual monomers (TEGDMA and Bis-GMA) of a set visible-light-cured dental composite resin when immersed in water. J. Oral Rehab. 1991, 18, 353–362. [Google Scholar] [CrossRef]
- Kleverlaan, C.J.; Feilzer, A.J. Polymerization shrinkage and contraction stress of dental resin composites. Dent. Mater. 2005, 21, 1150–1157. [Google Scholar] [CrossRef]
- Anseth, K.S.; Newman, S.M.; Bowman, C.N. Polymeric dental composites properties and reaction behavior of multimethacrylate dental restorations. Adv. Polym. Sci. 1995, 122, 177–217. [Google Scholar]
- Bowen, R.L. Use of epoxy resins in restorative materials. J. Dent. Res. 1956, 35, 360–369. [Google Scholar] [CrossRef]
- Beyth, N.; Yudovin-Farber, I.; Basu, A.; Weiss, E.I.; Domb, A.J. Antimicrobial nanoparticles in restorative composites. In Emerging Nanotechnology in Dentistry, 2nd ed.; Subramani, K., Ahmed, W., Eds.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2018; Chapter 3; pp. 41–58. [Google Scholar]
- Yudovin-Farber, I.; Neyth, N.; Weiss, E.I.; Domb, A.J. Antibacterial effect of composite resins containing quaternary ammonium polyethyleneimine nanoparticles. J. Nanopart. Res. 2010, 12, 591–603. [Google Scholar] [CrossRef]
- Farah, S.; Khan, W.; Farber, I.; Kesler-Shvero, D.; Beyth, N.; Weiss, E.I.; Domb, A.J. Crosslinked QA-PEI nanoparticles: Synthesis, reproductibility, chemical modification and stability study. Polym. Adv. Technol. 2013, 24, 446–452. [Google Scholar] [CrossRef]
- Beyth, N.; Houri-Haddad, Y.; Baraness-Hadar, L.; Yudovin-Farber, I.; Domb, A.J.; Weiss, E.I. Surface antimicrobial activity and biocompatibility of incorporated polyethyleneimine nanoparticles. Biomaterials 2008, 29, 4157–4163. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A.S.; Cheng, L.; Weir, M.D.; Hsia, R.C.; Rodrigues, L.K.A.; Xu, H.H.K. Novel dental adhesive containing antibacterial agents and calcium phosphate nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Melo, M.A.S.; Cheng, L.; Weir, M.D.; Bai, Y.; Xu, H.H.K. Effect of quaternary ammonium and silver nanoparticle containing adhesives on dentin bond strength and dental plaque microcosm biofilm. Dent. Mater. 2012, 28, 842–852. [Google Scholar] [CrossRef]
- Padovani, G.C.; Feitosa, V.P.; Sauro, S.; Tay, F.R.; Duran, G.; Paula, A.J.; Duran, N. Advances in dental materials through nanotechnology: Facts, Perspective and Toxicological aspects. Trends Biotechnol. 2015, 33, 621–636. [Google Scholar] [CrossRef] [PubMed]
- Wingo, K. A review of dental cements. JOVD 2018, 35, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Scraton, A.B.; Rangarajan, B.; Klier, J. Biomedical applications of polyelectrolytes. Adv. Polym. Sci. 1995, 122, 1–54. [Google Scholar]
- Wilson, A.D.; Kent, B.E. The glass ionomer cement, a new translucent dental filling material. J. Appl. Chem. Biotechnol. 1971, 21, 313. [Google Scholar] [CrossRef]
- Wilson, A.D.; Kent, B.E. A new translucent cement for dentistry. The glass ionomer cement. Br. Dent. J. 1972, 132, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, S.K.; Nicholson, J.W. A review of glass-ionomer cements for clinical dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef]
- Wilson, A.D.; Kent, B.E.; Clinton, D.; Miller, R.P. The formation and microstructure of dental silicate cements. J. Mater. Sci. 1972, 7, 220–238. [Google Scholar] [CrossRef]
- Barry, T.I.; Clinton, D.J.; Wilson, A.D. The structure of a glass-ionomer cement and its relationship to the setting process. J. Dent. Res. 1979, 58, 1072–1079. [Google Scholar] [CrossRef]
- Lohbauer, U. Dental glass ionomer cements as permanent filling materials? Properties, limitations and future trends. Materials 2010, 3, 76–96. [Google Scholar] [CrossRef]
- Wasson, E.A.; Nicholson, J.W. A study of the relationship between setting chemistry and properties of modified glass-poly(alkenoate) cements. Br. Polym. J. 1990, 23, 179–183. [Google Scholar] [CrossRef]
- Wasson, E.A.; Nicholson, J.W. Studies on the setting chemistry of glass ionomer cements. Clin. Mater. 1991, 7, 289–293. [Google Scholar] [CrossRef]
