Comparative Investigation of Yield and Quality of Bio-Oil and Biochar from Pyrolysis of Woody and Non-Woody Biomasses
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Preparation
2.2. Biomass Physicochemical Properties Determination
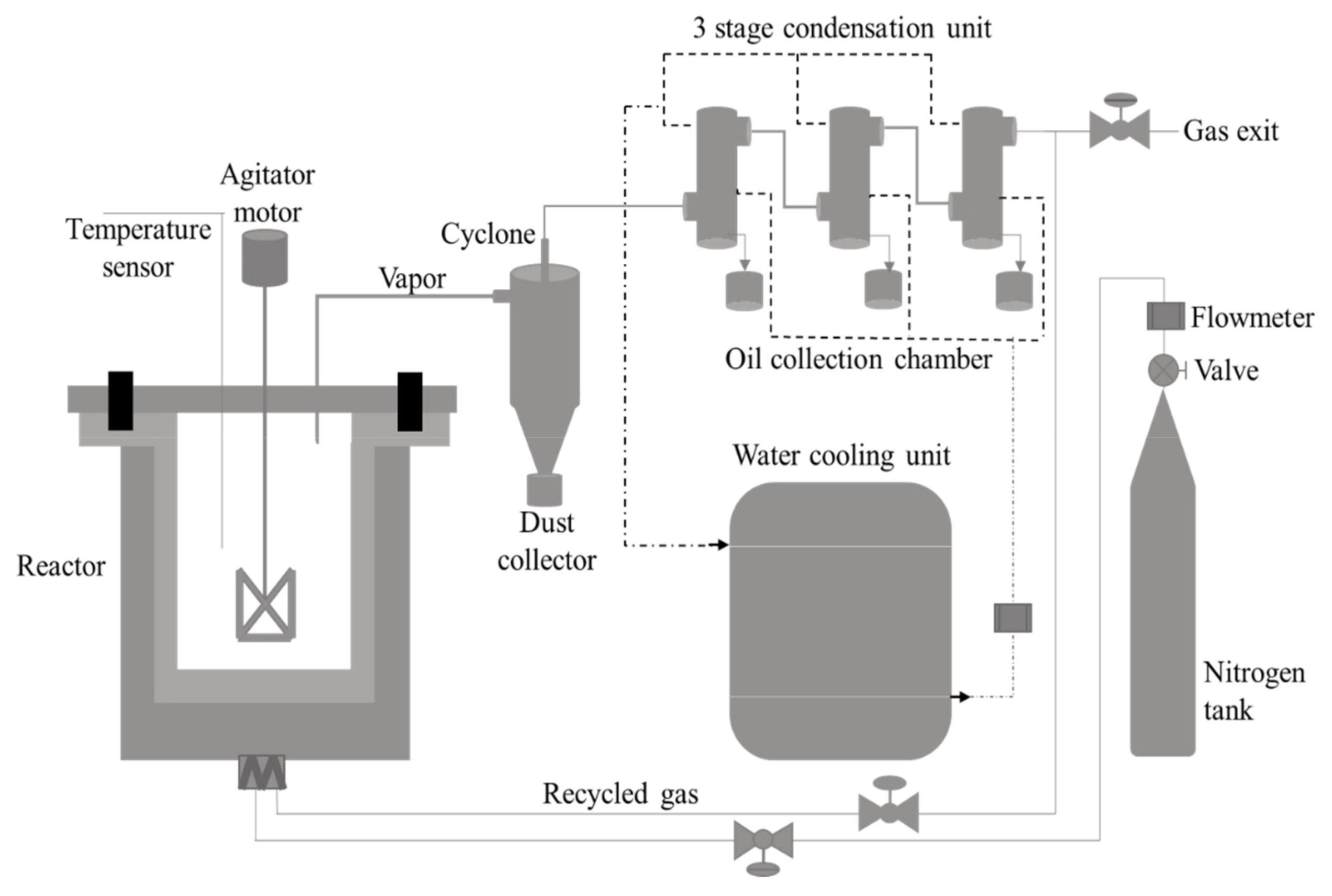
2.3. Experimental Setup
2.4. Experimental Procedure
2.5. Products Analyses
2.5.1. Determination of Liquid Product Properties
Basic Properties
Ultimate Analysis
Gas Chromatography Mass Spectrometry (GC-MS)
2.5.2. Determination of Biochar Properties
Proximate Analysis, Ultimate Analysis, and Higher Heating Value (HHV)
Carbon Sequestration and Recalcitrance
Ash Composition, Slagging and Fouling Indexes
Surface Morphology
BET Surface Area
3. Results
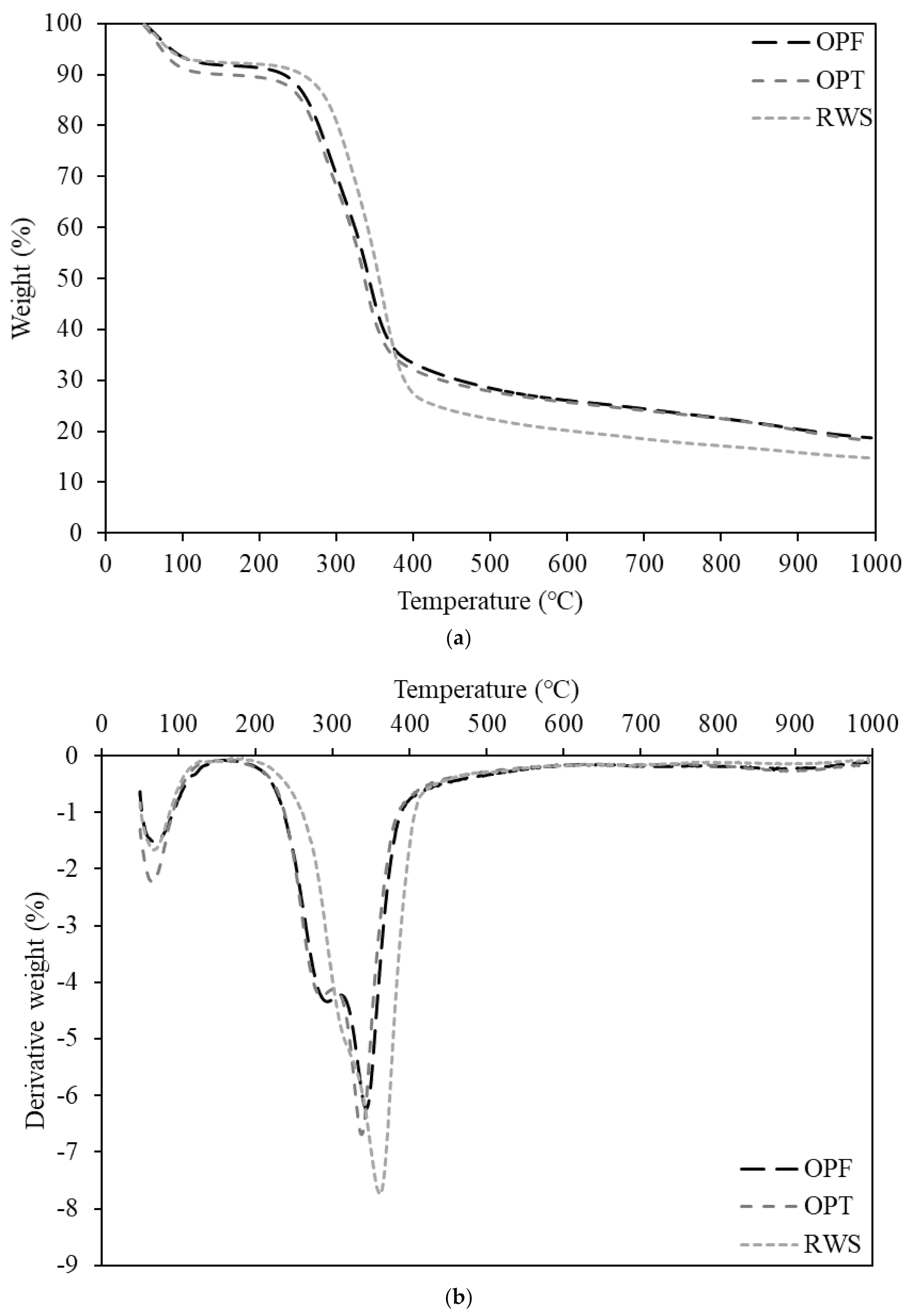
