Chemical Strategies towards the Synthesis of Betulinic Acid and Its More Potent Antiprotozoal Analogues
Abstract
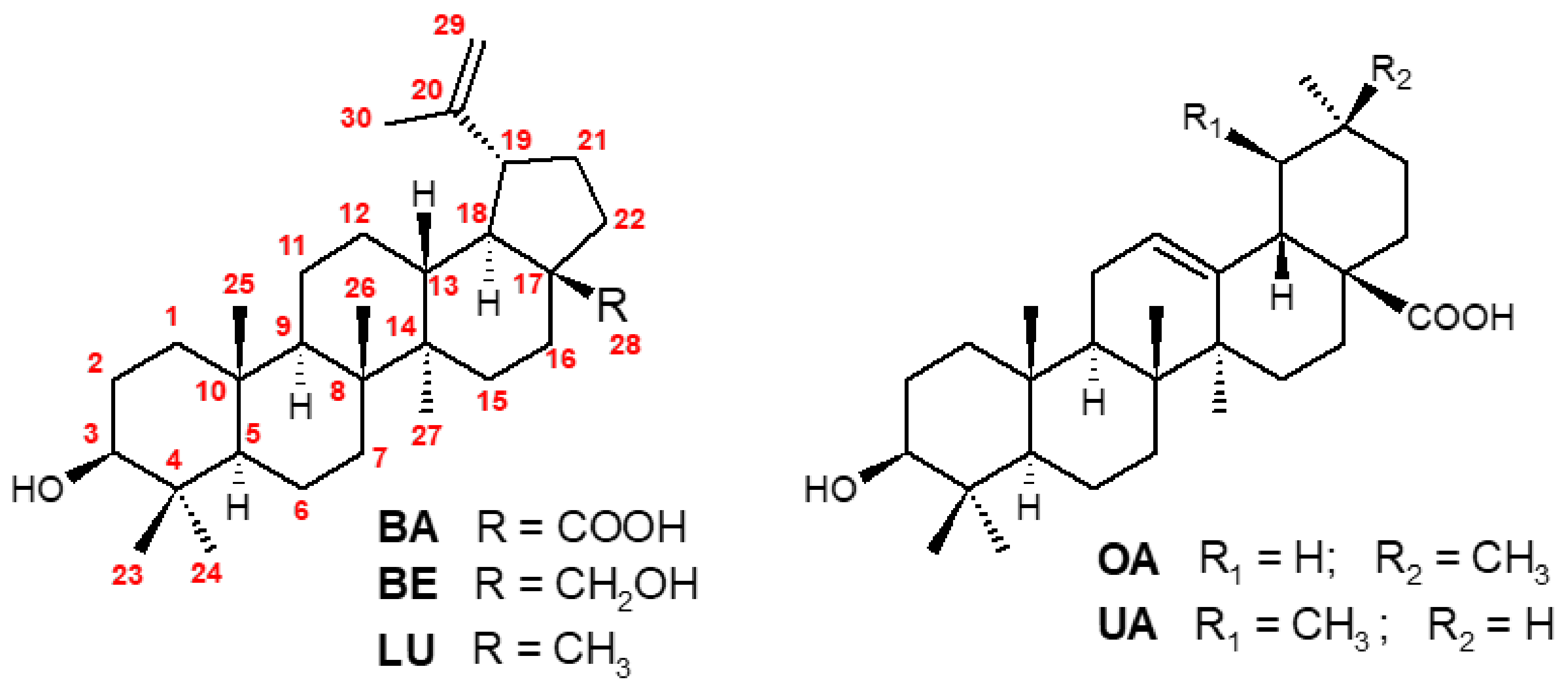
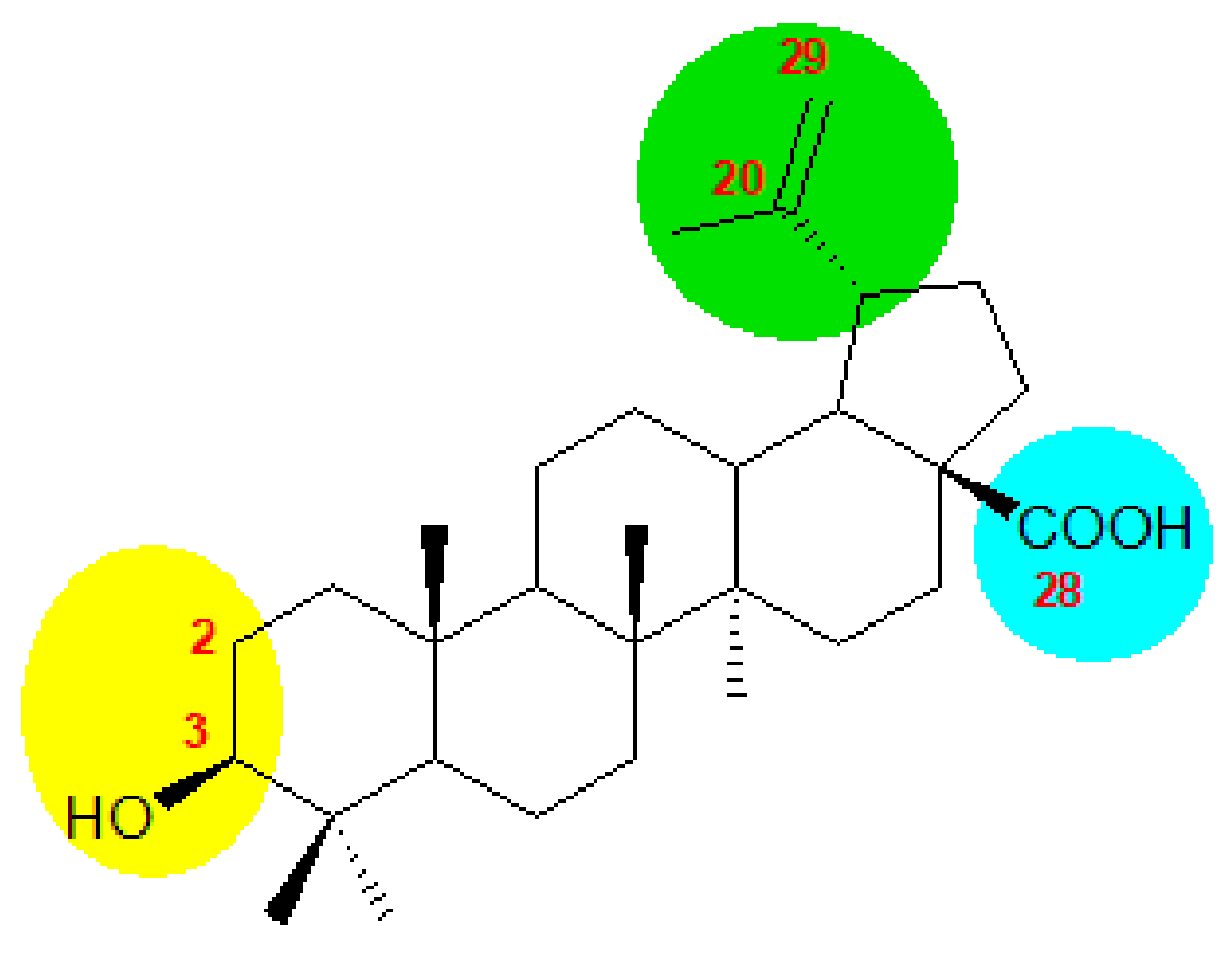
1. Introduction
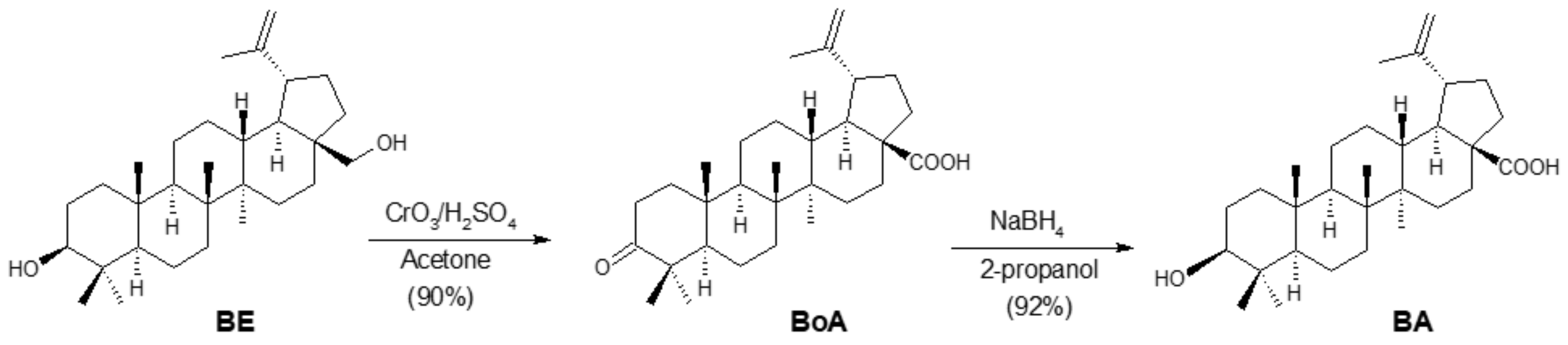
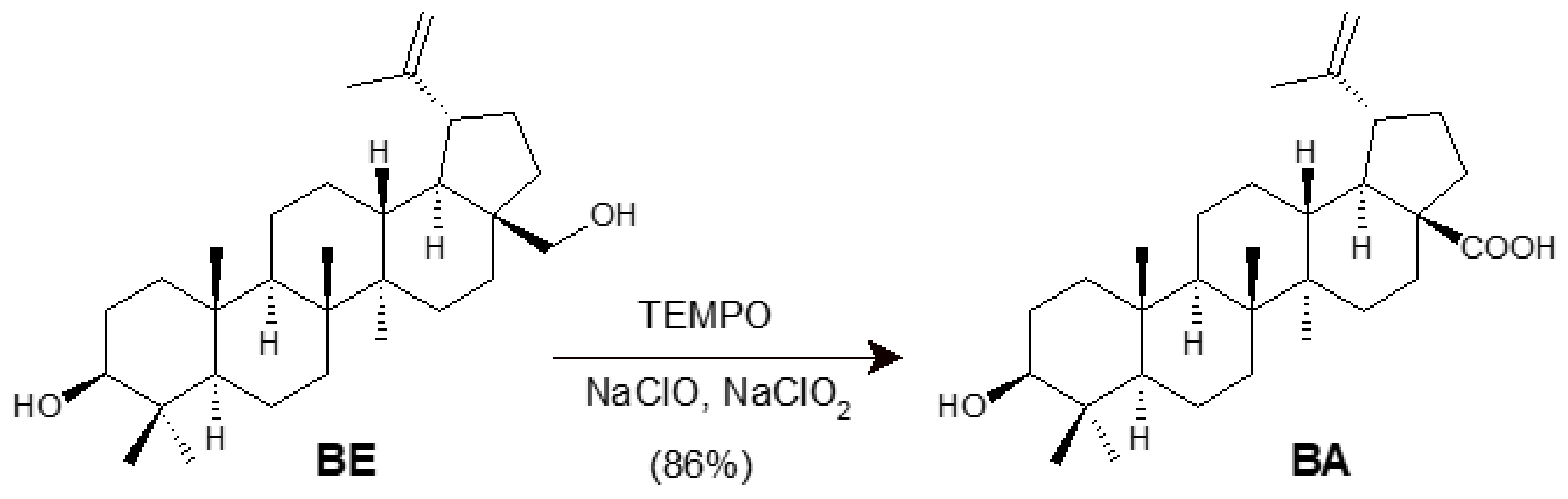
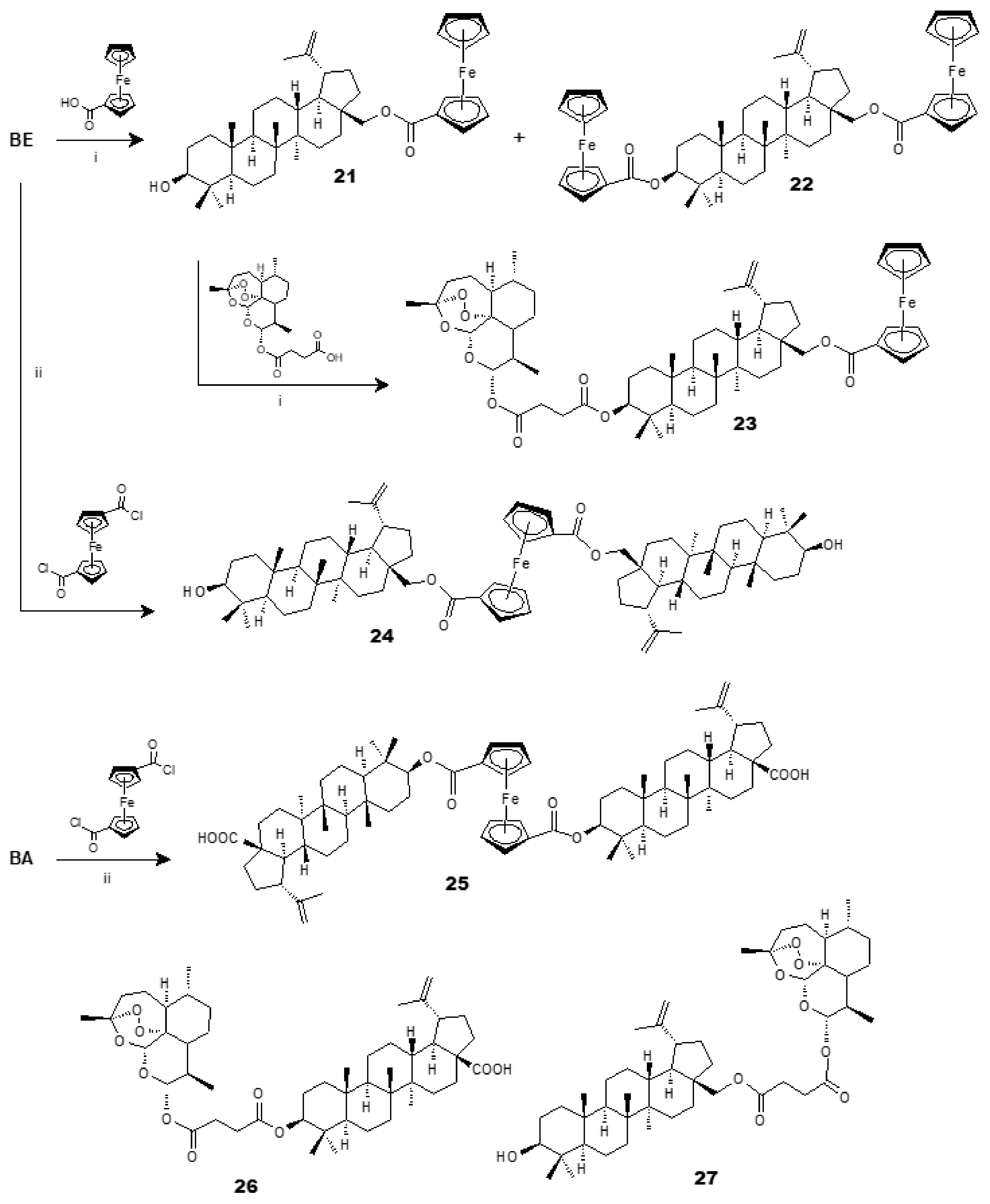
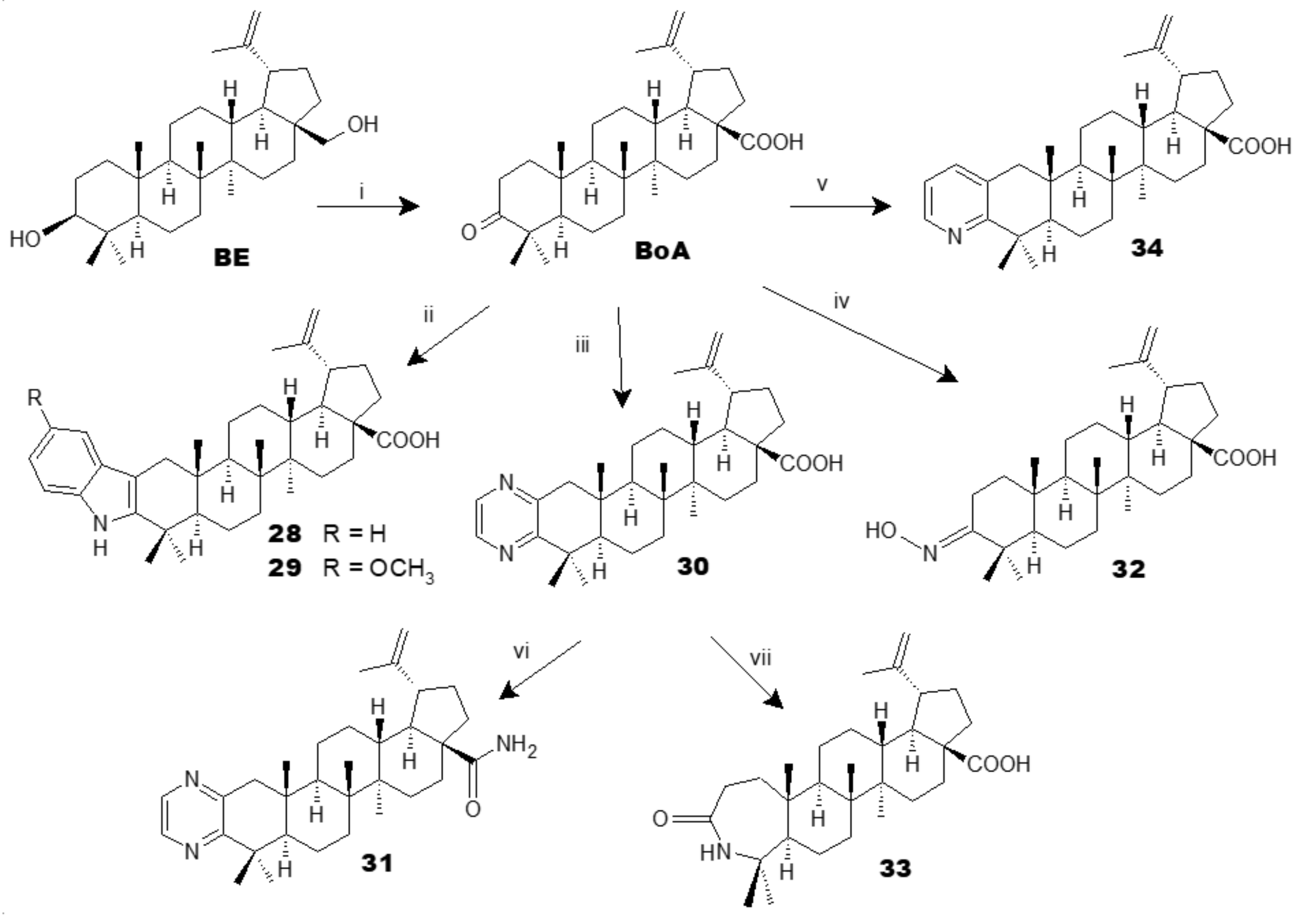
2. Synthesis of BA from Betulin
3. Synthesis of Antiprotozoal BA Analogues
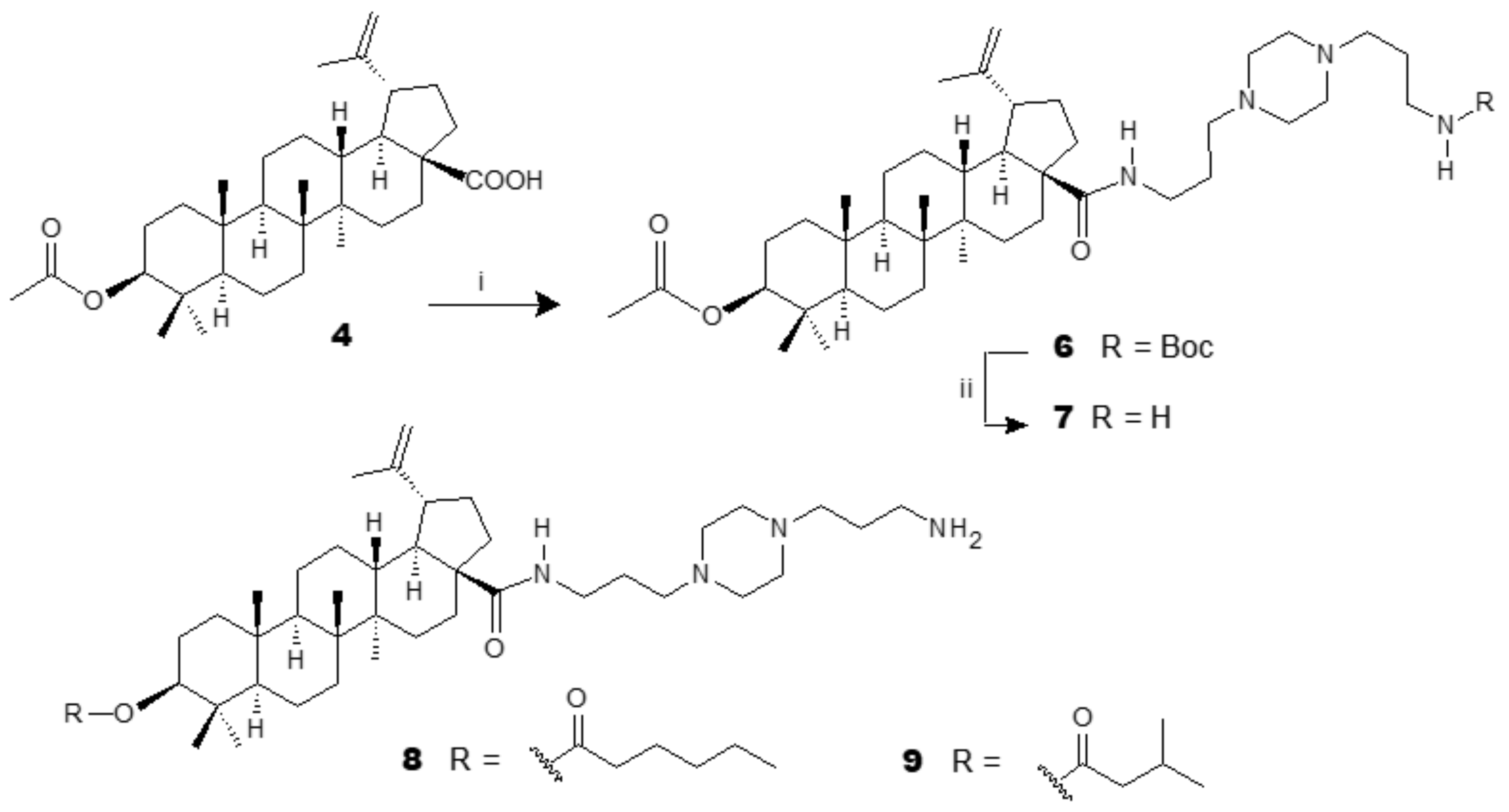
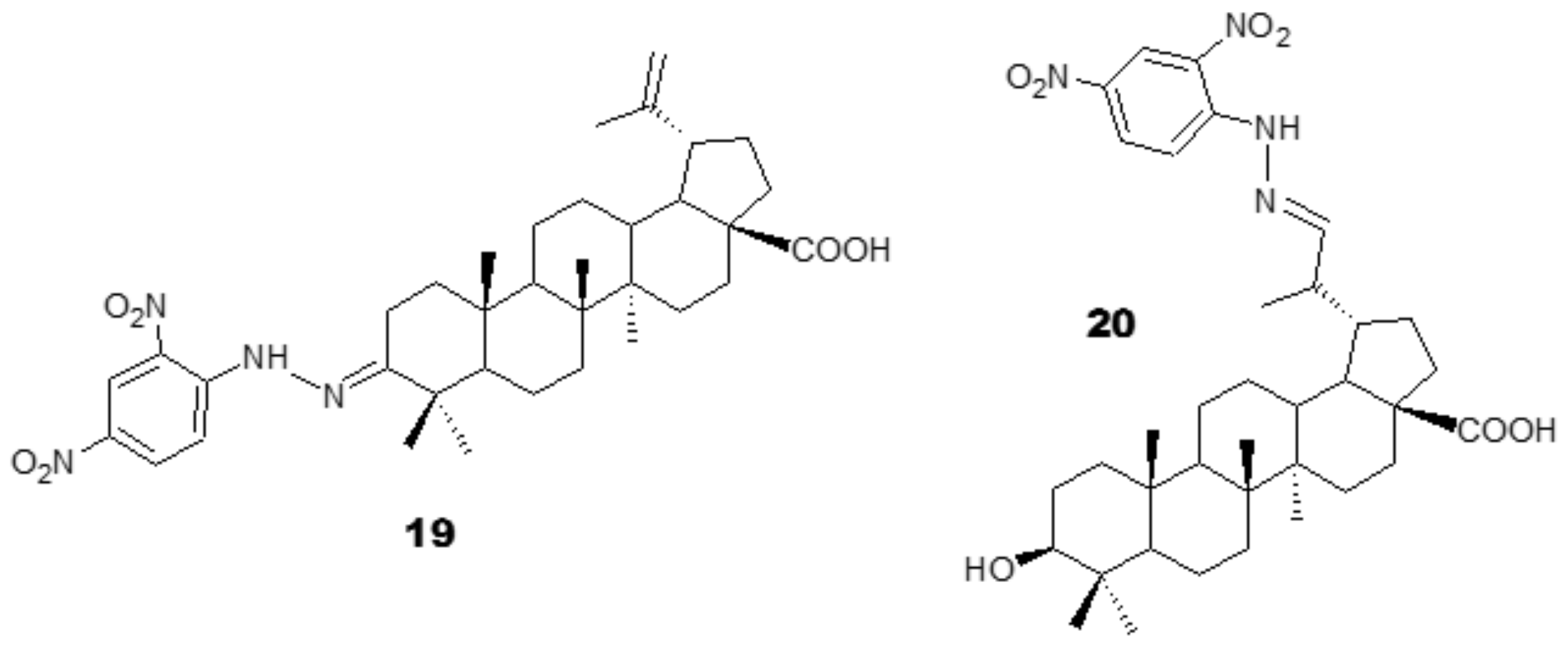
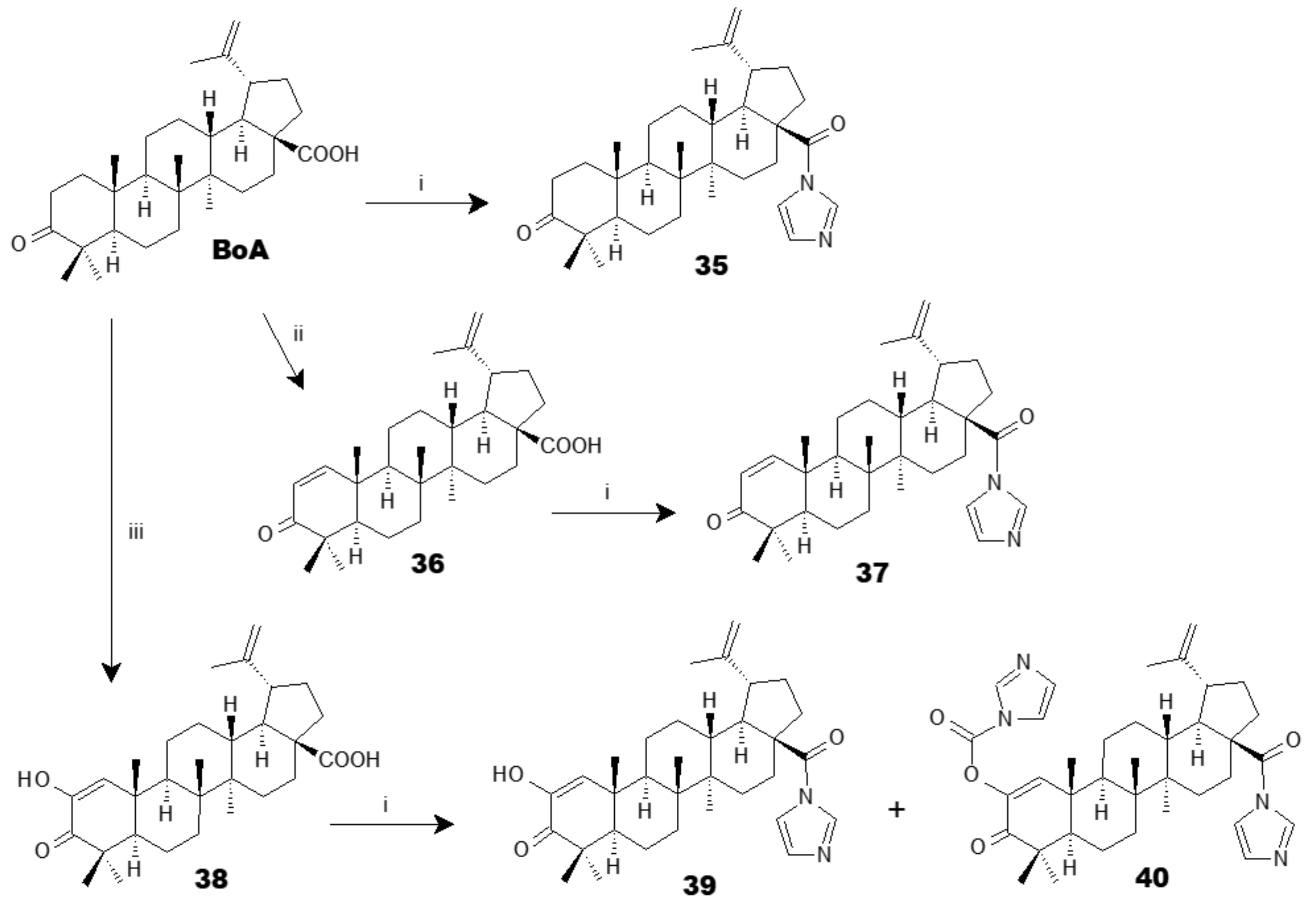
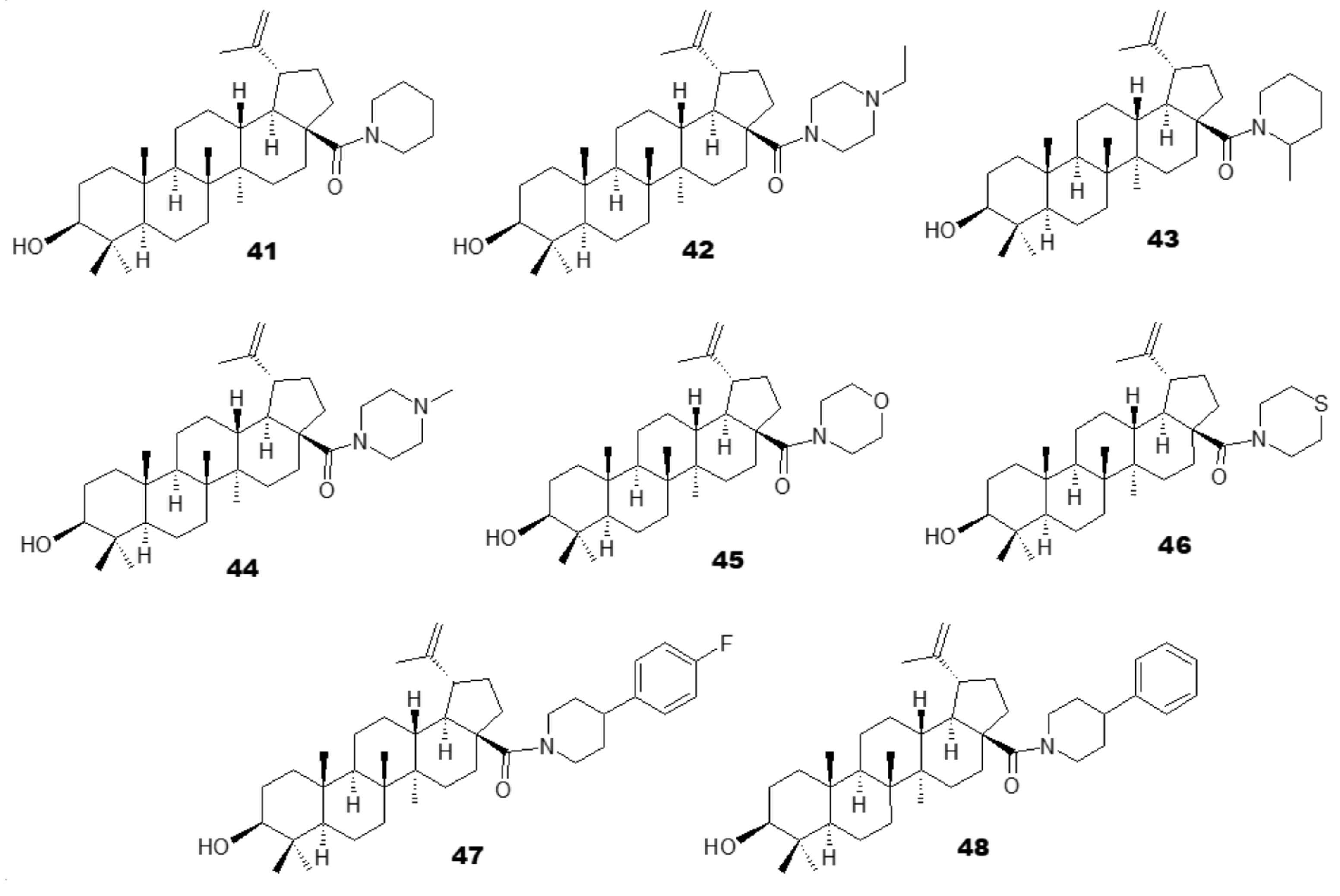
3.1. Antiplasmodial Analogues
3.2. Antileishmanial Analogues
3.3. Antitrypanosomal Analogues
4. Concluding Remarks and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robertson, A.; Soliman, G.; Owen, E.C. Polyterpenoid compounds. Part, I. Betulic acid from Cornus florida L. J. Chem. Soc. 1939, 1267–1273. [Google Scholar] [CrossRef]
