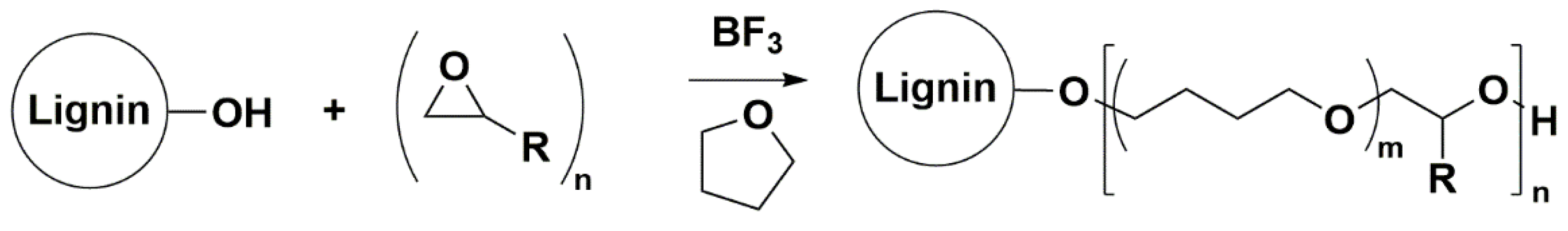
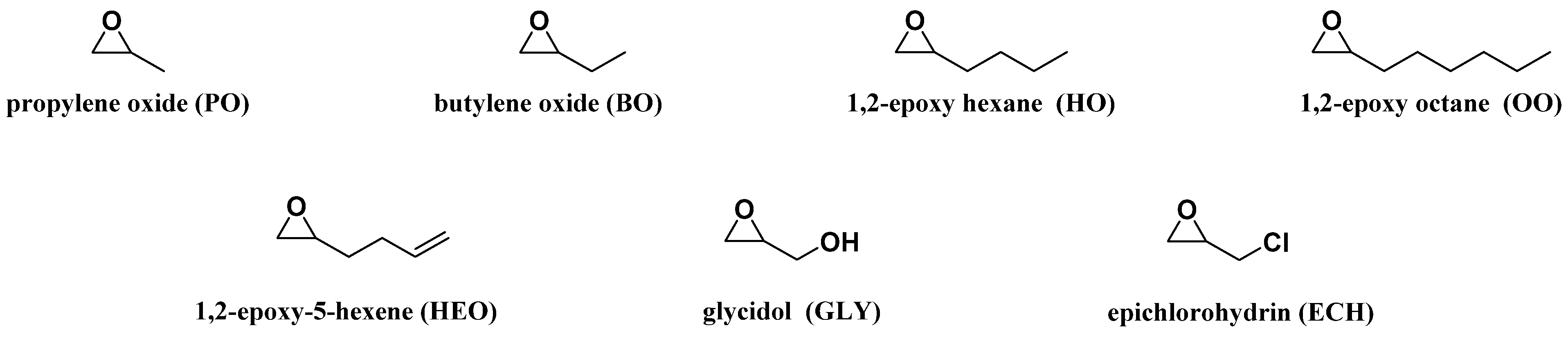Synthesis of the LPBs Family with Controlled Microstructure by CROP
LBPs were synthesized by CROP of oxiranes and THF in the presence of a OL source using a Lewis acid catalyst (BF
3·OEt
2) (
Scheme 1) being both the THF comonomer and solvent as previously discussed.
Based on our previous work [
17], the reaction parameters that showed little or negligible influence in the LBP microstructure, namely, the catalyst amount and the post-addition were selected. Therefore, the catalyst amount and the post-addition time were set at 0.125 catalyst/–OH–OL molar ratio and 1 h, respectively. Under the selected conditions, reaction parameters such as the oxirane/OH–OL molar ratio, the OL initial concentration, the reaction temperature, the specific flow rate of oxirane addition and the nature of the oxirane were studied to understand how they influence the LBPs’ microstructures using butylene oxide as the oxirane. However, before discussing the influence of these parameters, it is necessary to understand the propagation and termination step mechanisms of the CROP reaction in the presence of THF using lignin as the polyol initiator.