3.1. Characteristics of Raw Biomass
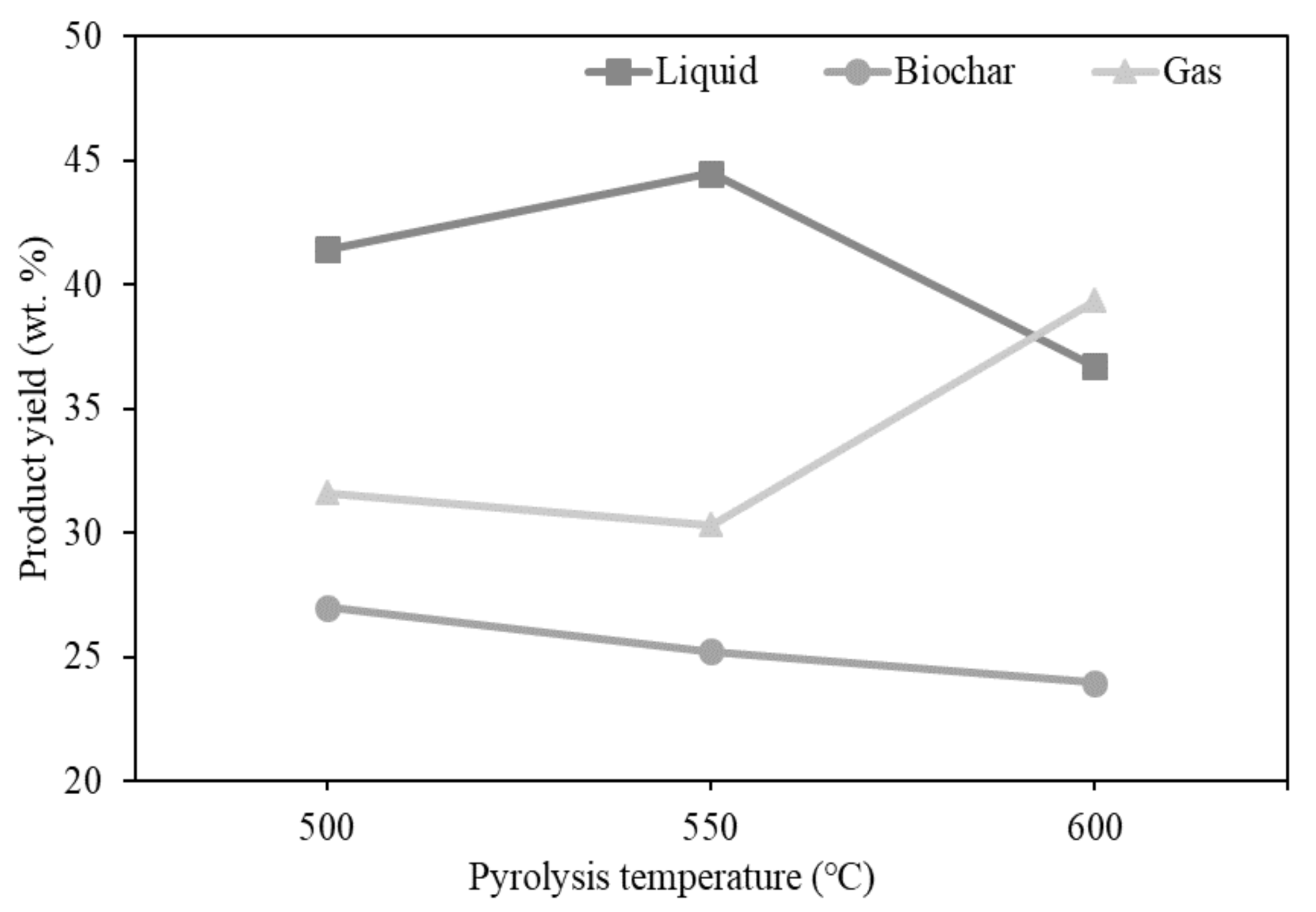
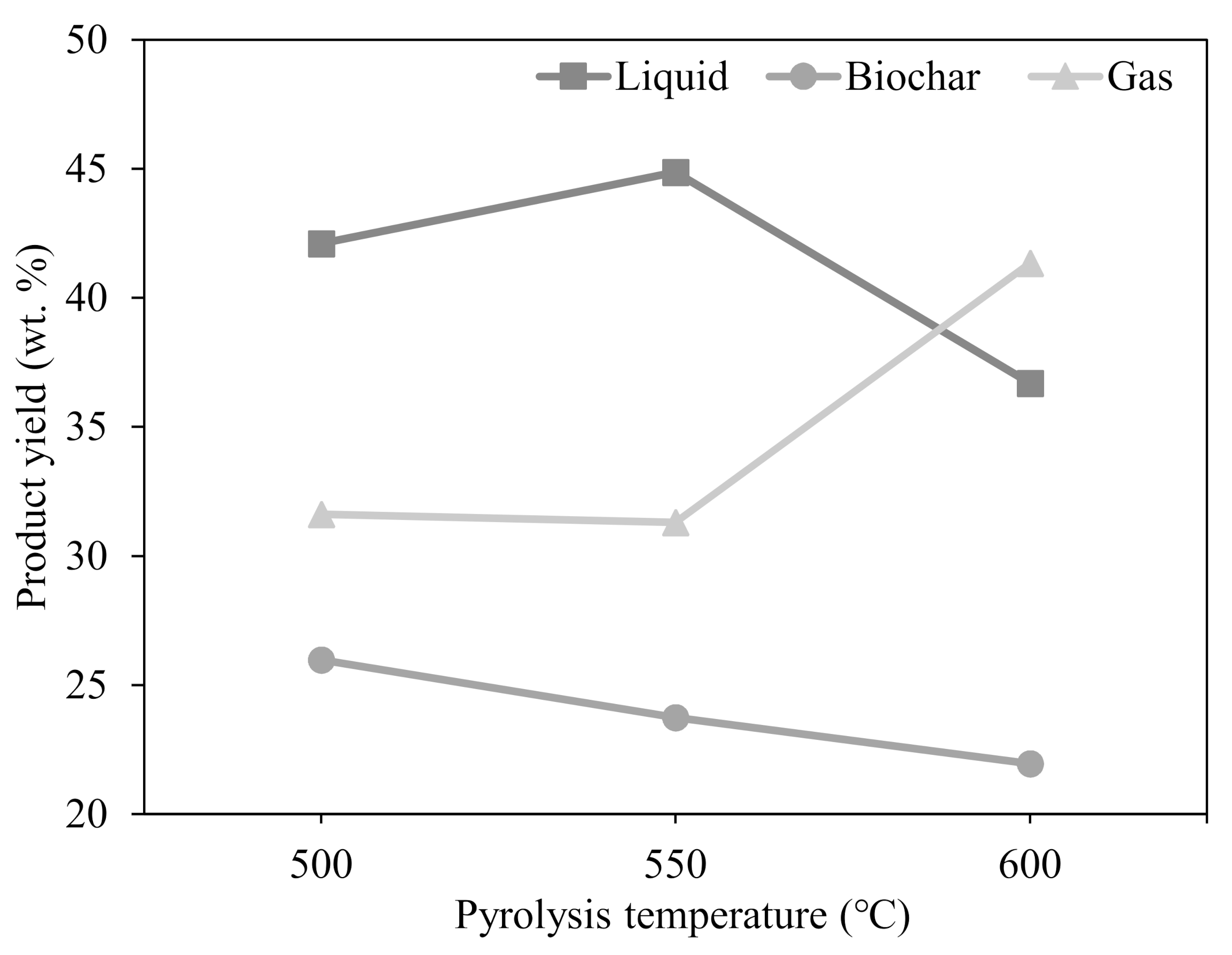
3.2. Effects of Temperature on Product Yields
3.3. Physicochemical Properties of Products
3.3.1. Liquid Product
Water Content
pH, Solids Content, Ash Content, and Density
Ultimate Analysis and Energy Content of the Liquid Product
Chemical Compounds
3.3.2. Biochar
Proximate Analysis
Ultimate Analysis and Energy Content
Surface Morphology and Specific Surface Area
Recalcitrance and Stability
Ash Composition, and Slagging and Fouling Indexes of Biochar
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- British Petroleum Company. BP Statistical Review of World Energy. 2019. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2019-full-report.pdf (accessed on 16 September 2020).