- Kawaguti, R.; Kim, K.W. The constituents of Zyzyphus vulgaris Lamark var. spinosus Bunge. II. Betulinic acid. Yakugaku Zasshi 1940, 60, 595–596. [Google Scholar] [CrossRef]
- Bruckner, G.J.; Kovacs, J.; Koczka, I. Occurrence of betulinic acid in the bark of the plane tree. J. Chem. Soc. 1948, 948–951. [Google Scholar] [CrossRef]
- Ralph, C.S.; White, D.E. Betulic acid from Syncarpia laurifolia. J. Chem. Soc. 1949, 3433–3434. [Google Scholar] [CrossRef]
- Retzlaff, F. Ueber Herba gratiolae. Arch. Pharm. (Weinh.). 1902, 240, 561–568. [Google Scholar] [CrossRef]
- Zellner, J.; Fajner, R. Wachsbestandteile in Hartriegelrinden (Cornaceae). Monatshefte 1925, 46, 611–630. [Google Scholar] [CrossRef]
- Stabursvik, A. Occurrence of betulinic acid in Menyanthes trifoliata L. Acta Chem. Scand. 1953, 7, 446–447. [Google Scholar] [CrossRef]
- Pai, S.R.; Joshi, R.K. Distribution of betulinic acid in plant kingdom. Plant. Sci. Today 2014, 1, 103–107. [Google Scholar] [CrossRef]