- Wasson, E.A.; Nicholson, J.W. New aspects on the setting of glass ionomer cements. J. Dent. Res. 1993, 72, 481–483. [Google Scholar] [CrossRef]
- Khan, A.S.; Khan, R.S.; Khan, M.; Rehman, I.U. Incorporation of nanoparticles in glass ionomer cements: Clinical applications properties and future perspective. In Nanobiomaterials in Clinical Dentistry, 2nd ed.; Subramani, K., Ahmed, W., Eds.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2019; Chapter 5; pp. 113–138. [Google Scholar]
- Moshaverinia, A.; Ansari, S.; Moshaverinia, M.; Roohpour, N.; Darr, J.A.; Rehman, I.U. Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional glass ionomer cements (GIC). Acta. Biomater. 2008, 4, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.W.; Yap, A.U.J.; Cheang, P.; Koh, Y.L.; Khor, K.A. Development of zirconia-glass ionomer cement composites. J. Non-Cryst. Solids 2010, 21, 499–507. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Ansari, S.; Movasaghi, Z.; Billington, R.W.; Darr, J.A.; Rehman, I.U. Modification of conventional glass-ionomer cements with N-vinylpyrrolidone containing polyacids, nano-hydroxy and fluoroapatite to improve mechanical properties. Dent. Mater. 2008, 24, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Caughman, W.F.; Caughman, G.B.; Dominy, W.T.; Schuster, G.S. Glass ionomer and composite resin cements: Effects on oral cells. J. Prosthet. Dent. 1990, 63, 513–521. [Google Scholar] [CrossRef]
- Mathis, R.; Ferracane, J.L. Properties of a glass ionomer/resin composite hybrid material. Dent. Mater. 1989, 5, 355–358. [Google Scholar] [CrossRef]
- Momoi, Y.; Hirosaki, K.; Kohno, A.; McCabe, J.F. Flexural properties of resin-modified “hybrid” glass ionomers in comparation with conventional acid-base glass ionomers. Dent. Mater. J. 1995, 14, 109–119. [Google Scholar] [CrossRef]
- Lawson, N.C.; Cakir, D.; Beck, P.; Ramp, L.; Burgess, J.O. Effect of light activation on resin-modified glass ionomer shear bond strength. Oper. Dent. 2012, 37, 380–385. [Google Scholar] [CrossRef]
- Mount, G.J.; Patel, C.; Makinson, O.F. Resin modified glass-ionomers, strength, cure depth and translucency. Aus. Dent. J. 2002, 47, 339–343. [Google Scholar] [CrossRef]
- Mitra, S.B. Adhesion to dentin and physical properties of a light-cured glass ionomer liner/base. J. Dent. Res. 1991, 70, 72–74. [Google Scholar] [CrossRef]
- Mitra, S.B. In vitro fluoride release from light-cured glass-ionomer liner/base. J. Dent. Res. 1991, 70, 75–78. [Google Scholar] [CrossRef]
- Nicholson, J.W. Glass ionomer dental cements: Uptade. Mater. Technol. Adv. Perform. Mater. 2010, 25, 8–13. [Google Scholar] [CrossRef]
- Wilson, A.D. Resin-modified glass-ionomer cements. Int. J. Prosthodont. 1990, 3, 425–429. [Google Scholar] [PubMed]
- Stanislawski, L.; Daniau, X.; Lautie, A.; Goldeberg, M. Factors responsible for pulp cell cytotoxicity induced by resin-modified glass ionomer cements. J. Biomed. Mater. Res. Appl. Biomater. 1999, 48, 277–288. [Google Scholar] [CrossRef]
- Xie, D.; Chung, I.D.; Wu, W.; Mays, J. Synthesis and evaluation of HEMA-free glass-ionomer cements for dental applications. Dent. Mater. 2004, 20, 470–478. [Google Scholar] [CrossRef]
- Varaprasad, D.; Rosthauser, J.; Winston, A. Hydroxamic acid polymers. Effect of structure on the chelation of iron in water. J. Polym. Sci. Polym. Chem. Ed. 1984, 22, 2131–3143. [Google Scholar] [CrossRef]
- Strassler, H.E.; Coviello, V. FujiCem resin-reinforced glass-ionomer cement. Contempt. Esthet. Restorat. Pract. 2001, 12, 1–2. [Google Scholar]
- Simon, J.; Darnell, L. Considerations for proper selection of dental cements. Compend. Contin. Educ. Dent. 2012, 33, 28–35. [Google Scholar]
- Loguercio, A.D.; Reis, A.; Mazzoeco, K.C.; Dias, A.L.; Busato, A.L.S.; La Motta Singer, J.; Rosa, P. Microleakage in Class II composite resin restorations: Total bonding and open sandwich technique. J. Adhes. Dent. 2002, 4, 137–144. [Google Scholar] [PubMed]
- FiltekTM Supreme XT Flowable Restorative, Technical Product Profile. Available online: https://multimedia.3m.com/mws/media/598213O/filtek-supreme-xt-fl-tpp.pdf (accessed on 24 February 2021).