- Kumar, A.; Kumar, N.; Baredar, P.; Shukla, A. A review on biomass energy resources, potential, conversion and policy in India. Renew. Sustain. Energy Rev. 2015, 45, 530–539. [Google Scholar] [CrossRef]
- Johnsson, F.; Kjärstad, J.; Rootzén, J. The threat to climate change mitigation posed by the abundance of fossil fuels. Clim. Policy 2019, 19, 258–274. [Google Scholar] [CrossRef]
- Rathore, D.; Nizami, A.-S.; Singh, A.; Pant, D. Key issues in estimating energy and greenhouse gas savings of biofuels: Challenges and perspectives. Biofuel Res. J. 2016, 3, 380–393. [Google Scholar] [CrossRef]
- Palamanit, A.; Khongphakdi, P.; Tirawanichakul, Y.; Phusunti, N. Investigation of yields and qualities of pyrolysis products obtained from oil palm biomass using an agitated bed pyrolysis reactor. Biofuel Res. J. 2019, 6, 1065–1079. [Google Scholar] [CrossRef]
- Davis, S.J.; Lewis, N.S.; Shaner, M.; Aggarwal, S.; Arent, D.; Azevedo, I.L.; Benson, S.M.; Bradley, T.; Brouwer, J.; Chiang, Y.-M.; et al. Net-zero emissions energy systems. Science 2018, 360, eaas9793. [Google Scholar] [CrossRef]
- Office of Agricultural Economics. Agricultural Statistics of Thailand. 2018. Available online: http://www.oae.go.th/view/1/Home/EN-US (accessed on 6 September 2020).
- Yusoff, S. Renewable energy from palm oil-innovation on effective utilization of waste. J. Clean. Prod. 2006, 14, 87–93. [Google Scholar] [CrossRef]
- Shuit, S.H.; Tan, K.T.; Lee, K.T.; Kamaruddin, A.H. Oil palm biomass as a sustainable energy source: A Malaysian case study. Energy 2009, 34, 1225–1235. [Google Scholar] [CrossRef]
- Sulaiman, F.; Abdullah, N.; Gerhauser, H.; Shariff, A. An outlook of Malaysian energy, oil palm industry and its utilization of wastes as useful resources. Biomass Bioenergy 2011. [Google Scholar] [CrossRef]
- Loh, S.K. The potential of the Malaysian oil palm biomass as a renewable energy source. Energy Convers. Manag. 2017, 141, 285–298. [Google Scholar] [CrossRef]
- Azwar, E.; Mahari, W.A.W.; Chuah, J.H.; Vo, D.-V.N.; Ma, N.L.; Lam, W.H.; Lam, S.S. Transformation of biomass into carbon nanofiber for supercapacitor application. A review. Int. J. Hydrogen Energy 2018, 43, 20811–20821. [Google Scholar] [CrossRef]
- Lam, S.S.; Su, M.H.; Nam, W.L.; Thoo, D.S.; Ng, C.M.; Liew, R.K.; Yek, P.N.Y.; Ma, N.L.; Vo, D.V.N. Microwave Pyrolysis with Steam Activation in Producing Activated Carbon for Removal of Herbicides in Agricultural Surface Water. Ind. Eng. Chem. Res. 2018, 58, 695–703. [Google Scholar] [CrossRef]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Sakulkit, P.; Palamanit, A.; Dejchanchaiwong, R.; Reubroycharoen, P. Characteristics of pyrolysis products from pyrolysis and co-pyrolysis of rubber wood and oil palm trunk biomass for biofuel and value-added applications. J. Environ. Chem. Eng. 2020, 8, 104561. [Google Scholar] [CrossRef]
- Shrivastava, P.; Khongphakdi, P.; Palamanit, A.; Kumar, A.; Tekasakul, P. Investigation of physicochemical properties of oil palm biomass for evaluating potential of biofuels production via pyrolysis processes. Biomass Convers. Biorefin. 2020, 1–15. [Google Scholar] [CrossRef]
- Pawar, A.; Panwar, N.L.; Salvi, B.L. Comprehensive review on pyrolytic oil production, upgrading and its utilization. J. Mater. Cycles Waste Manag. 2020, 22, 1712–1722. [Google Scholar] [CrossRef]
- Ronsse, F.; Van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2012, 5, 104–115. [Google Scholar] [CrossRef]
- Abhijeet, P.; Swagathnath, G.; Rangabhashiyam, S.; Rajkumar, M.A.; Balasubramanian, P. Prediction of pyrolytic product composition and yield for various grass biomass feedstocks. Biomass Convers. Biorefin. 2020, 10, 663–674. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Foong, S.Y.; Liew, R.K.; Yang, Y.; Cheng, Y.W.; Yek, P.N.Y.; Mahari, W.A.W.; Lee, X.Y.; Han, C.S.; Vo, D.-V.N.; Van Le, Q.; et al. Valorization of biomass waste to engineered activated biochar by microwave pyrolysis: Progress, challenges, and future directions. Chem. Eng. J. 2020, 389, 124401. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, P.; Zhang, H.; Yuan, W. Biochar production and applications in agro and forestry systems: A review. Sci. Total Environ. 2020, 723, 137775. [Google Scholar] [CrossRef] [PubMed]
- Veiga, T.R.L.A.; Lima, J.T.; Dessimoni, A.L.D.A.; Pego, M.F.F.; Soares, J.R.; Trugilho, P.F. Different Plant Biomass Characterizations for Biochar Production. Cerne 2017, 23, 529–536. [Google Scholar] [CrossRef]
- Rueangsan, K.; Suwapaet, N.; Pattiya, A. Bio-oil production by fast pyrolysis of cassava residues in a free-fall reactor using liquid media-assisted condensation. Energy Sources Part. A Recover. Util. Environ. Eff. 2018, 40, 615–622. [Google Scholar] [CrossRef]
- Güngör, A.; Önenç, S.; Uçar, S.; Yanik, J. Comparison between the “one-step” and “two-step” catalytic pyrolysis of pine bark. J. Anal. Appl. Pyrolysis 2012, 97, 39–48. [Google Scholar] [CrossRef]
- Kilic, M.; Pütün, E.; Pütün, A.E.; Kılıç, M. Optimization of Euphorbia rigida fast pyrolysis conditions by using response surface methodology. J. Anal. Appl. Pyrolysis 2014, 110, 163–171. [Google Scholar] [CrossRef]
- Oni, B.A.; Oziegbe, O.; Olawole, O.O. Significance of biochar application to the environment and economy. Ann. Agric. Sci. 2019, 64, 222–236. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A.V. Overview of Applications of Biomass Fast Pyrolysis Oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis—A Technological Review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Biswas, B.; Pandey, N.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Pyrolysis of agricultural biomass residues: Comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour. Technol. 2017, 237, 57–63. [Google Scholar] [CrossRef]