- Pavanasasivam, G.; Sultanbawa, M.U.S. Betulinic acid in the dilleniaceae and a review of its natural distribution. Phytochemistry 1974, 13, 2002–2006. [Google Scholar] [CrossRef]
- David, J.M.; Souza, J.C.; Guedes, M.L.S.; David, J.P. Estudo fitoquimico de Davilla rugosa: Flavonóides e terpenóides. Braz. J. Pharmacogn. 2006, 16, 105–108. [Google Scholar] [CrossRef]
- Moghaddam, M.G.; Ahmad, F.B.H. Various botanical sources of betulinic acid: A review. Asian J. Chem. 2012, 24, 4843–4846. [Google Scholar]
- Fukushima, E.O.; Seki, H.; Ohyama, K.; Ono, E.; Umemoto, N.; Mizutani, M.; Saito, K.; Muranaka, T. CYP716A subfamily members are multifunctional oxidases in triterpenoid biosynthesis. Plant. Cell Physiol. 2011, 52, 2050–2061. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, J.; Li, C.; Zhang, Y. Improvement of betulinic acid biosynthesis in yeast employing multiple strategies. BMC Biotechnol. 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Trumbull, E.R.; Bianchi, E.; Eckert, D.J.; Wiedhopf, R.M.; Cole, J.R. Tumor inhibitory agents from Vauquelinia corymbosa (Rosaceae). J. Pharm. Sci. 1976, 65, 1407–1408. [Google Scholar] [CrossRef]
- Pisha, E.; Chai, H.; Lee, I.-S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.W.; Fong, H.H.S.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xu, H.; Wang, L.; Li, Y.; Sun, P.; Wu, X.; Wang, G.; Chen, W.; Ye, W. Betulinic Acid and its Derivatives as Potential Antitumor Agents. Harv. Bus. Rev. 2008, 86, 84–92. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J.; Chen, Y. Betulinic acid and the pharmacological effects of tumor suppression (Review). Mol. Med. Rep. 2016, 14, 4489–4495. [Google Scholar] [CrossRef]
- Hoenke, S.; Heise, N.V.; Kahnt, M.; Deigner, H.P.; Csuk, R. Betulinic acid derived amides are highly cytotoxic, apoptotic and selective. Eur. J. Med. Chem. 2020, 207, 112815. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, H.; Luo, X.; Zhang, Y.; Yao, X.; Zheng, X. Structure and Anti-HIV Activity of Betulinic Acid Analogues. Curr. Med. Sci. 2018, 38, 387–397. [Google Scholar] [CrossRef]