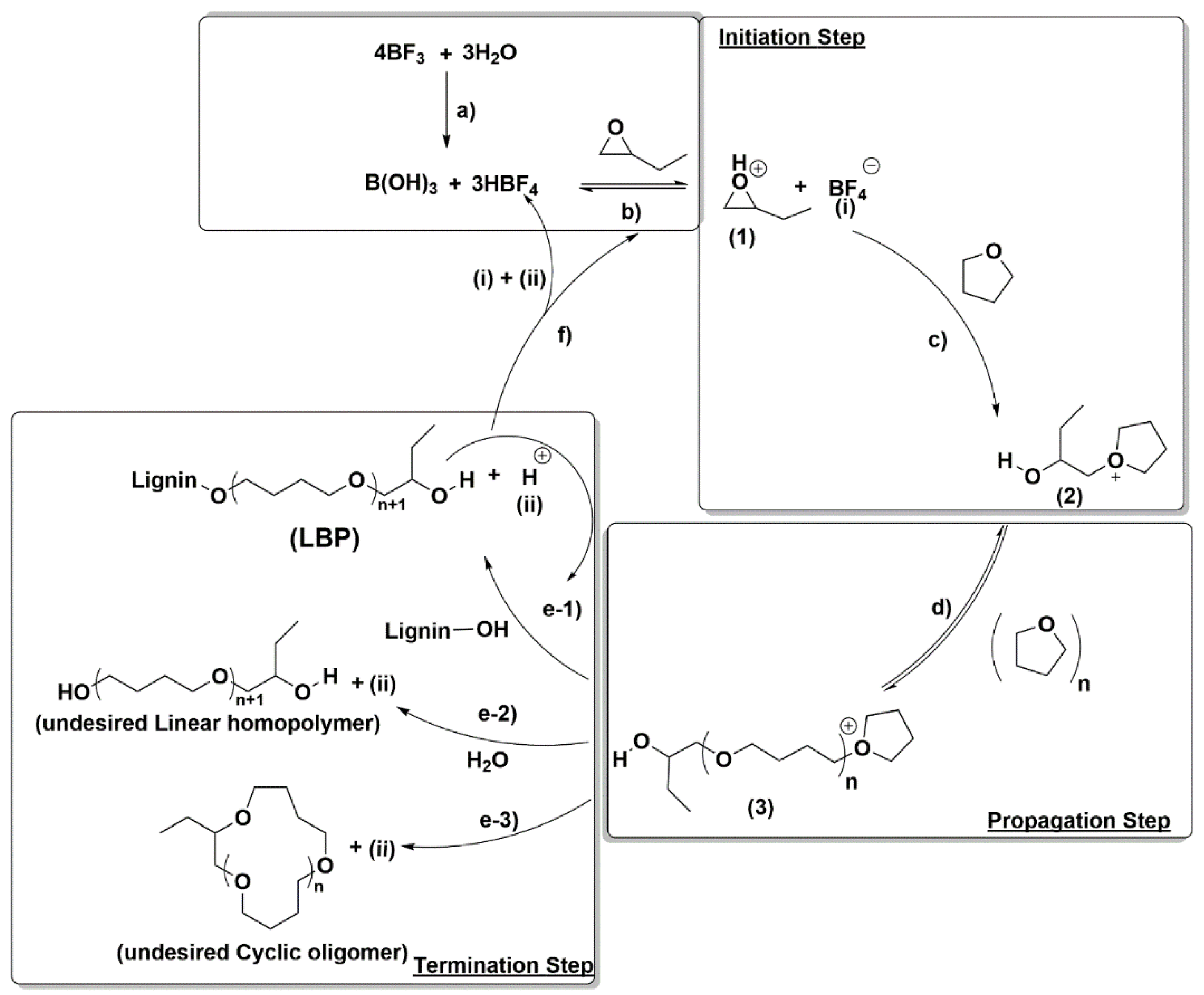
As depicted in
Scheme 2, after the initiation, the propagation step takes place by active chain end (ACE) mechanism by reaction of the formed cation 2 with THF leading to the growing polymer (3). This propagation step might also occur by reaction of cations 2 and 3 with the oxirane, but this is unlikely because THF is used as reaction media and therefore it is in a large excess relative to the oxirane due to the slow semicontinuous oxirane addition to the reaction mixture. On the other hand, the termination could potentially occur in three different ways: (1) by reaction of cation 3 with the –OH groups present in the lignin leading to the desired LBP or with an already formed LBP (path e-1); (2) by reaction with traces of water leading to an undesired linear homopolymer (path e-2); and (3) by back-biting reaction between the terminal –OH moiety in cations 3 with a carbon atom in the same cation leading to an undesired cyclic oligomer (COL) (path e-3). Consequently, in view of the CROP mechanism at the selected reaction conditions the competition between homopropagation (path d) and termination reactions (paths e-1, e-2 and e-3) will depend mainly on the THF and –OH concentrations as already reported in the literature [
19,
20]. Even if both reaction mechanisms, activated monomer (AM) and ACE mechanisms, occur simultaneously, one is preferred to the other depending on THF and –OH concentrations, allowing playing with those parameters to fine tune the final LBPs microstructure for a required application. Another variable playing a key role in the LBP microstructure is the reaction temperature; the higher temperature the lower THF incorporation in the final LBP [
21,
22]. On the basis of previously discussed premises, the influence of oxirane/–OH–OL molar ratio (affecting THF concentration), the influence of the lignin concentration (affecting the THF concentration and the –OH concentration), the effect of the temperature (affecting the THF incorporation), the influence of the oxirane specific addition flow rate (Q