- Mahari, W.A.W.; Zainuddin, N.F.; Chong, C.T.; Lee, C.L.; Lam, W.H.; Poh, S.C.; Lam, S.S. Conversion of waste shipping oil into diesel-like oil via microwave-assisted pyrolysis. J. Environ. Chem. Eng. 2017, 5, 5836–5842. [Google Scholar] [CrossRef]
- Kong, S.; Lam, S.S.; Yek, P.N.Y.; Liew, R.K.; Ma, N.L.; Osman, M.S.; Wong, C.C. Self-purging microwave pyrolysis: An innovative approach to convert oil palm shell into carbon-rich biochar for methylene blue adsorption. J. Chem. Technol. Biotechnol. 2019, 94, 1397–1405. [Google Scholar] [CrossRef]
- Abnisa, F.; Daud, W.M.A.W. A review on co-pyrolysis of biomass: An optional technique to obtain a high-grade pyrolysis oil. Energy Convers. Manag. 2014, 87, 71–85. [Google Scholar] [CrossRef]
- Mohammed, I.Y.; Abakr, Y.A.; Hui, J.N.X.; Alaba, P.A.; Morris, K.I.; Ibrahim, M.D. Recovery of clean energy precursors from Bambara groundnut waste via pyrolysis: Kinetics, products distribution and optimisation using response surface methodology. J. Clean. Prod. 2017, 164, 1430–1445. [Google Scholar] [CrossRef]
- Sareekam, N.; Kamarudin, S.K.; Kasmuri, N.H. Optimization of Bio Oil from Palm Oil Fronds Via Fast Pyrolysis. Indian J. Sci. Technol. 2016, 9. [Google Scholar] [CrossRef][Green Version]
- Asadullah, M.; Ab Rasid, N.S.; Kadir, S.A.S.A.; Azdarpour, A. Production and detailed characterization of bio-oil from fast pyrolysis of palm kernel shell. Biomass Bioenergy 2013, 59, 316–324. [Google Scholar] [CrossRef]
- Ruengvilairat, P.; Tanatavikorn, H.; Vitidsant, T. Bio-Oil Production by Pyrolysis of Oil Palm Empty Fruit Bunch in Nitrogen and Steam Atmospheres. J. Sustain. Bioenergy Syst. 2012, 2, 75–85. [Google Scholar] [CrossRef]
- Mao, Y.; Dong, L.; Dong, Y.; Liu, W.; Chang, J.; Yang, S.; Lv, Z.; Fan, P. Fast co-pyrolysis of biomass and lignite in a micro fluidized bed reactor analyzer. Bioresour. Technol. 2015, 181, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Imran, A.; Bramer, E.A.; Seshan, K.; Brem, G. An overview of catalysts in biomass pyrolysis for production of biofuels. Biofuel Res. J. 2018, 5, 872–885. [Google Scholar] [CrossRef]
- Chen, J.; Liang, J.; Wu, S. Lignin-rich biomass of cotton by-products for biorefineries via pyrolysis. Bioresour. Technol. 2016, 218, 402–409. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Z.; Tian, X.; Dai, L.; Jiang, L.; Zhang, S.; Wu, Q.; Wen, P.; Fu, G.; Liu, Y.; et al. Production of bio-oil from agricultural waste by using a continuous fast microwave pyrolysis system. Bioresour. Technol. 2018, 269, 162–168. [Google Scholar] [CrossRef]
- Guedes, R.E.; Luna, A.S.; Torres, A.R. Operating parameters for bio-oil production in biomass pyrolysis: A review. J. Anal. Appl. Pyrolysis 2018, 129, 134–149. [Google Scholar] [CrossRef]
- Leng, L.; Huang, H. An overview of the effect of pyrolysis process parameters on biochar stability. Bioresour. Technol. 2018, 270, 627–642. [Google Scholar] [CrossRef]
- Kurnia, J.C.; Jangam, S.V.; Akhtar, S.; Sasmito, A.; Mujumdar, A.S. Advances in biofuel production from oil palm and palm oil processing wastes: A review. Biofuel Res. J. 2016, 3, 332–346. [Google Scholar] [CrossRef]
- Guda, V.K.; Toghiani, H. Altering bio-oil composition by catalytic treatment of pinewood pyrolysis vapors over zeolites using an auger-packed bed integrated reactor system. Biofuel Res. J. 2016, 3, 448–457. [Google Scholar] [CrossRef][Green Version]
- Pattiya, A. Bio-oil production via fast pyrolysis of biomass residues from cassava plants in a fluidised-bed reactor. Bioresour. Technol. 2011, 102, 1959–1967. [Google Scholar] [CrossRef]
- Sirijanusorn, S.; Sriprateep, K.; Pattiya, A. Pyrolysis of cassava rhizome in a counter-rotating twin screw reactor unit. Bioresour. Technol. 2013, 139, 343–348. [Google Scholar] [CrossRef]
- Sukiran, M.A.; Loh, S.K.; Bakar, N.A. Production of Bio-oil from Fast Pyrolysis of Oil Palm Biomass using Fluidised Bed Reactor. J. Energy Technol. Policy 2016, 6, 52–62. [Google Scholar]
- Oasmaa, A.; Peacocke, C. A Guide to Physical Property Characterisation of Biomass-Derived Fast Pyrolysis Liquids; Technical Research Centre of Finland, Bergsmansgården: Espoo, Finland, 2001. [Google Scholar]
- Harvey, O.R.; Kuo, L.-J.; Zimmerman, A.R.; Louchouarn, P.; Amonette, J.E.; Herbert, B.E. An Index-Based Approach to Assessing Recalcitrance and Soil Carbon Sequestration Potential of Engineered Black Carbons (Biochars). Environ. Sci. Technol. 2012, 46, 1415–1421. [Google Scholar] [CrossRef]
- Tan, C.; Saritpongteeraka, K.; Kungsanant, S.; Charnnok, B.; Chaiprapat, S. Low temperature hydrothermal treatment of palm fiber fuel for simultaneous potassium removal, enhanced oil recovery and biogas production. Fuel 2018, 234, 1055–1063. [Google Scholar] [CrossRef]
- Pattiya, A.; Suttibak, S. Production of bio-oil via fast pyrolysis of agricultural residues from cassava plantations in a fluidised-bed reactor with a hot vapour filtration unit. J. Anal. Appl. Pyrolysis 2012, 95, 227–235. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 2): Conversion technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour. Technol. 2018, 251, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Widjaya, E.R.; Chen, G.; Bowtell, L.; Hills, C. Gasification of non-woody biomass: A literature review. Renew. Sustain. Energy Rev. 2018, 89, 184–193. [Google Scholar] [CrossRef]
- Pattiya, A.; Suttibak, S. Influence of a glass wool hot vapour filter on yields and properties of bio-oil derived from rapid pyrolysis of paddy residues. Bioresour. Technol. 2012, 116, 107–113. [Google Scholar] [CrossRef]