- Wu, H.F.; Morris-Natschke, S.L.; Xu, X.D.; Yang, M.H.; Cheng, Y.Y.; Yu, S.S.; Lee, K.H. Recent advances in natural anti-HIV triterpenoids and analogs. Med. Res. Rev. 2020, 40, 2339–2385. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Zheng, L.; Huang, X.; Wang, Y.; Chen, C.H.; Cheng, Y.Y.; Morris-Natschke, S.L.; Lee, K.H. Novel Betulinic Acid-Nucleoside Hybrids with Potent Anti-HIV Activity. Acs Med. Chem. Lett. 2020, 11, 2290–2293. [Google Scholar] [CrossRef]
- Ghaffari Moghaddam, M.; Bin, H.; Ahmad, F.; Samzadeh-Kermani, A. Biological Activity of Betulinic Acid: A Review. Pharmacol. Pharm. 2012, 03, 119–123. [Google Scholar] [CrossRef]
- Ríos, J.L.; Máñez, S. New Pharmacological Opportunities for Betulinic Acid. Planta Med. 2018, 84, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef]
- Saccoliti, F.; Di Santo, R.; Costi, R. Recent Advancement in the Search of Innovative Antiprotozoal Agents Targeting Trypanothione Metabolism. ChemMedChem 2020, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sarma, N.; Patouillard, E.; Cibulskis, R.E.; Arcand, J.L. The economic burden of Malaria: Revisiting the evidence. Am. J. Trop. Med. Hyg. 2019, 101, 1405–1415. [Google Scholar] [CrossRef]
- World Health Organization. Malaria. Available online: https://www.who.int/health-topics/malaria#tab=tab_1 (accessed on 15 December 2020).
- World Health Organization. Leishmaniasis. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab_1 (accessed on 15 December 2020).
- World Health Organization. Human African Trypanosomiasis (Sleeping Sickness). Available online: https://www.who.int/health-topics/human-african-trypanosomiasis#tab=tab_1 (accessed on 15 December 2020).
- World Health Organization. Chagas Disease (American Trypanosomiasis). Available online: https://www.who.int/health-topics/chagas-disease#tab=tab_1 (accessed on 15 December 2020).