s) (affecting the instant concentration-consumption of the oxirane) and once all these parameters were studied with the BO the influence of the oxirane nature (affecting mainly the thermal properties of the LBPs) was investigated as well.
This parameter was studied for values ranging from 1 to 5 using butylene oxide (BO) as an oxirane. As already stated, it is clear that the oxirane monomer acts as promotor in the CROP reaction and then it is expected that LBPs having higher molecular weights could be obtained by increasing the oxirane/OH–OL molar ratio. Experimental conditions of reactions carried out and results obtained are given in
Table 1 together with the characteristics of the starting OL (entry 1). The BO content in LBPs increases with BO/OH–OL molar ratio from 8 wt.% at a molar ratio of 1 to 16.7 wt.% at a molar ratio of 5. As expected, the OL content in the final LBP decreases from 24.5 wt.% at a BO/OH–OL molar ratio of 1 (entry 2) to 10.3 wt.% at a BO/OH–OL molar ratio of 5 (entry 3). Concerning THF incorporation into the final LBPs, it increases slightly with molar ratio. These results can be explained on the basis of the proposed CROP reaction mechanism since by increasing the oxirane content and consequently the relative ratio between oxirane/–OH–OL the propagation step is favored versus the termination one. On the other hand, the LBP hydroxyl number decreases by increasing the molar ratio from 81 to 35 mg KOH/g due to the increased amount of BO incorporated into the LBP because molecular mass increases with BO incorporation but OH groups introduced into a growing chain are independent of the number of BO units incorporated. The main difference between the three prepared LBPs at different molar ratios was observed in their crystallization and the melting temperatures, the lower the molar ratio the higher the crystallization temperature as a consequence of the lower length of flexible polyether chains. On the other hand, the melting temperature decreases when the molar ratio increases which can be attributed to the longer polyether moieties in the final LBP leading to a more flexible structure [
23].