- Uzun, B.B.; Varol, E.A.; Pütün, E. Pyrolysis: A Sustainable Way from Biomass to Biofuels and Biochar. In Biochar; Amsterdam University Press: Amsterdam, The Netherlands, 2016; pp. 239–265. [Google Scholar]
- Mishra, R.K.; Mohanty, K. Pyrolysis characteristics, fuel properties, and compositional study of Madhuca longifolia seeds over metal oxide catalysts. Biomass Convers. Biorefin. 2020. [Google Scholar] [CrossRef]
- Brar, J.S.; Singh, K.B.; Wang, J.; Kumar, S. Cogasification of Coal and Biomass: A Review. Int. J. For. Res. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Quan, C.; Gao, N.; Song, Q. Pyrolysis of biomass components in a TGA and a fixed-bed reactor: Thermo-chemical behaviors, kinetics, and product characterization. J. Anal. Appl. Pyrolysis 2016, 121, 84–92. [Google Scholar] [CrossRef]
- Basu, P. Analytical Techniques. Biomass Gasif. Pyrolysis Torrefaction 2018, 479–495. [Google Scholar] [CrossRef]
- Huang, X.; Cao, J.-P.; Zhao, X.-Y.; Wang, J.-X.; Fan, X.; Zhao, Y.-P.; Wei, X.-Y. Pyrolysis kinetics of soybean straw using thermogravimetric analysis. Fuel 2016, 169, 93–98. [Google Scholar] [CrossRef]
- Das, P.; Ganesh, A.; Wangikar, P. Influence of pretreatment for deashing of sugarcane bagasse on pyrolysis products. Biomass Bioenergy 2004, 27, 445–457. [Google Scholar] [CrossRef]
- Fahmi, R.; Bridgwater, A.; Darvell, L.; Jones, J.; Yates, N.; Thain, S.; Donnison, I. The effect of alkali metals on combustion and pyrolysis of Lolium and Festuca grasses, switchgrass and willow. Fuel 2007, 86, 1560–1569. [Google Scholar] [CrossRef]
- Müller-Hagedorn, M.; Bockhorn, H.; Krebs, L.; Müller, U. A comparative kinetic study on the pyrolysis of three different wood species. J. Anal. Appl. Pyrolysis 2003, 69, 231–249. [Google Scholar] [CrossRef]
- Kabir, G.; Din, A.M.; Hameed, B. Pyrolysis of oil palm mesocarp fiber and palm frond in a slow-heating fixed-bed reactor: A comparative study. Bioresour. Technol. 2017, 241, 563–572. [Google Scholar] [CrossRef]
- Bensidhom, G.; Ben Hassen-Trabelsi, A.; Alper, K.; Sghairoun, M.; Zaafouri, K.; Trabelsi, I. Pyrolysis of Date palm waste in a fixed-bed reactor: Characterization of pyrolytic products. Bioresour. Technol. 2018, 247, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Madhu, P.; Livingston, T.S.; Kanagasabapathy, H. Flash Pyrolysis of Lemon Grass (Cymbopogon flexuosus) for Bio-oil Production in an Electrically Heated Fluidized Bed Reactor. Waste Biomass Valorization 2017, 9, 1037–1046. [Google Scholar] [CrossRef]
- Şensöz, S.; Can, M. Pyrolysis of Pine (Pinus brutia Ten.) Chips: 1. Effect of Pyrolysis Temperature and Heating Rate on the Product Yields. Energy Sources 2002, 24, 347–355. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lee, M.K.; Chang, Y.M. Fast pyrolysis of rice straw, sugarcane bagasse and coconut shell in an induction-heating reactor. J. Anal. Appl. Pyrolysis 2006, 76, 230–237. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.; Ryu, C.; Gang, K.S.; Yang, W.; Park, Y.-K.; Jung, J.; Hyun, S. Comparison of biochar properties from biomass residues produced by slow pyrolysis at 500 °C. Bioresour. Technol. 2013, 148, 196–201. [Google Scholar] [CrossRef]
- Sokoto, M.A.; Biswas, B.; Kumar, J.; Bhaskar, T. Slow pyrolysis of Defatted Seeds Cakes of African star apple and silk cotton for production of bio-oil. Renew. Energy 2020, 146, 1710–1716. [Google Scholar] [CrossRef]
- Callegari, A.; Bolognesi, S.; Cecconet, D.; Capodaglio, A.G. Production technologies, current role, and future prospects of biofuels feedstocks: A state-of-the-art review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 384–436. [Google Scholar] [CrossRef]
- Sukumar, V.; Manieniyan, V.; Senthilkumar, R.; Sivaprakasam, S. Production of bio oil from sweet lime empty fruit bunch by pyrolysis. Renew. Energy 2020, 146, 309–315. [Google Scholar] [CrossRef]
- Kappler, G.; De Souza, D.M.; Moraes, C.A.M.; Modolo, R.C.E.; Brehm, F.A.; Wander, P.R.; da Cruz Tarelho, L.A. Conversion of Lignocellulosic Biomass Through Pyrolysis to Promote a Sustainable Value Chain for Brazilian Agribusiness. In Lignocellulosic Biorefining Technologies; Wiley: Hoboken, NJ, USA, 2020; pp. 265–283. [Google Scholar]
- Azni, A.A.; Ghani, W.A.W.A.K.; Idris, A.; Ja’Afar, M.F.Z.; Salleh, M.A.M.; Ishak, N.S. Microwave-assisted pyrolysis of EFB-derived biochar as potential renewable solid fuel for power generation: Biochar versus sub-bituminous coal. Renew. Energy 2019, 142, 123–129. [Google Scholar] [CrossRef]
- Maguyon-Detras, M.C.; Migo, M.V.P.; Van Hung, N.; Gummert, M. Thermochemical Conversion of Rice Straw. In Sustainable Rice Straw Management; Springer: Cham, Switzerland, 2019; pp. 43–64. [Google Scholar]
- Pimenidou, P.; Dupont, V. Characterisation of palm empty fruit bunch (PEFB) and pinewood bio-oils and kinetics of their thermal degradation. Bioresour. Technol. 2012, 109, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, C.; Liu, R.; Fei, W.; Liu, S. Effect of hot vapor filtration on the characterization of bio-oil from rice husks with fast pyrolysis in a fluidized-bed reactor. Bioresour. Technol. 2011, 102, 6178–6185. [Google Scholar] [CrossRef] [PubMed]
- Gómez, N.; Banks, S.; Nowakowski, D.; Rosas, J.; Cara, J.; Sánchez, M.; Bridgwater, A. Effect of temperature on product performance of a high ash biomass during fast pyrolysis and its bio-oil storage evaluation. Fuel Process. Technol. 2018, 172, 97–105. [Google Scholar] [CrossRef]
- Odetoye, T.E.; Onifade, K.R.; Abubakar, M.S.; Titiloye, J.O. Pyrolysis of Parinari polyandra Benth fruit shell for bio-oil production. Biofuel Res. J. 2014, 1, 85–90. [Google Scholar] [CrossRef]