- Manner, C.K.; Graef, K.M.; Dent, J. WIPO Re:Search: Catalyzing public-private partnerships to accelerate tropical disease drug discovery and development. Trop. Med. Infect. Dis. 2019, 4, 53. [Google Scholar] [CrossRef]
- Bernal, F.A.; Kaiser, M.; Wünsch, B.; Schmidt, T.J. Structure−Activity Relationships of Cinnamate Ester Analogues as Potent Antiprotozoal Agents. ChemMedChem 2020, 15, 68–78. [Google Scholar] [CrossRef]
- Chen, Q.-h.; Liu, J.; Zhang, H.-f.; He, G.-q.; Fu, M.-l. The betulinic acid production from betulin through biotransformation by fungi. Enzym. Microb. Technol. 2009, 45, 175–180. [Google Scholar] [CrossRef]
- Liu, J.; Fu, M.L.; Chen, Q.H. Biotransformation optimization of betulin into betulinic acid production catalysed by cultured Armillaria luteo-virens Sacc ZJUQH100-6 cells. J. Appl. Microbiol. 2011, 110, 90–97. [Google Scholar] [CrossRef]
- An, T.; Zha, W.; Zi, J. Biotechnological production of betulinic acid and derivatives and their applications. Appl. Microbiol. Biotechnol. 2020, 104, 3339–3348. [Google Scholar] [CrossRef] [PubMed]
- Stork, G.; Uyeo, S.; Wakamatsu, T.; Grieco, P.; Labovitz, J. The Total Synthesis of Lupeol. J. Am. Chem. Soc. 1971, 93, 4945–4947. [Google Scholar] [CrossRef]
- Surendra, K.; Corey, E.J. A short enantioselective total synthesis of the fundamental pentacyclic triterpene lupeol. J. Am. Chem. Soc. 2009, 131, 13928–13929. [Google Scholar] [CrossRef]
- Kim, D.S.H.L.; Chen, Z.; Van Nguyen, T.; Pezzuto, J.M.; Qiu, S.; Lu, Z.Z. A concise semi-synthetic approach to betulinic acid from betulin. Synth. Commun. 1997, 27, 1607–1612. [Google Scholar] [CrossRef]
- Laszczyk, M.N. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009, 75, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Csuk, R.; Schmuck, K.; Schäfer, R. A practical synthesis of betulinic acid. Tetrahedron Lett. 2006, 47, 8769–8770. [Google Scholar] [CrossRef]
- Ruzicka, L.; Lamberton, A.H.; Christie, E.W. Zur Kenntnis der Triterpene. 41. Mitteilung. Oxydation des Betulin-mono-acetats mit Chromtrioxyd zu sauren Produkten. Helv. Chim. Acta 1938, 1706–1717. [Google Scholar] [CrossRef]
- Baltina, L.A.; Flekhter, O.B.; Nigmatullina, L.R.; Boreko, E.I.; Pavlova, N.I.; Nikolaeva, S.N.; Savinova, O.V.; Tolstikov, G.A. Lupane triterpenes and derivatives with antiviral activity. Bioorganic Med. Chem. Lett. 2003, 13, 3549–3552. [Google Scholar] [CrossRef]
- Pichette, A.; Liu, H.; Roy, C.; Tanguay, S.; Simard, F.; Lavoie, S. Selective oxidation of betulin for the preparation of betulinic acid, an antitumoral compound. Synth. Commun. 2004, 34, 3925–3937. [Google Scholar] [CrossRef]
- Wickholm, N.; Alakurtti, S.; Yli-Kauhaluoma, J.; Koskimies, J. Method for preparation of betulinic acid 2013. WO2013038316A1, 21 March 2013. [Google Scholar]
- Domínguez-Carmona, D.B.; Escalante-Erosa, F.; García-Sosa, K.; Ruiz-Pinell, G.; Gutierrez-Yapu, D.; Chan-Bacab, M.J.; Giménez-Turba, A.; Peña-Rodríguez, L.M. Antiprotozoal activity of Betulinic acid derivatives. Phytomedicine 2010, 17, 379–382. [Google Scholar] [CrossRef]
- Bringmann, G.; Saeb, W.; Assi, L.A.; François, G.; Narayanan, A.S.S.; Peters, K.; Peters, E.-M. Betulinic acid: Isolation from Triphyophyllum peltatum and Ancistrocladus heyneanus, antimalarial activity, and crystal structure of the benzyl ester. Planta Med. 1997, 63, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.; Warhurst, D.; Kirby, G.; Simmonds, M. In vitro and in vivo evaluation of betulinic acid as an antimalarial. Phyther. Res. 1999, 13, 115–119. [Google Scholar] [CrossRef]
- Ziegler, H.L.; Franzyk, H.; Sairafianpour, M.; Tabatabai, M.; Tehrani, M.D.; Bagherzadeh, K.; Hägerstrand, H.; Stærk, D.; Jaroszewski, J.W. Erythrocyte membrane modifying agents and the inhibition of Plasmodium falciparum growth: Structure-activity relationships for betulinic acid analogues. Bioorganic Med. Chem. 2004, 12, 119–127. [Google Scholar] [CrossRef]
- De Sá, M.S.; Costa, J.F.O.; Krettli, A.U.; Zalis, M.G.; De Azevedo Maia, G.L.; Sette, I.M.F.; De Amorim CâMara, C.; Filho, J.M.B.; Giulietti-Harley, A.M.; Ribeiro Dos Santos, R.; et al. Antimalarial activity of betulinic acid and derivatives in vitro against Plasmodium falciparum and in vivo in P. berghei-infected mice. Parasitol. Res. 2009, 105, 275–279. [Google Scholar] [CrossRef]
- Innocente, A.M.; Silva, G.N.S.; Cruz, L.N.; Moraes, M.S.; Nakabashi, M.; Sonnet, P.; Gosmann, G.; Garcia, C.R.S.; Gnoatto, S.C.B. Synthesis and antiplasmodial activity of betulinic acid and ursolic acid analogues. Molecules 2012, 17, 12003–12014. [Google Scholar] [CrossRef]
- Diedrich, D.; Wildner, A.C.; Silveira, T.F.; Silva, G.N.S.; dos Santos, F.; da Silva, E.F.; do Canto, V.P.; Visioli, F.; Gosmann, G.; Bergold, A.M.; et al. SERCA plays a crucial role in the toxicity of a betulinic acid derivative with potential antimalarial activity. Chem. Biol. Interact. 2018, 287, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.N.S.; Schuck, D.C.; Cruz, L.N.; Moraes, M.S.; Nakabashi, M.; Gosmann, G.; Garcia, C.R.S.; Gnoatto, S.C.B. Investigation of antimalarial activity, cytotoxicity and action mechanism of piperazine derivatives of betulinic acid. Trop. Med. Int. Heal. 2015, 20, 29–39. [Google Scholar] [CrossRef]
- Da Silva, G.N.S.; Maria, N.R.G.; Schuck, D.C.; Cruz, L.N.; De Moraes, M.S.; Nakabashi, M.; Graebin, C.; Gosmann, G.; Garcia, C.R.S.; Gnoatto, S.C.B. Two series of new semisynthetic triterpene derivatives: Differences in anti-malarial activity, cytotoxicity and mechanism of action. Malar. J. 2013, 12, 1–7. [Google Scholar] [CrossRef]
- Cargnin, S.T.; Staudt, A.F.; Medeiros, P.; de Medeiros Sol Sol, D.; de Azevedo dos Santos, A.P.; Zanchi, F.B.; Gosmann, G.; Puyet, A.; Garcia Teles, C.B.; Gnoatto, S.B. Semisynthesis, cytotoxicity, antimalarial evaluation and structure-activity relationship of two series of triterpene derivatives. Bioorganic Med. Chem. Lett. 2018, 28, 265–272. [Google Scholar] [CrossRef]
- Ullah, A.; Baratto, L.C.; Paula, R.C.; Silva, L.H.V.; Soares, M.J.; Oliveira, B.H. Preparation of derivatives of betulinic acid, steviol and isosteviol and evaluation of antitrypanosomal and antimalarial activities. J. Braz. Chem. Soc. 2016, 27, 1245–1253. [Google Scholar] [CrossRef]