The hydroxyl numbers (OH#), are by far lower than the OH# of the employed OL (243 mg KOH/g) (entry 1,
Table 1) versus 81 mg KOH/g for a molar ratio of 1 (entry 2,
Table 1), 70 mg KOH/g for a molar ratio of 2 (entry 3,
Table 1) and 35 mg KOH/g for a molar ratio of 5 (entry 4,
Table 1) since the apparent average M
w of the final LBPs increased considerably with the molar ratio, M
w 989 g/mol being the apparent average for OL (entry 1,
Table 1) and 7172 g/mol, 11,263 g/mol and 30,855 g/mol for LBPs obtained with BO/OH–OL molar ratios of 1, 2 and 5, respectively (entries 2–4,
Table 1).
The LPBs were analyzed by ATR-FTIR as well, but no significant differences between them were found as it can be seen in supporting information (
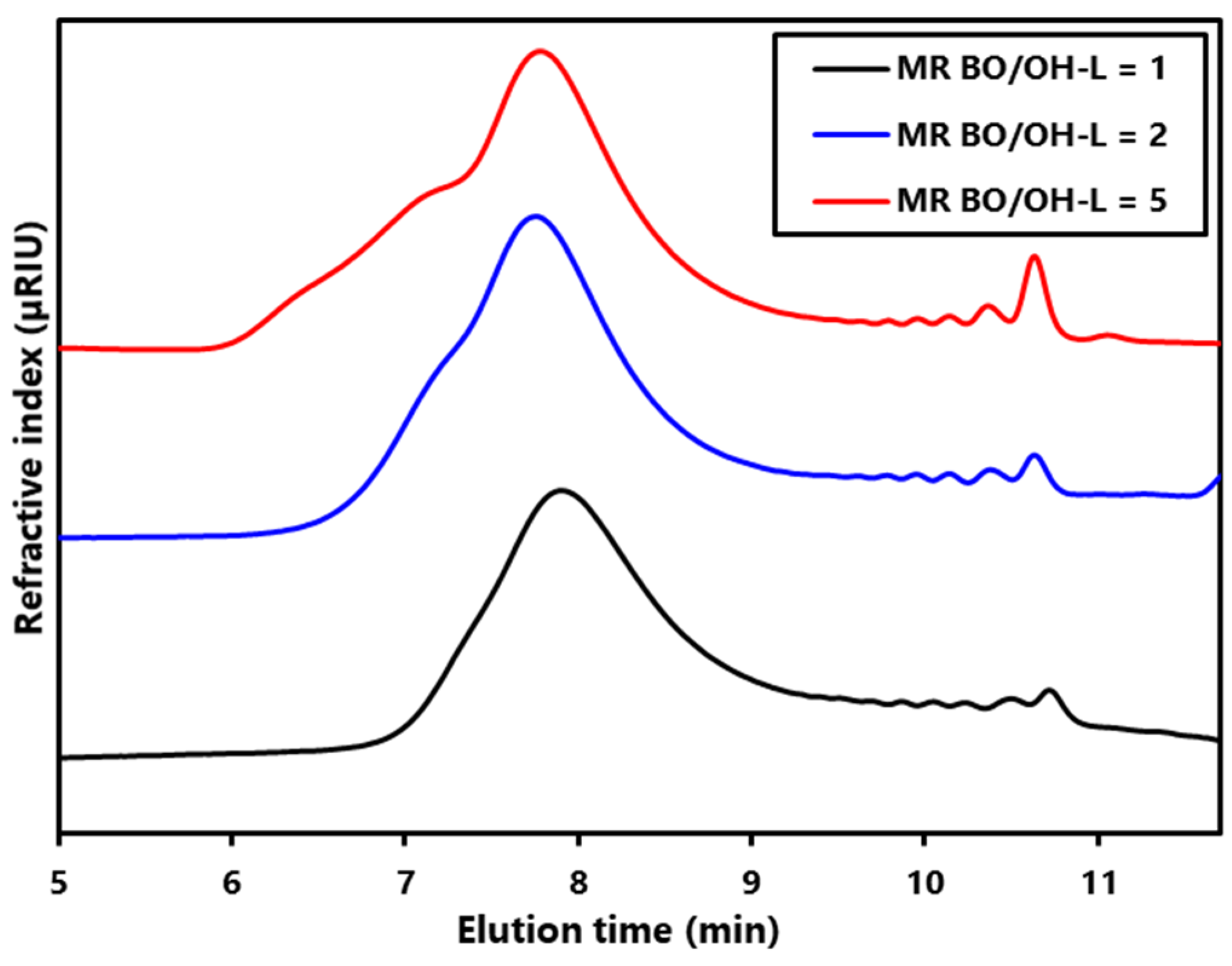
Figure S2). As expected, the MWD of the LBPs increased with the amount of BO incorporated (
Figure 1) [
24].