- Zhang, W.; Yuan, C.; Xu, J.; Yang, X. Beneficial synergetic effect on gas production during co-pyrolysis of sewage sludge and biomass in a vacuum reactor. Bioresour. Technol. 2015, 183, 255–258. [Google Scholar] [CrossRef]
- Bardalai, M.; Mahanta, D. Characterisation of Biochar Produced by Pyrolysis from Areca Catechu Dust. Mater. Today Proc. 2018, 5, 2089–2097. [Google Scholar] [CrossRef]
- Oasmaa, A.; Elliott, D.; Muller, S. Quality control in fast pyrolysis bio-oil production and use. Environ. Prog. Sustain. Energy 2009, 28, 404–409. [Google Scholar] [CrossRef]
- Feng, F.; Liu, W.Y.; Chen, Y.S. Study on derivatives of gambogic acid. J. China Pharm. Univ. 2005, 36, 302–305. [Google Scholar]
- Akhtar, J.; Amin, N.S. A review on operating parameters for optimum liquid oil yield in biomass pyroly-sis. Renew. Sustain. Energy Rev. 2012, 16, 5101–5109. [Google Scholar] [CrossRef]
- Alvarez, J.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Fast co-pyrolysis of sewage sludge and lignocellulosic biomass in a conical spouted bed reactor. Fuel 2015, 159, 810–818. [Google Scholar] [CrossRef]
- Suttibak, S.; Sriprateep, K.; Pattiya, A. Production of Bio-oil via Fast Pyrolysis of Cassava Rhizome in a Fluidised-Bed Reactor. Energy Procedia 2012, 14, 668–673. [Google Scholar] [CrossRef]
- Mushtaq, F.; Abdullah, T.A.T.; Mat, R.; Ani, F.N. Optimization and characterization of bio-oil produced by microwave assisted pyrolysis of oil palm shell waste biomass with microwave absorber. Bioresour. Technol. 2015, 190, 442–450. [Google Scholar] [CrossRef]
- Yoshida, T. Synthesis of polysaccharides having specific biological activities. Prog. Polym. Sci. 2001, 26, 379–441. [Google Scholar] [CrossRef]
- Thangalazhy-Gopakumar, S.; Adhikari, S.; Ravindran, H.; Gupta, R.B.; Fasina, O.; Tu, M.; Fernando, S.D. Physiochemical properties of bio-oil produced at various temperatures from pine wood using an auger reactor. Bioresour. Technol. 2010, 101, 8389–8395. [Google Scholar] [CrossRef]
- Nudri, N.A.; Bachmann, R.T.; Ghani, W.A.W.A.K.; Sum, D.N.K.; Azni, A.A. Characterization of oil palm trunk biocoal and its suitability for solid fuel applications. Biomass Convers. Biorefin. 2019, 10, 45–55. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Kalemba, I. Characterisation of renewable fuels’ torrefaction process with different instrumental techniques. Energy 2015, 87, 259–269. [Google Scholar] [CrossRef]
- Angin D Effect of pyrolysis temperature and heating rate on biochar obtained from pyrolysis of saf-flower seed press cake. Bioresour. Technol. 2013, 128, 593–597. [CrossRef]
- Tsai, W.-T.; Liu, S.-C.; Chen, H.-R.; Chang, Y.-M.; Tsai, Y.-L. Textural and chemical properties of swine-manure-derived biochar pertinent to its potential use as a soil amendment. Chemosphere 2012, 89, 198–203. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Liu, R. Effects of pyrolysis temperature and heating time on biochar obtained from the pyrolysis of straw and lignosulfonate. Bioresour. Technol. 2015, 176, 288–291. [Google Scholar] [CrossRef]
- Pereira, R.C.; Kaal, J.; Arbestain, M.C.; Lorenzo, R.P.; Aitkenhead, W.; Hedley, M.; Macias, F.D.; Hindmarsh, J.P.; Agulló, J.A.M. Contribution to characterisation of biochar to estimate the labile fraction of carbon. Org. Geochem. 2011, 42, 1331–1342. [Google Scholar] [CrossRef]
- Obernberger, I.; Thek, G. Physical characterisation and chemical composition of densified biomass fuels with regard to their combustion behaviour. Biomass Bioenergy 2004, 27, 653–669. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Hoekman, S.K.; Balasubramanian, R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Shaaban, A.; Se, S.-M.; Dimin, M.; Juoi, J.M.; Husin, M.H.M.; Mitan, N.M.M. Influence of heating temperature and holding time on biochars derived from rubber wood sawdust via slow pyrolysis. J. Anal. Appl. Pyrolysis 2014, 107, 31–39. [Google Scholar] [CrossRef]
- Lua, A.C. A detailed study of pyrolysis conditions on the production of steam-activated carbon derived from oil-palm shell and its application in phenol adsorption. Biomass Convers. Biorefin. 2019, 10, 523–533. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L. Transitional Adsorption and Partition of Nonpolar and Polar Aromatic Contaminants by Biochars of Pine Needles with Different Pyrolytic Temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef]
- Zimmerman, A.R. Abiotic and Microbial Oxidation of Laboratory-Produced Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1295–1301. [Google Scholar] [CrossRef]
- Spokas, K.; Koskinen, W.; Baker, J.; Reicosky, D. Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 2009, 77, 574–581. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Lehmann, J.; Thies, J.E.; Burton, S.D.; Engelhard, M.H. Oxidation of black carbon by biotic and abiotic processes. Org. Geochem. 2006, 37, 1477–1488. [Google Scholar] [CrossRef]
- Worasuwannarak, N.; Sonobe, T.; Tanthapanichakoon, W. Pyrolysis behaviors of rice straw, rice husk, and corncob by TG-MS technique. J. Anal. Appl. Pyrolysis 2007, 78, 265–271. [Google Scholar] [CrossRef]
- Mašek, O.; Brownsort, P.; Cross, A.; Sohi, S. Influence of production conditions on the yield and environmental stability of biochar. Fuel 2013, 103, 151–155. [Google Scholar] [CrossRef]
- Rankine I, Fairhurst TH Management of phosphorus, potassium and magnesium in mature oil palm. Better Crop Int. 1999, 13, 11.
- Wang, X.; Liu, Y.; Tan, H.; Ma, L.; Xu, T. Mechanism Research on the Development of Ash Deposits on the Heating Surface of Biomass Furnaces. Ind. Eng. Chem. Res. 2012, 51, 12984–12992. [Google Scholar] [CrossRef]