- Karagöz, A.Ç.; Leidenberger, M.; Hahn, F.; Hampel, F.; Friedrich, O.; Marschall, M.; Kappes, B.; Tsogoeva, S.B. Synthesis of new betulinic acid/betulin-derived dimers and hybrids with potent antimalarial and antiviral activities. Bioorganic Med. Chem. 2019, 27, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Sauvain, M.; Kunesch, N.; Poisson, J.; Gantier, J.C.; Gayral, P.; Dedet, J.P. Isolation of leishmanicidal triterpenes and lignans from the amazonian liana Doliocarpus dentatus (Dilleniaceae). Phyther. Res. 1996, 10, 1–4. [Google Scholar] [CrossRef]
- Chowdhury, A.R.; Mandal, S.; Goswami, A.; Ghosh, M.; Mandal, L.; Chakraborty, D.; Ganguly, A.; Tripathi, G.; Mukhopadhyay, S.; Bandyopadhyay, S.; et al. Dihydrobetulinic acid induces apoptosis in Leishmania donovani by targeting DNA topoisomerase I and II: Implications in antileishmanial therapy. Mol. Med. 2003, 9, 26–36. [Google Scholar] [CrossRef]
- Takahashi, M.; Fuchino, H.; Sekita, S.; Satake, M. In vitro leishmanicidal activity of some scarce natural products. Phyther. Res. 2004, 18, 573–578. [Google Scholar] [CrossRef]
- Haavikko, R.; Nasereddin, A.; Sacerdoti-Sierra, N.; Kopelyanskiy, D.; Alakurtti, S.; Tikka, M.; Jaffe, C.L.; Yli-Kauhaluoma, J. Heterocycle-fused lupane triterpenoids inhibit Leishmania donovani amastigotes. Medchemcomm 2014, 5, 445–451. [Google Scholar] [CrossRef]
- Santos, R.C.; Salvador, J.A.R.; Marín, S.; Cascante, M. Novel semisynthetic derivatives of betulin and betulinic acid with cytotoxic activity. Bioorganic Med. Chem. 2009, 17, 6241–6250. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.C.; Varandas, R.; Santos, R.C.; Santos-Rosa, M.; Alves, V.; Salvador, J.A.R. Antileishmanial activity of semisynthetic lupane triterpenoids betulin and betulinic acid derivatives: Synergistic effects with miltefosine. PLoS ONE 2014, 9, e89939. [Google Scholar] [CrossRef] [PubMed]
- Alakurtti, S.; Heiska, T.; Kiriazis, A.; Sacerdoti-Sierra, N.; Jaffe, C.L.; Yli-Kauhaluoma, J. Synthesis and anti-leishmanial activity of heterocyclic betulin derivatives. Bioorganic Med. Chem. 2010, 18, 1573–1582. [Google Scholar] [CrossRef]
- Hoet, S.; Pieters, L.; Muccioli, G.G.; Habib-Jiwan, J.L.; Opperdoes, F.R.; Quetin-Leclercq, J. Antitrypanosomal activity of triterpenoids and sterols from the leaves of Strychnos spinosa and related compounds. J. Nat. Prod. 2007, 70, 1360–1363. [Google Scholar] [CrossRef] [PubMed]
- Meira, C.S.; Barbosa-Filho, J.M.; Lanfredi-Rangel, A.; Guimarães, E.T.; Moreira, D.R.M.; Soares, M.B.P. Antiparasitic evaluation of betulinic acid derivatives reveals effective and selective anti-Trypanosoma cruzi inhibitors. Exp. Parasitol. 2016, 166, 108–115. [Google Scholar] [CrossRef]
- Borkova, L.; Hodon, J.; Urban, M. Synthesis of Betulinic Acid Derivatives with Modified A-Rings and their Application as Potential Drug Candidates. Asian J. Org. Chem. 2018, 7, 1542–1560. [Google Scholar] [CrossRef]
- Hodon, J.; Borkova, L.; Pokorny, J.; Kazakova, A.; Urban, M. Design and synthesis of pentacyclic triterpene conjugates and their use in medicinal research. Eur. J. Med. Chem. 2019, 182, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Grymel, M.; Zawojak, M.; Adamek, J. Triphenylphosphonium Analogues of Betulin and Betulinic Acid with Biological Activity: A Comprehensive Review. J. Nat. Prod. 2019, 82, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, M.; Liu, J.; Xu, T.Y.; Fang, R.S.; Chen, Q.H.; He, G.Q. A novel one-step microbial transformation of betulin to betulinic acid catalysed by Cunninghamella blakesleeana. Food Chem. 2013, 136, 73–79. [Google Scholar] [CrossRef]
- Chatterjee, P.; Kouzi, S.A.; Pezzuto, J.M.; Hamann, M.T. Biotransformation of the antimelanoma agent betulinic acid by Bacillus megaterium ATCC 13368. Appl. Environ. Microbiol. 2000, 66, 3850–3855. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Niu, Y.; Bakur, A.; Li, H.; Chen, Q. Cell-free production of pentacyclic triterpenoid compound betulinic acid from betulin by the engineered Saccharomyces cerevisiae. Molecules 2017, 22, 1075. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, S.T.; Nygren, H.; Maiorova, N.; Haavikko, R.; Alakurtti, S.; Yli-Kauhaluoma, J.; Rischer, H.; Oksman-Caldentey, K.M. Biotransformation of Cyclodextrine-Complexed Semisynthetic Betulin Derivatives by Plant Cells. Planta Med. 2018, 84, 743–748. [Google Scholar] [CrossRef]
- Cargnin, S.T.; Staudt, A.F.; Paula De Azevedo Dos Santos, A.; Gosmann, G.; Bioni, C.; Teles, G.; Gnoatto, S.B. Effective approach to semi-synthesis of lupane and ursane brominated derivatives and its effects on viability of Leishmania amazonensis. Ann. Med. Chem. Res. 2017, 3, 1–5. [Google Scholar]
- Tietze, L.F.; Bell, H.P.; Chandrasekhar, S. Natural product hybrids as new leads for drug discovery. Angew. Chem. Int. Ed. 2003, 42, 3996–4028. [Google Scholar] [CrossRef]
- Ojima, I. Modern Molecular Approaches to Drug Design and Discovery. Acc. Chem. Res. 2008, 41, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Bansal, Y.; Silakari, O. Multifunctional compounds: Smart molecules for multifactorial diseases. Eur. J. Med. Chem. 2014, 76, 31–42. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha, A.B.; Batista, R.; Castro, M.Á.; David, J.M. Chemical Strategies towards the Synthesis of Betulinic Acid and Its More Potent Antiprotozoal Analogues. Molecules 2021, 26, 1081. https://doi.org/10.3390/molecules26041081
Cunha AB, Batista R, Castro MÁ, David JM. Chemical Strategies towards the Synthesis of Betulinic Acid and Its More Potent Antiprotozoal Analogues. Molecules. 2021; 26(4):1081. https://doi.org/10.3390/molecules26041081
Chicago/Turabian StyleCunha, André Barreto, Ronan Batista, María Ángeles Castro, and Jorge Mauricio David. 2021. "Chemical Strategies towards the Synthesis of Betulinic Acid and Its More Potent Antiprotozoal Analogues" Molecules 26, no. 4: 1081. https://doi.org/10.3390/molecules26041081
APA StyleCunha, A. B., Batista, R., Castro, M. Á., & David, J. M. (2021). Chemical Strategies towards the Synthesis of Betulinic Acid and Its More Potent Antiprotozoal Analogues. Molecules, 26(4), 1081. https://doi.org/10.3390/molecules26041081