However, at long elution times, it can be observed that the small peaks attributed to cyclic oligomers (COLs) formation increase with molar ratio, as reported in the literature [
20]. In fact, it has been reported for the copolymerization of THF and ethylene oxide that the formation of COLs is lower or suppressed at THF conversion below 60% [
19]. As it can be seen in
Table 1, THF content in LBPs, and hence THF conversion, increases with molar ratio so that the lower the BO/OH–OL molar ratio the lower the amount of COL formed.
Concerning the decomposition temperature, it is observed that the LBPs have two degradation main peaks; one with the maximum weight lost ranging from ca. 360 to ca. 380 °C and another one with a maximum weight lost at ca. 540 °C (see
Figures S1 and S3). Relative to the decomposition of the initial OL, two decomposition peaks can be observed, the first one at 385 °C and the second and more intense one at around 530 °C (See
Figure S1). Therefore, the first weight lost in the LBP can be attributed to the polyether chain grafted to the OL core and the second one to the decomposition of the core OL. It seems clear that the thermal degradation pattern of the obtained LBPs at different molar ratios is very similar and it could be concluded that within the LBP composition range obtained the amount of added oxirane has no significant impact on the final LBPs thermal degradation; these results being consistent with those obtained in the copolymerization of styrene oxide with propylene oxide using diols as initiators [
25].
The OL concentration plays a crucial role in the polymerization process because OL concentration is directly related to the –OH groups available in the reaction media. The competition between the –OH groups and THF concentration is key in the CROP reaction because the prevalence of one of them has significant influence in the propagation and termination steps. In fact, the THF homopropagation competes with the termination by OL–OH groups as shown in
Scheme 2. Consequently, the OL initial concentration does affect the microstructure of the final LBPs allowing tuning of the final LBP properties. Several CROPs at four different initial OL concentrations (55 g/L, 110 g/L, 164 g/L and 274 g/L) at two different BO/OH–OL molar ratios (1 and 2) were carried out. Experimental conditions and results are given in
Table 2. A clear trend is observed at the two BO/OH–OL molar ratios studied, in other words, the lower the OL initial concentration the higher the apparent average M
w of the obtained LBP, which makes sense since by increasing the initial OL concentration there is an increase in the –OH concentration increasing the probability of the termination step by attack of the –OH to the growing polymer (
Scheme 2) avoiding the THF incorporation and consequently obtaining LBPs with lower apparent average Mw. Looking at the OH#, the lower the OH# the higher the apparent average M
w of the LBP obtained. The OH# is a crucial parameter for the polyol’s application scope determining, in most cases, their final application. In this sense, as it can be seen in
Table 2, different LBPs can be obtained with OH# ranging from 39 (entry 5,
Table 2) to 127 mg KOH/g (entry 4,
Table 2) by only tuning either the molar ratio, initial OL concentration, or both. The crystallization and melting temperatures of the resulting LBPs are also related to the OH# number and the apparent average M
w being both lower when increasing the OH#. It was found that there is not significant difference in the trend followed by the resulting LBPs at molar ratios 1 and 2 (entries 1–4 and entries 5–8,
Table 2). With initial OL concentrations of 55 g/L, 110 g/L and 164 g/L the main difference between the obtained LBPs at BO/OH–OL molar ratio of 1 or 2 is the apparent average M
w of the final LBPs, having those obtained at molar ratio 1 generally having a lower apparent average M
w (entries 1 and 5, entries 2 and 6 and entries 3 and 7,
Table 2). The lower apparent average M
w of the LBPs obtained at the lower molar ratio is attributed to the lower amount of oxirane used. Nevertheless, when the initial OL concentration is 274 g/mL almost no difference in the obtained LBPs at both studied molar ratios is observed (entries 4 and 8,
Table 2). This fact could be attributed to the large concentration of OL leading to a high amount of –OH groups ready to attack the growing polymer, thus predominating the termination step over the propagation one.