- Abdullah, N.; Sulaiman, F. The properties of the washed empty fruit bunches of oil palm. J. Phys. Sci. 2013, 24, 117–137. [Google Scholar]
- Zhu, Y.; Niu, Y.; Tan, H.; Wang, X. Short Review on the Origin and Countermeasure of Biomass Slagging in Grate Furnace. Front. Energy Res. 2014, 2. [Google Scholar] [CrossRef]
- Omar, F.N.; Mohammed, M.A.P.; Baharuddin, A.S. Effect of Silica Bodies on the Mechanical Behaviour of Oil Palm Empty Fruit Bunch Fibres. BioResources 2014, 9, 7041–7058. [Google Scholar] [CrossRef]


| Analysis | Procedure | Standard |
|---|---|---|
| Bulk density | Based on the mass and volume ratio, the bulk or apparent density of the ground biomass was determined. A cylindrical container with a particular inside diameter and volume (1000 mL) was used for this method. The samples were filled in the container up to a certain height and tapped down from top to maintain the proper top surface. The net weight was then reported for each sample, and the bulk density was determined. | - |
| Thermogravimetric analysis (TGA) | Thermogravimetric analysis (TGA) and derivative thermogravimetric analysis (DTG) were applied using a thermogravimetric analyzer (Perkin Elmer, Waltham, MA, USA). The observations were performed at temperatures ranging from 50 to 1000 °C and at a heating rate of 10 °C/min under a nitrogen gas atmosphere. | ASTM E1131 |
| Proximate analysis | A macro thermogravimetric analyzer (TGA 701, LECO, USA) was used for proximate analysis, including moisture content, volatile matter, fixed carbon content and ash content. | ASTM D7582 |
| Ultimate analysis | A CHNS/O Analyzer (FLASH 2000, Thermo Scientific, Monza, Italy) was used to analyze the basic elemental composition of each sample, including carbon (C), hydrogen (H), nitrogen (N), and sulfur (S). The oxygen content was determined by calculating a difference. | EN 15104 and ASTM D4239 |
| Lignocellulosic analysis | The acid detergent fiber (ADF), neutral detergent fiber (NDF), and acid detergent lignin (ADL) were used to determine the percentage of cellulose, hemicellulose, and lignin. Cellulose content = ADF–ADL Hemicellulose content = NDF–ADF Lignin content = ADL Extractives = 100 − (Cellulose + Hemicellulose + Lignin) | - |
| Higher heating value (HHV) | This was estimated by the following formula: where, C, H, O, N, S and Ash are from the elemental composition found by proximate analysis and ultimate analysis. | - |
| Lower heating value (LHV) | Estimated from the formula: where H is hydrogen (% wt.) on dry basis. | - |
| Property or Component | Type of Biomass | ||
|---|---|---|---|
| OPT | OPF | RWS | |
| Proximate Analysis | |||
| Moisture Content (wt. %, wet basis) | 7.07 ± 0.04 | 6.48 ± 0.03 | 7.13 ± 0.02 |
| Volatile Matter (wt. %, dry basis) | 71.14 ± 0.20 | 71.31 ± 0.30 | 75.98 ± 0.26 |
| Fixed Carbon (wt. %, dry basis) | 15.42 ± 0.22 | 16.68 ± 0.28 | 15.21 ± 0.24 |
| Ash (wt. %, dry basis) | 6.38 ± 0.02 | 5.24 ± 0.03 | 1.68 ± 0.01 |
| Ultimate Analysis (wt. %, Dry Basis) | |||
| Carbon | 45.79 ± 0.07 | 44.95 ± 0.15 | 47.55 ± 0.23 |
| Hydrogen | 6.15 ± 0.02 | 5.89 ± 0.06 | 6.22 ± 0.02 |
| Nitrogen | 1.47 ± 0.01 | 0.40 ± 0.01 | 0.32 ± 0.01 |
| Oxygen * | 46.33 ± 0.07 | 48.71 ± 0.01 | 45.91 ± 0.26 |
| Sulphur | 1.47 ± 0.01 | 0.06 ± 0.01 | 0.03 ± 0.01 |
| Lignocellulosic Component (wt. %, Dry Basis) | |||
| Cellulose | 47.81 ± 0.36 | 48.56 ± 0.28 | 56.91 ± 0.47 |
| Hemicellulose | 23.19 ± 0.14 | 22.41 ± 0.42 | 15.21 ± 0.28 |
| Lignin | 9.52 ± 0.09 | 13.15 ± 0.08 | 17.44 ± 0.16 |
| Extractives | 19.48 ± 0.16 | 15.88 ± 0.38 | 10.44 ± 0.44 |
| Element (mg/kg) | |||
| Silicon (Si) | 3560 | 1694 | 2538 |
| Iron (Fe) | 1589 | 2722 | 189 |
| Calcium (Ca) | 5432 | 7095 | 5164 |
| Magnesium (Mg) | 2789 | 2035 | 1058 |
| Sodium (Na) | 1065 | 250 | 68 |
| Potassium (K) | 9610 | 3240 | 5037 |
| Higher and Lower Heating Values (MJ/kg) | |||
| HHV | 23.22 ± 0.23 | 22.52 ± 0.41 | 23.77 ± 0.19 |
| LHV | 21.88 ± 0.18 | 21.24 ± 0.37 | 22.42 ± 0.47 |
| Other Properties | |||
| Molecular Formula | CH1.597O0.759 | CH1.559O0.813 | CH1.556O0.725 |
| H/C Molar Ratio | 1.59 | 1.55 | 1.55 |
| O/C Molar Ratio | 0.75 | 0.81 | 0.72 |
| Bulk Density (kg/m3) | 150.97 ± 0.36 | 220.68 ± 0.31 | 293.58 ± 0.29 |
| Type of Biomass | Pyrolysis Temperature (°C) | Properties of Liquid Product | ||||
|---|---|---|---|---|---|---|
| Water Content (wt. %) | Ash Content (wt. %) | pH Value | Density (kg/m3) | Solid Content (wt. %) | ||
| OPT | 500 | 70.84 ± 0.22 | 0.08 ± 0.02 | 2.91 ± 0.02 | 1011 ± 0.01 | 0.02 ± 0.01 |
| 550 | 67.99 ± 0.59 | 0.09 ± 0.01 | 2.96 ± 0.01 | 1008 ± 0.02 | 0.04 ± 0.02 | |
| 600 | 65.16 ± 0.81 | 0.09 ± 0.02 | 2.98 ± 0.01 | 1003 ± 0.01 | 0.05 ± 0.01 | |
| OPF | 500 | 67.96 ± 0.32 | 0.05 ± 0.01 | 2.67 ± 0.04 | 1013 ± 0.02 | 0.03 ± 0.02 |
| 550 | 65.11 ± 0.13 | 0.07 ± 0.02 | 2.59 ± 0.03 | 1007 ± 0.02 | 0.05 ± 0.03 | |
| 600 | 64.13 ± 0.30 | 0.08 ± 0.04 | 2.64 ± 0.02 | 1001 ± 0.01 | 0.06 ± 0.02 | |
| RWS | 500 | 59.30 ± 0.21 | 0.02 ± 0.01 | 2.15 ± 0.03 | 1012 ± 0.02 | 0.05 ± 0.05 |
| 550 | 54.80 ± 0.34 | 0.03 ± 0.01 | 2.18 ± 0.02 | 1009 ± 0.02 | 0.06 ± 0.03 | |
| 600 | 52.89 ± 0.70 | 0.05 ± 0.01 | 2.15 ± 0.02 | 1006 ± 0.01 | 0.07 ± 0.01 | |
| Biomass | Pyrolysis Temperature (°C) | Ultimate Analysis (wt. %, As Water Free Basis) | |||||
|---|---|---|---|---|---|---|---|
| Carbon | Hydrogen | Nitrogen | Sulphur | * Oxygen | ** HHV | ||
| OPT | 500 | 13.75 ± 0.20 | 6.20 ± 0.19 | 0.16 ± 0.00 | <0.01 | 12.99 ± 0.02 | 12.19 ± 0.15 |
| 550 | 14.62 ± 0.30 | 6.46 ± 0.21 | 0.23 ± 0.00 | <0.01 | 14.41 ± 0.24 | 14.28 ± 0.18 | |
| 600 | 15.37 ± 0.21 | 6.77 ± 0.17 | 0.24 ± 0.00 | ND | 16.10 ± 0.37 | 16.61 ± 0.23 | |