- KetacTM N 100 Light-Curing Nano-Ionomer Restorative, Ketac N100 Brochure. Available online: https://mws9.3m.com/mws/medi/4434800/ketac-n100-brochure-ebu.pdf (accessed on 25 February 2021).
- Croll, T.P.; Nicholson, J.W. Glass-ionomer cements in pediatric dentistry: Review of the literature. Pediatr. Dent. 2002, 24, 423–429. [Google Scholar]
- Sismanoglu, S.; Gumustas, B.; Yildirim-Bilmez, Z. Effect of polishing systems on fluoride release and surface roughness of different restorative materials. ODOVTOS Int. J. Dent. Sci. 2020, 22, 81–92. [Google Scholar] [CrossRef]
- Kumar, S.R.; Vijayalakshmi, R. Nanotechnology in dentistry. Indian J. Dent. Res. 2006, 17, 62–65. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Semmler-Behnke, M.; Chaudhry, Q. A complementary definition of nanomaterial. Nano Today 2010, 5, 165–168. [Google Scholar] [CrossRef]
- Akcan, R.; Aydogan, H.C.; Yildirim, M.S.; Tastekin, B.; Saglam, N. Nanotoxicity: A challenge for future medicine. Turk. J. Med. Sci. 2020, 50, 1180–1196. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerfaces 2007, 2, MR17–MR71. [Google Scholar] [CrossRef]
- Lecio, G.; Ribeiro, F.V.; Pimentel, S.P.; Reis, A.A.; Clima da Silva, R.V.; Nociti, F., Jr.; Moura, l.; Duek, E.; Casati, M.; Casarin, R.C.V. Novel 20% doxycycline-loaded PLGA nanospheres as adjuctive therapy in chronic periodontitis i.n type-2 diabetics: Randomized clinical, immune and microbiological trial. Clin. Oral Invest. 2020, 24, 1269–1279. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, Q.; Jiang, Z.; Wang, Y.; Yang, K.; Qiu, X. The effect of doxycycline-containing chitosan/carboxymethyl chitosan nanoparticles on NLRP 3 inflammasome in periodontal disease. Carbohydr. Polym. 2020, 237, 116163. [Google Scholar] [CrossRef] [PubMed]
- Olasen, I.; Yilmaz, O. Modulation of inflammasome activity by Porphyromonas gingivalis in periodontitis and associated systemic diseases. J. Oral Microbiol. 2016, 8, 30385. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasiliu, S.; Racovita, S.; Gugoasa, I.A.; Lungan, M.-A.; Popa, M.; Desbrieres, J. The Benefits of Smart Nanoparticles in Dental Applications. Int. J. Mol. Sci. 2021, 22, 2585. https://doi.org/10.3390/ijms22052585
Vasiliu S, Racovita S, Gugoasa IA, Lungan M-A, Popa M, Desbrieres J. The Benefits of Smart Nanoparticles in Dental Applications. International Journal of Molecular Sciences. 2021; 22(5):2585. https://doi.org/10.3390/ijms22052585
Chicago/Turabian StyleVasiliu, Silvia, Stefania Racovita, Ionela Aurica Gugoasa, Maria-Andreea Lungan, Marcel Popa, and Jacques Desbrieres. 2021. "The Benefits of Smart Nanoparticles in Dental Applications" International Journal of Molecular Sciences 22, no. 5: 2585. https://doi.org/10.3390/ijms22052585
APA StyleVasiliu, S., Racovita, S., Gugoasa, I. A., Lungan, M.-A., Popa, M., & Desbrieres, J. (2021). The Benefits of Smart Nanoparticles in Dental Applications. International Journal of Molecular Sciences, 22(5), 2585. https://doi.org/10.3390/ijms22052585