The ATR-FTIR spectra (see
Figure S4) of the obtained LBPs at the initial OL concentrations tested at molar ratios 1 and 2 were similar and no appreciable differences were found. The only difference is just the more intense –OH stretching band at around 3500 cm
−1 related to the intensity of the –OH group band in the LBPs with higher OL content.
Concerning the thermal degradation of obtained LBPs as a function of initial OL concentration, at both molar ratios it follows a similar trend as observed by TGA (see
Figure S5). In all cases, the decomposition temperature of the LBPs is in the range from 350 to 365 °C showing those obtained at a lower BO/OH–OL molar ratio’s slightly higher decomposition temperatures as observed before. Another weight loss between 490 and 530 °C is observed for all LBPs, attributed to the OL core whose maximum weight lost temperature is ca. 530 °C (see
Figure S1).
From a kinetic point of view, looking into the Arrhenius equation the rate constant (k) in a reaction increases with the temperature and this is the case of the CROP reaction with 3-member cyclic ethers (oxiranes) where the polymerization reaction is irreversible [
26]. Nevertheless, in the case of four or more-member cyclic ethers, such as THF, even if the polymerization rate is faster by increasing the CROP temperature, the equilibrium monomer concentration is higher and consequently the monomer conversion lower, thus obtaining higher molecular weight polymers at low temperatures [
22].
Consequently, if THF is employed as the solvent, it should be taken into account that its homopropagation is closely related to the reaction temperature. The higher the temperature, the higher the monomer equilibrium concentration and consequently less THF will be able to polymerize and will be incorporated into the final polymer [
20]. Therefore, the temperature could be used to control the THF homopolymerization, its incorporation into the final LBP and, as a consequence, the LBP final properties.
The influence on LBP microstructure of three reaction temperatures were investigated, 5 °C, 25 °C and 55 °C. Experimental conditions and results are given in
Table 3. As expected, the higher the temperature the lower the THF incorporation in the LBP, 75.3 wt.%, 71.5 wt.% and 59 wt.% at 5°C, 25 °C and 55 °C, respectively. The apparent average M
w of the obtained LBPs decreases drastically when temperature increases. Concerning the crystallization and melting temperatures, the LBPs with less THF incorporated, those obtained at higher temperatures, show both lower crystallization and melting temperatures. As in the case of the other studied variables, the ATR-FTIR spectra of the obtained LBPs varying the temperature do not show significant changes (
Figure S6). The decomposition temperature showed by TGA is quite similar in the three LBPs with a maximum weight lost at ca. 350–365 °C and another weight loss at around 440 °C attributed to the core OL decomposition, with this peak being more pronounced in the LBPs with higher OL content (
Figure S7), as expected.
The oxirane specific addition flow rate (Q
s) has a strong influence on the instant oxirane concentration in the reaction medium. Consequently, the formation of the undesired COLs could be avoided depending on how fast the oxirane is incorporated into the growing chain. The incorporation of the oxirane as well as its reactivity will then depend on the Q
s but also on the oxirane nature. To evidence the importance of the Q
s on the obtention of the final wanted LBP this study was done using BO as oxirane. Three different Q
s were tested (36.5, 73.0 and 146.0 mL/OH–OL/h). CROP experimental conditions and results obtained are given in
Table 4. As can be seen, there are no major differences in terms of OL, BO and THF contents in the resulting LBPs and, consequently, there are also no significant differences in their properties.