| OPF | 500 | 15.31 ± 0.18 | 6.40 ± 0.23 | 0.18 ± 0.18 | <0.01 | 13.48 ± 0.41 | 13.97 ± 0.19 |
| 550 | 17.30 ± 0.13 | 6.08 ± 0.12 | 0.20 ± 0.01 | <0.01 | 14.90 ± 0.24 | 16.01 ± 0.17 | |
| 600 | 17.34 ± 0.36 | 6.15 ± 0.26 | 0.12 ± 0.01 | ND | 15.83 ± 0.24 | 17.10 ± 0.09 | |
| 500 | 22.85 ± 0.25 | 6.09 ± 0.07 | 0.12 ± 0.01 | ND | 14.95 ± 0.28 | 18.53 ± 0.20 | |
| RWS | 550 | 24.64 ± 0.08 | 6.38 ± 0.21 | 0.13 ± 0.01 | ND | 17.12 ± 0.30 | 21.89 ± 0.14 |
| 600 | 26.54 ± 0.10 | 6.65 ± 0.18 | 0.12 ± 0.01 | ND | 16.74 ± 0.09 | 22.32 ± 0.13 | |
| Compounds | Peak Area (%) | ||
|---|---|---|---|
| OPT | OPF | RWS | |
| Phenol | 21.79 | 10.24 | 7.46 |
| 3-Penten-2-one, 3,4-dimethyl- | 9.87 | N/D | N/D |
| Butyric acid hydrazide | 4.19 | 2.65 | N/D |
| 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- | 3.81 | 4.12 | 5.02 |
| o-Cresol | 3.31 | 1.10 | 1.34 |
| p-Cresol | 2.83 | N/D | N/D |
| Phenol, 2-methoxy- | 2.84 | 4.40 | 4.99 |
| 1,3-Propanediamine | 2.62 | 1.86 | N/D |
| Creosol | 2.15 | 1.55 | 1.95 |
| Catechol | 2.10 | N/D | 1.68 |
| 1,2-Benzenediol, 3-methoxy- | 2.10 | 2.60 | 2.29 |
| Propanoic acid, 2-methyl-, propyl ester | 2.07 | N/D | 2.26 |
| Phenol, 4-ethyl-2-methoxy- | 1.92 | 1.88 | 2.16 |
| Phenol, 2,6-dimethoxy- | 1.83 | 11.32 | 11.72 |
| 3,5-Dimethoxy-4-hydroxytoluene | 1.59 | 3.21 | 4.23 |
| 1,3-Benzenediol | N/D | 5.12 | 5.09 |
| 2-Pentanone, 1-(2,4,6-trihydroxyphenyl) | 2.15 | 2.99 | 2.67 |
| 5-tert-Butylpyrogallol | 1.42 | 3.08 | 2.29 |
| (E)-2,6-Dimethoxy-4-(prop-1-en-1-yl) phenol | 1.42 | 1.54 | 1.62 |
| Anhydro-sugar | N/D | 8.14 | 7.79 |
| Type of Biomass | Pyrolysis Temperature (°C) | Proximate Analysis (wt. %, As Received Basis) | |||
|---|---|---|---|---|---|
| Moisture Content | Volatile Matter | Fixed Carbon | Ash Content | ||
| OPT | 500 | 3.85 ± 0.01 | 13.02 ± 0.75 | 71.69 ± 0.42 | 11.44 ± 0.87 |
| 550 | 3.74 ± 0.04 | 12.72 ± 0.25 | 72.51 ± 0.23 | 11.03 ± 0.29 | |
| 600 | 4.61 ± 0.04 | 11.41 ± 0.60 | 72.69 ± 0.65 | 11.29 ± 0.41 | |
| OPF | 500 | 4.58 ± 0.05 | 14.05 ± 1.14 | 74.06 ± 1.06 | 7.31 ± 0.30 |
| 550 | 4.13 ± 0.04 | 12.43 ± 0.18 | 75.42 ± 0.23 | 8.02 ± 0.20 | |
| 600 | 4.17 ± 0.13 | 10.25 ± 0.27 | 76.70 ± 0.74 | 8.88 ± 0.55 | |
| RWS | 500 | 3.41 ± 0.07 | 16.16 ± 0.18 | 73.29 ± 0.60 | 7.14 ± 0.43 |
| 550 | 3.76 ± 0.03 | 14.56 ± 0.28 | 74.48 ± 0.28 | 7.20 ± 0.11 | |
| 600 | 3.74 ± 0.03 | 13.06 ± 0.14 | 76.31 ± 0.27 | 6.89 ± 0.41 | |
| Type of Biomass | Pyrolysis Temperature (°C) | Ultimate Analysis (wt. %, Dry Basis) | |||||
|---|---|---|---|---|---|---|---|
| Carbon | Hydrogen | Nitrogen | Sulphur | Oxygen * | HHV | ||
| OPT | 500 | 77.53 ± 0.11 | 3.45 ± 0.13 | 0.43 ± 0.01 | 0.04 ± 0.01 | 18.63 ± 0.71 | 28.82 ± 0.52 |
| 550 | 79.35 ± 0.13 | 1.87 ± 0.05 | 1.78 ± 0.01 | 0.38 ± 0.01 | 16.65 ± 0.75 | 27.96 ± 0.38 | |
| 600 | 82.02 ± 0.14 | 1.59 ± 0.04 | 1.78 ± 0.01 | 0.40 ± 0.01 | 14.22 ± 0.42 | 28.79 ± 0.21 | |
| OPF | 500 | 75.07 ± 0.01 | 2.41 ± 0.01 | 0.43 ± 0.01 | 0.11 ± 0.01 | 22.00 ± 0.51 | 26.61 ± 0.22 |
| 550 | 76.41 ± 0.02 | 1.80 ± 0.04 | 0.40 ± 0.01 | 0.09 ± 0.01 | 21.31 ± 0.82 | 26.42 ± 0.39 | |
| 600 | 78.34 ± 0.01 | 1.91 ± 0.01 | 0.46 ± 0.01 | 0.11 ± 0.01 | 19.19 ± 0.51 | 27.43 ± 0.22 | |
| RWS | 500 | 76.65 ± 0.01 | 2.71 ± 0.06 | 0.41 ± 0.01 | 0.04 ± 0.01 | 20.21 ± 0.41 | 27.70 ± 0.23 |
| 550 | 78.59 ± 0.35 | 3.30 ± 0.02 | 0.49 ± 0.01 | 0.09 ± 0.01 | 17.63 ± 0.02 | 29.33 ± 0.02 | |
| 600 | 80.19 ± 0.02 | 2.09 ± 0.06 | 0.35 ± 0.01 | 0.03 ± 0.01 | 17.35 ± 0.23 | 28.50 ± 0.12 | |
| Name of Sample | BET Surface Area (m2/g) | Average Pore Diameter (nm) |
|---|---|---|
| OPT | 3.8034 | 99.9636 |
| OPT biochar | 0.3629 | 142.7742 |
| OPF | 3.127 | 96.4568 |
| OPF biochar | 0.3358 | 160.6406 |
| RWS | 4.7785 | 113.8839 |
| RWS biochar | 0.4854 | 175.7577 |
| Type of Biomass | Pyrolysis Temperature (°C) | Carbon Sequestration | |||
|---|---|---|---|---|---|
| Carbon (% wt.) | Fixed Carbon (wt. %) | R50 | CS (%) | ||
| OPT | 550 | 79.35 ± 0.13 | 72.51 ± 0.23 | 0.49 | 18.70 |
| OPF | 550 | 76.41 ± 0.02 | 75.42 ± 0.23 | 0.51 | 21.41 |
| RWS | 550 | 78.59 ± 0.35 | 74.48 ± 0.28 | 0.53 | 22.49 |
| Oxides (wt. %) | Biochar | ||
|---|---|---|---|
| OPT | OPF | RWS | |
| Fe2O3 | 2.991 | 4.92 | 1.124 |
| CaO | 12.625 | 14.069 | 11.361 |
| MgO | 5.155 | 5.383 | 9.096 |
| Na2O | 1.169 | 1.118 | 0.541 |
| K2O | 23.877 | 24.829 | 20.002 |
| SiO2 | 18.11 | 19.889 | 18.575 |
| Al2O3 | 0.686 | 0.643 | 0.676 |
| TiO2 | 1.411 | 0.672 | 0.16 |
| B/A | 2.27 | 2.37 | 2.17 |
| Sd | 0.09 | 0.09 | 0.38 |
| SI | 0.20 | 0.21 | 0.82 |
| FI | 56.79 | 61.57 | 44.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrivastava, P.; Kumar, A.; Tekasakul, P.; Lam, S.S.; Palamanit, A. Comparative Investigation of Yield and Quality of Bio-Oil and Biochar from Pyrolysis of Woody and Non-Woody Biomasses. Energies 2021, 14, 1092. https://doi.org/10.3390/en14041092
Shrivastava P, Kumar A, Tekasakul P, Lam SS, Palamanit A. Comparative Investigation of Yield and Quality of Bio-Oil and Biochar from Pyrolysis of Woody and Non-Woody Biomasses. Energies. 2021; 14(4):1092. https://doi.org/10.3390/en14041092
Chicago/Turabian StyleShrivastava, Pranshu, Anil Kumar, Perapong Tekasakul, Su Shiung Lam, and Arkom Palamanit. 2021. "Comparative Investigation of Yield and Quality of Bio-Oil and Biochar from Pyrolysis of Woody and Non-Woody Biomasses" Energies 14, no. 4: 1092. https://doi.org/10.3390/en14041092
APA StyleShrivastava, P., Kumar, A., Tekasakul, P., Lam, S. S., & Palamanit, A. (2021). Comparative Investigation of Yield and Quality of Bio-Oil and Biochar from Pyrolysis of Woody and Non-Woody Biomasses. Energies, 14(4), 1092. https://doi.org/10.3390/en14041092