Along the same lines, the ATR-FTIR spectra and the thermal degradation analysis (TGA) profiles of the LBPs do not show any significant difference between them as constated in
supporting information,
Figures S8 and S9, respectively.
Several oxiranes depicted in
Figure 2 were selected to investigate the influence on the LBPs microstructure of: (1) the length of the oxirane alkyl chain, PO (1 carbon atom), BO (2 carbon atoms), HO (4 carbon atoms) and OO (6 carbon atoms); and (2) the presence of a functional group pendant on the CH
3 carbon of the PO: an allylic moiety (HEO), a hydroxyl group (GLY) and a chlorine group (oxiranes). The influence of the oxirane nature on the LBP properties, namely, OH#, melting and crystallization temperatures and the apparent average M
w, is shown in
Table 5.
The aliphatic chain length was found to influence the THF monomer incorporation into the final LBP. Both the THF and OL contents on the final LBP decrease with the increase of the alkyl chain length while the oxirane content in the LBP increases with the length of the alkyl chain (entries 1–4,
Table 5). On the other hand, the crystallization temperature of the obtained LBPs decreases with the increase in aliphatic chain length up to 4 carbons while melting temperatures increase with increasing the number of carbons up to 4 into the aliphatic chain of the oxirane (entries 1–2,
Table 5). When the number of carbons in the aliphatic chain of the oxirane is 4 or 6, HO and OO, respectively, there was no significant difference found between the crystallization or melting temperatures (entries 3 and 4,
Table 5) [
23]. The presence in the oxirane of a –OH pendant group, as in the case of GLY, was found to strongly influence the melting temperature, 7.7 °C, (entry 6,
Table 5) compared to the other LBPs. The presence of a pendant double bond (HEO) or a –Cl group (oxiranes) leads to similar crystallization and melting temperatures (entries 5 and 7,
Table 5) but their melting temperatures (14.4 °C and 14.1 °C) are higher among the obtained LBPs, probably due to polar-polar interactions between polyether chains. Concerning the OH#, the LBPs obtained with the employed oxiranes show lower OH#, in the range from 58 to 79 mg KOH/g, than the starting OL as expected, except for GLY showing the corresponding LBP a OH# similar to the OL due to the presence of an extra –OH group in the LBP per GLY unit incorporated.
The presence of a double bond in oxirane HEO compared with oxirane HO (entries 3 and 5,
Table 5) clearly affects the composition of the final obtained LBP, in the presence of the double bond the content of THF increases from 72.3 to 78.8 wt.% while both OL and oxirane contents decrease in the LBP containing HEO. This fact could be explained because the double bond is withdrawing electron density on the carbon in two positions of the oxirane facilitating the THF incorporation. A similar behavior was observed with the oxiranes, an oxirane with another pendant electron-withdrawing group (–Cl). The apparent average M
w distributions of the prepared LBPs show similar molecular patterns, a main peak of higher apparent average M
w than that of the starting OL with a series of small peaks of lower apparent average M
w attributed to the formation of COLs (see
Figure S10). The LBPs with the highest apparent average M
w were obtained with HEO and oxiranes as the oxiranes, 52,900 and 35,594 g/mol, respectively, due to a higher THF incorporation into the LBP attributed to the presence of electron-withdrawing pending functionalities (entries 5 and 7,
Table 5). On the contrary, the LBP with the lowest apparent average M
w was obtained with GLY, 9373 g/mol, due to the lower THF incorporation into the final LBP (entry 6,
Table 5).
The ATR-FTIR spectra of these LBPs follow the same pattern with no significant variations but those attributed to stretching bands of C–Cl for oxiranes at 745 cm
−1 and of C=C for HEO at 910 cm
−1 and a more intense –OH stretching band for GLY (see
Figure S11). The thermal stability of the LBPs was followed by TGA and all LBPs have similar degradation profiles with two main mass loss peaks, the first one at ca. 350–380 °C attributed to the polyether chains grafted to the OL core and the second one at ca. 530 °C attributed to the degradation of the OL core (
Figure S12).